Abstract
Ageing leads to immune system dysfunction and the accumulation of autoantibodies. Because the rapid phagocytic clearance of apoptotic cells is required to prevent the development of autoimmunity, we examined the relative clearance of apoptotic material in young and aged mice using two independent assays. First, 2-year-old mice were found to be impaired in their ability to clear apoptotic keratinocytes following ultraviolet irradiation of the skin. Secondly, peritoneal macrophages exposed to apoptotic Jurkat T cells in vivo displayed diminished phagocytic activity in aged mice compared with 8-week-old mice. Consistent with these findings, aged mice exhibited signs of autoimmunity with the appearance of anti-nuclear antibodies and increased kidney glomerular size as well as complement deposits within the glomeruli. In vitro assays revealed that the pretreatment of macrophages with the serum from aged mice led to a reduction in their ability to phagocytose apoptotic bodies compared with macrophages treated with serum from young mice. These data show that the ageing process is accompanied by a diminished ability to clear apoptotic debris. This accumulation of apoptotic debris could contribute to immune system dysfunction that occurs in aged organisms.
Keywords: ageing, apoptosis, autoimmunity, phagocytosis
Introduction
Ageing is associated with immunosenescence which involves diminished responses to pathogens and increased susceptibility to infection [1,2]. Ageing leads to declines in T and B cell populations. Memory T cells from elderly patients exhibit a reduced ability to proliferate, presumably because of repeated clonal expansion in response to antigen stimulation over a lifetime [3]. In turn, these reductions in T cell responses contribute to the reduction in the ability of B cells to produce high-affinity antibodies. Ageing also impairs cells that are involved in initiating an innate immune response. For example, neutrophils and macrophages from aged organisms display reduced signal transduction, chemotaxis and superoxide production in response to stimuli [4,5]. Macrophages from ageing organisms show impaired capabilities to phagocytose both pathogenic bacteria and charcoal particles [6]. Furthermore, dendritic cells from elderly subjects are reported to display a reduced ability to phagocytose apoptotic bodies in vitro than dendritic cells from young subjects [7].
Whereas immune system function decreases with age, the elderly display a paradoxical increase in the incidence of autoimmune diseases [8]. For example, age is a risk factor for the development of rheumatoid arthritis, systemic lupus erythematosus (SLE), giant cell arteritis and monoclonal gammopathies [9–12]. Serological analyses also reveal an age-dependent increase in anti-nuclear antibody (ANA) titre and increases in the serum titre of rheumatoid factors in healthy individuals [13].
It is becoming increasingly appreciated that the clearance of dead cell debris from the body is required to maintain normal immune system function [14]. Typically, the uptake of apoptotic cells is rapid and non-inflammatory, but a disruption to this process can result in an accumulation of dead cells and elicit proinflammatory responses. It has been shown that organisms that are impaired in the clearance of apoptotic cells display systemic inflammation and a breach in self-tolerance in extreme cases [15–19]. Thus, this area of investigation has become a key area of focus in the study of autoimmune diseases.
We hypothesized that immune system dysfunction upon ageing may be accompanied by the accumulation of apoptotic cell debris in tissues. To test this hypothesis, we performed two independent in vivo assays of apoptotic clearance in young and elderly mice. Aged mice were found to be deficient in their ability to clear apoptotic cells in both assays, and this phenotype was associated with features of autoimmunity. Through further study we determined that this decrease in apoptotic cell clearance was linked to systemic factors in the aged mouse.
Materials and methods
Animals
Wild-type 8-week-old B6C3-F1 mice were purchased from Charles River Laboratories (Wilmington, MA, USA). Two-year-old B6C3-F1 mice were purchased from the aged colonies at the National Institute of Aging. Gld mice on C57BL/6 background (B6.gld) were purchased from Jackson Laboratories. Study protocols were approved by the Institutional Animal Care and Use Committee at Boston University School of Medicine.
Induction and analysis of keratinocyte apoptosis
Keratinocyte apoptosis in young or aged mice was performed as described previously [20]. The dorsal surface of young (2 months) and aged (24 months) mice (B6C3) was shaved the night before irradiation. The next morning, the mice were anaesthetized and irradiated with six fluorescent American Phillips F40 sunlamps (National Biological Corp., Twinsberg, OH, USA) mounted permanently above the animal cages, hence the distance between lamps and mice was fixed at all times and was 10 inches. Irradiance was metered with a research radiometer fitted with a UV probe at 285 ± 5 nm (model IL, 1700A; International Light, Newburyport, MA, USA), as described [21]. To measure the ultraviolet radiation (UVR) dose we used an UVR detector SED240/UVR-1/W with the peak at 285 nm (range 265–310 nm) and a dynamic range of 1 × 10−9−1 × 10−3 W/cm2. Using this UVR probe, we measured irradiance reliably at 285 ± 5 nm. Irradiance was adjusted to 9 × 10−5W/cm2, and mice were exposed to 450 mJ/cm2. Mice were killed at 24 h, and dorsal skin sections were fixed in 10% neutral buffered formalin overnight and then paraffin-embedded. Paraffin-embedded tissue was sectioned and haematoxylin and eosin staining was performed. Cells were characterized as apoptotic based on eosinophilic cytoplasm and hyperchromatic pyknotic nuclei. Data are expressed as the percentage of apoptotic keratinocytes.
Apoptotic cell phagocytosis in the peritoneum
B6C3F1 male mice were used at 8 weeks and 2 years of age. WT and B6.gld mice were used at 10–12 weeks of age. Injection of thioglycollate to the peritoneal cavity was performed to recruit inflammatory macrophages. At 3 days after injection, 5-[and 6-]carboxytetramethylrhodamine/succinimidyl ester (TAMRA/SE)-labelled apoptotic Jurkat T cells (1 × 107 cells) were injected into abdomen of mice. Early apoptotic Jurkat T cells were produced by UV exposure at 254 nm for 10 min, followed by incubation for 2 h in RPMI-1640/10% fetal bovine serum (FBS). The frequency of Jurkat T cell apoptosis was approximately 60–70% under these conditions, as determined by annexin V (R&D Systems, Minneapolis, MN, USA) binding using flow cytometric analysis as reported previously [22]. Early apoptotic Jurkat T cells were also assessed by their ability to exclude trypan blue upon microscopic analysis (typically > 95% for early apoptotic cells, whereas late apoptotic cells typically display < 30%). Apoptotic Jurkat T cells were labelled with TAMRA/SE (Molecular Probes, Eugene, OR, USA) by adding 50 μg of TAMRA (10 μg/ul) and incubating cells for 15 min on ice. Peritoneal cells were collected from the abdominal cavities 30 min after injection. Erythrocytes and unphagocytosed apoptotic bodies were removed by incubating on polystyrene dishes for 1 h and washed three times with phosphate-buffered saline (PBS). Cells were then stained with fluorescein isothiocyanate (FITC)-conjugated anti-mouse F4/80 antibody (Serotec, Kidlington, UK). Macrophage phagocytosis of apoptotic cells was determined by analysis of dual-labels by flow cytometric analysis for rhodamine, indicating TAMRA-positive apoptotic cells, and FITC, indicating F4/80 labelling of macrophages. Although these methods do not discern between adherence and uptake, for these assays we consider adherence to be part of the active phagocytic process leading to clearance. Peritoneal cell counts were analysed 3 days after thioglycollate intraperitoneal injection. Briefly, cells were recovered by peritoneal lavage with 5 ml PBS. Cells were counted initially on a haemocytometer, and macrophage levels were quantified on cytospins stained with Diff Quik (Dade Behring, Deerfield, IL, USA). A separate cohort of mice was injected with thioglycollate, and after 3 days peritoneal cells were collected and prepared for fluorescence activated cell sorter analysis by staining with F4/80 to determine macrophage number.
Apoptotic cell phagocytosis in vitro
B6C3F1 male mice were used at 8 weeks (young) and 2 years (aged) of age. Injection of thioglycollate to the peritoneal cavity was performed to recruit inflammatory macrophages. Peritoneal cells were collected from the abdominal cavities on day 3 after injection and plated on polystyrene dishes. Macrophages were allowed to adhere for 1 h and then TAMRA/SE-labelled apoptotic Jurkat T cells were added to the culture media (RPMI-1640 containing 2% FBS) for 30 min. In experiments where mouse serum was used in place of 2% FBS, young macrophages were treated with 45% serum from young and aged or young C57BL/6 and gld mice. Cells were washed with PBS to remove excess apoptotic cells and scraped to prepare for flow cytometry. Phagocytic activity of macrophages was determined by flow cytometry analysing for dual labelling of macrophages (F4/80-FITC) with TAMRA-positive apoptotic cells.
Kidney histology
Kidneys from each mouse were fixed in 10% neutral-buffered formalin overnight and processed immediately for embedding in paraffin. Paraffin-embedded tissue was sectioned (5 μm thick) and slides were stained with haematoxylin and eosin. Cross-sectional areas of at least 25 glomeruli were measured in each animal using computer-assisted pixel counting (Photoshop 7·0; Adobe, San Jose, CA, USA). Complement C3 immunofluorescent staining was performed on frozen tissue embedded in Tissue-Tek compound (Sakura Finetek, Torrence, CA, USA). Briefly, slides were fixed in acetone, washed and treated with 10% goat serum for blocking. A fluorescein-conjugated goat immunoglobulin G (IgG) fraction to mouse complement C3 antibody was used at a dilution of 1:400 (MP Biomedicals, Solon, OH, USA). Slides were examined under a fluorescent microscope by two blinded investigators.
Anti-nuclear antigen measurements
Circulating ANA levels were measured by immunofluorescence using human laryngeal tumour cells (HEp-2) coated slides (The Binding Site Inc., San Diego, CA, USA). Slides were incubated for 1 h with serial dilutions (1:40–1:2560) of mouse serum, washed in PBS, and then incubated with FITC-labelled goat anti-mouse IgG (whole molecule; Sigma-Aldrich, St Louis, MO, USA). Slides were viewed using fluorescent microscopy. The titre value is defined as the inverse value of the last positive dilution.
Statistical analysis
Results are shown as the mean ± standard error of the mean. Differences between groups were determined by Student's t-test, and were considered statistically significant for P < 0·05.
Results
Aged B6C3 mice develop signs of autoimmunity
Because autoreactive antibodies are found commonly in the serum of aged subjects [23,24], we determined if autoantibodies were also present in the aged mice used in our study. Untreated wild-type mice on the B6C3 background were killed at 8 weeks and 2 years of age. Aged mice had elevated levels of anti-nuclear antibodies, whereas these levels were undetectable in 8-week-old mice (Fig. 1a). Because autoimmunity is associated typically with renal dysfunction, formalin-fixed kidneys were sectioned and stained with haematoxylin and eosin to examine tissue morphology. Aged mice displayed larger glomeruli and tubular vacuolization compared with young mice (Fig. 1b and c). Quantification of glomeruli tuft size in histological sections indicated a statistically significant enlargement in aged mice (Fig. 1d). Aged, but not young, mice displayed complement C3 deposits, as indicated by a speckled pattern within glomeruli (Fig. 1d, arrows) that could be distinguished from the non-specific binding of the fluorescein-labelled antibody to the parietal envelope. Complement C3 deposits are consistent with immune-mediated kidney damage [25,26]. Collectively, these data document immune system dysfunction in B6C3 mice at 2 years of age.
Fig. 1.
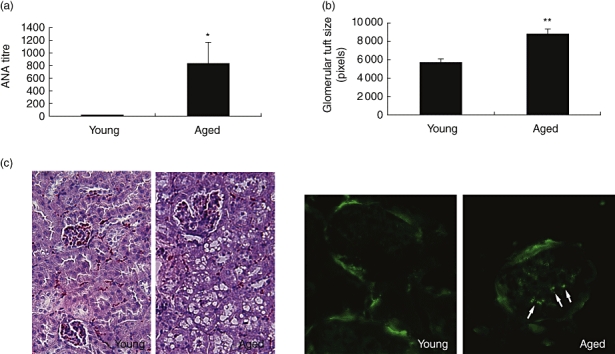
Development of autoreactive antibodies and renal dysfunction in aged B6C3 mice. (a) Blood was collected from 8-week- and 2-year-old B6C3F1 male mice. Serum anti-nuclear antibody titres were measured using human laryngeal tumour cells immunofluorescent slides and are reported as the inverse of the last positive dilution *P < 0·05 (n = 8–10). (b) Quantification of glomerular tuft size was performed (**P < 0·001; n = 8–10). (c) Histological sections of kidneys from young and aged mice stained with haematoxylin and eosin to determine glomerular tuft size (20× magnification). Immunofluorescence showing deposition of mouse complement C3 in glomeruli of aged mice (40× magnification). Arrows indicate complement deposition.
Impaired clearance of apoptotic cells in skin tissue of aged mice
Because impaired clearance of apoptotic cells has been shown to contribute to autoimmune disease in several mouse genetic models [15–19], we examined the clearance of apoptotic keratinocytes in young and old mice following an acute ‘sunburn’ injury in vivo using ultraviolet B (UVR) irradiation [20]. In this model, young and old B6C3 mice were irradiated with 4500 J/m2 UVR on their shaved dorsal surface for 5 min to trigger keratinocyte apoptosis. The dorsal skin was then harvested at 24 h following the UVR injury because this represents the time-point with the greatest apoptotic cell load in the skin tissue [20,27]. Keratinocytes were considered apoptotic based on morphological evidence such as eosinophilic cytoplasm and condensed pyknotic nuclei in histological sections stained with haematoxylin and eosin (Fig. 2a). In the absence of UVR irradiation, the low frequency of apoptotic keratinocytes within the dorsal skin of young and aged mice was similar. UVR irradiation led to an increase in the number of apoptotic keratinocytes in histological sections from both young and old mice, but the percentage of apoptotic keratinocytes was markedly higher in aged mice compared with young mice at the 24 h post-injury time-point (13·5 ± 2·0 versus 3·2 ± 1·0 respectively) (Fig. 2b). These results are consistent with the hypothesis that aged mice exhibit decreased phagocytic function following a synchronous apoptotic stimulus in skin.
Fig. 2.
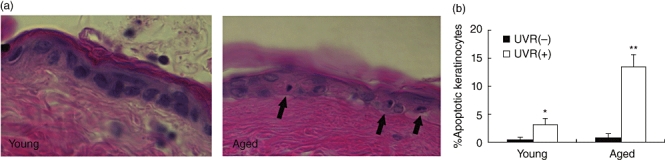
Aged mice have deficient clearance of apoptotic material after ultraviolet radiation (UVR) irradiation. Mice were killed 24 h following UVR irradiation treatment of 4500 J/m2. Skin was collected, fixed in formalin overnight, and embedded immediately in paraffin. (a) Two separate cohorts of mice (n = 5 for each experimental condition) were examined for keratinocyte morphology in skin sections stained with haematoxylin and eosin. Representative photomicrographs show increased apoptotic material remaining in skin of aged mice compared with young mice. (b) Quantification revealed a significant number of apoptotic keratinocytes after UVR administration in young mice (*P < 0·05) and a similar, impaired clearance of apoptotic keratinocytes in aged mice compared with young mice (**P < 0·01; n = 4–5).
Impaired clearance of apoptotic cells in peritoneum
To test more directly whether ageing leads to an impairment in apoptotic cell phagocytosis, a second assay involving the delivery of TAMRA/SE-labelled apoptotic Jurkat T cells into the peritoneal cavity of young and old mice was employed [19]. This assay has been used previously to document that autoimmune disease-prone mice exhibit an impaired ability to clear apoptotic cells [28]. To assess the effects of ageing on apoptotic cell clearance, inflammatory macrophages in the peritoneal cavity of young and old B6C3 mice were elicited by thioglycollate injection into young and old B6C3 mice and then challenged with apoptotic Jurkat T cells. After 30 min incubation, peritoneal cells were collected and analysed by differential cell counts on cytospin slides and by flow cytometry for macrophages labelled with the macrophage marker F4/80 and the apoptotic cell marker rhodamine fluorescent TAMRA/SE (Fig. 3a). Flow cytometric analyses revealed that 27·4% of macrophages from 2-year-old B6C3 mice double-stained with TAMRA, whereas 44·8% of macrophages from 8-week-old B6C3 mice double-stained with TAMRA (Fig. 3b). Thus, the phagocytic activity of old mice was reduced by 39%. However, ageing was not associated with a decline in macrophage cell count 3 days after thioglycollate injection in young versus old mice (9·3 × 105/ml in young mice versus 1·3 × 106/ml in aged mice, P = non-significant). These data indicate that ageing is associated with a reduction in the phagocytic activity of macrophages rather than by a difference in the ability to recruit macrophages to the peritoneum.
Fig. 3.
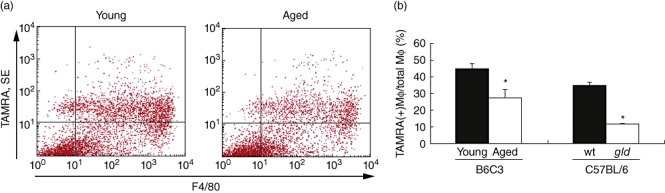
Aged mice have impaired clearance of apoptotic material in vivo. Young and aged mice received 5-[and 6-]carboxytetramethylrhodamine/ succinimidyl ester (TAMRA/SE)-labelled apoptotic Jurkat T cells to the peritoneal cavity 3 days after thioglycollate treatment. (a) Representative graphs from flow cytometry reveal that the number of macrophages co-localizing with TAMRA/SE was decreased in the aged mice compared with young. (b) Quantification of fluorescence activated cell sorter analysis shows a significant decrease in phagocytic activity in aged versus young mice, similar to that observed in young autoimmune gld mice when compared with wild-type (wt) (*P < 0·01; n = 8).
The peritoneal phagocytosis assay was also performed on 10–12-week-old gld mice in a C57BL/6 background that develop a mild lupus-like phenotype. Results of flow cytometry on cells collected from the peritoneum revealed that young gld mice also have an impaired ability to clear apoptotic material compared with wild-type C57BL/6 mice (12·0 ± 0·3% versus 35·1 ± 1·4%, respectively, P < 0·001) (Fig. 3b). Therefore, the decrease in phagocytic activity observed in aged mice is comparable to the decrease that occurs in a mouse model of autoimmune disease.
Impaired phagocytic activity of macrophages treated with aged serum in vitro
To address the mechanism by which aged macrophages display a decreased ability to clear apoptotic cells in vitro, apoptosis assays were performed with macrophages collected from young and aged B6C3 mice. No significant difference was found in the phagocytic activity of macrophages collected from the peritoneal cavity of young compared with aged mice (Fig. 4a). In addition, we detected no difference in phagocytic activity between macrophages isolated from wild-type C57BL/6 mice and gld mice on the C57BL/6 background (Fig. 4b). As there was no difference between young and aged macrophages in these assays, we sought to determine if systemic factors were contributing to the decreased clearance observed in the in vivo experiments. After incubation of young macrophages with serum from young or aged B6C3 mice, flow cytometric analyses revealed that 29·9% of macrophages treated with young serum and 20·9% of macrophages treated with aged serum double-stained with TAMRA (Fig. 4c). Thus, the phagocytic activity of young macrophages was reduced by 28% when treated with aged serum (P < 0·001). In addition, treating young macrophages with serum from 8-week-old gld mice resulted in a similar 35% reduction in apoptotic cell uptake compared with cells treated with wild-type C57BL/6 serum (Fig. 4d). These results suggest that there are systemic factors within aged or lupus-prone mice which can contribute to impaired apoptotic cell clearance.
Fig. 4.
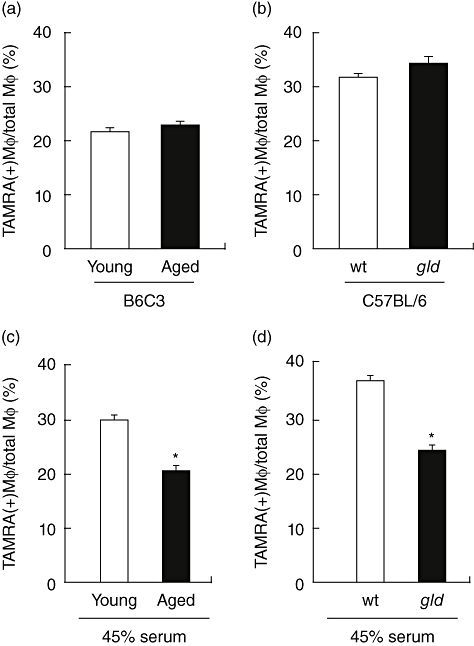
Young macrophages exhibit impaired clearance in vitro when exposed to aged serum. Peritoneal macrophages from young mice elicited after thioglycollate injection were collected and phagocytic activity was determined in vitro. Macrophages from young or aged B6C3 mice (a), and wild-type (wt) or gld mice in a C57BL/6 background (b), show no significant difference in ability to clear apoptotic Jurkat T cells in the presence of 2% fetal bovine serum. (c) Young macrophages, from B6C3 mice, pretreated with 45% serum from aged mice display impaired clearance of apoptotic cells compared with macrophages pretreated with serum from young mice (*P < 0·001). (d) Macrophages from young C57BL/6 mice show decreased clearance of apoptotic cells when treated with 45% serum from gld mice compared with serum from wt mice (*P < 0·001).
Discussion
Immune system dysfunction in the elderly involves an increased reactivity against self-antigens in spite of an overall decline in immune cell capacity to respond to foreign antigens [1,2,8]. A number of mechanisms have been proposed for immune system dysfunction upon ageing. For example, the decline in the response to foreign antigens is thought to occur largely from a depletion of haematopoietic lineage-specific stem cells [29]. On the other hand, the increased incidence of pathological autoimmunity in elderly populations is generally believed to be caused by the age-dependent accumulation of misfolded or modified proteins which may trigger autoimmune responses to self-proteins [30]. In addition, the mechanisms that participate in the clearance of modified or misfolded proteins may decline with age, leading to the prolonged exposure to autoantigens and lymphocyte activation.
Impaired apoptotic cell clearance has been been implicated recently in the development and progression of autoimmune diseases in some systems [31,32] but not others [33,34]. However, it has been well documented that reductions in an organism's ability to clear apoptotic bodies will promote an autoimmune phenotype. For example, defects in genes responsible for apoptotic clearance machinery lead to the development of an autoimmune phenotype in mouse models [15,16,18,22], and C1q and DNaseI deficiencies in humans are associated with the development of SLE [35,36]. Furthermore, apoptotic bodies and circulating DNA fragments are found in the sera of patients with SLE. Because the accumulation of apoptotic debris will produce an autoimmune phenotype and the elderly exhibit signs of autoimmunity, we hypothesized that the ageing process may impair the processes by which phagocytic cells eliminate apoptotic debris. It has been recognized that macrophages and other professional scavenger cells from elderly humans and aged rodents display a reduced capacity to engulf particles in vitro, indicative of a decline in general phagocytic mechanisms [6,37]. However, apoptotic cell clearance is mechanistically distinct from general phagocytosis. In this regard, nothing is known about how ageing affects the recognition of apoptotic cell-associated molecular motifs that function as ‘eat-me’ signals or the unique set of receptor and bridging molecules that control this process specifically [38]. Recently, Agrawal et al. showed that dendritic cells isolated from elderly subjects displayed an impaired capacity to engulf apoptotic cells in vitro[7], but in vivo evidence documenting the impaired clearance of apoptotic cells in elderly organisms has not been provided. Here, it is shown that aged mice have impaired phagocytic activity when challenged to clear apoptotic cells compared with young mice.
In this study, two models of apoptotic cell clearance in vivo were employed. The first assay examined the phagocytic activity of macrophages in skin tissue after UVR irradiation. This model involves an acute sunburn damage leading to synchronous cell death. Thus the relative efficiencies of clearance can be evaluated by analysing the numbers of residual apoptotic cells at time-points following the UVR irradiation. Previous studies have shown that maximal numbers of apoptotic keratinocytes are present at 24 h compared with 6, 12 and 48 h [20,27]. At this time-point, we found that aged mice exhibit a fourfold increase in residual apoptotic cells compared with young mice. A potential limitation of this assay is that keratinocytes from aged mice may display an increased susceptibility to apoptosis, and thus the increase in apoptotic cells in the aged mice may result from an increase in the frequency of cell death rather than a decrease in their clearance. Contrary to this hypothesis, we have reported that human fibroblasts obtained from older subjects display decreased frequencies of apoptosis in response to UB-induced DNA damage because of reductions in p53 levels [39]. In addition, it is reported that senescent keratinocytes are resistant to UV-induced apoptosis [40]. Thus, the UV-irradiation model employed here is likely to provide information about the relative rates of apoptotic cell clearance in vivo[20,27], although we cannot rule out the possibility that ageing alters keratinocyte susceptibility to apoptosis in vivo, either positively or negatively, thereby influencing the frequency of residual apoptotic cells detected at 24 h following UV irradiation.
To look more directly at the clearance of dead cells in young versus old mice, a second assay was employed where apoptotic Jurkat T cells labelled with TAMRA/SE were injected into the peritoneal cavity of thioglycolate-pretreated mice. Cells were then collected and macrophages positive for TAMRA/SE were quantified by flow cytometry analysis. In this assay, aged mice displayed reduced apoptotic cell uptake. The level of impairment in aged mice was similar to that in young gld mice in a C57BL/6 background that display a mild autoimmune phenotype. Because gld mice display systemic inflammation and titres of autoreactive antibodies, it is reasonable to speculate that the impaired clearance of apoptotic cells in the aged mice is functionally significant with regard to immune system dysfunction.
We did not observe a detectable difference between macrophages obtained from young and aged mice in their ability to ingest apoptotic cells in vitro. However, the uptake of apoptotic cells by macrophages was reduced when they were exposed to sera from aged mice compared with sera from young mice. In contrast, Agrawal et al. reported that dendritic cells isolated from elderly patients display reductions in both apoptotic cell and general mechanisms of phagocytosis because of impairments in intracellular signalling pathways. The reasons for the discrepancies between the two studies are unknown, but they could derive from differences in cell type (peripheral blood-derived dendritic cells versus peritoneal macrophages), species differences (human versus mouse) or the differences in life span of these two organisms. Our data suggest that the impaired clearance of apoptotic bodies in aged organisms may be due to an increase in the presence of systemic factors. For example, immune complexes, cytokines and oxidized low-density lipoproteins, which accumulate with ageing, have been shown to contribute to decreased phagocytic activity of macrophages [41–44]. Alternatively, ageing may be associated with the loss of pro-phagocytic factors, such as the opsonizing proteins that function to bridge the apoptotic cell with the macrophage [19,22]. It is possible that several of these circulating components participate in the decline of apoptotic cell uptake in aged mice, and it will be of interest to determine the identity of these factors.
In summary, we show that clearance of apoptotic cells is reduced in aged mice. This conclusion is supported by observations of impaired phagocytic activity in skin in response to UVR irradiation and in the peritoneal cavity after it is loaded with apoptotic cells. We also demonstrated that aged mice exhibited signs of autoimmunity similar to that observed in young mice with a mild lupus phenotype, and we provide evidence that systemic factors in aged and lupus-prone mice can contribute to the decreased phagocytic activity of macrophages in vitro. Because the accumulation of apoptotic debris contributes to an autoimmune phenotype, these data suggest that the immune system dysfunction observed in the elderly may result from defects in the ability of phagocytic cells to clear autoantigens that arise as a consequence of natural cell turnover.
Acknowledgments
This work was supported by National Institutes of Health Grant AG15052 to K. W. T. A. was supported by a National Heart, Lung and Blood Institute postdoctoral research training fellowship.
References
- 1.Aw D, Silva AB, Palmer DB. Immunosenescence: emerging challenges for an ageing population. Immunology. 2007;120:435–46. doi: 10.1111/j.1365-2567.2007.02555.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Linton PJ, Dorshkind K. Age-related changes in lymphocyte development and function. Nat Immunol. 2004;5:133–9. doi: 10.1038/ni1033. [DOI] [PubMed] [Google Scholar]
- 3.Murasko DM, Weiner P, Kaye D. Decline in mitogen induced proliferation of lymphocytes with increasing age. Clin Exp Immunol. 1987;70:440–8. [PMC free article] [PubMed] [Google Scholar]
- 4.Lord JM, Butcher S, Killampali V, Lascelles D, Salmon M. Neutrophil ageing and immunesenescence. Mech Ageing Dev. 2001;122:1521–35. doi: 10.1016/s0047-6374(01)00285-8. [DOI] [PubMed] [Google Scholar]
- 5.Gomez CR, Boehmer ED, Kovacs EJ. The aging innate immune system. Curr Opin Immunol. 2005;17:457–62. doi: 10.1016/j.coi.2005.07.013. [DOI] [PubMed] [Google Scholar]
- 6.Plowden J, Renshaw-Hoelscher M, Engleman C, Katz J, Sambhara S. Innate immunity in aging: impact on macrophage function. Aging Cell. 2004;3:161–7. doi: 10.1111/j.1474-9728.2004.00102.x. [DOI] [PubMed] [Google Scholar]
- 7.Yasuda K, Richez C, Maciaszek JW, et al. Murine dendritic cell type I IFN production induced by human IgG-RNA immune complexes is IFN regulatory factor (IRF) 5 and IRF7 dependent and is required for IL-6 production. J Immunol. 2007;178:6876–85. doi: 10.4049/jimmunol.178.11.6876. [DOI] [PubMed] [Google Scholar]
- 8.Prelog M. Aging of the immune system: a risk factor for autoimmunity? Autoimmun Rev. 2006;5:136–9. doi: 10.1016/j.autrev.2005.09.008. [DOI] [PubMed] [Google Scholar]
- 9.Kyle RA, Therneau TM, Rajkumar SV, et al. Prevalence of monoclonal gammopathy of undetermined significance. N Engl J Med. 2006;354:1362–9. doi: 10.1056/NEJMoa054494. [DOI] [PubMed] [Google Scholar]
- 10.Langford CA. Vasculitis in the geriatric population. Rheum Dis Clin North Am. 2007;33:177–95. doi: 10.1016/j.rdc.2006.12.002. [DOI] [PubMed] [Google Scholar]
- 11.Ramos-Casals M, Brito-Zeron P, Lopez-Soto A, Font J. Systemic autoimmune diseases in elderly patients: atypical presentation and association with neoplasia. Autoimmun Rev. 2004;3:376–82. doi: 10.1016/j.autrev.2003.12.002. [DOI] [PubMed] [Google Scholar]
- 12.Tutuncu Z, Kavanaugh A. Rheumatic disease in the elderly: rheumatoid arthritis. Rheum Dis Clin North Am. 2007;33:57–70. doi: 10.1016/j.rdc.2006.12.006. [DOI] [PubMed] [Google Scholar]
- 13.Tan EM, Feltkamp TE, Smolen JS, et al. Range of antinuclear antibodies in ‘healthy’ individuals. Arthritis Rheum. 1997;40:1601–11. doi: 10.1002/art.1780400909. [DOI] [PubMed] [Google Scholar]
- 14.Bleesing JJ, Brown MR, Novicio C, et al. a composite picture of TcR alpha/beta(+) CD4(−) CD8(−) T cells (alpha/beta-DNTCs) in humans with autoimmune lymphoproliferative syndrome. Clin Immunol. 2002;104:21–30. doi: 10.1006/clim.2002.5225. [DOI] [PubMed] [Google Scholar]
- 15.Bickerstaff MC, Botto M, Hutchinson WL, et al. Serum amyloid P component controls chromatin degradation and prevents antinuclear autoimmunity. Nat Med. 1999;5:694–7. doi: 10.1038/9544. [DOI] [PubMed] [Google Scholar]
- 16.Botto M, Dell'Agnola C, Bygrave AE, et al. Homozygous C1q deficiency causes glomerulonephritis associated with multiple apoptotic bodies. Nat Genet. 1998;19:56–9. doi: 10.1038/ng0598-56. [DOI] [PubMed] [Google Scholar]
- 17.Cohen PL, Caricchio R, Abraham V, et al. Delayed apoptotic cell clearance and lupus-like autoimmunity in mice lacking the c-mer membrane tyrosine kinase. J Exp Med. 2002;196:135–40. doi: 10.1084/jem.20012094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Napirei M, Karsunky H, Zevnik B, Stephan H, Mannherz HG, Moroy T. Features of systemic lupus erythematosus in Dnase1-deficient mice. Nat Genet. 2000;25:177–81. doi: 10.1038/76032. [DOI] [PubMed] [Google Scholar]
- 19.Taylor PR, Carugati A, Fadok VA, et al. A hierarchical role for classical pathway complement proteins in the clearance of apoptotic cells in vivo. J Exp Med. 2000;192:359–66. doi: 10.1084/jem.192.3.359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Okamoto H, Mizuno K, Itoh T, Tanaka K, Horio T. Evaluation of apoptotic cells induced by ultraviolet light B radiation in epidermal sheets stained by the TUNEL technique. J Invest Dermatol. 1999;113:802–7. doi: 10.1046/j.1523-1747.1999.00757.x. [DOI] [PubMed] [Google Scholar]
- 21.Allan AE, Archambault M, Messana E, Gilchrest BA. Topically applied diacylglycerols increase pigmentation in guinea pig skin. J Invest Dermatol. 1995;105:687–92. doi: 10.1111/1523-1747.ep12324466. [DOI] [PubMed] [Google Scholar]
- 22.Takemura Y, Ouchi N, Shibata R, et al. Adiponectin modulates inflammatory reactions via calreticulin receptor-dependent clearance of early apoptotic bodies. J Clin Invest. 2007;117:375–86. doi: 10.1172/JCI29709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hallgren HM, Buckley CE, III, Gilbertsen VA, Yunis EJ. Lymphocyte phytohemagglutinin responsiveness, immunoglobulins and autoantibodies in aging humans. J Immunol. 1973;111:1101–7. [PubMed] [Google Scholar]
- 24.Rowley MJ, Buchanan H, Mackay IR. Reciprocal change with age in antibody to extrinsic and intrinsic antigens. Lancet. 1968;2:24–6. doi: 10.1016/s0140-6736(68)92893-6. [DOI] [PubMed] [Google Scholar]
- 25.Bondanza A, Zimmermann VS, Dell'Antonio G, et al. Requirement of dying cells and environmental adjuvants for the induction of autoimmunity. Arthritis Rheum. 2004;50:1549–60. doi: 10.1002/art.20187. [DOI] [PubMed] [Google Scholar]
- 26.Bondanza A, Zimmermann VS, Dell'Antonio G, et al. Cutting edge: dissociation between autoimmune response and clinical disease after vaccination with dendritic cells. J Immunol. 2003;170:24–7. doi: 10.4049/jimmunol.170.1.24. [DOI] [PubMed] [Google Scholar]
- 27.O'Brien BA, Geng X, Orteu CH, et al. A deficiency in the in vivo clearance of apoptotic cells is a feature of the NOD mouse. J Autoimmun. 2006;26:104–15. doi: 10.1016/j.jaut.2005.11.006. [DOI] [PubMed] [Google Scholar]
- 28.Potter PK, Cortes-Hernandez J, Quartier P, Botto M, Walport MJ. Lupus-prone mice have an abnormal response to thioglycolate and an impaired clearance of apoptotic cells. J Immunol. 2003;170:3223–32. doi: 10.4049/jimmunol.170.6.3223. [DOI] [PubMed] [Google Scholar]
- 29.Ogawa T, Kitagawa M, Hirokawa K. Age-related changes of human bone marrow: a histometric estimation of proliferative cells, apoptotic cells, T cells, B cells and macrophages. Mech Ageing Dev. 2000;117:57–68. doi: 10.1016/s0047-6374(00)00137-8. [DOI] [PubMed] [Google Scholar]
- 30.Cloos PA, Christgau S. Post-translational modifications of proteins: implications for aging, antigen recognition, and autoimmunity. Biogerontology. 2004;5:139–58. doi: 10.1023/B:BGEN.0000031152.31352.8b. [DOI] [PubMed] [Google Scholar]
- 31.Gaipl US, Voll RE, Sheriff A, Franz S, Kalden JR, Herrmann M. Impaired clearance of dying cells in systemic lupus erythematosus. Autoimmun Rev. 2005;4:189–94. doi: 10.1016/j.autrev.2004.10.007. [DOI] [PubMed] [Google Scholar]
- 32.Munoz LE, Gaipl US, Franz S, et al. SLE − a disease of clearance deficiency? Rheumatology (Oxford) 2005;44:1101–7. doi: 10.1093/rheumatology/keh693. [DOI] [PubMed] [Google Scholar]
- 33.Perruche S, Kleinclauss F, Angonin R, et al. a single intravenous infusion of apoptotic cells, an alternative cell-based therapy approach facilitating hematopoietic cell engraftment, did not induce autoimmunity. J Hematother Stem Cell Res. 2003;12:451–9. doi: 10.1089/152581603322286088. [DOI] [PubMed] [Google Scholar]
- 34.Stuart LM, Takahashi K, Shi L, Savill J, Ezekowitz RA. Mannose-binding lectin-deficient mice display defective apoptotic cell clearance but no autoimmune phenotype. J Immunol. 2005;174:3220–6. doi: 10.4049/jimmunol.174.6.3220. [DOI] [PubMed] [Google Scholar]
- 35.Botto M, Walport MJ. Hereditary deficiency of C3 in animals and humans. Int Rev Immunol. 1993;10:37–50. doi: 10.3109/08830189309051170. [DOI] [PubMed] [Google Scholar]
- 36.Yasutomo K, Horiuchi T, Kagami S, et al. Mutation of DNASE1 in people with systemic lupus erythematosus. Nat Genet. 2001;28:313–4. doi: 10.1038/91070. [DOI] [PubMed] [Google Scholar]
- 37.Izgut-Uysal VN, Ozkaya YG, Ozdemir S, Yargicoglu P, Agar A. Effect of 1-arginine on age-related changes in macrophage phagocytic activity. Immunol Invest. 2004;33:287–93. doi: 10.1081/imm-120037276. [DOI] [PubMed] [Google Scholar]
- 38.Savill J, Dransfield I, Gregory C, Haslett C. A blast from the past: clearance of apoptotic cells regulates immune responses. Nat Rev Immunol. 2002;2:965–75. doi: 10.1038/nri957. [DOI] [PubMed] [Google Scholar]
- 39.Goukassian D, Gad F, Yaar M, Eller US, Nehal MS, Gilchrest BA. Mechanisms and implications of the age-associated decrease in DNA repair capacity. FASEB J. 2000;14:1325–34. doi: 10.1096/fj.14.10.1325. [DOI] [PubMed] [Google Scholar]
- 40.Chaturvedi V, Qin JZ, Stennett L, Choubey D, Nickoloff BJ. Resistance to UV-induced apoptosis in human keratinocytes during accelerated senescence is associated with functional inactivation of p53. J Cell Physiol. 2004;198:100–9. doi: 10.1002/jcp.10392. [DOI] [PubMed] [Google Scholar]
- 41.Sambrano GR, Parthasarathy S, Steinberg D. Recognition of oxidatively damaged erythrocytes by a macrophage receptor with specificity for oxidized low density lipoprotein. Proc Natl Acad Sci USA. 1994;91:3265–9. doi: 10.1073/pnas.91.8.3265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Sambrano GR, Steinberg D. Recognition of oxidatively damaged and apoptotic cells by an oxidized low density lipoprotein receptor on mouse peritoneal macrophages: role of membrane phosphatidylserine. Proc Natl Acad Sci USA. 1995;92:1396–400. doi: 10.1073/pnas.92.5.1396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Gardai SJ, McPhillips KA, Frasch SC, et al. Cell-surface calreticulin initiates clearance of viable or apoptotic cells through trans-activation of LRP on the phagocyte. Cell. 2005;123:321–34. doi: 10.1016/j.cell.2005.08.032. [DOI] [PubMed] [Google Scholar]
- 44.Roccatello D, Coppo R, Piccoli G, et al. Circulating Fc-receptor blocking factors in IgA nephropathies. Clin Nephrol. 1985;23:159–68. [PubMed] [Google Scholar]


