Abstract
Local humoral and cellular immune responses modulate the inflammatory processes involved in the development of atherosclerotic lesions, as well as in the evolution of brain infarcts in stroke patients. The role of systemic adaptive immunity on the progression of such disease manifestations is less clear. In the current study, we evaluated the percentages of T helper 1 (Th1) [interleukin (IL)-2, interferon (IFN)-γ] and Th2 (IL-4, IL-10) cytokine-producing peripheral blood CD4+ and CD8+ T cells in 23 patients with a history of ischaemic stroke (IS) at the chronic stable phase of the disease (median post-stroke time 34·5 months). Seven stroke-free individuals matched for age and vascular risk factors (matched controls, MC) were collected for comparison. To measure cytokine values at baseline and after stimulation, we used a flow cytometry method of intracellular cytokine staining. Intrinsic Th1 and Th2 cytokine production in unstimulated T cells was negligible in all study participants. Following mitogenic stimulation with phorbol 12-myristate13-acetate/ionomycin, both the IS and the MC groups exhibited a similarly strong Th1 response; IL-2 production predominated in the CD4+ T cells and IFN-γ in the CD8+ T cells. However, when measuring the Th2 cytokine-production capacity post-stimulation, a significant increase in the percentage of IL-4-producing T cells was observed in the IS groups, compared with the MC group, resulting in a significantly lower ratio of IFN-γ-/IL-4-producing T cells. No such Th2 enhancement could be confirmed for the case of IL-10. We propose that in IS patients there is a systemic shift of the immune system towards Th2 responses at the late post-acute phase of stroke.
Keywords: atherosclerosis, cytokines/interleukins, flow cytometry/FACS, intracellular cytokine staining, T cells
Introduction
Mechanisms of the immune system govern the inflammatory processes which are related to stroke. Atherosclerotic transformation of intra- and/or extracranial vessels is the most common cause of acute ischaemic cerebral events, and has become recognized widely as an inflammatory disorder [1]. Moreover, the ischaemic brain lesion and its fate (i.e. either necrosis and expansion or salvation and restriction) are mediated by the actions of immunocompetent cells and their induced soluble components (e.g. cytokines) [2].
Atherosclerosis is a chronic inflammatory vascular disorder involving mechanisms of both innate and adaptive immunity [3,4]. Atherosclerotic lesions are infiltrated by T cells and macrophages from the very early to more advanced stages of the disease [5]. Specific humoral and cellular immune responses against atherosclerosis-associated antigens modulate lesion development [6,7]. Studies on both experimental animals and human patients have shown that lesional CD4+ T cells express relatively high levels of the T helper 1 (Th1) proinflammatory cytokines interferon (IFN)-γ and interleukin (IL)-2, and relatively low levels of the Th2 anti-inflammatory cytokines IL-4 and IL-10 [8]. Polarization of T cells towards a Th1 immune response has been demonstrated to be pro-atherogenic, because proinflammatory cytokines promote macrophages and other immune cells to ultimately exhibit their cytotoxic properties [9]. Recently, it has been suggested that genetic variations of inflammatory cytokines may predispose to either strong or weak immune responses, and could therefore contribute to the risk of cerebrovascular atherosclerotic complications (reviewed in [10]).
Cerebral infarction generates a local inflammatory response [11], which is responsible for ischaemia/reperfusion injury and mediates potently both detrimental and beneficial tissue complications [12]. The T cell repertoire in evolving stroke lesions includes predominantly CD8+ cytotoxic T cells as well as CD4+ Th1 cells and, to a lesser extent, CD4+ anti-inflammatory Th2 cells [13]. Lesional Th1 cells secrete proinflammatory cytokines [e.g. IL-2, IL-12, IFN-γ, tumour necrosis factor (TNF)-α], known to sustain the deleterious tissue effects of an otherwise normally induced immune response. In mice, intracerebroventricular injection of IL-1β or TNF-α enhances brain ischaemic damage [14,15], and such action can be counteracted effectively by molecular antagonism of these cytokines [16,17].
The implication of circulating T cells in the pathogenesis of atherosclerosis has been demonstrated directly in the hypercholesterolaemic apoE knock-out (apoE°) mouse model. In their study, Zhou et al. reported that transfer of CD4+ T cells to atherosclerotic apoE°/SCID mice aggravated their disease [18]. Others showed that transfer of B, but not of T, cells in splenectomized apoE° mice conferred protection against atherosclerosis advance [19]. Interestingly, under experimental conditions, a shift in T cell polarization from a Th1 to a Th2 cytokine-production profile, was found to be equally beneficial to a net T cell depletion [20,21]. A systemic suppression of the proinflammatory cytokines IL-12, IFN-γ and IL-18 or a systemic increase of the anti-inflammatory cytokines IL-4 and IL-10 were reported to have a beneficial effect on disease progression [20,22–24]. Furthermore, naturally arising CD4+, CD25+ T regulatory cells seem to be powerful inhibitors of atherosclerosis in several mouse models [25].
Shortly after focal cerebral ischaemia, the peripheral immune system is being activated massively [26]. Offner et al. reported a pronounced increase in splenic CD4+ forkhead box P3+ (T regulatory cells) soon after experimental stroke, resulting presumably in inhibition of CD4+ and CD8+ Th1 cells [27]. The reciprocal systemic Th2 enhancement of the immune response has been suggested to be a beneficial process in neuroprotection and axonal regeneration following acute central nervous system injuries in animal models [28]. However, the price for this protective Th2 inflammatory shift is increased susceptibility to infection, mainly because of impaired cell-mediated immunity [28]. Stroke-induced immunodeficiency has also been described in animals, and has been associated with increased bacterial infections in the early post-stroke phase [29].
In the present study, we investigated the intracellular inflammatory potential of systemic T cells, with the intention of elucidating whether such a Th2 immune shift could be replicated in the case of human stroke. In particular, the Th1/Th2 cytokine profiles of peripheral blood CD4+ and CD8+ T cells were determined in subjects with a history of ischaemic stroke (IS) during the stable chronic phase of the disease, and compared with the Th1/Th2 profiles of age-matched patients with similar vascular risk factors but without a history of stroke.
Materials and methods
Study populations
Twenty-three subjects with a previous IS, 21 (91%) male, mean age 63·9 ± 8·9 years, were studied. All patients had been admitted through the accident and emergency department in the neurology ward of Patras University Hospital (PUH), Greece. Seven individuals of the same age group, four (57%) male, mean age 66·0 ± 9·1 years, with similar vascular risk factors but with a past medical history free of stroke were recruited and used as a matched control group (MC). In Table 1 their demographic data and vascular risk factors of MC are compared with those of the IS group.
Table 1.
Demographic data and vascular risk factors of ischaemic stroke (IS) and matched control (MC) groups.
| IS group n = 23 | MC group n = 7 | P* | |
|---|---|---|---|
| Age [mean (± s.d.)] | 63·9 (±8·9) | 66·0 (±9·1) | 0·36† |
| Sex (female) | 2 (9%) | 3 (43%) | 0·07 |
| Arterial hypertension | 14 (78%) | 6 (60%) | 0·4 |
| Atrial fibrillation | 1 (4%) | 0 | 1·0 |
| Ischaemic heart disease | 4 (17%) | 1 (14%) | 1·0 |
| Hypercholesterolaemia (taking statins) | 6 (26%) | 0 | 0·29 |
| Current smoking | 11 (48%) | 2 (29%) | 0·42 |
| Anti-platelet treatment | |||
| None | 2 (9%) | 4 (57%) | |
| Aspirine | 10 (43%) | 2 (29%) | 1·0‡ |
| Ticlopidine/clopidogrel | 11 (48%) | 1 (14%) | |
Fisher's exact two-tailed P-value for the comparison of proportions.
Mann–Whitney test for two groups.
P-value refers to the comparison between patients and controls that were on anti-platelet treatment.
The diagnosis of stroke was made according to World Health Organization criteria [30]. A brain imaging scan (at least one computed tomography or magnetic resonance imaging within 3 days from admission to hospital) was carried out in all cases. Patients with intracerebral or subarachnoid haemorrhage, diabetes mellitus or autoimmune disorders, as well as with history of any acute or chronic infectious disease, were excluded. Exclusion criteria for both patients and controls also included trauma, surgery or any febrile episode (T > 36·8°C), either documented or simply reported, during the last 2 weeks prior to blood sampling.
Demographic data and risk factors were recorded on admission. Vascular risk factors (past medical history of arterial hypertension, atrial fibrillation, ischaemic heart disease, diabetes mellitus, hypercholesterolaemia) were considered present according to standard clinical criteria, and were reconfirmed during their follow-up appointments at the stroke out-patient clinic. Smoking history was coded as never, previous (at least 6 months after smoking cessation) and current.
The median time between the acute event and our investigation was 34·5 months (range 8–48 months). All patients had a good long-term functional outcome (<2 in the modified Oxford Handicap scale (Rankin) [31]). Six of the IS patients (26%) had hypercholesterolaemia and were receiving treatment with statins (ISs+), whereas none of the remaining 17 patients were on any lipid-modifying regimen (ISs–). At the time of the investigation, 10 of the patients (43%) were receiving aspirin, 11 (48%) clopidogrel and two (9%) were not on any anti-platelet treatment because of contraindications.
Blood samples were collected in the morning, under fasting conditions. All subjects enrolled in the study gave their informed consent. The study was approved by the ethical and scientific committees of PUH.
Intracellular cytokine staining
The method used for intracellular cytokine determination in CD4+ and CD8+ T cells is a modification of previously described methods [32,33]. Heparinized blood was diluted 1 : 1 with RPMI-1640 culture medium and stimulated with 25 ng/ml phorbol 12-myristate13-acetate (PMA) and 1 μM ionomycin in the presence of 10 μg/ml brefeldin A (BFA) (all from Sigma Chemical Co., St Louis, MO, USA) in 1 ml total volume. Unstimulated samples were prepared in parallel, with the addition of BFA. After a 5-h culture, 100 μl of sample were incubated with the following mouse anti-human antibodies: the monoclonal antibody (mAb) CD8 ± phycoerythrin (PE)-cyanin 5·1 (clone B9·11; Immunotech, Marseille, France) and the polyclonal antibody CD4 ± fluorescein isothiocyanate (clone Leu3a-3b; Becton Dickinson Immunocytometry System, San Jose, CA, USA). After completion of the surface staining, the red blood cells were lysed, the samples permeabilized by incubation with Cytofix/Cytoperm Buffer (PharMingen, San Diego, CA, USA) and incubated with mAbs against different cytokines. The mAbs used were: mouse anti-human IFN-γ-PE (clone 45·15), anti-IL-2-PE (clone N7·48A), anti-IL-4-PE (clone 4D9) from Immunotech and rat anti-human IL-10-PE (clone JES3–9D7) (PharMingen). The appropriate isotype-MC were also used. The mAb CD69-PE (clone TP1·55·3, Immunotech) was used as a marker of early cell activation [34]. After stimulation with PMA/ionomycin, the intracellular expression of the CD69 antigen was >95% in both CD4+ and CD8+ T cell subsets.
The analysis was performed by a Coulter Epics-XL-MCL flow cytometer (Coulter, Miami, FL, USA). The lymphocytic population was defined initially in a forward- and side-scatter (SSC) gate. A second gate (logical) was then created around the CD4+ cells in a CD4+versus SSC dot plot, or around the CD8+bright cells in a CD8+versus SSC dot plot. The percentages of cytokine-producing cells were calculated in the total CD4+ or CD8+ population and not in the total T cell population. The ratio of Th1-/Th2-producing cells was calculated as the percentage of IFN-γ-producing divided by the percentage of IL-4-producing cells.
Statistical analysis
The data were checked with Kolmogorov–Smirnov's test and found to be distributed normally; therefore, parametric tests were used to compare values. For variables in Table 1, Mann–Whitney U-test for two samples was used in non-parametric comparisons, and χ2with Fisher's exact two-tailed P-value in the comparison of proportions. In analysis of the study results, initial comparisons between the three groups were performed by one-way analysis of variance test. When significance arose, a post hoc analysis of dyads with Tukey's test for multiple comparisons was used for pairwise analyses. The spss software, version 14, was used for the analysis. Data were expressed as mean ± standard deviation and P-values < 0·05 were considered statistically significant.
Results
We studied the intracytoplasmic expression of Th1 and Th2 cytokines in peripheral blood CD4+ and CD8+ T cells in stroke patients at the chronic stable phase of the disease, as well as in MC. Because treatment with statins has been related previously to a Th2 shift in experimental studies [35–38], the IS group was divided further into patients receiving and those not receiving statin therapy (ISs+ and ISs- respectively).
As shown in Table 2, the constitutive intracytoplasmic production of the Th1 and Th2 cytokines studied in unstimulated CD4+and CD8+ peripheral blood T cells was negligible, and did not differ significantly between IS patients (IS ± statins) and MC. Following stimulation with PMA/ionomycin, a strong Th1 profile emerged in all study groups (Table 3 and Fig. 1). In particular, IL-2 production predominated in the CD4+T cell group and IFN-γ in the CD8+ T cell group.
Table 2.
Unstimulated T helper 1 (Th1)- and Th2-producing CD4+ and CD8+ T-cells from peripheral blood of ischaemic stroke patients and controls.
| Cytokine | Group | CD4+ T cells (%) | P-value* | CD8+ T cells (%) | P-value* |
|---|---|---|---|---|---|
| IFN-γ | MC | 0·06 ± 0·04 | n.s. | 0·09 ± 0·05 | n.s. |
| ISs- | 0·08 ± 0·04 | 0·11 ± 0·04 | |||
| ISs+ | 0·10 ± 0·05 | 0·09 ± 0·02 | |||
| IL-2 | MC | 0·18 ± 0·10 | n.s. | 0·17 ± 0·10 | n.s. |
| ISs- | 0·15 ± 0·09 | 0·18 ± 0·15 | |||
| ISs+ | 0·16 ± 0·09 | 0·17 ± 0·11 | |||
| IL-4 | MC | 0·09 ± 0·04 | n.s. | 0·08 ± 0·03 | n.s. |
| ISs- | 0·12 ± 0·06 | 0·16 ± 0·15 | |||
| ISs+ | 0·13 ± 0·07 | 0·17 ± 0·14 | |||
| IL-10 | MC | 0·27 ± 0·14 | n.s. | 0·43 ± 0·22 | n.s. |
| ISs- | 0·22 ± 0·19 | 0·34 ± 0·31 | |||
| ISs+ | 0·29 ± 0·29 | 0·29 ± 0·23 |
Whole blood was cultured for 5 h in plain medium in the presence of brefeldin A. The values of cytokine-producing cells were calculated as the percentage of CD4+ and CD8+ peripheral T cells. Data are expressed as mean ± standard deviation.
P-values refer to the comparison between the three study groups (MC, ISs -, ISs+) using one-way analysis of variance test. IL, interleukin; IFN, interferon; ISs-, ischaemic stroke patients not receiving statins (n = 17); ISs+, IS patients receiving statins (n = 6); MC, matched controls (n = 7); n.s., not statistically significant.
Table 3.
Induced T helper 1 (Th1) and Th2 cytokine-producing CD4+ and CD8+ T cells from peripheral blood of ischaemic stroke patients and controls, following mitogenic stimulation.
| Cytokine | Group | CD4+ T cells (%) | P-value* | CD8+ T cells (%) | P-value* |
|---|---|---|---|---|---|
| IFN-γ | MC | 29·10 ± 9·14 | n.s. | 63·97 ± 17·59 | n.s. |
| ISs- | 25·72 ± 10·59 | 64·79 ± 15·83 | |||
| ISs+ | 33·23 ± 9·29 | 68·16 ± 14·71 | |||
| IL-2 | MC | 63·77 ± 11·26 | n.s. | 21·50 ± 8·47 | n.s. |
| ISs- | 63·32 ± 12·34 | 20·07 ± 7·79 | |||
| ISs+ | 63·61 ± 10·93 | 24·64 ± 3·62 | |||
| IL-4 | MC | 0·23 ± 0·18 | 0·17 ± 0·10 | ||
| ISs- | 1·09 ± 0·95 | 0·027 | 0·90 ± 0·97 | n.s. | |
| ISs+ | 1·04 ± 0·39 | 0·028 | 1·33 ± 1·15 | 0·015 | |
| IL-10 | MC | 0·41 ± 0·21 | n.s. | 0·48 ± 0·28 | n.s. |
| ISs- | 0·37 ± 0·22 | 0·28 ± 0·20 | |||
| ISs+ | 0·56 ± 0·36 | 0·30 ± 0·11 |
Whole blood samples were cultured with phorbol 12-myristate13-acetate/ionomycin for 5 h in the presence of brefeldin A. The values of cytokine-producing cells were calculated as the percentage of CD4+ and CD8+ peripheral T cell populations. The data are expressed as mean ± standard deviation.
The post-hoc Tukey test was used to compare MC with ISs- and MC with ISs+. IL, interleukin; IFN, interferon; ISs-, ischaemic stroke patients not receiving statins (n = 17); ISs+, IS patients receiving statins (n = 6); MC, matched controls (n = 7); n.s., not statistically significant.
Fig. 1.

A representative fluorescence activated cell sorter experiment showing the percentage of interferon-γ- and interleukin-4-producing CD4+ and CD8+ T cells in an ischaemic stroke patient not receiving statins (middle), an ischaemic stroke patients receiving statins (bottom) and a matched control (top), following stimulation with phorbol 12-myristate13-acetate/ionomycin for 5 h in the presence of brefeldin A. MC, match control; IS, ischaemic stroke.
The percentages of Th2 cytokine-producing CD4+ or CD8+ T cells remained relatively low after mitogenic stimulation in all study groups (Table 3 and Fig. 1). However, a significant increase was observed in the percentage of IL-4-producing CD4+T cells of the IS patients ± statins and IL-4-producing CD8+ T cells of the IS patients ± statins, compared with the MC group (Fig. 1). The percentage of the IL-4-producing CD8+ T cells of the IS patients ± statins was also higher compared with the MC group, although this increase did not reach statistical significance. The above differences remained significant even when patients were divided into two treatment groups, receiving either aspirin (n = 10) or clopidogrel (n = 11), and then compared separately with the MC group (data not shown). In contrast, the above observation could not be replicated for the case of IL-10-producing clones. The percentages of IL-10-producing CD4+ and CD8+ T cells did not show any statistical difference between the two study groups and, interestingly, the values for the CD8+ groups were almost identical to those obtained in their unstimulated counterparts (Table 3).
Figure 2 depicts the ratios of the percentages of IFN-γ-/IL-4-producing cells. In IS patients ± statins, the ratio of IFN-γ-/IL-4-producing unstimulated CD4+ or CD8+ T cells did not differ from that of the control group. In contrast, following mitogenic stimulation, the ratios of IFN-γ-/IL-4-producing CD4+ or CD8+ cells were significantly lower in IS patients ± statins than in the MC group.
Fig. 2.
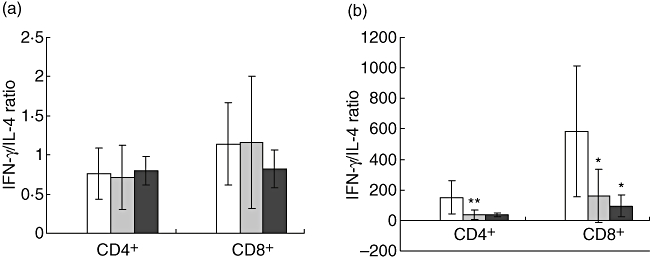
Ratio of interferon (IFN)-γ/interleukin (IL)-4-producing CD4+ and CD8+ T cells in ischaemic stroke patients not receiving statins (Iss-) (n = 17), in IS patients receiving statins (ISs+) (n = 6), and in matched controls (MC) (n = 7) (a) without stimulation and (b) following stimulation with phorbol 12-myristate13-acetate/ionomycin. Results are depicted as mean ± standard deviation. □ = MC, ○ = ISs-, □ = ISs+. Statistical significance is shown as *P < 0·05.
Discussion
In the present study, we investigated the Th1/Th2 cytokine profiles of peripheral blood CD4+ and CD8+ T cells of IS patients, long after the acute ischaemic event (median time 34·5 months), using a flow cytometry technique that allows for the determination of cytokine production at a single cell level.
No constitutive peripheral T cell activation was observed, either in the IS patients ± statin therapy or in those stroke-free subjects with risk factors for cerebrovascular complications (MC group). Upon stimulation with thepharmacological agents PMA and ionomycin, which can bypass proximal signalling pathways [39,40], the percentage of IFN-γ-producing CD8+ T cells was significantly higher both in the IS ± statins and in the MC group. These findings indicate that vascular risk factors result in long-term priming of CD8+ T cells to express proinflammatory cytokines. However, patients having experienced a cerebral ischaemic event showed a delayed, significant increase of CD4+ and CD8+ IL-4-producing T cells post-stimulation. This finding could not be replicated for IL-10-producing T cells, and suggested a long-term shift towards a systemic Th2 response attributed only to IL-4. The increase of CD4+ and CD8+ IL-4-producing T cells post-stimulation remained significant even after adjustment for anti-platelet treatment (aspirin or clopidogrel), implying that our result could not be attributed to the anti-inflammatory effects of aspirin. A consequent reduction in the ratio of IFN-γ-/IL-4-producing CD4+ and CD8+ T cells was established for the IS group, as opposed to the MC group.
Interleukin-4 is a pleiotropic anti-inflammatory cytokine [41], known to be responsible for the differentiation and stability of T cells into the Th2 phenotype [42]. In their work, Torres et al. argued that Th1 cytokine production is the default expression pattern after stimulation of naive CD4+ T cells, which precedes Th2 cytokine release [43]. Their results also showed that expression of Th2 genes (i.e. Th2 cytokine shift) depended markedly on the presence of both IFN-γ and IL-4 [43]. Furthermore, others have suggested that specialization to a specific lymphokine-producing pattern may not be completed until relatively long into the differentiation history of a T cell clone [44]. The above studies are in consistence with our results which demonstrate that, in the post-acute phase of a cerebrovascular event, T cells express a reduced IFN-γ/IL-4 ratio after stimulation. This reduction is due to an increase in the IL-4 production potential of these cells, and not in IFN-γ suppression, implying a clear skew in the polarization of the immune response towards the Th2 type.
A shift towards a Th2 response in atherosclerotic animal models has been associated with a favourable outcome [20,21,29]. Overexpression of IL-10-producing T cells in transgenic mice resulted in a significant reduction of advanced atherosclerotic lesions, an effect that was associated with a parallel decrease in IFN-γ production by monocytes, and a shift in the subclass of immunoglobulin against oxidized low-density lipoproteins from IgG2 to IgG1 [20]. Administration of pentoxyllin, a known inhibitor of Th1 differentiation pathway, resulted in a reduction of atherosclerotic lesions, with a parallel increase in IL-10- but not of IFN-γ-producing T cells [19].
In their study, Pelidou et al. demonstrated an increase of the IL-10-secreting monocytes in stroke patients as early as 10 days after the acute event, although they were not able to do so for the case of IL-4 [45]. IL-10 is another anti-inflammatory cytokine, whose cerebrospinal and serum levels have been found to peak around day 7 after stroke onset and then to decrease gradually [46–48]. This suspected early contribution of IL-10 to neuroprotection in stroke has also been demonstrated by Vila et al., who showed that low acute IL-10 but not IL-4 levels correlate with worse clinical outcome after stroke [49]. These studies indicate that IL-4 has negligible effects in the early phase of an acute cerebrovascular event but, critically, mediates a Th2 immune response shift during the late course of the disease. Moreover, a previous study in patients with unstable angina, using methods similar to ours, showed a marked increase of IFN-γ-producing CD4+ and CD8+ T cells in the early phase of unstable angina, followed by a significant increase in IL-4-producing CD4+ T cells during the waning of the symptoms [50]; nevertheless, this shift towards a Th2 immune response was only transient (lasting for 6–8 weeks). This latter result, along with that demonstrated by Pelidou et al. [45] and Vila et al. [49], seems not to contradict our findings of an IL-4-mediated Th2 immune shift during the advanced phase after an acute cerebrovascular event (range 8–48 months, median 34·5 months).
To our knowledge, this is the first study on human stroke to imply that there could be a protective, IL-4-mediated, delayed post-stroke modification of the peripheral T cells towards the anti-inflammatory Th2 immune response. This Th1/Th2 profile rearrangement may be due to CD4+ regulatory T cells that expand upon activation, and together with the plaque-reactive memory CD8+ T cells counteract the default proinflammatory Th1 systemic response after in vivo antigenic stimulation. Clearly, the identification of the degree of T regulatory cells' involvement in the Th2 immune shift would have increased the significance of our results considerably. Such an assessment should be the object of a newly designed study on stroke survivors. Furthermore, one could argue that our IS patients were studied a relatively long time after stroke and had a good performance status; therefore, they belonged to a group with a favourable outcome. This fact remains supportive of our argument for a protective immune response, initiated during the acute ischaemic event and lasting for some time, rather than introducing a selection bias per se.
Whether a systemic shift towards a Th2 response in these patients has long-term effects in systemic immunity (immunosuppression), and therefore in the occurrence of immune-mediated diseases, is still unknown. A low IFN-γ/IL-4 ratio has been reported in an experimental mouse model of severe cerebral ischaemia; in this study, the authors linked the decreased IFN-γ/IL-4 ratio with impaired IFN-γ production, which resulted in a compromised anti-bacterial defence, and related their findings to lower survival and decreased resistance to infections after stroke [29]. Through its immunoregualtory role, IL-4 participates in exacerbation of asthmatic attacks [51], containment of parasitic gastrointestinal infections [52], prevention of diabetes in mice [53] and spontaneous recovery from experimental autoimmune encephalomyelitis [54]. The possible post-stroke effect of the above-demonstrated Th2-skewed immune response in atopic, infectious and autoimmune diseases remains to be clarified.
Another interesting point that emerged from this study is that treatment with statins does not influence the Th1/Th2 profiles of the IS patients. Statins, apart from their cholesterol-reducing effect, have been shown to exert pleiotropic beneficial properties, including lowering the levels of inflammatory markers of innate immunity such as hsCRP [55]. Moreover, it has been demonstrated that statins promote Th2, and reduce Th1, immune responses under experimental conditions [35–38]. However, their effect on circulating T cells in stroke patients has not been investigated. Our results suggest that post-stroke T cell priming to an increased IL-4 expression post-stimulation may be a more robust reason for a Th2 immune bias, rather than treatment with statins itself.
In conclusion, patients in the post-acute phase of stroke seem to have acquired circulating T cells of both CD4+ and CD8+ phenotype, which upon activation can secrete IL-4 readily to counterbalance a hazardous IFN-γ-dominated systemic immune response. Further studies will elucidate if the IL-4-secreting cells belong to a T regulatory subset and, if so, whether their circulation levels could be associated with emerging clinical manifestations and stroke outcome.
Acknowledgments
This study has been supported partly by Karatheodoris grant B.705 from the University of Patras.
References
- 1.Ross R. Atherosclerosis − an inflammatory disease. N Engl J Med. 1999;340:115–26. doi: 10.1056/NEJM199901143400207. [DOI] [PubMed] [Google Scholar]
- 2.Tan KT, Lip GY, Blann AD. Post-stroke inflammatory response: effects of stroke evolution and outcome. Curr Atheroscler Rep. 2003;5:245–51. doi: 10.1007/s11883-003-0046-6. [DOI] [PubMed] [Google Scholar]
- 3.Hansson GK, Libby P, Schonbeck U, Yan ZQ. Innate and adaptive immunity in the pathogenesis of atherosclerosis. Circ Res. 2002;91:281–91. doi: 10.1161/01.res.0000029784.15893.10. [DOI] [PubMed] [Google Scholar]
- 4.Lindsberg PJ, Grau AJ. Inflammation and infections as risk factors for ischaemic stroke. Stroke. 2003;34:2518–32. doi: 10.1161/01.STR.0000089015.51603.CC. [DOI] [PubMed] [Google Scholar]
- 5.van der Wal AC, Das PK, Bentz van de Berg D, et al. Atherosclerotic lesions in humans. In situ immunophenotypic analysis suggesting an immune mediated response. Lab Invest. 1989;61:166–70. [PubMed] [Google Scholar]
- 6.Stemme S, Faber B, Holm J, et al. T lymphocytes from human atherosclerotic plaques recognize oxidized low density lipoprotein. Proc Natl Acad Sci USA. 1995;92:3893–7. doi: 10.1073/pnas.92.9.3893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zhou X, Caligiuri G, Hamsten A, et al. LDL immunization induces T-cell-dependent antibody formation and protection against atherosclerosis. Arterioscler Thromb Vasc Biol. 2001;21:108–14. doi: 10.1161/01.atv.21.1.108. [DOI] [PubMed] [Google Scholar]
- 8.Frostegard J, Ulfgren AK, Nyberg P, et al. Cytokine expression in advanced human atherosclerotic plaques: dominance of pro-inflammatory (Th1) and macrophage-stimulating cytokines. Atherosclerosis. 1999;145:33–43. doi: 10.1016/s0021-9150(99)00011-8. [DOI] [PubMed] [Google Scholar]
- 9.Hansson GK. Immune mechanisms in atherosclerosis. Arterioscler Thromb Vasc Biol. 2001;21:1876–90. doi: 10.1161/hq1201.100220. [DOI] [PubMed] [Google Scholar]
- 10.Marousi S, Ellul J, Karakantza M. Genetic polymorphisms of Type-1 and Type-2 inflammatory cytokines in ischaemic stroke in press. Vasc Dis Prev. 2008;5 in press. [Google Scholar]
- 11.Wang Q, Tang XN, Yenari MA. The inflammatory response in stroke. J Neuroimmunol. 2007;184:53–68. doi: 10.1016/j.jneuroim.2006.11.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Stoll G, Jander S, Schroeter M. Detrimental and beneficial effects of injury-induced inflammation and cytokine expression in the nervous system. Adv Exp Med Biol. 2002;513:87–113. doi: 10.1007/978-1-4615-0123-7_3. [DOI] [PubMed] [Google Scholar]
- 13.Arumugam TV, Granger DN, Mattson MP. Stroke and T-cells. Neuromol Med. 2005;7:229–42. doi: 10.1385/NMM:7:3:229. [DOI] [PubMed] [Google Scholar]
- 14.Stroemer RP, Rothwell NJ. Exacerbation of ischaemic brain damage by localized striatal injection of interleukin-1beta in the rat. J Cereb Blood Flow Metab. 1998;18:833–9. doi: 10.1097/00004647-199808000-00003. [DOI] [PubMed] [Google Scholar]
- 15.Barone FC, Arvin B, White RF, et al. Tumor necrosis factor-alpha. A mediator of focal ischaemic brain injury. Stroke. 1997;28:1233–44. doi: 10.1161/01.str.28.6.1233. [DOI] [PubMed] [Google Scholar]
- 16.Relton JK, Martin D, Thompson RC, Russell DA. Peripheral administration of interleukin-1 receptor antagonist inhibits brain damage after focal cerebral ischaemia in the rat. Exp Neurol. 1996;138:206–13. doi: 10.1006/exnr.1996.0059. [DOI] [PubMed] [Google Scholar]
- 17.Nawashiro H, Martin D, Hallenbeck JM. Inhibition of tumor necrosis factor and amelioration of brain infarction in mice. J Cereb Blood Flow Metab. 1997;17:229–32. doi: 10.1097/00004647-199702000-00013. [DOI] [PubMed] [Google Scholar]
- 18.Zhou X, Nicoletti A, Elhage R, Hansson GK. Transfer of CD4(+) T cells aggravates atherosclerosis in immunodeficient apolipoprotein E knockout mice. Circulation. 2000;102:2919–22. doi: 10.1161/01.cir.102.24.2919. [DOI] [PubMed] [Google Scholar]
- 19.Caligiuri G, Nicoletti A, Poirier B, Hansson GK. Protective immunity against atherosclerosis carried by B cells of hypercholesterolemic mice. J Clin Invest. 2002;109:745–53. doi: 10.1172/JCI07272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Pinderski LJ, Fischbein MP, Subbanagounder G, et al. Overexpression of interleukin-10 by activated T lymphocytes inhibits atherosclerosis in LDL receptor-deficient mice by altering lymphocyte and macrophage phenotypes. Circ Res. 2002;90:1064–71. doi: 10.1161/01.res.0000018941.10726.fa. [DOI] [PubMed] [Google Scholar]
- 21.Laurat E, Poirier B, Tupin E, et al. In vivo downregulation of T helper cell 1 immune responses reduces atherogenesis in apolipoprotein E-knockout mice. Circulation. 2001;104:197–202. doi: 10.1161/01.cir.104.2.197. [DOI] [PubMed] [Google Scholar]
- 22.Lee TS, Yen HC, Pan CC, Chau LY. The role of interleukin 12 in the development of atherosclerosis in ApoE-deficient mice. Arterioscler Thromb Vasc Biol. 1999;19:734–42. doi: 10.1161/01.atv.19.3.734. [DOI] [PubMed] [Google Scholar]
- 23.Elhage R, Jawien J, Rudling M, et al. Reduced atherosclerosis in interleukin-18 deficient apolipoprotein E-knockout mice. Cardiovasc Res. 2003;59:234–40. doi: 10.1016/s0008-6363(03)00343-2. [DOI] [PubMed] [Google Scholar]
- 24.Davenport P, Tipping PG. The role of interleukin-4 and interleukin-12 in the progression of atherosclerosis in apolipoprotein E-deficient mice. Am J Pathol. 2003;163:1117–25. doi: 10.1016/S0002-9440(10)63471-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ait-Oufella H, Salomon BL, Potteaux S, et al. Natural regulatory T cells control the development of atherosclerosis in mice. Nat Med. 2006;12:178–80. doi: 10.1038/nm1343. [DOI] [PubMed] [Google Scholar]
- 26.Offner H, Subramanian S, Parker SM, et al. Experimental stroke induces massive, rapid activation of the peripheral immune system. J Cereb Blood Flow Metab. 2006;26:654–65. doi: 10.1038/sj.jcbfm.9600217. [DOI] [PubMed] [Google Scholar]
- 27.Offner H, Subramanian S, Parker SM, et al. Splenic atrophy in experimental stroke is accompanied by increased regulatory T cells and circulating macrophages. J Immunol. 2006;176:6523–31. doi: 10.4049/jimmunol.176.11.6523. [DOI] [PubMed] [Google Scholar]
- 28.Hendrix S, Nitsch R. The role of T helper cells in neuroprotection and regeneration. J Neuroimmunol. 2007;184:100–12. doi: 10.1016/j.jneuroim.2006.11.019. [DOI] [PubMed] [Google Scholar]
- 29.Prass K, Meisel C, Hoflich C, et al. Stroke-induced immunodeficiency promotes spontaneous bacterial infections and is mediated by sympathetic activation reversal by poststroke T helper cell type 1-like immunostimulation. J Exp Med. 2003;198:725–36. doi: 10.1084/jem.20021098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hatano S. Experience from a multicentre stroke register: a preliminary report. Bull World Health Organ. 1976;54:541–53. [PMC free article] [PubMed] [Google Scholar]
- 31.Bamford JM, Sandercock PA, Warlow CP, Slattery J. Interobserver agreement for the assessment of handicap in stroke patients. Stroke. 1989;20:828. doi: 10.1161/01.str.20.6.828. [DOI] [PubMed] [Google Scholar]
- 32.Picker LJ, Singh MK, Zdraveski Z, et al. Direct demonstration of cytokine synthesis heterogeneity among human memory/effector T cells by flow cytometry. Blood. 1995;86:1408–19. [PubMed] [Google Scholar]
- 33.Karakantza M, Theodorou GL, Mouzaki A, et al. In vitro study of the long-term effects of post-traumatic splenectomy on cellular immunity. Scand J Immunol. 2004;59:209–19. doi: 10.1111/j.0300-9475.2004.01379.x. [DOI] [PubMed] [Google Scholar]
- 34.Risso A, Smilovich D, Capra MC, et al. CD69 in resting and activated T lymphocytes. Its association with a GTP binding protein and biochemical requirements for its expression. J Immunol. 1991;146:4105–14. [PubMed] [Google Scholar]
- 35.Youssef S, Stuve O, Patarroyo JC, et al. The HMG-CoA reductase inhibitor, atorvastatin, promotes a Th2 bias and reverses paralysis in central nervous system autoimmune disease. Nature. 2002;420:78–84. doi: 10.1038/nature01158. [DOI] [PubMed] [Google Scholar]
- 36.Hakamada-Taguchi R, Uehara Y, Kuribayashi K, et al. Inhibition of hydroxymethylglutaryl-coenzyme a reductase reduces Th1 development and promotes Th2 development. Circ Res. 2003;93:948–56. doi: 10.1161/01.RES.0000101298.76864.14. [DOI] [PubMed] [Google Scholar]
- 37.Aktas O, Waiczies S, Smorodchenko A, et al. Treatment of relapsing paralysis in experimental encephalomyelitis by targeting Th1 cells through atorvastatin. J Exp Med. 2003;197:725–33. doi: 10.1084/jem.20021425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Arora M, Chen L, Paglia M, et al. Simvastatin promotes Th2-type responses through the induction of the chitinase family member Ym1 in dendritic cells. Proc Natl Acad Sci USA. 2006;103:7777–82. doi: 10.1073/pnas.0508492103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Truneh A, Albert F, Golstein P, Schmitt-Verhulst AM. Early steps of lymphocyte activation bypassed by synergy between calcium ionophores and phorbol ester. Nature. 1985;313:318–20. doi: 10.1038/313318a0. [DOI] [PubMed] [Google Scholar]
- 40.Truneh A, Albert F, Golstein P, Schmitt-Verhulst AM. Calcium ionophore plus phorbol ester can substitute for antigen in the induction of cytolytic T lymphocytes from specifically primed precursors. J Immunol. 1985;135:2262–7. [PubMed] [Google Scholar]
- 41.Paul WE. Interleukin-4: a prototypic immunoregulatory lymphokine. Blood. 1991;77:1859–70. [PubMed] [Google Scholar]
- 42.Nakamura T, Lee RK, Nam SY, et al. Roles of IL-4 and IFN-gamma in stabilizing the T helper cell type 1 and 2 phenotype. J Immunol. 1997;158:2648–53. [PubMed] [Google Scholar]
- 43.Torres KC, Dutra WO, Gollob KJ. Endogenous IL-4 and IFN-gamma are essential for expression of Th2, but not Th1 cytokine message during the early differentiation of human CD4+ T helper cells. Hum Immunol. 2004;65:1328–35. doi: 10.1016/j.humimm.2004.06.007. [DOI] [PubMed] [Google Scholar]
- 44.Street NE, Schumacher JH, Fong TA, et al. Heterogeneity of mouse helper T cells. Evidence from bulk cultures and limiting dilution cloning for precursors of Th1 and Th2 cells. J Immunol. 1990;144:1629–39. [PubMed] [Google Scholar]
- 45.Pelidou SH, Kostulas N, Matusevicius D, et al. High levels of IL-10 secreting cells are present in blood in cerebrovascular diseases. Eur J Neurol. 1999;6:437–42. doi: 10.1046/j.1468-1331.1999.640437.x. [DOI] [PubMed] [Google Scholar]
- 46.Tarkowski E, Rosengren L, Blomstrand C, et al. Early intrathecal production of interleukin-6 predicts the size of brain lesion in stroke. Stroke. 1995;26:1393–8. doi: 10.1161/01.str.26.8.1393. [DOI] [PubMed] [Google Scholar]
- 47.Perini F, Morra M, Alecci M, et al. Temporal profile of serum anti-inflammatory and pro-inflammatory interleukins in acute ischaemic stroke patients. Neurol Sci. 2001;22:289–96. doi: 10.1007/s10072-001-8170-y. [DOI] [PubMed] [Google Scholar]
- 48.Waje-Andreassen U, Krakenes J, Ulvestad E, et al. IL-6: an early marker for outcome in acute ischaemic stroke. Acta Neurol Scand. 2005;111:360–5. doi: 10.1111/j.1600-0404.2005.00416.x. [DOI] [PubMed] [Google Scholar]
- 49.Vila N, Castillo J, Davalos A, et al. Levels of anti-inflammatory cytokines and neurological worsening in acute ischaemic stroke. Stroke. 2003;34:671–5. doi: 10.1161/01.STR.0000057976.53301.69. [DOI] [PubMed] [Google Scholar]
- 50.Liuzzo G, Kopecky SL, Frye RL, et al. Perturbation of the T-cell repertoire in patients with unstable angina. Circulation. 1999;100:2135–9. doi: 10.1161/01.cir.100.21.2135. [DOI] [PubMed] [Google Scholar]
- 51.Coleman JW, Holliday MR, Kimber I, et al. Regulation of mouse peritoneal mast cell secretory function by stem cell factor, IL-3 or IL-4. J Immunol. 1993;150:556–62. [PubMed] [Google Scholar]
- 52.Else KJ, Finkelman FD, Maliszewski CR, Grencis RK. Cytokine-mediated regulation of chronic intestinal helminth infection. J Exp Med. 1994;179:347–51. doi: 10.1084/jem.179.1.347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Rapoport MJ, Jaramillo A, Zipris D, et al. Interleukin 4 reverses T cell proliferative unresponsiveness and prevents the onset of diabetes in nonobese diabetic mice. J Exp Med. 1993;178:87–99. doi: 10.1084/jem.178.1.87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Khoury SJ, Hancock WW, Weiner HL. Oral tolerance to myelin basic protein and natural recovery from experimental autoimmune encephalomyelitis are associated with downregulation of inflammatory cytokines and differential upregulation of transforming growth factor beta, interleukin 4, and prostaglandin E expression in the brain. J Exp Med. 1992;176:1355–64. doi: 10.1084/jem.176.5.1355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Miida T, Hirayama S, Nakamura Y. Cholesterol-independent effects of statins and new therapeutic targets: ischaemic stroke and dementia. J Atheroscler Thromb. 2004;11:253–64. doi: 10.5551/jat.11.253. [DOI] [PubMed] [Google Scholar]


