Abstract
Mesangioproliferative glomerulonephritis (MsPGN) is a disease of high incidence in humans. Rats with Thy-1 nephritis (Thy-1 N) are used as an animal model for studying MsPGN. Although several studies have demonstrated that many pathological factors could cause the injury of glomerular mesangial cells (GMCs) in Thy-1 N, changes of profile and the molecular mechanism of the disease (i.e. the role of transcription factors) at intervals remain unclear. The purpose of this study was to identify the changes in gene expression profile and to observe the role of nuclear factor kappa B (NF-κB) on the pathological change of renal tissue in Thy-1 N rats. Our results showed that the pathological changes of GMCs in Thy-1 N included three phases: apoptosis (40 min), necrosis (24 h) and proliferation (7 days). Concomitantly, at 40 min and on day 7, the up-regulation of 341 genes and 250 genes were observed, while 392 genes and 119 genes were down-regulated in Thy-1 N. Expression of interleukin (IL)-1β, IL-6, proliferating cell nuclear antigen, α-smooth muscle actin, collagen type IV and excretion of urinary protein was increased in rats with Thy-1 N and decreased in pyrrolidine dithiocarbamate-treated rats with Thy-1 N. These data indicated that the significant changes in the gene profile were coupled with the pathological changes of Thy-1 N, and activation of NF-κB may contribute to the pathogenesis of GMCs apoptosis, proliferation, extracelluar matrix accumulation and proteinuria in Thy-1 N.
Keywords: extracelluar matrix, glomerular mesangial cells, mesangioproliferative glomerulonephritis, nuclear factor kappa B, Thy-1 nephritis
Introduction
Mesangioproliferative glomerulonephritis (MsPGN), characterized by the production of a variety of cytokines, glomerular mesangial cells (GMCs) damage and extracelluar matrix (ECM) accumulation, is a disease of high incidence in humans. Thy-1 nephritis (Thy-1 N), induced by Thy-1 antibody binding to the corresponding antigen on the membrane of GMCs, is a well-known model of human MsPGN [1]. Previous studies of Thy-1 N have revealed that glomerular injury is complement-dependent and neutrophil-independent. The complement C5b-9 complexes induced injury of GMCs by increasing several inflammatory mediators or cytokines [2–4]. However, a comprehensive overview on the mechanism governing the initiation and progression of MsPGN has not been well elucidated.
Microarray analysis is a powerful technique that allows the simultaneous detection of thousands of differently expressed genes [5,6]. The value of evaluating gene expression profiles has been documented in animal models of several diseases [7]. Microarray analysis can be applied to kidney disease in providing more efficient tools in clinical glomerulonephritis [6–8]. In this study, we first created large-scale gene expression profiles for a rat MsPGN model to find pathological genes which are potential contributors to GMC dysfunction in Thy-1 N. Up-regulation of the nuclear factor kappa B (NF-κB) gene was found in the process of Thy-1 N. NF-κB is a pivotal transcription factor that regulates genes that control multiple immune and inflammatory responses, as well as cell proliferation and apoptosis [7]. NF-κB is activated in several forms of experimental and human glomerulonephritis, including immune complex kidney disease [8–11], crescentic glomerulonephritis [12] and lupus nephritis [9]. We then explored the role of NF-κB on the pathological changes including GMC apoptosis, necrosis, proliferation, ECM accumulation and proteinuria of rats with Thy-1 N.
Materials and methods
Induction to Thy-1 N and experimental protocols
Thy-1 antibody against Thy-1 antigen of rat thymocyte was prepared as documented previously [1]. Using the immunohistochemical method, the titre of Thy-1 antibody was assessed to be 1:320 [1].
Twenty-four normal female Sprague–Dawley rats (180–200 g) with normal complement activity were maintained in the laboratory animal centre of Nanjing Medical University under controlled-environment conditions and were given access to food and water ad libitum. They were divided randomly into three groups (eight in each group), as follows: (i) in the Thy-1 N model group, the rats were administered Thy-1 antibody (0·5 ml/100 g body weight) by a single intravenous injection; (ii) in the pyrrolidine dithiocarbamate (PDTC) + Thy-1 N group, the rats were treated intraperitoneally with PDTC (12 mg/100 g body weight; Sigma, St Louis, MO, USA) prior to Thy-1 antibody injection and were then given the same dose each day from days 1–7; (iii) in the control group the rats were injected with normal rabbit serum (0·5 ml/100 g body weight).
Samples of the rat renal cortices were obtained at 40 min, 24 h or on day 7 (7 days) by kidney biopsies under ether anaesthesia. Some samples were collected and pooled for microarray analysis and reverse transcription–polymerase chain reaction (RT–PCR), and others were embedded in octreotide (OCT), paraffin and Epon 812 respectively.
Microarray analysis
Total RNA isolation, cDNA synthesis, in vitro transcription and microarray analysis were performed as reported previously [13]. Briefly, total RNA from rat renal cortices with or without Thy-1 N was extracted and sent to Shanghai Gene Company for analysis. The chips were scanned by an Agilent scanner and read with Imagene software to analyse the intensities of the fluorescent signals. The data were normalized by Genespring, and the Cy3/Cy5 ratios of the two groups (Thy-1 N and control) were obtained to screen out differently expressed genes: the down-regulated genes with a ratio of lower than 0·5 and the up-regulated genes with a ratio higher than 2.
Reverse transcription–polymerase chain reaction
The mRNA levels of renal tissue in rats with Thy-1 N at 40 min, 24 h and on day 7 after administration of Thy-1 antibody were assayed by RT–PCR. As described above, equal amounts of total RNA (2 μg) from each sample were converted to cDNA. The RT reaction was subject to PCR amplification in a 20-μl reaction volume with 0·5 μmol/l of each primer. The primer sequences were as follows: interleukin (IL)-1β, forward primer, 5′-CTGCAGCTGGAGAGTGTGG-3′ and reverse primer, 5′-CAT CCC ATA CAC ACG GAC AAC TAG-3′; IL-6, forward primer, 5′-AGA GGA TAC CAC CCA CAA C-3′ and reverse primer, 5′-GTT TCG GTC TCA GTA AGT C-3′; and β-actin, forward primer 5′-TGA CGT TGA CAT CCG TAA AG-3′ and reverse primer 5′-ACA GTG AGG CCA GGA TAG AG-3′. PCR was performed at 94°C for 10 min followed by 28 cycles of denaturation, annealing and extension at 94°C for 30 s, 58°C for 30 s and 72°C for 1 min, respectively, and the final extension at 72°C for 10 min. PCR reactions for each sample were performed in duplicate. Amplication products were run on 1% agarose gel. Ratios for IL-1β and IL-6/β-actin mRNA were calculated for each sample.
Immunohistochemical examination
The paraffin-embedded samples of the tissues from renal cortices (4 μm) were examined to detect NF-κB p65, proliferatiny cell nuclear antigen (PCNA), α-smooth muscle actin (α-SMA) and collagen type IV (CL-IV) protein at 40 min, 24 h and on day 7 by indirect immunohistochemistry [14]. In order to investigate NF-κB activation, we used a monoclonal antibody (anti-NF-κB, p65 subunit: MAB3026; Chemicon International, Inc., Temecula, CA, USA) that recognizes specifically an epitope on the p65 subunit that is masked by bound IκB. Several studies have shown that this antibody detects activated NF-κB exclusively, because it recognizes p65 only in the absence of IκB [15–17]. In brief, the sections were incubated with monoclonal antibodies of NF-κB p65 (1:150), PCNA (1:75; Dako, Copenhagen, Denmark), α-SMA (1:50; Sigma) or CL-IV (1:40; Neumarker, Fremont, CA, USA) and followed incubation with horseradish peroxidase-conjugated secondary antibody (1:500; Jackson, West Grove, PA, USA) or biotinylated anti-mouse IgG for 30 min and visualized by avidin–biotin peroxidase reaction (Vector, Philadelphia, PA, USA). Negative controls were incubated without primary antibody. NF-κB p65-positive and PCNA-positive cells were counted in 20 glomeruli from each section. The semiquantitative analysis on optical density (OD) value of α-SMA and CL-IV staining by immunohistochemistry was completed by NYD-1000 colour picture analysis through detecting the OD value of 20 glomeruli per section densitometry under microscope. These proteins were determined by calculating the mean OD value from all specimens in the three groups.
Renal histological examination
The samples of the renal cortices of each rat were obtained at 40 min, 24 h and on day 7 by kidney biopsy and embedded in OCT, Epon 812 or paraffin. For light microscopy (LM), histological sections (4 μm) of all samples were stained with haematoxylin and eosin, and the glomerular cellularity was evaluated by counting the total number of nuclei in 10 representative glomeruli from each section. With electron microscopy (EM), ultrathin sections of all samples were stained with uranyl acetate and lead citrate, and the ultrastructural changes were examined.
Measurement of urinary protein
Urine samples from the three groups of rats were collected at −24 h, 24 h and on day 7 after different treatments. The concentration of urinary protein was detected by total protein UC FS kit (DiaSys Diagnostic Systems, Holzheim, Gemany).
Statistical analysis
All results are given as means ± standard deviation. Statistical analysis was performed by one-way analysis of variance with simultaneous multiple comparisons between groups by the Scheffé method. The P-value of < 0·05 was considered to be statistically significant.
Results
Gene expression profile of renal tissues in rats with Thy-1 N
To draw a composite map of the gene expression profile, we performed microarray experiments for the MsPGN model (Thy-1 N) at 40 min and on day 7. Of 10 002 clone spots 341 genes were up-regulated, 392 genes were down-regulated at 40 min and 250 genes were up-regulated, and 119 genes were down-regulated on day 7 after administration of Thy-1 antibody. Some up-regulated genes involved in the progression of Thy-1 N were presented in Table 1. Based on searches of the biological functions in PubMed, the up-regulated genes which may be associated with the development of Thy-1 N were categorized into six groups: (i) genes related to apoptosis and necrosis, such as nerve growth factor induced protein I-B, growth arrest and DNA-damage-inducible protein 45 gamma (Gadd45γ); (ii) genes related to cell proliferation, such as regucalcin, microtubule-associated protein 1a, cyclin D3; (iii) genes related to fibrosis, such as S100 calcium-binding protein A4 (S100A4), connective tissue growth factor (CTGF) and collagen, type 1, alpha 1; (iv) genes related to monocyte cytokines, such as IL-1β and IL-6, tumour necrosis factor (TNF)-α, small inducible cytokine family such as monocyte chemoattractant protein-1 and chemokine receptors (Ccr2 and Ccr3); (v) genes related to transcription factors, such as NF-κB, interferon regulatory factor 1 and activating transcription factor 3; and (vi) others.
Table 1.
Genes up-regulated during the progression of Thy-1 nephritis (Thy-1 N).
| Ratio (Cy3/Cy5) | ||||
|---|---|---|---|---|
| Symbol | Gene description | Gene accession | 40 min | 7 days |
| NGFI-B | Nerve growth factor-induced protein I-B | NM_024388 | 15.62 | 1.00 |
| Gadd45γ | Growth arrest and DNA-damage-inducible protein 45 gamma | AB0202978 | 5.40 | 0.96 |
| TNFR1a | Tumour necrosis factor-receptor, member la | NM_013091 | 5.20 | 3.71 |
| PCD 6 | Programmed cell death 6 | BC026823 | 2.41 | 0.87 |
| C-fos | c-fos oncogene | X06769 | 4.85 | 6.39 |
| CCND3 | Cyclin D3 | NM_012766 | 4.01 | 5.26 |
| MAP1a | Microtubule-associated protein la | NM_030995 | 6.32 | 3.25 |
| EGR1 | Early growth response 1 | NM_012551 | 2.22 | 1.21 |
| IGFBP1 | Insulin-like growth factor binding protein 1 | NM_013144 | 3.60 | 2.95 |
| Regucalcin | Regucalcin | NM_031546 | 4.29 | 5.82 |
| CL1A1 | Collagen, type 1, alpha 1 | M12199 | 1.50 | 3.33 |
| CL8 | Procollagen, type XIII | XM_122043 | 3.01 | 4.21 |
| S100A9 | S100 calcium-binding protein A9 | NM_053587 | 2.14 | 3.11 |
| CTGF | Connective tissue growth factor | NM_022266 | 10.56 | 1.40 |
| Laminin receptor 1 | Laminin receptor 1 | NM_017138 | 3.48 | 3.26 |
| IL-1β | Interleukin-1β | NM_031512 | 2.00 | 4.80 |
| IL-6 | Interleukin-6 | NM_012589 | 2.20 | 2.50 |
| Ccr2 | Chemokine (C-C motif) receptor 2 | NM_053647 | 2.12 | 2.81 |
| Ccr3 | Chemokine (C-C motif) receptor 3 | U22414 | 1.90 | 2.31 |
| ScyA2(MCP-1) | Small inducible cytokine A2/monocyte chemoattractant peptide-1 | NM_031530 | 8.57 | 5.76 |
| ScyA4 | Small inducible cytokine A4 | NM_U06434 | 3.25 | 2.19 |
| NF-κB | Nuclear factor kappa B | AA858801 | 0.52 | 2.01 |
| ATF-3 | Activating transcription factor 3 | NM_012912 | 2.40 | 1.73 |
| IRF-1 | Interferon regulatory factor 1 | NM_012591 | 2.00 | 0.54 |
| etc. | ||||
Activation of NF-κB in renal tissues of rats with Thy-1 N
The mRNA transcripts of NF-κB were found to be up-regulated slightly at 40 min and up-regulated significantly on day 7 by microarray experiments compared with the control group. In order to ensure the location of the activation NF-κB protein, the NF-κB p65 was detected by immunohistochemistry in renal tissues of rats with Thy-1 N. The NF-κB p65 protein began to express in nuclei at 40 min, then increased gradually at 24 h and peaked on day 7 (Fig. 1a). However, in the control group, only faint positive staining was visible at the same time. Quantitative analysis of the NF-κB p65-positive cells in renal tissue revealed that the number of NF-κB p65-positive cells in the Thy-1 N group at 40 min, 24 h and on day 7 was much greater than in the control group (Fig. 1b). The protein expression of NF-κB p65 by immunohistochemistry was consistent with the results of the microarray. These results implied that transcript factor NF-κB was activated consistently along the whole Thy-1 N progression including apoptosis, necrosis and proliferation.
Fig. 1.
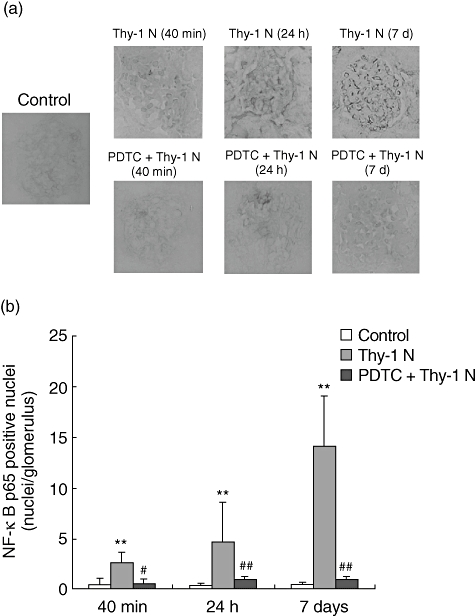
Results of immunohistochemical staining for nuclear factor kappa B (NF-κB) p65. The rats were administered rabbit serum intravenously (control) or Thy-1 nephritis (Thy-1 N) antibody, or treated intraperitoneally with pyrrolidine dithiocarbamate (PDTC) prior to Thy-1 antibody injection and were then given the same dose each day from days 1–7 (PDTC + Thy-1 N). (a) Representative micrographs of glomeruli showed changes of NF-κB p65 by immunohistochemistry at 40 min, 24 h and on day 7 (7 days) in control rats or Thy-1 N rats or PDTC-treated rats on day 7. In Thy-1 N rats, the NF-κB p65 protein began to express in nuclei and staining-positive cells presented at 40 min, then increased gradually at 24 h and peaked on day 7, compared with the control group. Fewer positive cells were seen in the PDTC + Thy-1 N group rats (×400). (b) Quantitative analysis of the NF-κB p65-positive cells in glomerulus shows that the number of NF-κB-positive cells was clearly increased at 40 min, 24 h and on day 7 in the Thy-1 N group compared with the control group (**P < 0·01), and there were fewer positive cells in the glomeruli of PDTC + Thy-1 N group rats at the corresponding time, compared with the Thy-1 N group (#P < 0·05, ##P < 0·01).
Effects of PDTC on NF-κB, IL-1β and IL-6 production in renal tissue of rats with Thy-1 N
Pyrrolidine dithiocarbamate is a potent and specific inhibitor of NF-κB. PDTC administered to rats with Thy-1 N inhibited the NF-κB activation in glomeruli. Immunohistochemistry showed that there was significant reduction of NF-κB-positive cells in the PDTC + Thy-1 N group at 40 min, 24 h and on day 7 compared with the Thy-1 N group (Fig. 1). To investigate expression of the NF-κB-dependent genes IL-1β and IL-6 in Thy-1 N rats and PDTC-treated rats, RT–PCR for IL-1β and IL-6 mRNA was performed on glomerular RNA. Densitometry showed a significant increase of IL-1β and IL-6 in Thy-1 N rats compared with the control rats, and a marked decrease in PDTC-treated rats compared with Thy-1 N rats on day 7 (Fig. 2).
Fig. 2.
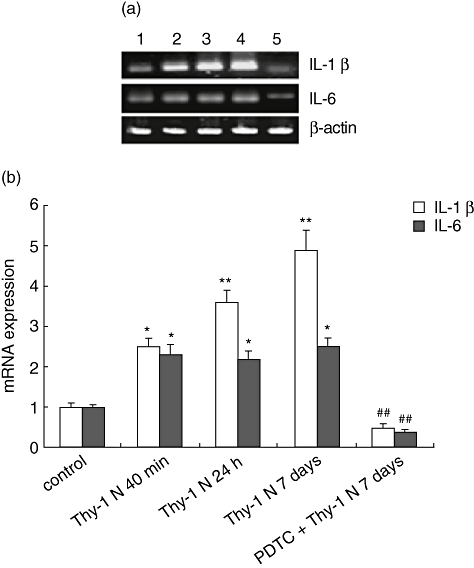
Glomerular expression of interleukin (IL)-1β and IL-6 mRNA in Thy-1 nephritis (Thy-1 N) rats and pyrrolidine dithiocarbamate (PDTC)-treated Thy-1 N rats (PDTC + Thy-1 N, as described in Methods) by reverse transcription–polymerase chain reaction. PCR amplified over a spectrum of cycles. (a) Amplified products were analysed by agarose gel electrophoresis. Representative bands showed expression of IL-1β, IL-6 and β-actin mRNA in glomerular RNA of control (lane 1), Thy-1 N 40 min (lane 2), Thy-1 N 24 h (lane 3), Thy-1 N day 7 (lane 4) and PDTC + Thy-1 N day 7 (lane 5) groups. (b) Densitomertry of bands was completed and values represented fold expression ± standard deviation of IL-1β or IL-6 to β-actin ratio. Semiquantitative analysis showed that expression of IL-1β and IL-6 in the Thy-1 N group at 40 min, 24 h and on day 7 after Thy-1 antibody administration was higher than in the control group (*P < 0·05, **P < 0·01), and there was a striking reduction of IL-1β and IL-6 in the PDTC-treated rats in comparison with Thy-1 N rats on day 7 (##P < 0·01).
Effects of PDTC on glomerular PCNA, α-SMA and CL-IV expression in renal tissue of rats with Thy-1 N
Cytokine expression plays a key role in the pathogenesis of kidney disease, which is involved in tissue remodelling and repair by mesangial cell proliferation and ECM accumulation. PCNA and α-SMA are two of the most extensively investigated parameters of GMCs proliferation, and their up-regulation in renal tissue of rats with Thy-1 N were confirmed using immunohistochemistry (Fig. 3a). On day 7, ECM proteins were accumulated in the glomeruli (Fig. 4). CL-IV protein was increased in renal tissue of rats with Thy-1 N, as shown by immunohistochemistry (Fig. 3a). However, in the PDTC + Thy-1 N group, the number of PCNA-positive cells was markedly less than that in the Thy-1 N group at 24 h and on day 7, but at 40 min there were no significant differences in the three groups. Semiquantitative analysis indicated that at 40 min and 24 h, the relative levels of α-SMA and CL-IV protein were not obviously lower in the PDTC + Thy-1 N group compared with that in the Thy-1 N group. On day 7, however, there were clear reductions in the PDTC + Thy-1 N group compared with the Thy-1 N group (Fig. 3b).
Fig. 3.
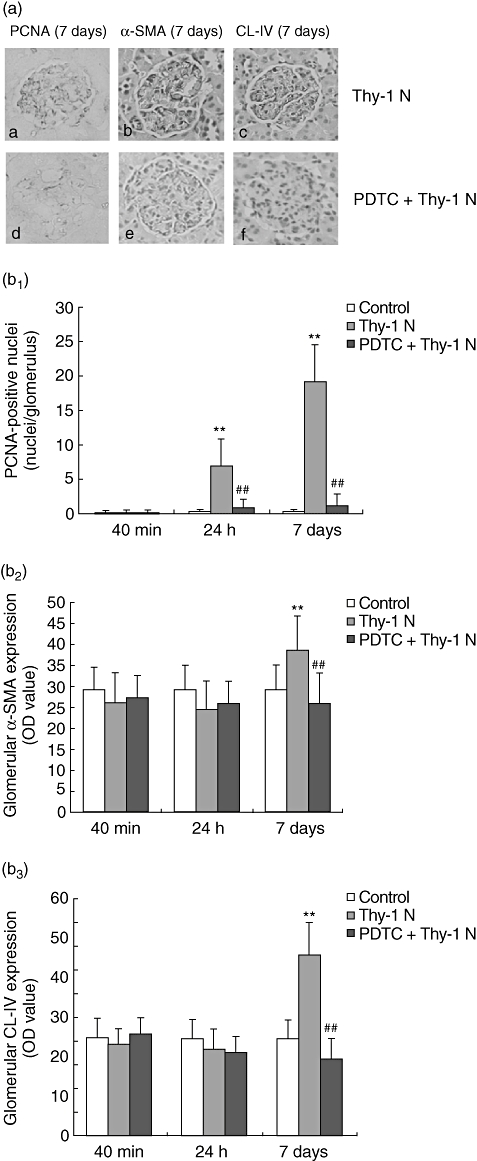
Results of immunohistochemical staining for proliferating cell nuclear antigen (PCNA), α-smooth muscle actin (α-SMA) and collagen type IV (CL-IV). (a) Representative micrographs of immunohistochemical staining for PCNA, α-SMA and CL-IV in glomerulus of Thy-1 nephritis (Thy-1 N) rats (a, b, c) or pyrrolidine dithiocarbamate (PDTC)-treated rats (d, e, f) on day 7 (×400). PCNA-positive cells were decreased and α-SMA and CL-IV were weakly positive in glomeruli of PDTC-treated rats compared with Thy-1 N rats. (b1) Quantitative analysis of the PCNA-positive cells disclosed that the number of PCNA-positive cells was clearly increased at 24 h and on day 7 in Thy-1 N group compared with the control group (**P < 0·01), and there were fewer positive cells in the PDTC + Thy-1 N group rats at the above-mentioned time compared with the Thy-1 N group (##P < 0·01). (b2, b3) Semiquantitative analysis on OD value of α-SMA and CL-IV staining by immunohistochemistry showed that the relative levels of α-SMA and CL-IV protein in Thy-1 N group on day 7 were clearly greater than in the control group (**P < 0·01). However, in the PDTC + Thy-1 N group, there were striking reductions of α-SMA and CL-IV proteins on day 7, compared with the Thy-1 N group (##P < 0·01).
Fig. 4.
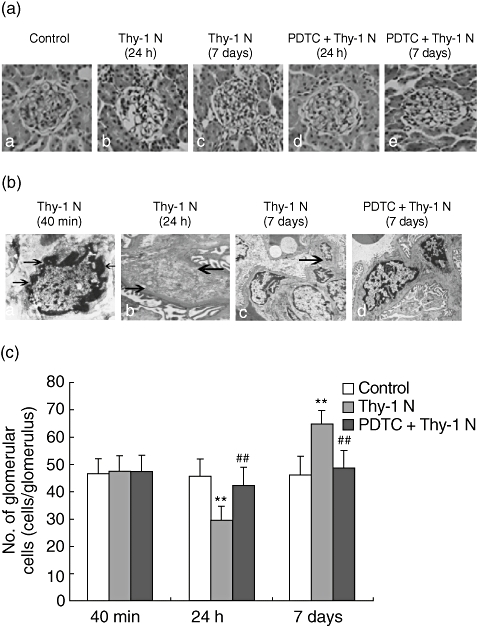
Glomerular morphological changes in Thy-1 nephritis (Thy-1 N) rats and pyrrolidine dithiocarbamate (PDTC)-treated (PDTC + Thy-1 N) rats (a). Representative micrographs show morphological changes of glomeruli at 24 h and on day 7 in rats (haematoxylin and eosin staining). In Thy-1 N rats, glomerular cellularity was reduced concurrently with focal capillary destruction and ballooning of the glomerular tuft at 24 h (b), and marked segmental mesangial hypercellularity was found on day 7 (c). In PDTC + Thy-1 N rats, no marked pathological changes were seen at 24 h (d) and on day 7 (e), compared with control rats (a) (×400). (b) Representative electron micrographs showed ultrastructural changes of glomeruli at 40 min, 24 h and on day 7 in Thy-1 N rats and PDTC-treated rats with Thy-1 N on day 7. (a) Irregular aggregation of chromatin in the periphery of the nucleus and clear condensation of the nuclear chromatin in glomerular mesangial cells (GMCs) were shown in the Thy-1 N group at 40 min after Thy-1 antibody injection (×6000). (b) Mesangial cells lysised and disappeared with mesangial matrix destruction at 24 h (×6000). (c) Proliferation of GMCs and accumulation of extracelluar matrix (ECM) were observed on day 7 (×3500). (d) In the PDTC + Thy-1 N group, the glomeruli were enlarged with less mesangial hypercellularity and ECM accumulation compared with the Thy-1 N group on day 7 (×4000). (c) Comparison of the total number of glomerular cells among the three groups indicated that the number of glomerular cells in the Thy-1 N group was much lower than in the control group at 24 h, and remarkably higher than that in the control group on day 7 (**P < 0·01). In the PDTC + Thy-1 N group, the glomerular cell number was significantly higher at 24 h and clearly lower on day 7, compared with the Thy-1 N group (##P < 0·01).
Effect of PDTC on the renal damage in Thy-1 N rats
Following the previously described protocols, signs of glomerular morphological changes were observed in the control group, the Thy-1 N group and the PDTC + Thy-1 N group. Using LM, the glomerular cells began to decrease at 40 min and decreased significantly 24 h after administration of Thy-1 antibody. However, glomerular focal hypercellularity was remarkable on day 7 (Fig. 4a). Using EM, GMCs of rats with Thy-1 N exhibited clear condensation of nuclear chromatin and gathering at the nuclear margins at 40 min, demonstrating that the mesangial cell had entered apoptosis. At 24 h, some GMCs began to lyse and showed a reduction of electron density in the mesangial region. On day 7, the glomeruli were enlarged with mesangial hypercellularity and accumulation of ECM. However, in the PDTC + Thy-1 N group, there was less mesangial hypercellularity and ECM accumulation in the glomeruli (Fig. 4b). Rats in the control group at the same phases showed normal glomerular histology. The results of glomerular cell counts indicated that there was no significant difference in the three groups at 40 min and an obvious reduction at 24 h in Thy-1 N group, compared with the PDTC + Thy-1 N group. With the progress of Thy-1 N, on day 7 the number of glomerular cells in the PDTC + Thy-1 N group were significantly fewer than in the Thy-1 N model (Fig. 4c).
Effect of PDTC on the urinary protein excretion of rats with Thy-1 N
Administration of PDTC reduced NF-κB activation in Thy-1 N rats, as mentioned above, so its effect on the development of proteinuria was also examined. The level of urinary protein excretion in the PDTC + Thy-1 N group was much lower than in the Thy-1 N model group at 24 h and at 7 days (Fig. 5).
Fig. 5.
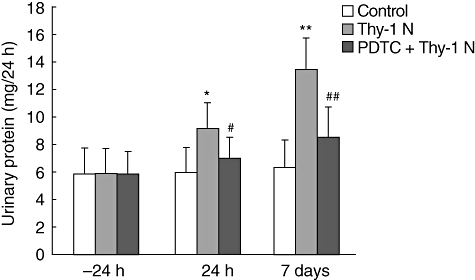
Measurement of urinary protein level. Urinary protein excretion is shown for 24 h and day 7 following induction of Thy-1 nephritis (Thy-1 N) rats. The amount of total urinary protein in Thy-1 N group was higher than that in the control group at 24 h and on day 7 (P < 0·05, P < 0·01), and in the pyrrolidine dithiocarbamate + Thy-1 N group the amount of total urinary protein was remarkably lower at 24 h and on day 7, compared with the Thy-1 N group (#P < 0·05, ##P < 0·01).
Discussion
Rats with Thy-1 N are used as an animal model for studying human MsPGN, characterized by a series of immunological events [18–20]. In our experiment, at 40 min the damaged GMCs showed typical morphological apoptotic features, and at 24 h there was an apparent reduction in total glomerular cellularity, indicating that necrotic degeneration began to emerge in injured GMCs and to disappear from glomeruli. On day 7, proliferation of GMCs and accumulation of ECM were observed under LM and EM. These results suggest that the course of Thy-1 N involves three phases, namely apoptosis, necrosis and proliferation, consistent with previous studies [21–25].
Glomerular mesangial cells damage in Thy-1 N is associated with pathological factors such as Thy-1 antibody, complement C5b-9 complex and inflammatory mediators, including IL-1β or TNF-α[18,21,22,26], but the mechanism of Thy-1 N has not been understood fully. In order to explore the cause and pathogenesis of the disease, we used microarray analysis to monitor the temporal changes in gene expression together with pathological changes of GMCs in rats with Thy-1 N. Our data showed that, at 40 min after administration of Thy-1 antibody, the up-regulation of 341 genes and down-regulation of 392 genes, and on day 7 the up-regulation of 250 genes and down-regulation of 119 genes, were exhibited in 10 002 clone spots. Previous studies on gene expression in experimental kidney disease have also shown that renal mRNA levels for some cytokines and ECM components can be used as a prognostic marker [27–29]. On the other hand, NF-κB regulates the transcription of many genes, including cytokines and proteases as well as enzymes involved in reactive oxygen species (ROS) and prostaglandin synthesis [30–33]. In the experiment, NF-κB gene was up-regulated by microarray. Molecules regulated transcriptionally by NF-κB have been reported to be a central response of inflammatory models of renal diseases and various human glomerulonephritis [34,35]. Therefore, the focus of our study was to determine whether NF-κB was activated in Thy-1 N and whether it might contribute to the development of pathogenic progress of Thy-1 N.
As an inactive heterodimer (p50/p65), NF-κB is present in the cytoplasm bound by an inhibitor, IκB, which prevents the transcription factor from migrating into the nucleus. Inflammatory signals induce phosphorylation of IκB by specific kinases, followed by degradation through the ubiquitin–proteasome pathway. Activated NF-κB translocates into the nucleus where it regulates the transcription of target genes containing NF-κB consensus sequences in their promoter region. Using immunohistochemistry, we found that the NF-κB p65 protein localized in the nucleus presented at 40 min, increased gradually at 24 h and peaked on day 7 after administration of Thy-1 antibody. The up-regulation of NF-κB target genes [36,37] in our microarray are shown partly in Table 1. In glomerulonephritis, activation of NF-κ B led to expression of proinflammatory genes including inducible nitric oxide synthase (iNOS) [38], cytokines, chemokines and adhesion molecules. Activity of the iNOS had been identified in several models of glomerulonephritis [39–41]. In Thy-1 N, the iNOS induction was complement-dependent [4,42], the mRNA level of iNOS was up-regulated by dot-blot [4] and iNOS-positive cells in glomeruli were confirmed by double-labelled immunohistochemistry [43]. Based on these previous findings, the essential role of iNOS in Thy-1 N was clear and we may pay attention to expression of cytokines such as IL-1β and IL-6 to be regulated by NF-κB in this study. RT–PCR analysis of glomerular RNA showed increased levels of IL-1β and IL-6 mRNA at 40 min, 24 h and on day 7 in Thy-1 N rats, and immunohistochemistry analysis of glomerular protein showed significant increased expression of PCNA, α-SMA and CL-IV on day 7. These results indicated that NF-κB was activated in Thy-1 N and it is possible that disruption of NF-κB-activating pathways may effectively influence activation of GMCs and accumulation of ECM. PDTC, a potent inhibitor of NF-κB activation, was used in the study. As a scavenger of free radicals and a chelator of heavy metalions, when inhibiting the activation of NF-κB PDTC is thought to interfere with the signalling of intracellular hydroxyl radicals, which precedes phosphorylation of IκBa [44–47] and, to our knowledge, there is no effect of PDTC on the immune response. Our study showed that PDTC inhibited the Thy-1 antibody-induced increase of glomerular NF-κB, followed by the inhibition of mRNA expression of IL-1β and IL-6. PDTC also decreased expressions of PCNA, α-SMA and CL-IV and prevented urinary protein excretion in rats with Thy-1 N. From another viewpoint, these results also suggested that NF-κB was activated in Thy-1 N and might play a crucial role in the pathogenic setting of Thy-1 N.
It is uncertain that the mechanism leads to activation of NF-κB in Thy-1 N. As has been reported in other cell types, complement injury to mensigal cells might activate NF-κB directly [31]. However, activation of NF-κB could also occur indirectly. Complement injury might result in stimulation of GMCs by inflammatory mediators such as ROS [47]. Therefore, such stimulation might activate NF-κB, amplifying acute renal injury by increasing transcription of some cytokines and chemokines regulated by NF-κB. In previous studies on Thy-1 N, the roles of cytokines such as IL-1β[48], IL-6 [49], platelet-derived growth factor (PDGF), basic fibroblast growth factor (bFGF), transforming growth factor-β (TGF-β) [50] and CTGF [28] in the mesangioproliferative response have been discussed. All these cytokines were present in various inflammatory cells and/or in GMCs themselves, thereby allowing these factors to exert paracrine and/or autocrine regulatory functions on GMCs. In vivo studies have shown that PDGF, bFGF and TGF-β participated in either the GMCs proliferation directly and indirectly (via macrophages), or ECM expansion that followed GMC injury with Thy-1 antibody. In many systems, CTGF had been shown clearly to promote ECM accumulation and was a key promoter of fibrosis. The precise mechanism responsible for activation of NF-κB in Thy-1 N requires further investigation.
In summary, we have created a large-scale gene expression profile for rat Thy-1 N model and demonstrated activation of NF-κB in Thy-1 N. The ability of PDTC to reduce GMC proliferation, ECM accumulation and urinary protein excretion in rats with Thy-1 N suggest that NF-κB activation might contribute to pathogenesis of Thy-1 N. These findings provide a comprehensive overview on the mechanism governing the initiation and development of Thy-1 N for studying human MsPGN in the future.
Acknowledgments
This work is funded by grants numbers 30571728 and 30471615 from the National Natural Science Foundations of China and no. 20060312005 from National Doctoral Foundation of China. The authors are grateful to Dr Bin Sun and Mrs Tao Peng for technical help.
References
- 1.Yamamoto T, Wilson CB. Complement dependence of antibody-induced mesangial cell injury in the rat. J Immunol. 1987;138:3758–65. [PubMed] [Google Scholar]
- 2.Couser WG, Pippin JW, Shankland SJ. Complement (C5b-9) induces DNA synthesis in rat mesangial cells in vitro. Kidney Int. 2001;59:905–12. doi: 10.1046/j.1523-1755.2001.059003905.x. [DOI] [PubMed] [Google Scholar]
- 3.Abe K, Li K, Sacks SH, Sheerin NS. The membrane attack complex, C5b-9, up-regulates collagen gene expression in renal tubular epithelial cells. Clin Exp Immunol. 2004;136:60–6. doi: 10.1111/j.1365-2249.2004.02411.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wang Y, He Q, Qin H, et al. The complement C5b-9 complexes induced injury of glomerular mesangial cells in rats with Thy-1 nephritis by increasing nitric oxide synthesis. Life Sci. 2006;79:182–92. doi: 10.1016/j.lfs.2005.12.053. [DOI] [PubMed] [Google Scholar]
- 5.Lu XC, Williams AJ, Yao C, et al. Microarray analysis of acute and delayed gene expression profile in rats after focal ischemic brain injury and reperfusion. J Neurosci Res. 2004;77:843–57. doi: 10.1002/jnr.20218. [DOI] [PubMed] [Google Scholar]
- 6.Preston GA, Waga I, Alcorta DA, et al. Gene expression profiles of circulating leukocytes correlate with renal disease activity in IgA nephropathy. Kidney Int. 2004;65:420–30. doi: 10.1111/j.1523-1755.2004.00398.x. [DOI] [PubMed] [Google Scholar]
- 7.Schwab K, Witte DP, Aronow BJ, Devarajan P, Potter SS, Patterson LT. Microarray analysis of focal segmental glomerulosclerosis. Am J Nephrol. 2004;24:438–47. doi: 10.1159/000080188. [DOI] [PubMed] [Google Scholar]
- 8.Izumi Y, Izumiya Y, Shiota M, et al. Gene expression profile in experimental mesangial proliferative glomerulonephritis. J Pharmacol Sci. 2004;96:91–4. doi: 10.1254/jphs.rc0040012. [DOI] [PubMed] [Google Scholar]
- 9.Ghosh S, Karin M. Missing pieces in the NF-kappaB puzzle. Cell. 2002;109:S81–S96. doi: 10.1016/s0092-8674(02)00703-1. [DOI] [PubMed] [Google Scholar]
- 10.Gomez-Guerrero C, Lopez-Franco O, Suzuki Y, et al. Nitric oxide production in renal cells by immune complexes: role of kinases and nuclear factor-kappaB. Kidney Int. 2002;62:2022–34. doi: 10.1046/j.1523-1755.2002.00653.x. [DOI] [PubMed] [Google Scholar]
- 11.Tsukinoki T, Sugiyama H, Sunami R, et al. Mesangial cell Fas ligand: upregulation in human lupus nephritis and NFkappaB-mediated expression in cultured human mesangial cells. Clin Exp Nephrol. 2004;8:196–205. doi: 10.1007/s10157-004-0301-3. [DOI] [PubMed] [Google Scholar]
- 12.Sakai N, Wada T, Furuichi K, et al. p38 MAPK phosphorylation and NF-kappaB activation in human crescentic glomerulonephritis. Nephrol Dial Transplant. 2002;17:998–1004. doi: 10.1093/ndt/17.6.998. [DOI] [PubMed] [Google Scholar]
- 13.Yano N, Endoh M, Fadden K, et al. Comprehensive gene expression profile of the adult renal cortex: analysis by cDNA array hybridization. Kidney Int. 2000;57:1452–9. doi: 10.1046/j.1523-1755.2000.00990.x. [DOI] [PubMed] [Google Scholar]
- 14.Huo R, Zhu Y, Ma X, Lin M, Zhou Z, Sha J. Differential expression of glucose-regulated protein 78 during spermatogenesis. Cell Tissue Res. 2004;316:359–67. doi: 10.1007/s00441-004-0885-7. [DOI] [PubMed] [Google Scholar]
- 15.Brand K, Page S, Rogler G, et al. Activated transcription factor nuclear factor-kappa B is present in the atherosclerotic lesion. J Clin Invest. 1996;97:1715–22. doi: 10.1172/JCI118598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Nonaka M, Chen XH, Pierce JE, et al. Prolonged activation of NF-kappaB following traumatic brain injury in rats. J Neurotrauma. 1999;16:1023–34. doi: 10.1089/neu.1999.16.1023. [DOI] [PubMed] [Google Scholar]
- 17.Hickenbottom SL, Grotta JC, Strong R, Denner LA, Aronowski J. Nuclear factor-kappaB and cell death after experimental intracerebral hemorrhage in rats. Stroke. 1999;30:2472–7. doi: 10.1161/01.str.30.11.2472. [DOI] [PubMed] [Google Scholar]
- 18.Nauta AJ, Daha MR, Tijsma O, Van de Water B, Tedesco F, Roos A. The membrane attack complex of complement induces caspase activation and apoptosis. Eur J Immunol. 2002;32:783–91. doi: 10.1002/1521-4141(200203)32:3<783::AID-IMMU783>3.0.CO;2-Q. [DOI] [PubMed] [Google Scholar]
- 19.Quigg RJ. Complement and autoimmune glomerular diseases. Curr Dir Autoimmun. 2004;7:165–80. doi: 10.1159/000075692. [DOI] [PubMed] [Google Scholar]
- 20.Turrnberg D, Cook HT. Complement and glomerulonephritis: new insights. Curr Opin Nephrol Hypertens. 2005;14:223–8. doi: 10.1097/01.mnh.0000165887.75501.24. [DOI] [PubMed] [Google Scholar]
- 21.Amere A, Coppo R. Role of apoptosis in pathogenesis and progression of renal diseases. Nephron. 2000;86:99–104. doi: 10.1159/000045725. [DOI] [PubMed] [Google Scholar]
- 22.Shimizu A, Kitamura H, Masada Y, et al. Complement-mediated killing of mesangial cells in experimental glomerulonephritis, cell death by a combination of apoptosis and necrosis. Nephron. 2000;86:152–60. doi: 10.1159/000045734. [DOI] [PubMed] [Google Scholar]
- 23.Sakai N, Iseki K, Suzuki S, et al. Uninephrectomy induces progressive glomerulosclerosis and apoptosis in anti-Thy1 glomerulonephritis. Pathol Int. 2005;55:19–26. doi: 10.1111/j.1440-1827.2005.01781.x. [DOI] [PubMed] [Google Scholar]
- 24.Wu SH, Wu XH, Lu C, Dong L, Zhou GP, Chen ZQ. Lipoxin A4 inhibits connective tissue growth factor-induced production of chemokines in rat mesangial cells. Kidney Int. 2006;69:248–56. doi: 10.1038/sj.ki.5000025. [DOI] [PubMed] [Google Scholar]
- 25.Krishnaswami S, Ly QP, Rothman VL, Tuszynski GP. Thrombospondin-1 promotes proliferative healing through stabilization of PDGF. J Sur Res. 2002;107:124–30. doi: 10.1006/jsre.2002.6485. [DOI] [PubMed] [Google Scholar]
- 26.Trachtman H. Nitric oxide and glomerulonephritis. Semin Nephrol. 2004;24:324–32. doi: 10.1016/j.semnephrol.2004.04.004. [DOI] [PubMed] [Google Scholar]
- 27.Dinarello CA. Interleukin-1 and interleukin-1 antagonism. Blood. 1991;77:1627–52. [PubMed] [Google Scholar]
- 28.Gupta S, Clarkson MR, Duggan J, Brady HR. Connective tissue growth factor: potential role in glomerulosclerosis and tubulointerstitial fibrosis. Kidney Int. 1996;49:1120–6. [Google Scholar]
- 29.Ranieri E, Gesualdo L, Petrarulo F, Schena FP. Urinary IL-6/EGF ratio: a useful prognostic marker for the progression of renal damage in IgA nephropathy. Kidney Int. 2001;50:1990–2001. doi: 10.1038/ki.1996.521. [DOI] [PubMed] [Google Scholar]
- 30.Neale TJ, Ojha PP, Exner M, et al. Proteinuria in passive Heymann nephritis is associated with lipid peroxidation and formation of adducts on type IV collagen. J Clin Invest. 1994;94:1577–84. doi: 10.1172/JCI117499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kilgore KS, Schmid E, Shanley TP, et al. Sublytic concentrations of the membrane attack complex of complement induce endothelial interleukin-8 and monocyte chemoattractant protein-1 through nuclear factor-κB activation. Am J Pathol. 1997;150:2019–31. [PMC free article] [PubMed] [Google Scholar]
- 32.Long J, Song N, Liu XP, Guo KJ, Guo RX. Nuclear factor-kappaB activation on the reactive oxygen species in acute necrotizing pancreatitic rats. World J Gastroenterol. 2005;11:4277–80. doi: 10.3748/wjg.v11.i27.4277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kim MJ, Ryu GR, Kang JH, et al. Inhibitory effects of epicatechin on interleukin-1beta-induced inducible nitric oxide synthase expression in RINm5F cells and rat pancreatic islets by down-regulation of NF-kappaB activation. Biochem Pharmacol. 2004;68:1775–85. doi: 10.1016/j.bcp.2004.06.031. [DOI] [PubMed] [Google Scholar]
- 34.Yoshioka K, Takemura T, Murakami K, et al. In situ expression of cytokines in IgA nephritis. Kidney Int. 1993;44:825–33. doi: 10.1038/ki.1993.317. [DOI] [PubMed] [Google Scholar]
- 35.Collins T, Read MA, Neish AS. Transcriptional regulation of endothelial cells adhesion molecules: NF-κB and cytokine-inducible enhancers. FEBS Lett. 1995;9:899–909. [PubMed] [Google Scholar]
- 36.Baldwin ASJ. The NFκB and IκB proteins: new discoveries and insights. Annu Rev Immunol. 1996;14:649–81. doi: 10.1146/annurev.immunol.14.1.649. [DOI] [PubMed] [Google Scholar]
- 37.Barnes PJ, Karin M. Nuclear factor-κB: a pivotal transcription factor in chronic inflammatory diseases. N Engl J Med. 1997;336:1066–71. doi: 10.1056/NEJM199704103361506. [DOI] [PubMed] [Google Scholar]
- 38.Lopez-Franco O, Suzuki Y, Sanjuan G, et al. Nuclear factor-κB inhibitors as potential novel anti-inflammatory agents for the treatment of immune glomerulonephritis. Am J Pathol. 2002;161:1497–505. doi: 10.1016/s0002-9440(10)64425-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Jansen A, Cook T, Taylor GM, et al. Induction of nitric oxide synthase in rat immune complex glomerulonephritis. Kidney Int. 1994;45:1215–19. doi: 10.1038/ki.1994.161. [DOI] [PubMed] [Google Scholar]
- 40.Cook HT, Ebrahim H, Jansen AS, Foster GR, Largen P, Cattell V. Expression of the gene for inducible nitric oxide synthase in experimental glomerulonephritis in the rat. Clin Exp Immunol. 1994;97:315–20. doi: 10.1111/j.1365-2249.1994.tb06087.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Cook HT, Sullivan R. Glomerular nitrite synthesis in in situ immune complex glomerulonephritis in the rat. Am J Pathol. 1991;139:1047–52. [PMC free article] [PubMed] [Google Scholar]
- 42.Mosley K, Waddington SN, Ebrahim H, Cook T, Cattell V. Inducible nitric oxide synthase induction in Thy 1 glomerulonephritis is complement and reactive oxygen species dependent. Exp Nephrol. 1999;7:26–34. doi: 10.1159/000020581. [DOI] [PubMed] [Google Scholar]
- 43.Goto S, Yamamoto T, Feng L, et al. Expression and localisation of inducible nitric oxide synthase in anti-thy-1 glomerulonephritis. Am J Pathol. 1995;147:1133–41. [PMC free article] [PubMed] [Google Scholar]
- 44.Schreck R, Meier B, Männel DN, Dröge W, Baeuerle PA. Dithiocarbamates as potent inhibitors of nuclear factor kappa B activation in intact cells. J Exp Med. 1992;175:1181–94. doi: 10.1084/jem.175.5.1181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Nurmi A, Vartiainen N, Pihlaja R, Goldsteins G, Yrjänheikki J, Koistinaho J. Pyrrolidine dithiocarbamate inhibits translocation of nuclear factor kappa-B in neurons and protects against brain ischaemia with a wide therapeutic time window. J Neurochem. 2004;91:755–65. doi: 10.1111/j.1471-4159.2004.02756.x. [DOI] [PubMed] [Google Scholar]
- 46.Parodi FE, Mao D, Ennis TL, Bartoli MA, Thompson RW. Suppression of experimental abdominal aortic aneurysms in mice by treatment with pyrrolidine dithiocarbamate, an antioxidant inhibitor of nuclear factor-kappaB. J Vasc Surg. 2005;41:479–89. doi: 10.1016/j.jvs.2004.12.030. [DOI] [PubMed] [Google Scholar]
- 47.Gao LJ, Qiu W, Wang YW, Xu WH, Xu J, Tong JX. Sublytic complement C5b-9 complexes induce thrombospondin-1 production in rat glomerular mesangial cells via PI3-k/Akt: association with activation of latent transforming growth factor-β1. Clin Exp Immunol. 2006;144:326–34. doi: 10.1111/j.1365-2249.2006.03069.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Tesch GH, Lan HY, Atkins RC, et al. Role of interleukin-1 in mesangial cell proliferation and matrix deposition in experimental mesangioproliferative nephritis. Am J Pathol. 1997;151:141–50. [PMC free article] [PubMed] [Google Scholar]
- 49.Eitner F, Westerhuis R, Burg M, et al. Role of interleukin-6 in mediating mesangial cell proliferation and matrix production in vivo. Kidney Int. 1997;51:69–78. doi: 10.1038/ki.1997.9. [DOI] [PubMed] [Google Scholar]
- 50.Floege J, Eng E, Yo ung BA, Johnson RJ. Factors involved in the regulation of mesangial cell proliferation in vitro and in vivo. Kidney Int Suppl. 1993;39:S47–54. [PubMed] [Google Scholar]


