Abstract
We have demonstrated previously that liver allograft tolerance is associated with the immunosuppressive activity of anti-histone H1 autoreactive antibodies induced in the serum of liver transplantation. Furthermore, we and others have shown that nuclear proteins such as histone H1 and high mobility group box 1 play an important role in maturation of dendritic cells (DCs), although the precise mechanisms are still unknown. In the present study, we focus upon the significance of histone H1 on DCs in terms of the intracellular signalling pathway of DCs. Our immunostaining and immunoblot studies demonstrated that histone H1 was detected in cytoplasm and culture supernatants upon the activation of DCs. Histone H1 blockage by anti-histone H1 antibody down-regulated the intracellular activation of mitogen-activated protein kinases (MAPKs) (p38) and IκBα of DCs, and inhibited DC activity in the proliferation of CD4+ T cells. On the other hand, the addition of histone H1 without endotoxin stimulation up-regulated major histocompatibility complex class II, the CD80 and CD86 surface markers of DCs and the activation of MAPKs (p38 and extracellular-regulated kinase 1/2) and IκBα. These results suggest that the translocation of histone H1 from nuclei to cytoplasm and the release of their own histone H1 are necessary for the maturation of DCs and the activation for T lymphocytes.
Keywords: antigen-presenting cells; dendritic cells (myeloid, plasmacytoid, monocyte-derived); flow cytometry/FACS; immune regulation; proliferation
Introduction
Certain immunosuppressive serum factors contribute to liver allograft tolerance by naturally overcoming rejection without requiring the use of any immunosuppressive drugs. Our recent work has demonstrated that anti-histone H1 antibody, which increases transiently during the rejection phase (post-operative days 14–21) in a tolerogenic rat orthotopic liver transplantation (OLT) [a Dark Agouti (DA) liver into a Piebald Virol Glaxo (PVG)] model, serves as an immunosuppressive immunoglobulin G (IgG) protein [1,2]. We have also demonstrated that rabbit polyclonal antibody directed histone H1 not only suppresses mixed leucocyte reaction, but also prolongs heart allograft survival while immunodepletion of anti-histone H1 autoantibodies from post-OLT serum causes immunosuppressive activity to vanish [1,2]. Furthermore, we found that anti-nuclear antibodies [histone H1 and high mobility group box 1 (HMGB1)] induced in the serum after cessation of immunosuppressants in clinical and experimental drug-induced tolerance [3]. We also demonstrated that up-regulation of anti-histone H1 antibody by histone H1 vaccination induced alloreactive T cell unresponsiveness and prolonged heterotopic heart allograft survival [4]. In our previous study, the mechanisms underlying the immunosuppressive activity induced by anti-histone H1 antibody were explored with particular focus upon dendritic cells (DCs), T cells, lymphokine-activated killer (LAK) cells and natural killer (NK) cells [5]. We have shown that non-specific cytotoxic cells and cytotoxic cell activity are affected by histone H1 [5]. The addition of anti-histone H1 antibody to LAK cultures was found to decrease the percentages of NK cell receptor protein 1 populations and to down-regulate levels of inducible nitric oxide synthase (iNOS), interleukin (IL)-2 and interferon (IFN)-γ in reverse transcription–polymerase chain reaction. The cytotoxicity of LAK and NK cells was lower when pretreated with anti-histone H1 antibody than when pretreated with control IgG [5]. Furthermore, the addition of anti-histone H1 antibody to concanavalin A (ConA) blasts inhibited the proliferation of lymphocytes without toxicity but increased the population of CD4+ CD25+ T cells. DCs treated with anti-histone H1 antibody expressed lower levels of CD80/CD86, IL-1β and IL-6. These results suggest the possibility that a blockade of histone H1 modulates DCs towards tolerogenic status and then may induce CD4+ CD25+ T cells [5].
Dendritic cells are the most important antigen-presenting cells that play a significant role in bridging innate resistance and adaptive immunity [6,7]. Immature DCs reside in peripheral tissues where during infection they release soluble mediators (cytokines, chemokines and IFNs) that participate in innate inflammatory responses [7,8]. Although the maturation mechanism of DCs remains unknown, recent reports have focused upon the important role of nuclear proteins such as HMGB1 and histone H1 on the maturation, differentiation and function of DCs [5,9]. Exogenous HMGB1 promotes DC maturation [10,11] and also controls effectively the activation of T lymphocytes [10]. Gabrilovich et al. has suggested that histone H1 may be an important factor in normal DC differentiation because DC production and differentiation in histone h1°-knock-out mice were reduced significantly [12]. We have also demonstrated in a previous study that DCs treated with anti-histone H1 antibody down-regulate iNOS and histone h1° gene expression through decreased nuclear factor kappa B (NF-κB)-dependent transcription [5]. These findings suggest that NF-κB signalling inactivation because of the inhibition of histone H1 may result in the suppression of DC maturation, although the exact nature of this suppression is unknown. Histones, which bind to the linker DNA between nucleosomal cores, facilitate the formation of higher-order chromatin structures with the nucleosome dyad [13]. These structures, which were once believed to arise only in chromatin gathering and remodelling, are now recognized to carry out a multitude of functions in various cellular and extracellular locations. These functions include acting as an innate immunity effector and cellular receptor as well as participating in both the signalling and advertisement of apoptosis [14,15]. Therefore, the main aim of the present study was to investigate how the blockade of histone H1 affects innate immunity and intracellular signalling pathways during DCs maturation and subsequent T cell activation.
Materials and methods
Animals
Male DA (major histocompatibility complex haplotype RT1a) and PVG (RT1c) rats were obtained from Japan SLC (Hamamatsu, Japan) and the Institute of Laboratory Animals of the Graduate School of Medicine, Kyoto University (Kyoto, Japan) respectively. All animals were maintained in specific pathogen-free animal facilities with water and commercial rat food provided ad libitum. All the experiments were carried out following institutional animal ethical committee guidelines.
Dendritic cells purified from bone marrow cultures
Thigh bones obtained from DA rats were cleaned of muscle tissue and placed in sterile Petri dishes containing complete medium [RPMI-1640 with 10% fetal bovine serum (FBS), penicillin/streptomycin 100 U/ml and 100 μg/ml and 2 mM l-glutamine], following the procedures described elsewhere [5]. On day 8, the non-adherent cells were aspirated for DCs culture. Briefly, the wells were then washed once with medium, and the cells were pooled. DCs were placed in fresh DC culture medium (DCM) containing 50 ng/ml recombinant granulocyte-macrophage colony-stimulating factor (rGM-CSF), 50 ng/ml rIL-4 (CytoLab, Ltd, Rehovot, Israel) and either endotoxin [lipopolysaccharide (LPS); 1 μg/ml; Sigma, St Louis, MO, USA] or calf thymus histone H1 (10 μg/ml; Upstate, Charlottesville, VA, USA) and cultured in a new six-well plate with or without the addition of anti-histone H1 antibody (10 μg/ml) (Santa Cruz Biotechnology, Santa Cruz, CA, USA) and rabbit IgG (10 μg/ml) (Santa Cruz Biotechnology) respectively. The DCs were then transferred onto adhesion slides for immunoassays. Bone marrow (BM)-derived adherent cells were cultured for 8 days in six-well tissue culture plates in DCM supplemented with 50 ng/ml rGM-CSF and 50 ng/ml rIL-4 with or without anti-histone H1 antibody. The harvested cells were stained with fluorescein isothiocyanate (FITC)-conjugated anti-histocompatibility complex class (MHC) class II, CD86 and CD80 (BD Biosciences Pharmingen, San Diego, CA, USA) and then analysed by an Epics® ALTRA™ flow cytometer (Beckman Coulter, Fullerton, CA, USA) using EXPO32 software. The supernatants and cells were harvested and stored at −70°C until used.
Isolation of naive CD4+ T cells from rat splenocytes
Rat CD4+ T cells were isolated from mononuclear cells using the MagCellect Rat CD4+ T cell isolation kit (R&D Systems, Inc., Minneapolis, MN, USA), following the manufacturer's instructions. Briefly, mononuclear cells were obtained from the spleen of PVG rats after centrifugation in Lymphoprep™ medium (density 1·077; Gibco™ Invitrogen Corporation, Carlsbad, CA, USA) at 400 g for 20 min. The cells were resuspended in phosphate-buffered saline (PBS)/0·1% bovine serum albumin and washed three times. The mononuclear cells were incubated with MagCellect Rat CD4+ T cell antibody cocktail (CD4+ T cell kit) for 15 min, and MagCellect GAM Ferrofluid was then added to the cell suspension for 15 min. The reaction tube was placed in the MagCellect magnet (Dynal Biotech, ASA, Oslo, Norway) and incubated for 10 min at room temperature. Magnetically tagged cells were migrated towards the magnet, leaving the untouched desired cells in suspension in the supernatant. The purity of the naïve CD4+ T cells was typically >90%.
Culture of CD4+ T cells with DCs
CD4+ T cells were labelled by carboxyfluorescein succinimidyl ester (CFSE; Sigma), as described previously [5]. DCs were incubated with LPS alone or in the presence of anti-histone H1 antibody or control rabbit IgG cultured with 5-carboxy-succinimidyl-fluorescein-ester (CFSE)-labelled CD4+ T cells. DCs were washed twice with culture medium (RPMI-1640 with 10% FBS, penicillin/streptomycin 100 U/ml and 100 μg/ml) (to rule out the direct effect of LPS and/or anti-histone H1 antibody on CD4+ T cells) prior to mixing with CD4+ T cells. The T cells were mixed with allogeneic DCs at a ratio of 10:1 and were plated at 5 × 105 cells/ml in a 48-well plate. In the positive control, CFSE-labelled T cells were stimulated with 1 μg/ml anti-CD3 antibody or 2·5 μg/ml ConA. In some experiments, supernatants of various DC cultures were added to anti-CD3 antibody-stimulated CFSE-labelled CD4+ T cells. Cultured cells from each well were harvested after 5 days and preincubated with mouse anti-rat CD32 (FcγII receptor) (BD Biosciences Pharmingen) to block non-antigen-specific binding of Igs. Cells were incubated at 4°C for 30 min with allophycocyanin-conjugated mouse anti-rat CD4 antibody (BD Biosciences Pharmingen). Two-colour flow cytometery was performed on an Epics® ALTRA™ flow cytometer (Beckman Coulter) using EXPO32 software.
Immunostaining for histone H1 of rat BM-derived DCs
Cellular localization of histone H1 was assayed by immunostaining of rat BM-derived DCs using rabbit anti-histone H1 polyclonal antibodies (Santa Cruz Biotechnology). After 24 h of culturing, DCs were transferred onto adhesion slides and incubated at 37°C for 1 h to allow adherence. The adhesion cells were fixed with phosphate-buffered formaldehyde (4%; pH 7.4) at 4°C for 10 min and permeabilized with Triton X-100. After the slides were washed three times with PBS buffer, the cells were incubated sequentially with anti-OX-62 (Serotec, Oxford, UK) and anti-histone H1 antibody. Binding of the primary monoclonal antibody (mAb) was detected with FITC-conjugated anti-rabbit IgG and phycoerythrin-conjugated anti-mouse IgG (antibody diluted with antibody dilution buffer; Dako, Glostrup, Denmark). The nuclei were counterstained with 4′,6-diamidino-2-phenylindole (Molecular Probes, Invitrogen Corporation).
Immunoblot analysis of histone H1
The levels of histone H1 in the supernatants of various cultured DCs were assessed by Western blotting. The supernatants were electrophoresed on 10% sodium dodecyl sulphate-polyacrylamide gel electrophoresis (SDS-PAGE) gels and transferred onto polyvinylidene difluoride (PVDF) membranes (Bio-Rad Laboratories, Inc., Hercules, CA, USA). Membranes were blocked with 5% (w/v) skimmed milk/0·1% (v/v) Tween 20 in PBS for 30 min at room temperature, incubated overnight with anti-histone H1 antibody (Santa Cruz Biotechnology) at 4°C and detected with horseradish peroxidase-coupled secondary antibodies (anti-rabbit IgG; Santa Cruz Biotechnology). Colour development was performed with an enhanced chemiluminescence (ECL) plus detection kit (Millipore Corporation, Bedford, MA, USA). Relevant bands were quantified by densitometry using UVI photo version 99 (UVItec, Cambridge, UK).
Western blot analysis of cellular protein kinases
Cell lysates (30–40 μg) were electrophoresed on 10% SDS-PAGE gels and transferred onto PVDF membranes (Bio-Rad). Membranes were blocked with 5% (w/v) skimmed milk/0.1% (v/v) Tween 20 in PBS for 30 min at room temperature and incubated overnight with primary antibody at 4°C. Antibodies to phospho- and total p38, phospho- and total extracellular signal-regulated kinase (ERK), phospho- and stress-activated protein kinase/c-Jun N-terminal kinase (SAPK/JNK) and phospho- and total IκBα were all purchased from Cell Signalling (Cell Signalling Technology, Inc., Danvers, MA, USA). Antibody to β-actin were obtained from Santa Cruz Biotechnology. Detection was performed with an ECL plus detection kit (Millipore), and relevant bands were quantified by densitometry using UVI photo version 99 and TotalLab software version 1·00 (Nonlinear USA Inc., Durham, NC, USA).
Lactate dehydrogenase assay
Cells damage was measured by a cytotoxicity detection kit (Roche Applied Science, Mannheim, Germany) in the various DCs cultured. DCs treated with 1% Triton X-100 were used as a positive control of cell damage. The supernatants were harvested by centrifuge at 250 g for 10 min. One hundred μl of supernatant were removed carefully and transferred into the wells of an optically clear 96-well flat-bottomed plate. One hundred μl of reaction mixture (as per the manufacturer's instructions) were added to each well and the mixture was incubated for up to 30 min at room temperature, after which absorbance was measured at 490 nm using a MRX Microplate Reader (Dynatech Laboratories, Chantilly, VA, USA).
Statistical analysis
Descriptive statistics, means, standard deviations (s.d.) and ranges were used where appropriate. For comparison of groups, one-way analysis of variance and Duncan's post hoc test were used where appropriate. The results are given as mean values ± s.d. of the mean. A value of P < 0·05 was considered to indicate statistical significance.
Results
Rat BM-derived maturing DCs translocate nuclear histone H1 into cytosol and secrete it in the extracellular milieu
When BM cells obtained from DA rats were cultured with rGM-CSF and rIL-4, high levels of histone H1 were expressed in the nuclei of iDCs, as shown by immunocytochemistry (Fig. 1a). We also confirmed the DC-specific morphology of cultured cells in our assay system, and that most histone H1-translocating cells expressed a rat DC lineage marker OX62 (data not shown). At 24 h after the addition of endotoxin, iDCs were found to change the localization of histone H1; histone H1 leaves the nucleus and reaches the cytoplasmic vesicles (Fig. 1b). The immunoblot analyses of histone H1 in various DC cultured supernatants are shown in Fig. 1c. The translocation of nuclear histone H1 to the cytoplasmic vesicles precedes its release in the medium (Fig. 1c), while the addition of anti-histone H1 antibody induces histone H1 down-regulation. Moreover, lactate dehydrogenase (LDH) is not released in the medium (Fig. 1d), suggesting that the accumulation of histone H1 in the culture supernatants was not correlated with cell damage or release of LDH.
Fig. 1.
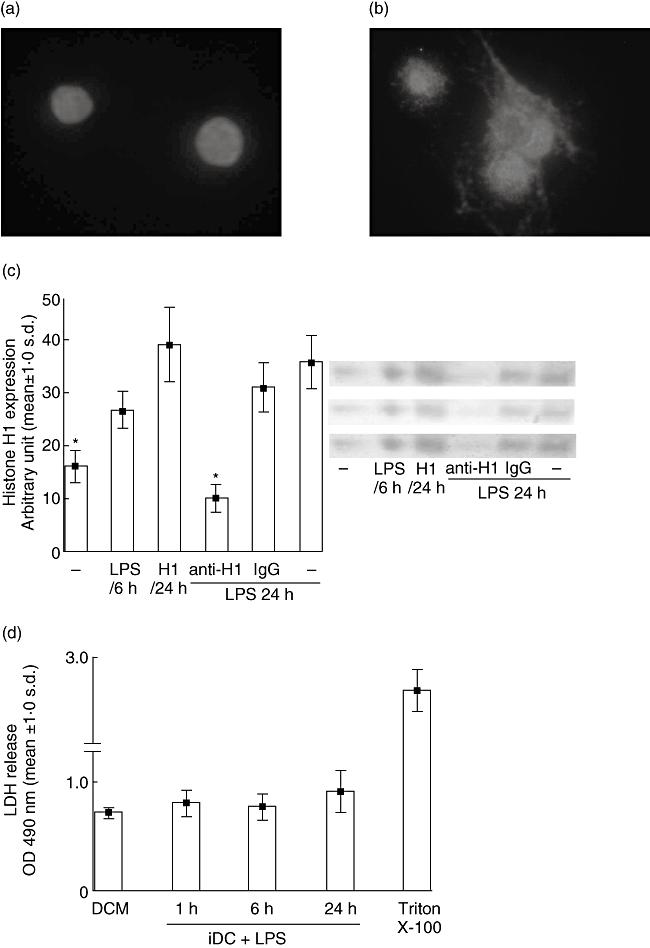
Maturing dendritic cells (DCs) secrete histone H1. (a) Histone H1 staining (white) is present in the nuclei of immature DCs (iDCs). (b) Upon maturation of DCs with endotoxin [lipopolysaccharide (LPS)], histone H1 staining is seen predominantly in the cytoplasm. (c) DCs were stimulated with histone H1 alone or stimulated with an endotoxin in the presence of either anti-histone H1 antibody or control immunoglobulin G (IgG), and cell-conditioned culture supernatants were assayed for levels of histone H1 protein by Western blotting analysis. The extracellular levels of histone H1 increased with time, while anti-histone H1 added into DCs cultures decreased histone H1 levels. (d) Cell damage was detected by lactate dehydrogenase (LDH). DCs treated with 1% Triton X-100 were used as a positive control of cell damage [mean ± standard deviation (s.d.): 2·70 ± 0·19]. The accumulation of histone H1 in the culture supernatants was not correlated with the release of LDH. Results are representative of three independent experiments with essentially similar tendencies. DCM, DC cultures medium (DCM) alone. Representative examples of three independent experiments are shown as the mean ± s.d. of duplicate cultures. *P < 0·05. LPS, lipopolysaccharide.
Histone H1 modulates the maturation of DCs
In our previous studies, endotoxin-treated DCs failed to mature when anti-histone H1 antibody was added [5]. This suggests that sustained histone H1 is necessary for DC maturation. We therefore evaluated the expression of MHCII, CD80 and CD86 molecules after endotoxin or histone H1 stimulation by flow cytometry. Although DCs appended with endotoxin up-regulate the expression of all markers (Fig. 2), anti-histone H1 antibody significantly hindered CD80 and CD86, while leaving the expression of MHCII unaffected (Fig. 2). DCs appended with histone H1 expressed all markers without endotoxin treatment compared with iDCs (Fig. 2).
Fig. 2.
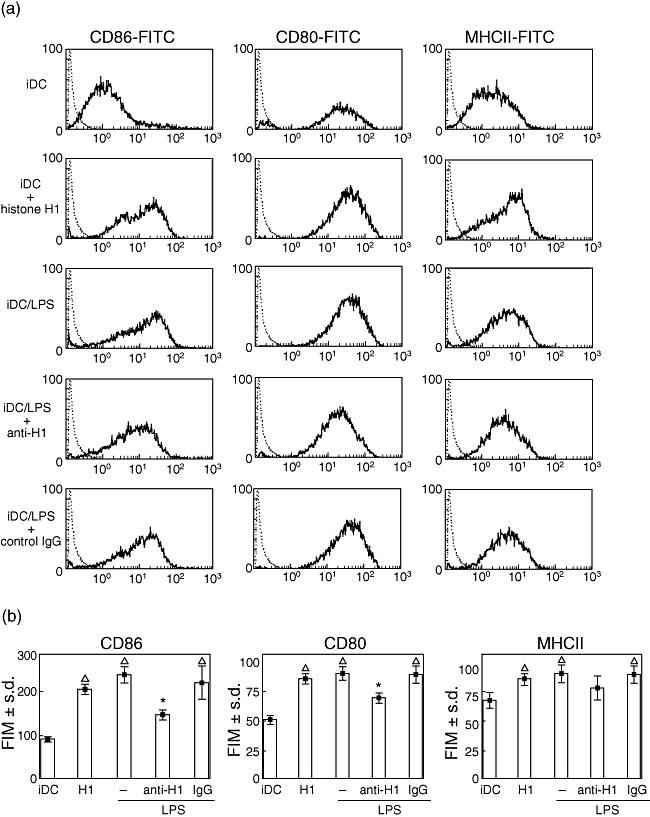
Expression of co-stimulatory molecules on dendritic cells (DCs). DCs were propagated from Dark Agouti bone marrow in recombinant interleukin-4 and recombinant granulocyte–macrophage colony-stimulating factor, and on day 8 were stimulated by endotoxin [lipopolysaccharide (LPS)] in the presence of anti-histone H1 antibody or rabbit immunoglobulin G (IgG). In order to measure the effect of histone H1 in DCs maturation, the immature DCs (iDCs) were cultured with histone H1 without endotoxin stimulation on day 8. The expression of the DCs maturation surface markers including CD 80, CD86 and major histocompatibility complex (MHC) II was determined by flow cytometric analysis. (a) Surface expression of the indicated markers is overlaid between isotype control (dotted lines) and iDCs treated with either endotoxin or histone H1 in various systems. (b) Representative examples of three independent experiments are shown as the mean ± standard deviation of duplicate cultures. *P < 0·05 versus IgG; Δ, P < 0·05 versus iDCs. FITC, fluorescein isothiocyanate, LPS, lipopolysaccharide.
Anti-histone H1 antibody suppresses DC activity
We next analysed whether the activity of maturing DCs treated with anti-histone H1 antibody influences the cross-talk with naive CD4+ T cells. CFSE-labelled CD4+ T cells were cultured with DCs treated with anti-histone H1 antibody or DCs treated with control rabbit IgG. All cells were harvested after 5 days, and CFSE fluorescence was detected by flow cytometry. Daughter T cells, derived from the responder splenocytes following DC stimulation, were differentiated from undivided T cells and identified based on the intensity of CFSE staining (Fig. 3a). Maturing DCs induced the expansion of CD4+ T cells (46·1 ± 3·6%), and a histone H1 blockade induced by antibodies abrogated T cell expansion (33·2 ± 3·2%, P < 0·01). Irrelevant control IgG was ineffective (Fig. 3b).
Fig. 3.
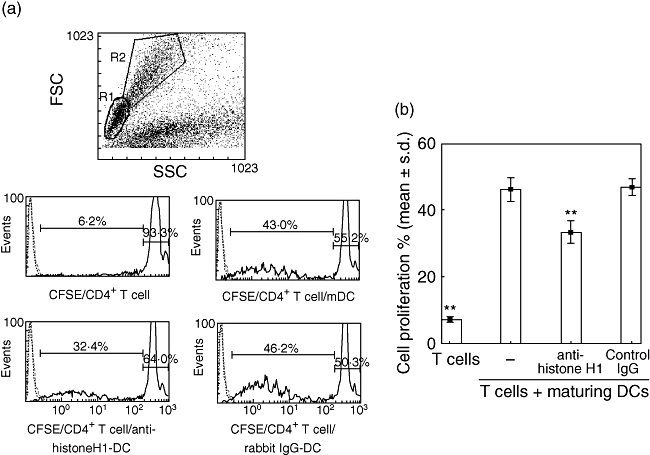
Histogram plots show the cell division associated with carboxyfluorescein succinimidyl ester (CFSE)-labelling of Piebald Virol Glaxo CD4+ T cells stimulated by mature dendritic cells (DCs) alone or by a DC culture in the presence of either anti-histone H1 antibody or control immunoglobulin G (IgG). (a) All plots were gated on CD4-positive cells (histograms not shown), while the histograms were also gated to include both resting lymphocytes (R1) and blasts (R2). CFSE-labelling of CD4+ T cells alone or T cells stimulated by mature DCs, DCs treated with anti-histone H1 antibody or DCs treated with control IgG. (b) A histone H1 blockade induced by anti-histone H1 antibody abrogates T cell proliferation. Data are representative of three independent experiments with essentially similar tendencies. **P < 0·01.
Secretion of histone H1 by DCs is required for CD4+ T cell proliferation
The proliferation of CFSE-labelled CD4+ T cells stimulated by CD3 mAb and cultured with various DC-cultured supernatants was analysed by flow cytometry (Fig. 4a). The effect of secreted histone H1 on CD3-triggered T cell activation was inhibited by a histone H1 blockade induced by anti-histone H1 antibodies compared with control IgG (Fig. 4b).
Fig. 4.
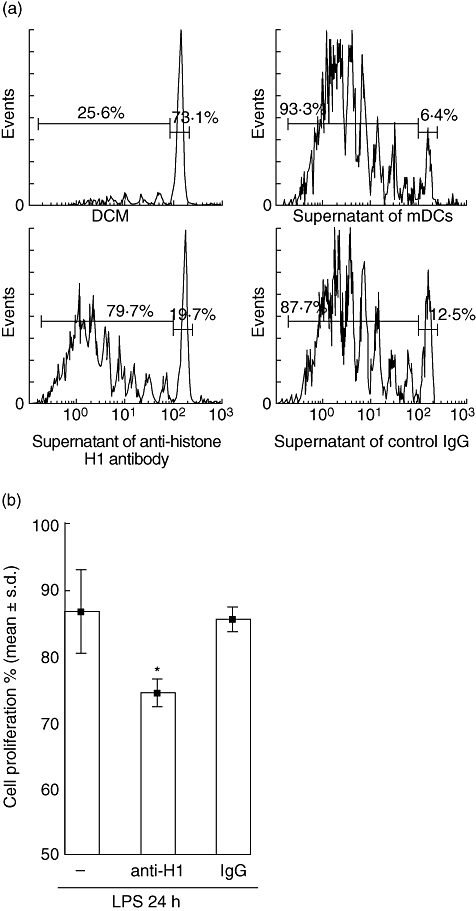
Secretion of histone H1 by dendritic cells (DCs) is required for CD4+ T cell proliferation. (a) Histogram plots show the cell division associated with carboxyfluorescein succinimidyl ester-labelling of Piebald Virol Glaxo CD4+ T cells stimulated with anti-CD3 monoclonal antibody in the presence of supernatants of DCs alone or supernatant of anti-H1 antibody-DCs, supernatant of control immunoglobulin G-DCs. (b) A secreted histone H1 blockade induced by anti-histone H1 antibody reduced T cell proliferation. Data are representative of three independent experiments with essentially similar tendencies. *P < 0·05. LPS, lipopolysaccharide.
Dendritic cell activation is suppressed by anti-histone H1 antibody via the inactivation of mitogen-activated protein kinases p38 and IκB
We assessed the phosphorylation of p38, ERK1/2, SAPK/JNK and IκBα in DCs after endotoxin stimulation in the presence or absence of a histone H1 blockade. Toll-like receptor 4 (TLR-4) activation induced an early and transient phosphorylation of p38, ERK1/2 and IκBα. Indeed, the activation of p38, ERK1/2, SAPK/JNK and IκBα was observed after 1 h endotoxin stimulation in DCs (Fig. 5a). We also observed the comparable phosphorylation of p38, ERK1/2, SAPK/JNK and IκBα in iDCs cultured with histone H1 but without endotoxin stimulation. In this early phosphorylation of ERK1/2 and SAPK/JNK was not influenced by anti-histone H1 antibody, while phosphorylation of p38 and IκBα was found to be decreased significantly by anti-histone H1 antibody at 1 h (Fig. 5a). Six hours of endotoxin stimulation significantly increased the phosphorylation of p38 and IκBα (Fig. 5b). Anti-histone H1 antibodies induced a significant decrease in the phosphorylation of p38 and IκBα, but not in that of ERK1/2 or SAPK/JNK (Fig. 5b) at 6 h after TLR triggering, while control antibodies had no effect (Fig. 5b).
Fig. 5.

Dendritic cell (DC) activation suppressed by anti-histone H1 antibody via the inactivation of mitogen-activated protein kinases p38, IκBα and nuclear factor kappa B. Immature DCs (iDCs) were incubated with histone H1 or endotoxin [lipopolysaccharide (LPS)] alone (–) or in the presence of anti-histone H1 antibodies (anti-H1) or control immunoglobulin G (IgG). The samples were analysed by Western blotting with anti-phospho-p38 (P-p38), anti-phospho-extracellular-regulated kinase (ERK)1/2 (P-ERK1/2), anti-phospho-stress-activated protein kinase/c-Jun N-terminal kinase (SAPK/JNK) or anti-phospho-IκBα (P-IκB) antibodies. Total amounts of proteins were evaluated after stripping the membranes and blotting with antip38, -ERK1/2, -SAPK/JNK, -IκBα or -β-actin antibodies. (a) We observed an increase in the phosphorylation of p38, IκB, ERK1/2 and SAPK/JNK in DCs 1 h after endotoxin stimulation. Anti-histone H1 antibodies decreased the amounts of phosphorylated p38 and IκBα. (b) iDCs were stimulated with endotoxin (LPS) for 6 h alone or in the presence of anti-histone H1 antibody or control IgG. Treatment with control IgG did not alter the phosphorylation of proteins after endotoxin stimulation. However, the phosphorylation of p38 and IκBα still inhibited by anti-histone H1 antibody. Densitometric quantifications are shown at the bottom as ratios. Results are represented as the mean of three independent experiments ± standard deviation. *P < 0·05.
Discussion
The presence of histones in cytoplasm has already been demonstrated [16] and the role of histone H1 has been transformed from one of cytoplasmic loitering to one of active intracellular signalling in apoptosis studies [17]. In fact, the production of DCs in h1°-deficient mice is affected specifically, while it appears normal with respect to the function of granulocytes and macrophages [12]. Additionally, it has been reported that histone H1 accumulates after cell proliferation stops during culturing [18] and that, in histone h1° knock-out mice, DCs maturation is down-regulated and is unable to stimulate a proliferative response in naive T-cells, while T cells from histone h1°-deficient mice show normal function [12]. In our previous studies, we have provided evidence that autoreactive antibodies against nuclear histone H1 are a major immunosuppressive factor induced in serum during a rejection phase after OLT [1,2]. Additionally, our later study suggested that anti-histone H1 antibody inhibited the maturation of DCs and also suppressed the proliferation of lymphocytes [5]. In the present study, we have demonstrated that histone H1 is present in the cytoplasm after endotoxin stimulation, and that there is an inhibitory effect of anti-histone H1 antibody on DCs via a blockade of p38 and IκB.
Dendritic cell fractions from knock-out mice were unable to stimulate such a response in T cells from knock-out mice, and only DCs from naive mice were capable of inducing a primary T cell response. Additionally, T cells from h1° knock-out mice responded well to stimulation with control DCs [12]. Our experiments have shown that DCs suppressed their action on naive allogeneic CD4+ T cells through a blockade of histone H1. The mechanism of association between h1° and DC development remains unclear. We assume that DCs release their own histone H1 actively into the milieu upon activation. This event may be necessary for the maturation of DCs themselves and for cross-talk with CD4+ T cells. This speculation is supported by the following results shown in Fig. 1. In DCs that had undergone endotoxin stimulation, histone H1 was translocated into cytoplasm and released into culture medium. Under neutralizing antibodies, histone H1 levels decreased in the culture medium. The activation of T cell proliferation induced by CD3 antibody decreased in a histone H1 blockade environment. Our data also show that histone H1 is involved in controlling NF-κB expression. DCs cultured with anti-histone H1 antibody down-regulated NF-κB expression [5]. As NF-κB activation is critically important for DC differentiation, it is possible that increased expression of histone H1 at the down-stream region of NF-κB is required for suppression of the genes inhibiting DC maturation [19].
Interleukin-12 secretion, ERK1/2 and p38 SAPK (p38), together with the transcription factor NF-κB, are involved in the maturation of DCs [20,21]. The activation of NF-κB can be evaluated by the NF-κB binding inhibitory protein, IκB. Degradation after the phosphorylation of IκB allows the nuclear translocation of NF-κB and the subsequent activation of transcription. NF-κB activation may be controlled by histone H1 because NF-κB is down-regulated significantly in histone h1°-deficient mice [12,22,23]. HMGB1 is another nuclei protein that interacts not only with TLR-4 but also with TLR-2 and the receptor for advanced glycation end products (RAGE), leading to the activation of p38, ERK1/2 and NF-κB in maturing DCs [9,24]. In the present study, we found that the phosphorylation of p38 and IκBα were down-regulated in the presence of anti-histone H1 antibody for 1 h and 6 h compared with that of mDCs treated with control IgG. Our data suggest that an appropriate time of exposure to histone H1 may be necessary for DC maturation via the activation of mitogen-activated protein kinases (p38 and IκBα) and NF-κB. Our data show that DCs cultured with either anti-histone H1 antibody or histone H1 did not affect the RAGE level based on immunoblot analysis (data not shown), suggesting that another receptor for histone H1 may be present in DCs. Our previous study strongly implies the existence of histone H1 or histone H1-like antigens on the splenic cell surface and its expression level was increased after Con-A stimulation [1,2]. Recently, we have produced monoclonal histone H1 antibody [25] and is now sequencing membrane receptors reacted by this mAb. Therefore, we hypothesize that anti-histone H1 antibody might cross-react with cell surface histone H1(-like) molecules on DCs and inhibit DC maturation through intracellular signalling pathways.
In conclusion, we have demonstrated that the translocation and secretion of histone H1 may play an important role in DC maturation and activation for T cell proliferation. Although this mechanism can be explained in part via the intracellular signalling pathway, further investigation and the identification of a histone H1 receptor would help in establishing novel immunotherapies for cancer, allergies, autoimmune diseases and allograft rejection.
Acknowledgments
This work was supported by grants from the National Health Research Institute (NHRI-EX94-9228SP to C.-L. C.), National Science Council (NSC 95-2314-B-182A-089 to C.-L. C.; NSC 95-2314-B-182A-132 to Y.-F. C.) and Chang Gung Memorial Hospital (Chang Gung Medical Research Project; CMRPG850052 to T. N.; CMRPG850071 to C.-L. C.; CMRPG860181 to Y.-C. H.) of Taiwan.
References
- 1.Nakano T, Kawamoto S, Lai CY, et al. Liver transplantation-induced antihistone H1 autoantibodies suppress mixed lymphocyte reaction. Transplantation. 2004;77:1595–603. doi: 10.1097/01.tp.0000123079.10650.71. [DOI] [PubMed] [Google Scholar]
- 2.Nakano T, Kawamoto S, Lai CY, et al. Characterization of immunosuppressive factors expressed in serum by rat tolerogenic liver transplantation. Transplant Proc. 2005;37:80–1. doi: 10.1016/j.transproceed.2004.12.290. [DOI] [PubMed] [Google Scholar]
- 3.Nakano T, Goto S, Lai CY, et al. Experimental and clinical significance of antinuclear antibodies in liver transplantation. Transplantation. 2007;83:1122–5. doi: 10.1097/01.tp.0000258646.54562.c7. [DOI] [PubMed] [Google Scholar]
- 4.Nakano T, Goto S, Lai CY, et al. Impact of vaccine therapy using nuclear histone H1 on allograft survival in experimental organ transplantation. Transpl Immunol. 2007;17:147–52. doi: 10.1016/j.trim.2007.01.003. [DOI] [PubMed] [Google Scholar]
- 5.Hsu LW, Goto S, Nakano T, et al. The effects of anti-histone H1 antibody on immune cells responsible for rejection reaction. Mol Immunol. 2005;42:1155–64. doi: 10.1016/j.molimm.2004.11.010. [DOI] [PubMed] [Google Scholar]
- 6.Steinman RM, Kaplan G, Witmer MD, Cohn ZA. Identification of a novel cell type in peripheral lymphoid organs of mice. V. Purification of spleen dendritic cells, new surface markers, and maintenance in vitro. J Exp Med. 1979;149:1–16. doi: 10.1084/jem.149.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Banchereau J, Steinman RM. Dendritic cells and the control of immunity. Nature. 1998;392:245–52. doi: 10.1038/32588. [DOI] [PubMed] [Google Scholar]
- 8.Lanzavecchia A, Sallusto F. Regulation of T cell immunity by dendritic cells. Cell. 2001;106:263–6. doi: 10.1016/s0092-8674(01)00455-x. [DOI] [PubMed] [Google Scholar]
- 9.Dumitriu IE, Baruah P, Valentinis B, et al. Release of high mobility group box 1 by dendritic cells controls T cell activation via the receptor for advanced glycation end products. J Immunol. 2005;174:7506–15. doi: 10.4049/jimmunol.174.12.7506. [DOI] [PubMed] [Google Scholar]
- 10.Rovere-Querini P, Capobianco A, Scaffidi P, et al. HMGB1 is an endogenous immune adjuvant released by necrotic cells. EMBO Rep. 2004;5:825–30. doi: 10.1038/sj.embor.7400205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Messmer D, Yang H, Telusma G, et al. High mobility group box protein 1: an endogenous signal for dendritic cell maturation and Th1 polarization. J Immunol. 2004;173:307–13. doi: 10.4049/jimmunol.173.1.307. [DOI] [PubMed] [Google Scholar]
- 12.Gabrilovich DI, Cheng P, Fan Y, et al. H1(0) histone and differentiation of dendritic cells. A molecular target for tumor-derived factors. J Leukoc Biol. 2002;72:285–96. [PubMed] [Google Scholar]
- 13.Misteli T, Gunjan A, Hock R, Bustin M, Brown DT. Dynamic binding of histone H1 to chromatin in living cells. Nature. 2000;408:877–81. doi: 10.1038/35048610. [DOI] [PubMed] [Google Scholar]
- 14.Seuss D. Oh, the places you'll go! New York: Random House; 1990. [Google Scholar]
- 15.Parseghian MH, Luhrs KA. Beyond the walls of the nucleus: the role of histones in cellular signaling and innate immunity. Biochem Cell Biol. 2006;84:589–604. doi: 10.1139/o06-082. [DOI] [PubMed] [Google Scholar]
- 16.Zlatanova JS, Srebreva LN, Banchev TB, Tasheva BT, Tsanev RG. Cytoplasmic pool of histone H1 in mammalian cells. J Cell Sci. 1990;96:461–8. doi: 10.1242/jcs.96.3.461. [DOI] [PubMed] [Google Scholar]
- 17.Konishi A, Shimizu S, Hirota J, et al. Involvement of histone H1.2 in apoptosis induced by DNA double-strand breaks. Cell. 2003;114:673–88. doi: 10.1016/s0092-8674(03)00719-0. [DOI] [PubMed] [Google Scholar]
- 18.Lennox RW, Cohen LH. The histone H1 complements of dividing and nondividing cells of the mouse. J Biol Chem. 1983;258:262–8. [PubMed] [Google Scholar]
- 19.Gabrilovich D, Ishida T, Oyama T, et al. Vascular endothelial growth factor inhibits the development of dendritic cells and dramatically affects the differentiation of multiple hematopoietic lineages in vivo. Blood. 1998;92:4150–66. [PubMed] [Google Scholar]
- 20.Ardeshna KM, Pizzey AR, Devereux S, Khwaja A. The PI3 kinase, p38 SAP kinase, and NF-kappaB signal transduction pathways are involved in the survival and maturation of lipopolysaccharide-stimulated human monocyte-derived dendritic cells. Blood. 2000;96:1039–46. [PubMed] [Google Scholar]
- 21.Kikuchi K, Yanagawa Y, Iwabuchi K, Onoe K. Differential role of mitogen-activated protein kinases in CD40-mediated IL-12 production by immature and mature dendritic cells. Immunol Lett. 2003;89:149–54. doi: 10.1016/s0165-2478(03)00134-2. [DOI] [PubMed] [Google Scholar]
- 22.Cruz MT, Goncalo M, Figueiredo A, Carvalho AP, Duarte CB, Lopes MC. Contact sensitizer nickel sulfate activates the transcription factors NF-κB and AP-1 and increases the expression of nitric oxide synthase in a skin dendritic cell line. Exp Dermatol. 2004;13:18–26. doi: 10.1111/j.0906-6705.2004.00105.x. [DOI] [PubMed] [Google Scholar]
- 23.Crispe IN. Hepatic T cells and liver tolerance. Nat Rev Immunol. 2003;3:51–62. doi: 10.1038/nri981. [DOI] [PubMed] [Google Scholar]
- 24.Park JS, Svetkauskaite D, He Q, et al. Involvement of Toll-like receptors 2 and 4 in cellular activation by high mobility group box 1 protein. J Biol Chem. 2004;279:7370–7. doi: 10.1074/jbc.M306793200. [DOI] [PubMed] [Google Scholar]
- 25.Shimada Y, Goto T, Kawamoto S, et al. Development of a two-step chromatography procedure that allows the purification of a high-purity anti-histone H1 monoclonal immunoglobulin M antibody with immunosuppressant activity. Biomed Chromatogr. 2008;22:13–9. doi: 10.1002/bmc.887. [DOI] [PubMed] [Google Scholar]


