Abstract
Mikulicz's disease (MD) is gaining acceptance as an immunoglobulin G4 (IgG4)-related disease characterized by bilateral lacrimal and salivary gland swelling. The aetiology of MD and other IgG4-related diseases is still unclear. The present work was performed to study the clonality of infiltrating IgG4-positive plasma cells in lacrimal glands and circulating peripheral blood cells in patients with MD, and compare the clonal relationship between infiltrating and circulating IgG4 positive cells. Total cellular RNA was extracted from the lacrimal glands and peripheral blood in five MD patients. Reverse transcription polymerase chain reaction was performed with primers specific for activation-induced cytidine deaminase (AID) and for Ig VH and IgG4. Sequences of Ig VH were compared with the structure of Ig VH of the lacrimal glands and the peripheral blood cells. AID was expressed to varying degrees in lacrimal glands of all MD patients. Most IgG4-positive cells infiltrating lacrimal glands and in peripheral blood were polyclonal, although several clonally related pairs were detected. In one patient, two of the circulating IgG4 VH4-59 clones shared identical CDR3 sequences with the clones within the lacrimal glands. In conclusion, while most tissue-infiltrating and circulating IgG4-positive cells in MD are polyclonal, some clonally related IgG4 positive cells exist between lacrimal gland and peripheral blood, accounting for the clinical features of MD as an IgG4-related disease involving multiple organs.
Keywords: activation-induced cytidine deaminase, IgG4, immunoglobulin, Milulicz's disease, somatic hypermutation
Introduction
Mikulicz's disease (MD) is a chronic inflammatory disorder characterized by symmetrical swelling of the lacrimal and/or salivary glands [1,2]. Although MD and Sjögren's syndrome (SS) have been considered to be the same disease since Morgan and Castleman demonstrated their histological similarity [3], recent studies have indicated that they exhibit differences with respect to the following points: (i) serum immunoglobulin G4 (IgG4) elevation and infiltration of IgG4-positive plasma cells in lacrimal glands and salivary glands are characteristic findings in MD, but are absent in SS; (ii) autoantibodies, such as anti-nuclear antibody, anti-SSA antibody and anti-SSB antibody are usually negative in MD; and (iii) steroid sensitivity is marked in MD, but not in SS [4,5].
Recently, MD has been considered to be an IgG4-related disease and shares some histopathological features with other such diseases such as sclerosing pancreatitis [6], retroperitoneal fibrosis [7], tubulointerstitial nephritis [8] and inflammatory pseudotumour of the lung [9]. However, the aetiology of MD as well as that of IgG4-related diseases is still unclear.
Ectopic germinal centre (GC)-like structures are found in the affected organs in some autoimmune diseases such as salivary glands in SS [10–12], synovia in rheumatoid arthritis [13–15] and thymus in myasthenia gravis [16]. This structure is also found in lacrimal glands and salivary glands in MD. Class switch recombination (CSR) and somatic hypermutation (SHM) are found in GC-like structures. Activation-induced cytidine deaminase (AID) is a member of the RNA editing cytidine deaminase family and is required for both CSR and SHM [17,18]. AID is expressed in not only GC in secondary lymphoid organs but also ectopic GC-like structures in SS [19], allergic rhinitis [20] and bronchial asthma [21].
Previous studies have demonstrated mutated V gene rearrangements in SS, mucosa-associated lymphoid tissue lymphoma [22,23], cryoglobulinaemia [24,25] and possibly other diseases, suggesting an antigen-driven local immune response. However, to our knowledge, few reports analysing Ig V gene of infiltrating IgG4-producing plasma cells in IgG4-related disease are available [26]. This prompted us to examine the expression of AID in lacrimal glands in MD and analyse the Ig heavy chain clonality of IgG4-positive plasma cells infiltrating lacrimal glands and IgG4-positive circulating peripheral blood cells. We found that SHM of IgG4-positive plasma cells had occurred in the lacrimal glands, suggesting an antigen-driven local immune response in MD. Furthermore, the presence of genetically related B cells or plasma cells in the lacrimal glands and systemic pool may reflect the multi-organ involvement in IgG4-related diseases including MD.
Materials and methods
Patients
After informed consent had been obtained, five patients with MD (three females and two males) aged 54·4 ± 9·6 years were analysed in this study. Their clinical data are shown in Table 1. Elevated IgG4 (561·6 ± 397·0) was seen in all patients; the normal values of IgG4 is 4·8–105 mg/dl. Lacrimal glands and peripheral blood samples were obtained from these patients. MD was diagnosed according to previously described criteria [4], namely (i) persistent (≥ 3 months) symmetrical swelling of more than two lacrimal and major salivary glands; (ii) prominent mononuclear infiltration of lacrimal and salivary glands; and (iii) exclusion of other diseases associated with glandular swelling, such as sarcoidosis and lymphoproliferative disease. In addition to these diagnostic criteria, we determined any elevation of the serum IgG4 level and infiltration of IgG4 positive plasma cells in the lacrimal glands by immunostaining. SS was diagnosed according to the European [27] and Japanese criteria [28].
Table 1.
Profile of patients with Mikulicz's disease.
| IgG | IgG4 | (%) | IgE | ANA | SSA | SSB | C3 | C4 | CH50 | |||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 65 | M | 1960 | 164 | (8·4) | 1621 | <×20 | <10 | <15 | 91 | 9 | 43 |
| 2 | 64 | F | 1820 | 486 | (26·7) | 90 | <×20 | <10 | <15 | 129 | 15 | 51 |
| 3 | 45 | F | 2350 | 1000 | (42·5) | 183 | <×20 | <10 | <15 | n.t. | n.t. | n.t. |
| 4 | 46 | F | 1350 | 209 | (15·5) | 170 | ×40 | <10 | n.t. | 157 | 27 | 64 |
| 5 | 52 | M | 3440 | 949 | (27·6) | 973 | <×20 | n.t. | n.t. | 89 | 6 | 24 |
Elevated immunoglobulin G4 (IgG4) (561·6 ± 397·0) was seen in all patients. Elevated IgE and decreased complement were seen in cases 1 and 5. Anti-nuclear antibody, anti-Sjögren's syndrome A (SSA) antibody and anti-Sjögren's syndrome A (SSB) SSB antibody were negative in all patients. ANA, anti-nuclear antibody; CH50, haemolytic assay of classical pathway of complement; n.t., not tested.
Reverse transcription–polymerase chain reaction
Total cellular RNA was extracted from the lacrimal glands and peripheral blood using TRIzol reagent (Invitrogen, Carlsbad, CA, USA). The cDNA was synthesized using the oligo d(T) 16 (Applied Biosystems, Foster City, CA, USA). We performed reverse transcription–polymerase chain reaction (RT–PCR) for AID. The oligonucleotide sequences of AID are shown in Table 2[29]. The amplification programme consisted of one cycle of 94°C for 10 min, followed by 30 cycles of 94°C for 15 s, 54°C for 30 s and 72°C for 30 s. After 30 cycles all products received a final extension of 5 min at 72°C. In the second PCR, the annealing temperature was 60°C and we then performed Ig subclass-specific RT–PCR amplification using primer for Ig Framework (FR) 3-specific primer [30] and Ig subclass-specific primer [31]. The oligonucleotide sequences of Ig subclass-specific reverse primers are shown in Table 2. VH of IgG4 was analysed using Ig heavy chain (VH) gene family-specific primers and IgG4-specific primer. Primers used for VH gene family-specific amplification have been described previously [32]. VH of IgM was analysed using IgM-specific primer [30]. The amplification programme consisted of one cycle of 94°C for 10 min, followed by 30 cycles of 94°C for 15 s, 59°C for 30 s and 72°C for 30 s. After 30 cycles all products received a final extension of 5 min at 72°C. Cloning of the VH gene PCR products was performed using the Topo TA Cloning Kit (Invitrogen, Carlsbad, CA, USA).
Table 2.
Sequences of primers.
| AidF1 | 5′-GAGGCAAGAAGACACTCTGG-3′ |
| AidR1 | 5′-GTGACATTCCTGGAAGTTGC-3′ |
| AidF2 | 5′-TAGACCCTGGCCGCTGCTACC-3′ |
| AidR2 | 5′-CAAAAGGATGCGCCGAAGCTGTCTGGAG-3′ |
| FR3 | 5′-CTGAGGACACGGCCGTGTATTACTG-3′ |
| IgG1 | 5′-GCACGGTGGGCATGTGTGAGTTTTGTCACAAGATTTGGGCTC-3′ |
| IgG2 | 5′-TGGGCACGGTGGGCACTCGACACAACATTTGCGCTCAACTGT-3′ |
| IgG3 | 5′-AAGATTTGGGCTCTGGGCACCGTGGGCATGTGTGAGTTGT-3′ |
| IgG4 | 5′-AACTCAGGTGCTGGGCATGATGGGGGACCATA-3′ |
| hM3 | 5′-GGAAAAGGGTTGGGGCGGAT-3′ |
AID, activation-induced cytidine deaminase; IgG, immunoglobulin G.
Sequence analysis
Sequences were analysed with an automated DNA sequencer (ABI PRISM 310 Genetic Analyser; Applied Biosystems). Primers for sequence analysis were M13F, M13R or VH gene-specific primers.
Data analysis
Sequences were compared with those in the IMGT/V-QUEST (http://imgt.cines.fr/IMGT_vquest/share/textes/index.html) and compared with the structure of Ig VH of the lacrimal glands and the peripheral blood cells.
Calculation of the number of expected replacement mutations (R mutations) in CDRs or FRs was based on a computer algorithm developed by Chang and Casali [33]. This algorithm calculates the inherent susceptibility to amino acid replacement given any single nucleotide change. The number of expected R mutations was calculated as follows: n × (CDR Rf or FR Rf) × (CDRrelor FRrel), where n = total number of observed mutations, Rf = replacement frequency inherent to CDR or FR sequences and CDRrel or FRrel = relative size of the CDRs or FRs. The probability (P) that excess or scarcity of R mutations in the CDRs or FRs was due to chance alone was calculated using a binomial distribution model, as reported by Chang and Casali [33], as follows: P = {n!/[k!(n − k)!]} × qk × (1 − q)n–k, where n = total number of observed mutations, k = number of observed R mutations in the CDRs or FRs and q = probability that an R mutation localizes to CDRs or FRs (q = CDRrel × CDR Rf or FRrel × FR Rf). In analysing in FRs, we exclude FR1 because the sequence primer was located in the middle of FR1.
Results
Detection of AID in patients with MD
By analysing the expression of AID in the lacrimal glands of the five MD patients using RT–PCR, we confirmed that AID was expressed to varying degrees in all of them (Fig. 1a), and speculated that both SHM and CSR occur in lacrimal glands.
Fig. 1.
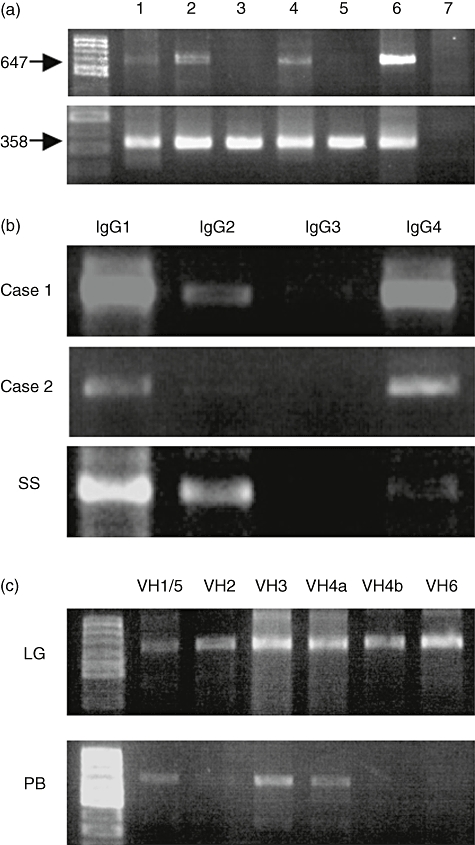
(a) Expression of activation-induced cytidine deaminase (AID) in lacrimal glands in patients with Mikulicz's disease (MD). Lanes 1–5: patients; lane 6: RAMOS cell (Burkitt's lymphoma-derived cell line) as a positive control; lane 7: hybridoma producing immunoglobulin M (IgM) monoclonal rheumatoid factor as a negative control. AID was expressed to varying degrees in all MD patients. (b) IgG subclass of infiltrating cells in lacrimal glands in patients with MD. SS is minor salivary glands in a patient with Sjögren's syndrome. IgG4 was detected at the same level as IgG1 in the lacrimal glands of MD. (c) Analysis of VH family of IgG4-positive cells infiltrating lacrimal glands and peripheral blood in case 1.
Analysis of VH gene
We examined the SHM in VH of IgG4 in lacrimal glands and peripheral blood in two MD patients. First, we performed Ig subclass-specific PCR. IgG4 was detected at the same level as IgG1 in the lacrimal glands of MD, but not in SS (Fig. 1b). We then performed VH family-specific PCR of IgG4, and detected VH1, 3, 4, 5 and 6 gene products in the lacrimal glands and peripheral blood in both patients (Fig. 1c). Among the VH4 family of IgG4-positive cells, VH4-59 was the most common gene in the lacrimal glands in both cases (49·1% and 71·9% respectively) (Table 3).
Table 3.
Sequence analysis of VH4 in lacrimal glands and peripheral blood.
| Case 1 | Case 2 | |||||||
|---|---|---|---|---|---|---|---|---|
| LG | PB | LB | PB | |||||
| n | (%) | n | (%) | n | (%) | n | (%) | |
| VH4-4 | 3 | (5·3) | 5 | (11·1) | 1 | (3·1) | 0 | (0·0) |
| VH4-31 | 13 | (22·8) | 12 | (26·7) | 0 | (0·0) | 0 | (0·0) |
| VH4-39 | 7 | (12·3) | 1 | (2·2) | 7 | (21·9) | 13 | (40·6) |
| VH4-55P | 1 | (1·8) | 0 | (0·0) | 0 | (0·0) | 0 | (0·0) |
| VH4-59 | 28 | (49·1) | 27 | (60·0) | 23 | (71·9) | 17 | (53·1) |
| VH4-61 | 3 | (5·3) | 0 | (0·0) | 1 | (3·1) | 2 | (6·3) |
| VH4-80p | 2 | (3·5) | 0 | (0·0) | 0 | (0·0) | 0 | (0·0) |
| Total | 55 | 45 | 32 | 32 | ||||
Immunoglobulin (Ig) VH of VH4 gene was sequenced. In both patients, VH4-59 was the major gene in LG and PB. LG, lacrimal glands; PB, peripheral blood.
To investigate the clonality of infiltrating IgG4-positive plasma cells, sequence analysis of the VH4-59 was performed. Most IgG4-positive cells infiltrating lacrimal glands were polyclonal, although several clonally related pairs were detected (three pairs of 22 clones and three pairs of 24 clones, respectively, were identical in each patient). VH4-59 was also predominant among the peripheral blood IgG4-positive cells in both patients, and most IgG4-positive cells in peripheral blood were polyclonal except for some clones (one pair of 18 clones, and two pairs of 24 clones, respectively, shared identical sequences in each patient) (Fig. 2a and b). We also analysed the sequence of IgG1-positive cells infiltrating the lacrimal glands in case 1, indicating that all clones were polyclonal, with no genetically related clone existing between IgG1-positive cells and IgG4-positive cells (data not shown).
Fig. 2.


(a) Analysis of CDR3 of VH4-59 in case 1. Clonally related pairs were indicated in upper panel. Three pairs of 22 clones in LG and one pair of 19 clones, respectively, were identical. Clonally related clones between lacrimal glands and peripheral blood were indicated in the middle panel. Each clone's name was as follows: (1) eye-55; (2) eye-96; (3) PB-57; and (4) PB-24. Other clones were polyclonal (lower panel). (b) Analysis of CDR3 of VH4-59 in case 2. Clonally related pairs were indicated in the upper panel. Three pairs of 24 clones in lacrimal glands and two pairs of 24 clones in peripheral blood, respectively, were identical. Clonally related clones between lacrimal glands and peripheral blood were not detected in case 2. Other clones were polyclonal (lower panel). LG, lacrimal glands; PB, peripheral blood.
We also analysed whether common or related clones between lacrimal glands and peripheral blood were present. In one patient, two of the circulating IgG4 VH4-59 clones shared identical third complementarity determining region (CDR3) sequences with the clones in the lacrimal glands (Fig. 3a). These clones did not share identical sequences in any other region, such as CDR1, CDR2, FR2 or FR3 (Fig. 3b). These data indicated that the IgG4-positive cells migrate between peripheral blood and lacrimal glands (Fig. 3c). In one pair, the R/S ratio in the CDRs was statistically significantly higher than would be expected by chance alone (eye-96; P = 0·015, PB-24; P < 0·001). In another pair, the R/S ratio was higher than would be expected by chance alone, although not statistically significantly so (eye-55; P = 0·132, PB-57; P = 0·055). In the FRs, the R/S ratio was statistically significantly lower than would be expected by chance alone in peripheral blood (PB-24; P = 0·001, PB-96; P = 0·043). The R/S ratio in lacrimal glands was also lower, although not statistically significantly so (eye-55; P = 0·076, eye-96; P = 0·067) (Table 4).
Fig. 3.
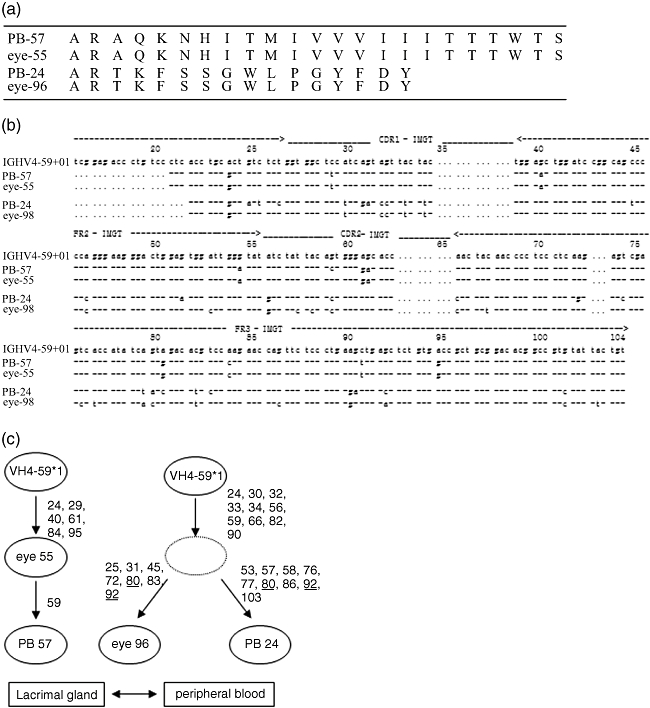
(a) CDR3 of genetically related clones between lacrimal glands and peripheral blood in case 1. (b) Sequence analysis of CDR1, CDR2, FR2 and FR3. Somatic hypermutation (SHM) were seen in these clones but did not share identical sequences. (c) Intraclonal diversification of genetically related clones between lacrimal glands and peripheral blood. Numbers beside the arrows refer to the number of amino acid exchanges. Underlined numbers such as 80 and 92 indicated different nucleotide exchanges. These data indicated that the IgG4-positive cells migrate between peripheral blood and lacrimal glands. PB, peripheral blood.
Table 4.
Mutation analysis of VH gene in genetically related clones between lacrimal glands and peripheral blood.
| Observed R/S | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Expected R/S | CDRs | FRs | |||||||
| Similarity (%) | CDRs ratio | FRs ratio | No | Ratio | P | No | Ratio | P | |
| eye-55 | 95·7 | 3·59 | 3·19 | 3/0 | ∞ | 0·13 | 3/3 | 1·00 | 0·08 |
| PB-57 | 95·2 | 3·59 | 3·19 | 4/0 | ∞ | 0·06 | 3/3 | 1·00 | 0·04 |
| eye-96 | 89·5 | 3·59 | 3·19 | 8/0 | ∞ | 0·02 | 10/4 | 2·50 | 0·07 |
| PB-24 | 89·0 | 3·59 | 3·19 | 9/0 | ∞ | < 0·01 | 8/6 | 1·33 | < 0·01 |
In the second pair (eye-96 and PB-24), both replacement/silent (R/S) ratios were statistically significantly higher than expected R/S in CDRs. In both pairs, R/S ratios in peripheral blood clones were statistically significantly lower than expected R/S in FRs. R/S ratios in lacrimal glands were also lower than expected, although the difference was not statistically significant. P is the probability for obtaining the observed number of R mutations by chance.
Discussion
We confirmed that AID mRNA transcripts were expressed in lacrimal glands of MD. Since AID has been shown to be involved in the regulation of both CSR and SHM, several reports have demonstrated that AID mRNA transcripts are present not only in secondary lymphoid organs but also in local inflammatory tissues. Recently, Bombardieri et al. reported that AID was expressed within salivary and parotid glands in SS [19]. These findings suggest that local CSR and SHM in inflammatory tissues may exist in various inflammatory diseases, including autoimmune ones such as SS. Our data suggest that GC-reaction, such as B cell maturation represented by CSR and SHM, occurs in lacrimal glands and salivary glands in MD. To clarify the importance of the any contribution of AID to the pathogenesis of MD and SS, further studies evaluating the amount and the distribution of AID in salivary and lacrimal glands in MD are needed.
Few reports have analysed Ig clonality in the affected organs in IgG4-related disease. Recently, Kojima et al. demonstrated that B cells or plasma cells around pancreatic interlobular ducts were polyclonal in 10 of 11 patients with autoimmune pancreatitis, while one patient showed oligoclonal expansion [26]. However, they analysed Ig heavy chain clonality using VH-FR3 and JH primers, which were not specific for IgG4 but were common in all IgG subclasses. Therefore, our work is the first to show IgG4 polyclonality in the affected organs in IgG4-related diseases, although there are limitations with regard to the number of patients analysed and VH family. Moreover, we also show that IgG1-positive B cells or plasma cells infiltrating lacrimal glands are also polyclonal (data not shown). Both our results and their data, indicating polyclonality of infiltrating B cells or plasma cells, suggest that there are many antigen responses in IgG4-positive plasma cell-infiltrated lesions.
We found that two of the circulating IgG4 VH4-59 clones shared identical CDR3 sequences with the clones in the lacrimal glands. One of these pairs had some different SHM other than CDR3, suggesting that these clones had originated from the same ancestor. In another pair, the circulating clone had one additional mutation in comparison with lacrimal glands, suggesting the possibility that the circulating clone was downstream and migrated from the lacrimal glands to peripheral blood pool (Fig. 3c). While most plasma cells are short-lived and are developed from memory B cells through plasmablasts by a chronic antigen-driven immune reaction, some memory B cells may become long-lived plasma cells [34,35]. The presence of genetically related B cells or plasma cells in the lacrimal glands and peripheral blood might explain the characteristic multi-organ involvement in IgG4-related disease including MD through the following mechanism: migration of memory B cells or long-lived plasma cells from lacrimal or salivary glands to bone marrow or directly to other target organs.
We have reported recently that the number of T helper 2 (Th2) cells and regulatory T cells was increased in the lesions of IgG4-related diseases including autoimmune pancreato-cholangitis (AIPC) [36]. In addition, the levels of Th2-related cytokines represented by interleukin (IL)-4 and regulatory cytokines represented by IL-10 were increased in AIPC and related IgG4 diseases, leading to IgG4 and IgE production. Although these cytokines can explain the histopathological features shared by IgG4-related diseases, the mechanism underlying the preferential involvement of different organs in individual patients is still unclear. To clarify this characteristic organ involvement in IgG4-related diseases, analyses from aspects of unknown antigens are also required. It is hoped that the knowledge gained from such studies will suggest novel therapeutic strategies for these diseases.
Acknowledgments
We thank Professor Muramatsu and Dr Kurosu for their kind advice, Mr J. S. Gelblum for his critical reading of the manuscript and Mrs Y. Kubo for her secretarial assistance.
References
- 1.Mikulicz J. Über eine eigenartige symmetrische Erkrankung der Thränen-und Mundspeicheldrüsen [Concerning a peculiar symmetrical disease of the lacrimal and salivary glands] Beitr Chir Fortsch Gewidmet Theodor Billroth. 1892:610–30. [Google Scholar]
- 2.Schaffer A, Jacobsen A. Mikulicz's syndrome: a report of ten cases. Am J Dis Child. 1927;34:327–46. [Google Scholar]
- 3.Morgan WS, Castleman B. A clinicopathologic study of Mikulicz's disease. Am J Pathol. 1953;29:471–503. [PMC free article] [PubMed] [Google Scholar]
- 4.Yamamoto M, Harada S, Ohara M, et al. Clinical and pathological differences between Mikulicz's disease and Sjogren's syndrome. Rheumatology (Oxford) 2005;44:227–34. doi: 10.1093/rheumatology/keh447. [DOI] [PubMed] [Google Scholar]
- 5.Yamamoto M, Ohara M, Suzuki C, et al. Elevated IgG4 concentrations in serum of patients with Mikulicz's disease. Scand J Rheumatol. 2004;33:432–3. doi: 10.1080/03009740410006439. [DOI] [PubMed] [Google Scholar]
- 6.Hamano H, Kawa S, Horiuchi A, et al. High serum IgG4 concentrations in patients with sclerosing pancreatitis. N Engl J Med. 2001;344:732–8. doi: 10.1056/NEJM200103083441005. [DOI] [PubMed] [Google Scholar]
- 7.Hamano H, Kawa S, Ochi Y, et al. Hydronephrosis associated with retroperitoneal fibrosis and sclerosing pancreatitis. Lancet. 2002;359:1403–4. doi: 10.1016/s0140-6736(02)08359-9. [DOI] [PubMed] [Google Scholar]
- 8.Takeda S, Haratake J, Kasai T, Takaeda C, Takazakura E. IgG4-associated idiopathic tubulointerstitial nephritis complicating autoimmune pancreatitis. Nephrol Dial Transplant. 2004;19:474–6. doi: 10.1093/ndt/gfg477. [DOI] [PubMed] [Google Scholar]
- 9.Zen Y, Kitagawa S, Minato H, et al. IgG4-positive plasma cells in inflammatory pseudotumor (plasma cell granuloma) of the lung. Hum Pathol. 2005;36:710–17. doi: 10.1016/j.humpath.2005.05.011. [DOI] [PubMed] [Google Scholar]
- 10.Salomonsson S, Jonsson MV, Skarstein K, et al. Cellular basis of ectopic germinal center formation and autoantibody production in the target organ of patients with Sjogren's syndrome. Arthritis Rheum. 2003;48:3187–201. doi: 10.1002/art.11311. [DOI] [PubMed] [Google Scholar]
- 11.Amft N, Curnow SJ, Scheel-Toellner D, et al. Ectopic expression of the B cell-attracting chemokine BCA-1 (CXCL13) on endothelial cells and within lymphoid follicles contributes to the establishment of germinal center-like structures in Sjogren's syndrome. Arthritis Rheum. 2001;44:2633–41. doi: 10.1002/1529-0131(200111)44:11<2633::aid-art443>3.0.co;2-9. [DOI] [PubMed] [Google Scholar]
- 12.Daridon C, Pers JO, Devauchelle V, et al. Identification of transitional type II B cells in the salivary glands of patients with Sjogren's syndrome. Arthritis Rheum. 2006;54:2280–8. doi: 10.1002/art.21936. [DOI] [PubMed] [Google Scholar]
- 13.Weyand CM, Goronzy JJ. Ectopic germinal center formation in rheumatoid synovitis. Ann N Y Acad Sci. 2003;987:140–9. doi: 10.1111/j.1749-6632.2003.tb06042.x. [DOI] [PubMed] [Google Scholar]
- 14.Han S, Cao S, Bheekha-Escura R, Zheng B. Germinal center reaction in the joints of mice with collagen-induced arthritis: an animal model of lymphocyte activation and differentiation in arthritis joints. Arthritis Rheum. 2001;44:1438–43. doi: 10.1002/1529-0131(200106)44:6<1438::AID-ART239>3.0.CO;2-Q. [DOI] [PubMed] [Google Scholar]
- 15.Wagner UG, Kurtin PJ, Wahner A, et al. The role of CD8+ CD40L+ T cells in the formation of germinal centers in rheumatoid synovitis. J Immunol. 1998;161:6390–7. [PubMed] [Google Scholar]
- 16.Meraouna A, Cizeron-Clairac G, Panse RL, et al. The chemokine CXCL13 is a key molecule in autoimmune myasthenia gravis. Blood. 2006;108:432–40. doi: 10.1182/blood-2005-06-2383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Muramatsu M, Kinoshita K, Fagarasan S, Yamada S, Shinkai Y, Honjo T. Class switch recombination and hypermutation require activation-induced cytidine deaminase (AID), a potential RNA editing enzyme. Cell. 2000;102:553–63. doi: 10.1016/s0092-8674(00)00078-7. [DOI] [PubMed] [Google Scholar]
- 18.Honjo T, Kinoshita K, Muramatsu M. Molecular mechanism of class switch recombination: linkage with somatic hypermutation. Annu Rev Immunol. 2002;20:165–96. doi: 10.1146/annurev.immunol.20.090501.112049. [DOI] [PubMed] [Google Scholar]
- 19.Bombardieri M, Barone F, Humby F, et al. Activation-induced cytidine deaminase expression in follicular dendritic cell networks and interfollicular large B cells supports functionality of ectopic lymphoid neogenesis in autoimmune sialoadenitis and MALT lymphoma in Sjogren's syndrome. J Immunol. 2007;179:4929–38. doi: 10.4049/jimmunol.179.7.4929. [DOI] [PubMed] [Google Scholar]
- 20.Coker HA, Durham SR, Gould HJ. Local somatic hypermutation and class switch recombination in the nasal mucosa of allergic rhinitis patients. J Immunol. 2003;171:5602–10. doi: 10.4049/jimmunol.171.10.5602. [DOI] [PubMed] [Google Scholar]
- 21.Takhar P, Corrigan CJ, Smurthwaite L, et al. Class switch recombination to IgE in the bronchial mucosa of atopic and nonatopic patients with asthma. J Allergy Clin Immunol. 2007;119:213–18. doi: 10.1016/j.jaci.2006.09.045. [DOI] [PubMed] [Google Scholar]
- 22.Qin Y, Greiner A, Trunk MJ, Schmausser B, Ott MM, Muller-Hermelink HK. Somatic hypermutation in low-grade mucosa-associated lymphoid tissue-type B-cell lymphoma. Blood. 1995;86:3528–34. [PubMed] [Google Scholar]
- 23.Bahler DW, Miklos JA, Swerdlow SH. Ongoing Ig gene hypermutation in salivary gland mucosa-associated lymphoid tissue-type lymphomas. Blood. 1997;89:3335–44. [PubMed] [Google Scholar]
- 24.Mondelli MU, Zorzoli I, Cerino A, et al. Clonality and specificity of cryoglobulins associated with HCV: pathophysiological implications. J Hepatol. 1998;29:879–86. doi: 10.1016/s0168-8278(98)80114-1. [DOI] [PubMed] [Google Scholar]
- 25.Antonescu C, Mayerat C, Mantegani A, Frei PC, Spertini F, Tissot JD. Hepatitis C virus (HCV) infection: serum rheumatoid factor activity and HCV genotype correlate with cryoglobulin clonality. Blood. 1998;92:3486–7. [PubMed] [Google Scholar]
- 26.Kojima M, Sipos B, Klapper W, et al. Autoimmune pancreatitis: frequency, IgG4 expression, and clonality of T and B cells. Am J Surg Pathol. 2007;31:521–8. doi: 10.1097/01.pas.0000213390.55536.47. [DOI] [PubMed] [Google Scholar]
- 27.Vitali C, Bombardieri S, Moutsopoulos HM, et al. Assessment of the European classification criteria for Sjogren's syndrome in a series of clinically defined cases: results of a prospective multicentre study. The European Study Group on Diagnostic Criteria for Sjogren's Syndrome. Ann Rheum Dis. 1996;55:116–21. doi: 10.1136/ard.55.2.116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Fujibayashi K, Sugai S, Miyasaka N, et al. Revised Japanese criteria for Sjogren's syndrome. A research report for the promotion of immunologic work on specific diseases Research Committee. Tokyo: Ministry of Health, Labour, and Welfare, Japan; 1999. [in Japanese]. [Google Scholar]
- 29.Takhar P, Smurthwaite L, Coker HA, et al. Allergen drives class switching to IgE in the nasal mucosa in allergic rhinitis. J Immunol. 2005;174:5024–32. doi: 10.4049/jimmunol.174.8.5024. [DOI] [PubMed] [Google Scholar]
- 30.Ivanovski M, Silvestri F, Pozzato G, et al. Somatic hypermutation, clonal diversity, and preferential expression of the VH 51p1/VL kv325 immunoglobulin gene combination in hepatitis C virus-associated immunocytomas. Blood. 1998;91:2433–42. [PubMed] [Google Scholar]
- 31.Sideras P, Nilsson L, Islam KB, Ericsson H, Hammarstrom L, Smith CI. Quantitative and qualitative analysis of human IgG subclass specific mRNA using solution hybridization. Scand J Immunol. 1991;34:557–64. doi: 10.1111/j.1365-3083.1991.tb01579.x. [DOI] [PubMed] [Google Scholar]
- 32.Bahler DW, Swerdlow SH. Clonal salivary gland infiltrates associated with myoepithelial sialadenitis (Sjogren's syndrome) begin as nonmalignant antigen-selected expansions. Blood. 1998;91:1864–72. [PubMed] [Google Scholar]
- 33.Chang B, Casali P. The CDR1 sequences of a major proportion of human germline Ig VH genes are inherently susceptible to amino acid replacement. Immunol Today. 1994;15:367–73. doi: 10.1016/0167-5699(94)90175-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Schittek B, Rajewsky K. Maintenance of B-cell memory by long-lived cells generated from proliferating precursors. Nature. 1990;346:749–51. doi: 10.1038/346749a0. [DOI] [PubMed] [Google Scholar]
- 35.Radbruch A, Muehlinghaus G, Luger EO, et al. Competence and competition: the challenge of becoming a long-lived plasma cell. Nat Rev Immunol. 2006;6:741–50. doi: 10.1038/nri1886. [DOI] [PubMed] [Google Scholar]
- 36.Zen Y, Fujii T, Harada K, et al. Th2 and regulatory immune reactions are increased in immunoglobin G4-related sclerosing pancreatitis and cholangitis. Hepatology. 2007;45:1538–46. doi: 10.1002/hep.21697. [DOI] [PubMed] [Google Scholar]


