Abstract
Studies on physiology and pathology as they relate to the immune system draw heavily upon rodent models. With the increasing impetus provided by initiatives in translational medicine, the demand for ever more sophisticated, ‘humanized’ murine models is greater than ever. However, the design and implementation of studies in such mice is far from trivial. Here we provide a technical perspective on the increasing interest in developing humanized mice. We give examples of primary data starting with the routine procurement of human donor material, through CD34+ cell purification prior to engraftment to injection into immunocompromised mice. Our goal is to provide practical advice to the many investigators who may be commencing or considering such studies.
Keywords: CD34, cord blood, humanized mouse
The need for humanized mice
Studies on basic human immunology and immunopathology, and the translation of research findings into experimental therapies, are constrained by limitations on the scope of experimentation in vitro. Murine animal models of human disease can be highly informative but carry their own limitations, which have been the subject of excellent recent reviews [1–3]. Non-human primate studies can bridge some of the gaps in understanding, but their use is restricted by high cost, limited availability, lack of inbred strains and ethical issues. In the era of translational medicine, complex biological mechanisms require analysis in vivo so that manipulable rate-determining steps can be defined. This has provoked a growing demand for new animal models that are able to sustain studies of human tissues and cells in vivo. Humanized mice, defined here as immunodeficient mice engrafted with human CD34+ haematopoietic stem cells (HSCs), have emerged in recent years as potentially powerful tools with which to gain greater insights into many infectious and inflammatory human diseases.
Focus on the model
Several models have been described since the first successful xenogeneic graft into severe combined immune deficient (SCID) mice by Mosier et al. in 1988 [4] (see Table 1). The discovery of the SCID mutation, which results in the lack of T and B cells in mice [5], provided the first experimental animal model in which to study in vivo the haematopoietic potential of peripheral blood mononuclear cells (PBMCs) [4], fetal haematopoietic tissues [6] and HSCs [7]. The failure of early studies to achieve better than short-term engraftment success was attributed to ‘leaky’ emergence of functional host T and B cells [8] and high levels of innate immunity, mediated most notably by natural killer (NK) cells [9,10]. Other genetically modified backgrounds, such as engineered deletion of recombination-activating genes (Rag) 1 and 2, offered incremental improvements but problems with NK cell activity remained [9]. Use of the non-obese diabetic (NOD) strain, which has defects in NK cell activity and other innate immune mechanisms [11], supports better levels of engraftment with HSCs [12,13] and has been adopted widely in recent years as an appropriate model to study human immunity into a mouse recipient.
Table 1.
Key features of humanized mouse reconstitution studies.
| Mouse strain | Mouse phenotype | Reconstituting cells | Sites of reconstitution | Evidence of engraftment and immune reactivity | Duration of functional immune responses and limitations of model | References |
|---|---|---|---|---|---|---|
| SCID | Lack of mature T and B cells | Fetal haematopoietic tissues | Peripheral blood | Very low level of engraftment | Short-term engraftment because of leakiness | [4,6,7] |
| High levels of NK cells | PBMCs | Spleen | Low level of T cells | Residual NK cells and innate immunity | ||
| HSCs | Lymph nodes | B cells, Ig | ||||
| Rag1null or Rag2null | Lack of mature T and B cells | HSCs | Peripheral blood | Low and variable level of engraftments | Residual NK cells and innate immunity | [9] |
| Spleen | Low level of T cells | |||||
| Lymph nodes | B cells, Ig | |||||
| NOD/SCID | Lack of mature T and B cells | PBMCs | Peripheral blood | Increased level of engraftment | Residual innate immunity | [11–13] |
| Decreased innate immune mechanisms | HSCs | Spleen | Increased level of T cells | Low number but functional NK cells | ||
| Bone marrow | B cells, Ig | Short life span | ||||
| NOD/SCID/ IL-2rgnull or BALB/c Rag2null/IL-2rgnull | Lack of mature T and B cells | PBMCs | Peripheral blood | High level of engraftment | Long life span | [19–24] |
| Many innate immune defects | HSCs | Spleen | T cells, B cells, Ig, dendritic cells | |||
| Lymph nodes | Functional human immune system | |||||
| Bone marrow | ||||||
| Thymus |
HSCs, haematopoietic stem cells; Ig, immunoglobulin; NK, natural killer; NOD/SCID, non-obese diabetic/severe combined immune deficient; PBMC, peripheral blood mononuclear cells.
Recent advances in our understanding of NK cell development, and especially the reliance of these cells upon interleukin (IL)-15 as a development and survival factor [14,15], allowed better immunodepleting gene knock-outs to be designed. The IL-15 receptor γ chain is also shared by other high-affinity IL receptors such as those for IL-2, IL-4, IL-7, IL-9 and IL-21 and is therefore termed the IL-2 receptor common γ chain (IL-2rg) [16]. Absence of the IL-2rg leads to severe impairments in T and B cell development and, above all, completely abrogates NK cell development [17,18]. Thus, a new generation of NOD/SCID/IL-2rgnull[19–23] and BALB/c Rag2null/IL-2rgnull[24] models has emerged, promising better engraftment of human HSCs and PBMCs compared with the original humanized mouse models [24], and providing new insights into human primary and secondary lymphoid organ development and the induction of adaptive immune responses. It is unclear why immunodeficient strains with equivalent immunodeficiency-related mutations differ in their ability to support stem cell engraftment. It is likely that gene expression and cytokine environment are critical. Indeed, a previously unknown Sirpa gene polymorphism has been identified recently as a determinant influencing the efficacy of human HSC engraftment into the NOD/SCID strain [25].
Focus on the graft
An equally important component to the development of humanized mice is the choice of engrafting cells. Although human PBMCs have been used by many investigators, the best reconstitution has resulted from the introduction of human HSCs. These are rare cells that can be found in peripheral blood, bone marrow and umbilical cord blood (UCB) and have multi-potential, are self-renewing and/or undergo commitment to common myeloid or lymphoid progenitors [26]. One of the first models of HSC engraftment was described by Lapidot et al. in which human HSCs were injected into irradiated SCID mice [7]. Since then early-stage stem cell markers have been identified on human progenitors, including CD34 [27] and more recently CD133 (formerly AC133 or proninin-1 [28]). CD133 is an alternative to CD34 in the selection of human HSCs and identifies a more primitive population [28,29]. Expression of CD38 reveals lineage commitment and a loss of generative and repopulating capacity [30], and different combinations of cell subsets expressing these have been evaluated to identify the most immature human stem cell subset that has pluri-potential and is sufficiently abundant to allow exploitation. Of late, human CD34+[21,24,31], CD133+[32], CD34+ CD133+[33], CD34+ CD38–[23,34] and Lin– CD34+ CD38–[35] HSCs have been used to repopulate immunodeficient mice, most of them isolated from UCB and enriched by magnetic separation, cell sorting or both. From these studies it is now well established that newborn/fetal HSCs have more potential than adult HSCs [36], and that UCB is a better source than bone marrow of HSCs with high engraftment level [37].
More recently, with the emergence of expertise, reagents and technologies in the arena of stem cell research, several teams have focused on improving methods deployed in vitro to maintain and/or expand human HSCs without compromising their development and engraftment capacity. Different expansion methods using various cytokine cocktails and media have been developed to culture human HSCs, especially UCB CD34+ cells [38,39]. Cells isolated from UCB and enriched in short- or long-term culture have then been engrafted into NOD/SCID mice, showing a retained capacity to repopulate T lymphocytes [with a broad T cell receptor (TCR) β chain repertoire][40] and dendritic cells [33,40].
To date, human HSCs have been injected into newborn [23] or adult [22] irradiated recipients via the intravenous route. Conditioned newborn immune deficient mice have also been repopulated after intraperitoneal [41], and more recently after intrahepatic, injections [24]. Other protocols designed to enhance the development and the differentiation of human HSCs have involved direct injection of HSCs into stem cell niches, such as the bone marrow [42], or into the uterus [43]. Placing human HSCs in suitable haematopoietic microenvironments, such as newborn liver or bone marrow, may help progenitors to survive and engraft in the absence of murine homing receptors.
The challenge
With so many options for experimentation, there is as yet little consensus on the optimal conditions for humanization of mice using human HSCs, in terms of the best strain, age, dose of irradiation, route of injection, stem cell subset and source and culturing cells for grafting. Undoubtedly such a consensus will emerge, but establishing the framework for such studies takes time. In light of this, we provide some practical tips for setting up a logistical basis for routine engraftment studies.
The necessary practical steps
We present here a flowchart (Fig. 1) identifying the steps that require practical consideration if the transfer of HSCs into mice of an appropriate stage of development is to be achieved. Inviting women to participate and provide cord blood samples is clearly the first hurdle and is not trivial, as at the latter stages of pregnancy there are many distractions. There is also a burgeoning interest in creating banks of cord blood for use in the event of subsequent disease, which may compete with the supply for research. Human samples must be obtained according to institutional guidelines approved by local ethics committees, and this procedure may differ from one country to another. Obtaining consent is also time-consuming and the approved procurement of blood needs to be co-ordinated temporally with the availability of recipient mice (Fig. 1), emphasizing the need to optimize the steps in between: a failure to prepare HSC properly will risk the unnecessary generation of litters of mice for which no grafts are available, while unreliability in the mouse breeding programme may leave the researcher with cells for which there is no recipient. In our own experience, focusing upon women undergoing delivery by planned Caesarean section during weekly operating theatre lists provides the most stable footing on which to obtain consent and samples.
Fig. 1.
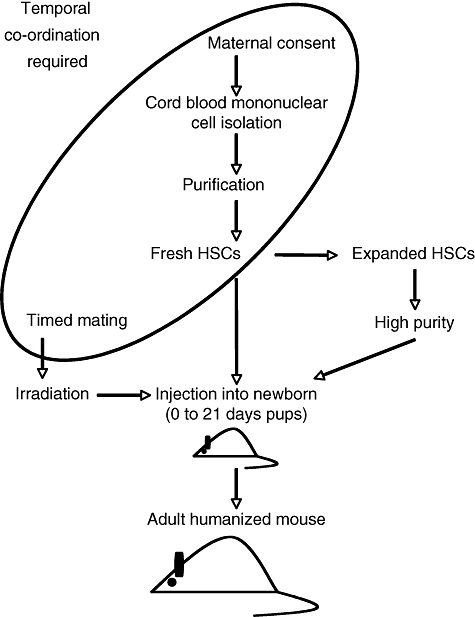
Temporal coordination of steps to obtain humanized mice. Schematic showing the organizational challenges in obtaining cord blood from elective Caesarean sections and obtaining and using haematopoietic stem cells to reconstitute newborn pups in order to obtain humanized adult mice. HSC, haematopoietic stem cell.
Isolation of UCB mononuclear cells
The second challenge is whether the yield of UCB mononuclear cells will be sufficient to permit experimental planning. Based on our experience of 43 UCB samples, we suggest that the researcher may anticipate a median blood volume of 35 ml (range: 15–60 ml), containing a median total number of 2 × 108 cord blood mononuclear cells (range: 1 × 108−6 × 108). Several factors may affect the mononuclear cell yield. First, one needs to find a density gradient matrix that works well for UCB, which is not necessarily the same as is efficacious for adult peripheral blood. Moreover, during the purification of cells away from red blood cells, which we perform on Ficoll-Paque premium gradients (Amersham, Little Chalfont, Buckinghamshire, UK; density = 1·077 g/ml), retention of red blood cells can occur at the interface or just below if there is a delay of several hours between withdrawal of blood from the cord and its collection, or if the pH of the phosphate-buffered saline–anti-coagulant citrate dextrose-formula A buffer is lower than 7·40 (PBS–ACD-A).
Isolation, analysis and culture of human CD34+ HSCs
The next critical steps are the purification of HSCs, and the validation of purity. Magnetic separation is a commonly employed approach (see additional practical data) to isolate CD34+ cells. One can expect a median frequency of CD34+ cells from unmanipulated UCB of ∼0·27% (range: 0·03–0·56%) expressed as a percentage of mononuclear cells. Because CD34+ cells are very rare, they cannot be distinguished by flow cytometry as a clear population on standard forward- and side-scatter density plots of UCB mononuclear cells (Fig. 2a). However, after magnetic separation CD34+ HSCs cells show greater cell size than the lymphocyte population and reduced granularity when compared with monocytes or granulocytes (Fig. 2b). To assess the efficacy of magnetic bead isolation, a flow cytometry comparison of UCB mononuclear cells is made before and after isolation for the expression of CD3, CD34 and CD133. Prior to purification, the abundance of T cells in the UCB will be reflected by high expression of CD3 (Fig. 3a and b), with negligible capacity to detect any CD34+ or CD133+ cells (Fig. 3c and d). After isolation, cells are highly enriched for expression of CD34, with almost complete depletion of CD3+ cells (Fig. 3e). A significant proportion of CD34+ cells should co-express CD133 (Fig. 3g). An aggregate summary of the distribution of CD34, CD133 and CD3 expression after enrichment of human CD34+ HSCs is shown in Table 2.
Fig. 2.
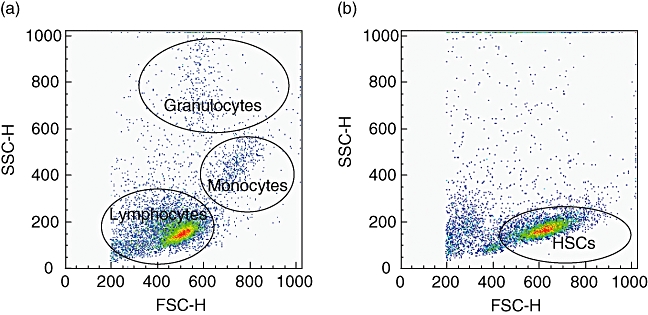
Human CD34+ cells do not share the same size and internal complexity patterns as lymphocytes. Typical forward-scatter (FSC) and side-scatter (SSC) density plot of umbilical cord blood mononuclear cells before immunomagnetic enrichment, showing lymphocytes, monocytes and granulocytes. Here, the number of haematopoietic stem cells (HSCs) is too low to allow their direct detection (a). FSC and SSC density plot of human HSCs from the positive fraction after CD34 immunomagnetic enrichment using the AutoMACS apparatus. The lymphocytes and granulocytes are almost entirely depleted (a).
Fig. 3.
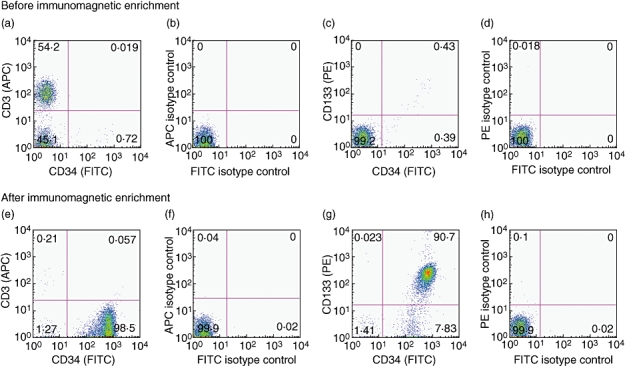
Immunomagnetic enrichment provides a good purification of CD34+ as well as CD133+ cells and depletes the CD3+ subset. Flow cytometry expression dot plots representing CD34, CD133 and CD3 surface expression before and after immunomagnetic enrichment. The numbers shown in quadrants are mean percentages. Expression of CD34 and CD3 antigens on umbilical cord blood mononuclear cells (a) and relevant isotype controls (b). Expression of CD34 and CD133 antigens on UCB mononuclear cells (c) and relevant isotype controls (d). Expression of CD34 and CD3 antigens on haematopoietic stem cells of the positive fraction following a Posseld2 immunomagnetic purification programme (e) and relevant isotype controls (f). Expression of CD34 and CD133 antigens on HSCs of the positive fraction after immunomagnetic enrichment (g) and relevant isotype controls (h). APC, antigen-presenting cell; FITC, fluorescein isothiocyanate; PE, phycoerythrin.
Table 2.
Mean expression of CD34, CD133 and CD3 markers from 21 umbilical cord blood mononuclear cell preparations after immunomagnetic enrichment of CD34+ cells.
| Marker | Mean expression (%) | Standard deviation |
|---|---|---|
| CD34 | 97·44 | 2·26 |
| CD133 | 85·15 | 9·34 |
| CD3 | 0·68 | 0·45 |
The challenge of obtaining freshly purified HSC routinely in complete synchrony with the availability of newborn mice has provoked approaches to maintaining and expanding CD34+ HSCs in vitro. We have tested two different media both supplemented with the same cytokine cocktail. After 4 days of culture in Iscove's modified Dulbecco's medium (IMDM), CD34+ cells showed a slight expansion in cell numbers (Fig. 4b and d) compared with a greater expansion (∼threefold) in Stemline II medium (Fig. 4c and d). In both culture systems, the size of the CD34+ HSCs also tends to increase compared with freshly isolated cells (Fig. 4a–c). Of note, surface expression of CD34 and CD133 was decreased in culture, and the degree to which this occurs may vary with the culture conditions employed (Fig. 5a and b).
Fig. 4.
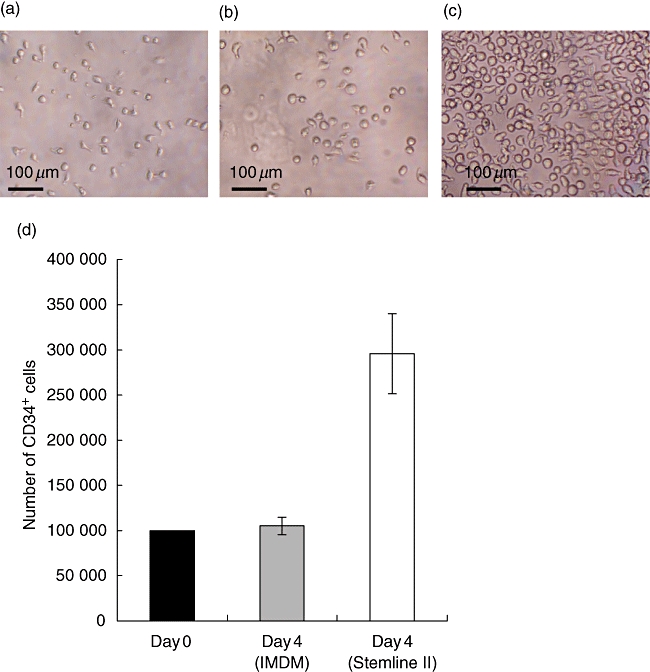
Ex vivo expansion of purified human CD34+ umbilical cord blood cells: comparison between Iscove's modified Dulbecco's medium (IMDM) and Stemline II media. Liquid culture of human CD34+ haematopoietic stem cells over 4 days in IMDM and Stemline II media stimulated with recombinant human Flk-2 ligand recombinant human thrombopoietin (10 μg/ml) and recombinant human stem cell factor (20 μg/ml). Photomicrograph of fresh CD34+ progenitors (concentration: 100 000 cells/ml). Bar represents 100 μm (a). Photomicrographs of the same CD34+ stem cells after 4 days in culture in IMDM (b) and Stemline II (c) media, both supplemented with cytokines. Cells growing in IMDM show a very low expansion after 4 days. On the contrary, CD34+ progenitors expend at least three times in Stemline II medium supplemented with the same cytokine cocktail. Histogram shows data from four different cord blood samples, showing the average number of CD34+ cells and corresponding standard deviation (d). Day 0 (▪), day 4 using IMDM ( ) and day 4 using Stemline II (□).
) and day 4 using Stemline II (□).
Fig. 5.
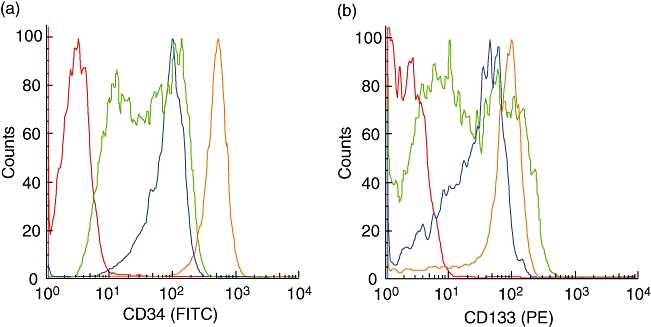
CD34+ cells difference of expression of CD34 and CD133 antigens after using Iscove's modified Dulbecco's medium (IMDM) or Stemline II media during 4 days of culture. Flow cytometry histograms. (a) Comparison of CD34 expression between fresh CD34+ cells ( ), CD34+ after 4 days in IMDM medium (
), CD34+ after 4 days in IMDM medium ( ) and CD34+ after 4 days in Stemline II medium (
) and CD34+ after 4 days in Stemline II medium ( ). Isotype control (
). Isotype control ( ). (b) Comparison of CD133 expression between fresh CD34+ cells (
). (b) Comparison of CD133 expression between fresh CD34+ cells ( ), CD34+ after 4 days in IMDM medium (
), CD34+ after 4 days in IMDM medium ( ) and CD34+ after 4 days in Stemline II medium (
) and CD34+ after 4 days in Stemline II medium ( ). Isotype control (
). Isotype control ( ). FITC, fluorescein isothiocyanate; PE, phycoerythrin.
). FITC, fluorescein isothiocyanate; PE, phycoerythrin.
Fate of human HSCs after engraftment
As a target organ for injection, the liver of newborn mice is relatively large and visible through the skin, and an administration aliquot of ∼30 μl is appropriate, delivered by 30 g needle. Presumably to create or prime appropriate niches for stem cell survival and differentiation, even newborn immunodeficient mice require a sublethal dose of irradiation before any transplantation, although the required dose of irradiation differs according to whether the recipient is newborn or adult [22,23]. Two doses of 2·5 G separated by 4 h is within the optimal range, although SCID mice show higher radiosensitivity than either Rag1null or Rag2null mice [11,44]. Sensitivity to irradiation is increased in unhealthy pups, which is common in immunodeficient strains, particularly among first litters. Once cells are injected, the species barrier will inevitably limit their survival, expansion and differentiation in the recipient mice. Therefore, specific human cytokines, growth factors and adhesion molecules, provided by injection or by transgenic expression, may improve the function and appropriate trafficking of human lymphocytes in a neo-formed immune system. As mentioned earlier, the genetic background of the immunodeficient recipients is important, with no report to date of successful reconstitution after HSC engraftment in C57BL6 Rag2null/IL-2rgnull mice. Inevitably, the initial attempts at engraftment will require that mice be killed after 4–6 weeks in order to track cells in different organs: reactivity to anti-human CD45 can provide the primary criterion of engraftment, followed by further phenotyping. Finally, because there is no heritability of each humanized mouse, it is worth considering that the life span of mice varies between strains: thus, NOD/SCID/IL-2rgnull mice live longer compared with NOD/SCID mice, that suffer a high rate of lymphomas.
Current limitations of humanized mouse models
Humanized mice have been optimized intensively since the first successful graft into SCID mice [4], and are now used as original tools for human biomedical research. They offer new opportunities to study human infectious diseases such as human immunodeficiency virus [45–49], Dengue haemorrhagic fever [50] and Epstein–Barr virus [51,52], as well as inflammatory conditions such as autoimmune disease [53], organ graft rejection [54], human therapeutics [55] and inflammatory conditions not replicated easily in conventional models [56,57]. They also provide new insights into the behaviour of human cells in vivo, vis-à-vis challenges that would not be ethical to administer to volunteers [58]. Among the obstacles currently receiving attention, the most obvious is the lack of human leucocyte antigen (HLA) class I and class II selecting elements for shaping the T cellrepertoire, albeit that Ishikawa et al. showed that someHLA-restricted T cell clones are produced in NOD/SCID/IL-2rgnull mice reconstituted with human CD34+ HSCs [23]. Moreover, no xenoreactivity against mouse tissues has been reported in this model, indicating that some central tolerance vis-à-vis mouse major histocompatibility complex apparently occurs. As an example of the promise of HLA-transgenic humanized mice one can consider psoriasis, a moderately common, highly debilitating disease for which there is no universally accepted animal model. Among the prime candidates for genes associated strongly with the disease is HLA-C, for which there is no obvious mouse orthologue. The critical, pathognomonic interactions of HLA-C may be with the TCR on CD8+ T cells, and with NK receptors on NK cells and on subsets of T cells. Hence, the analysis of psoriasis in humanized mice optimally requires a balanced differentiation of lymphocyte subtypes that may interact with a single transgene-encoded human element. Although this may sound daunting, it is feasible. The vigilant respect for practical experimentation, coupled with a high level of team coordination between obstetricians, scientists and veterinarians, is essential.
Additional practical data
Umbilical cord blood sampling and purification of cord blood mononuclear cells
We have collected UCB samples after obtaining consent from mothers scheduled to undergo elective Caesarean section at the St Thomas' Hospital Birth Centre, according to institutional guidelines approved by the Local Research Ethics Committee. Blood was manipulated routinely within 4–6 h after being collected using preheparinized 10 ml syringes equipped with 21 g needles and deposited directly into 50 ml polypropylene tubes containing 40 ml of sterile (PBS)−0·6% ACD-A at pH = 7·4 and kept at room temperature. Twenty-five ml of diluted anti-coagulated UCB was then layered carefully onto 15 ml of Ficoll-Paque Premium (Amersham, density = 1·077 g/ml) to deplete red blood cells through centrifugation at 400 g for 30 min (20°C) without brake. The mononuclear cell layer at the interface was collected and washed twice with PBS–ACD-A at 300 g for 10 min. Cells were resuspended in 5 ml PBS–ACD-A and a small aliquot taken for cell enumeration and viability using trypan blue.
Purification of CD34+ cells from UCB
Human UCB CD34+ cells were enriched using the Miltenyi CD34 MicroBead Kit and the AutoMACS apparatus, according to the manufacturer's instructions (Miltenyi Biotec, Bergish Gladbach, Germany), with slight modifications. Briefly, the cell suspension was resuspended in 300 μl/108 cells of cold (4°C) PBS–ACD-A/0·5% bovine serum albumin (BSA). The cell suspension was left on ice for 10 min and 100 μl/108 cells of FcR blocking reagent from Miltenyi CD34 MicroBead Kit added by mixing gently. After incubation on ice for 5 min, 100 μl/108 cells of magnetic magnetic antibody cell sorter MicroBeads conjugated to monoclonal mouse anti-human CD34 antibody were added and incubated on ice for 30 min. Cells were washed twice using 10 ml/108 cells PBS–ACD-A/0·5% BSA (300 g for 10 min) and resuspended in 500 μl/108 cells PBS–ACD-A/0·5% BSA and maintained on ice prior to separation. The AutoMACS apparatus was primed with cold, sterile running (PBS–ACD-A/0·5% BSA) and rinsing (PBS–ACD-A) buffers, and a ‘clean’ programme run prior to or between magnetic separations. The most sensitive programme (Posseld2) was used and CD34+ cells selected positively, counted and analysed by flow cytometry to determine yield and purity. Cells were then either cryopreserved and stored in liquid nitrogen or used for expansion cultures ex vivo.
Expansion of CD34+ UCB cells ex vivo
The logistics of coordinating maternal delivery and UCB CD34+ HSC separation with the birth of litters of immune deficient mice in order to conduct engraftment studies led us to examine two serum-free expansion media, both supplemented with cytokines. IMDM (Invitrogen, Paisley, UK) and Stemline II medium (Stemline II HSC expansion; Sigma Aldrich, Poole, Dorset, UK) both supplemented with recombinant human Flt-3/Flk-2 ligand (50 μg/ml), recombinant human thrombopoietin (10 μg/ml), recombinant human c-kit ligand or stem cell factor (20 μg/ml) (R&D Systems, Abingdon, UK) were compared. CD34+ cells at 105/ml in flat-bottomed 48-well plates were incubated in a fully humidified atmosphere (5% CO2) at 37°C. After 4 days, cells were resuspended by vigorous pipetting and expression of CD34, CD133 and CD3 markers assessed by flow cytometry.
Flow cytometry analysis of human CD34+ HSCs
The purity of UCB CD34+ cells was evaluated by three-colour flow cytometry using a fluorescence activated cell sorter (FACS)Calibur (Becton-Dickinson, San Jose, CA, USA). Cells (0·05–1 × 106) were incubated for 5 min at 4°C with FcR blocking reagent (Miltenyi Biotec) to prevent non-specific labelling and then stained with fluorescein isothiocyanate-conjugated anti-human CD34 (clone AC136; Miltenyi Biotec), phycoerythrin-conjugated anti-human CD133/2 (clone 293C3; Miltenyi Biotec) and antigen-presenting cell-conjugated anti-human CD3 (clone UCHT1; eBiosciences, San Diego, CA, USA) for 15 min at 4°C. Cells were washed twice with PBS 2% fetal calf serum (500 g for 5 min). A replicate sample was stained with appropriate isotype-matched control antibodies to determine the level of background staining and data analysed with FlowJo software.
Acknowledgments
This work was supported by grants from MedImmune, the Dunhill Medical Trust and the Welcome Trust. The authors acknowledge financial support from the Department of Health via the National Institute for Health Research comprehensive Biomedical Research Centre award to Guy's and St Thomas' NHS Foundation Trust in partnership with King's College London. We are grateful to Professor Andrew Shennan and colleagues for assistance in collecting human cord blood samples and to mothers attending the Birth Centre at St Thomas' Hospital for their participation in these studies.
References
- 1.Serreze DV, Chen YG. Of mice and men: use of animal models to identify possible interventions for the prevention of autoimmune type 1 diabetes in humans. Trends Immunol. 2005;26:603–7. doi: 10.1016/j.it.2005.08.012. [DOI] [PubMed] [Google Scholar]
- 2.Roep BO, Atkinson M. Animal models have little to teach us about type 1 diabetes: 1. In support of this proposal. Diabetologia. 2004;47:1650–6. doi: 10.1007/s00125-004-1517-1. [DOI] [PubMed] [Google Scholar]
- 3.Roep BO, Atkinson M, von Herrath M. Satisfaction (not) guaranteed: re-evaluating the use of animal models of type 1 diabetes. Nat Rev Immunol. 2004;4:989–97. doi: 10.1038/nri1502. [DOI] [PubMed] [Google Scholar]
- 4.Mosier DE, Gulizia RJ, Baird SM, Wilson DB. Transfer of a functional human immune system to mice with severe combined immunodeficiency. Nature. 1988;335:256–9. doi: 10.1038/335256a0. [DOI] [PubMed] [Google Scholar]
- 5.Bosma GC, Custer RP, Bosma MJ. A severe combined immunodeficiency mutation in the mouse. Nature. 1983;301:527–30. doi: 10.1038/301527a0. [DOI] [PubMed] [Google Scholar]
- 6.McCune JM, Namikawa R, Kaneshima H, Shultz LD, Lieberman M, Weissman IL. The SCID-hu mouse: murine model for the analysis of human hematolymphoid differentiation and function. Science. 1988;241:1632–9. doi: 10.1126/science.241.4873.1632. [DOI] [PubMed] [Google Scholar]
- 7.Lapidot T, Pflumio F, Doedens M, Murdoch B, Williams DE, Dick JE. Cytokine stimulation of multilineage hematopoiesis from immature human cells engrafted in SCID mice. Science. 1992;255:1137–41. doi: 10.1126/science.1372131. [DOI] [PubMed] [Google Scholar]
- 8.Bosma GC, Fried M, Custer RP, Carroll A, Gibson DM, Bosma MJ. Evidence of functional lymphocytes in some (leaky) scid mice. J Exp Med. 1988;167:1016–33. doi: 10.1084/jem.167.3.1016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Greiner DL, Hesselton RA, Shultz LD. SCID mouse models of human stem cell engraftment. Stem Cells. 1998;16:166–77. doi: 10.1002/stem.160166. [DOI] [PubMed] [Google Scholar]
- 10.Christianson SW, Greiner DL, Schweitzer IB, et al. Role of natural killer cells on engraftment of human lymphoid cells and on metastasis of human T-lymphoblastoid leukemia cells in C57BL/6J-scid mice and in C57BL/6J-scid bg mice. Cell Immunol. 1996;171:186–99. doi: 10.1006/cimm.1996.0193. [DOI] [PubMed] [Google Scholar]
- 11.Shultz LD, Schweitzer PA, Christianson SW, et al. Multiple defects in innate and adaptive immunologic function in NOD/LtSz-scid mice. J Immunol. 1995;154:180–91. [PubMed] [Google Scholar]
- 12.Lowry PA, Shultz LD, Greiner DL, et al. Improved engraftment of human cord blood stem cells in NOD/LtSz-scid/scid mice after irradiation or multiple-day injections into unirradiated recipients. Biol Blood Marrow Transplant. 1996;2:15–23. [PubMed] [Google Scholar]
- 13.Pflumio F, Izac B, Katz A, Shultz LD, Vainchenker W, Coulombel L. Phenotype and function of human hematopoietic cells engrafting immune-deficient CB17-severe combined immunodeficiency mice and nonobese diabetic-severe combined immunodeficiency mice after transplantation of human cord blood mononuclear cells. Blood. 1996;88:3731–40. [PubMed] [Google Scholar]
- 14.Lodolce JP, Boone DL, Chai S, et al. IL-15 receptor maintains lymphoid homeostasis by supporting lymphocyte homing and proliferation. Immunity. 1998;9:669–76. doi: 10.1016/s1074-7613(00)80664-0. [DOI] [PubMed] [Google Scholar]
- 15.Kennedy MK, Glaccum M, Brown SN, et al. Reversible defects in natural killer and memory CD8 T cell lineages in interleukin 15-deficient mice. J Exp Med. 2000;191:771–80. doi: 10.1084/jem.191.5.771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sugamura K, Asao H, Kondo M, et al. The interleukin-2 receptor gamma chain: its role in the multiple cytokine receptor complexes and T cell development in XSCID. Annu Rev Immunol. 1996;14:179–205. doi: 10.1146/annurev.immunol.14.1.179. [DOI] [PubMed] [Google Scholar]
- 17.Cao X, Shores EW, Hu-Li J, et al. Defective lymphoid development in mice lacking expression of the common cytokine receptor gamma chain. Immunity. 1995;2:223–38. doi: 10.1016/1074-7613(95)90047-0. [DOI] [PubMed] [Google Scholar]
- 18.DiSanto JP, Muller W, Guy-Grand D, Fischer A, Rajewsky K. Lymphoid development in mice with a targeted deletion of the interleukin 2 receptor gamma chain. Proc Natl Acad Sci USA. 1995;92:377–81. doi: 10.1073/pnas.92.2.377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ito M, Hiramatsu H, Kobayashi K, et al. NOD/SCID/gamma(c)(null) mouse: an excellent recipient mouse model for engraftment of human cells. Blood. 2002;100:3175–82. doi: 10.1182/blood-2001-12-0207. [DOI] [PubMed] [Google Scholar]
- 20.Yahata T, Ando K, Nakamura Y, et al. Functional human T lymphocyte development from cord blood CD34+ cells in nonobese diabetic/Shi-scid, IL-2 receptor gamma null mice. J Immunol. 2002;169:204–9. doi: 10.4049/jimmunol.169.1.204. [DOI] [PubMed] [Google Scholar]
- 21.Hiramatsu H, Nishikomori R, Heike T, et al. Complete reconstitution of human lymphocytes from cord blood CD34+ cells using the NOD/SCID/gammacnull mice model. Blood. 2003;102:873–80. doi: 10.1182/blood-2002-09-2755. [DOI] [PubMed] [Google Scholar]
- 22.Shultz LD, Lyons BL, Burzenski LM, et al. Human lymphoid and myeloid cell development in NOD/LtSz-scid IL2R gamma null mice engrafted with mobilized human hemopoietic stem cells. J Immunol. 2005;174:6477–89. doi: 10.4049/jimmunol.174.10.6477. [DOI] [PubMed] [Google Scholar]
- 23.Ishikawa F, Yasukawa M, Lyons B, et al. Development of functional human blood and immune systems in NOD/SCID/IL2 receptor {gamma} chain(null) mice. Blood. 2005;106:1565–73. doi: 10.1182/blood-2005-02-0516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Traggiai E, Chicha L, Mazzucchelli L, et al. Development of a human adaptive immune system in cord blood cell-transplanted mice. Science. 2004;304:104–7. doi: 10.1126/science.1093933. [DOI] [PubMed] [Google Scholar]
- 25.Takenaka K, Prasolava TK, Wang JCY, et al. Polymorphism in Sirpa modulates engraftment of human hematopoietic stem cells. Nature Immunology. 2007;8:1313. doi: 10.1038/ni1527. [DOI] [PubMed] [Google Scholar]
- 26.Metcalf D. On hematopoietic stem cell fate. Immunity. 2007;26:669–73. doi: 10.1016/j.immuni.2007.05.012. [DOI] [PubMed] [Google Scholar]
- 27.Strauss LC, Brovall C, Fackler MJ, et al. Antigenic analysis of hematopoiesis. IV. The My-11 hematopoietic cell surface antigen is expressed by myelomonocytic and lymphoid, but not erythroid, progenitor cells. Exp Hematol. 1986;14:935–45. [PubMed] [Google Scholar]
- 28.Yin AH, Miraglia S, Zanjani ED, et al. AC133, a novel marker for human hematopoietic stem and progenitor cells. Blood. 1997;90:5002–12. [PubMed] [Google Scholar]
- 29.Pasino M, Lanza T, Marotta F, et al. Flow cytometric and functional characterization of AC133+ cells from human umbilical cord blood. Br J Haematol. 2000;108:793–800. doi: 10.1046/j.1365-2141.2000.01949.x. [DOI] [PubMed] [Google Scholar]
- 30.Payne KJ, Crooks GM. Immune-cell lineage commitment: translation from mice to humans. Immunity. 2007;26:674–7. doi: 10.1016/j.immuni.2007.05.011. [DOI] [PubMed] [Google Scholar]
- 31.Ueda T, Yoshino H, Kobayashi K, et al. Hematopoietic repopulating ability of cord blood CD34(+) cells in NOD/Shi-scid mice. Stem Cells. 2000;18:204–13. doi: 10.1634/stemcells.18-3-204. [DOI] [PubMed] [Google Scholar]
- 32.Kuci S, Wessels JT, Buhring HJ, et al. Identification of a novel class of human adherent CD34– stem cells that give rise to SCID-repopulating cells. Blood. 2003;101:869–76. doi: 10.1182/blood-2002-03-0711. [DOI] [PubMed] [Google Scholar]
- 33.de Wynter EA, Buck D, Hart C, et al. CD34+AC133+ cells isolated from cord blood are highly enriched in long-term culture-initiating cells, NOD/SCID-repopulating cells and dendritic cell progenitors. Stem Cells. 1998;16:387–96. doi: 10.1002/stem.160387. [DOI] [PubMed] [Google Scholar]
- 34.Bhatia M, Wang JC, Kapp U, Bonnet D, Dick JE. Purification of primitive human hematopoietic cells capable of repopulating immune-deficient mice. Proc Natl Acad Sci USA. 1997;94:5320–5. doi: 10.1073/pnas.94.10.5320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Bonnet D, Bhatia M, Wang JC, Kapp U, Dick JE. Cytokine treatment or accessory cells are required to initiate engraftment of purified primitive human hematopoietic cells transplanted at limiting doses into NOD/SCID mice. Bone Marrow Transplant. 1999;23:203–9. doi: 10.1038/sj.bmt.1701564. [DOI] [PubMed] [Google Scholar]
- 36.Ueda T, Yoshida M, Yoshino H, et al. Hematopoietic capability of CD34+ cord blood cells: a comparison with CD34+ adult bone marrow cells. Int J Hematol. 2001;73:457–62. doi: 10.1007/BF02994007. [DOI] [PubMed] [Google Scholar]
- 37.Kim DK, Fujiki Y, Fukushima T, Ema H, Shibuya A, Nakauchi H. Comparison of hematopoietic activities of human bone marrow and umbilical cord blood CD34 positive and negative cells. Stem Cells. 1999;17:286–94. doi: 10.1002/stem.170286. [DOI] [PubMed] [Google Scholar]
- 38.Kawada H, Ando K, Tsuji T, et al. Rapid ex vivo expansion of human umbilical cord hematopoietic progenitors using a novel culture system. Exp Hematol. 1999;27:904–15. doi: 10.1016/s0301-472x(99)00012-0. [DOI] [PubMed] [Google Scholar]
- 39.Forraz N, Pettengell R, McGuckin CP. Characterization of a lineage-negative stem-progenitor cell population optimized for ex vivo expansion and enriched for LTC-IC. Stem Cells. 2004;22:100–8. doi: 10.1634/stemcells.22-1-100. [DOI] [PubMed] [Google Scholar]
- 40.Kobari L, Giarratana MC, Gluckman JC, Douay L, Rosenzwajg M. Ex vivo expansion does not alter the capacity of umbilical cord blood CD34+ cells to generate functional T lymphocytes and dendritic cells. Stem Cells. 2006;24:2150–7. doi: 10.1634/stemcells.2006-0102. [DOI] [PubMed] [Google Scholar]
- 41.Gimeno R, Weijer K, Voordouw A, et al. Monitoring the effect of gene silencing by RNA interference in human CD34+ cells injected into newborn RAG2–/– gammac–/– mice: functional inactivation of p53 in developing T cells. Blood. 2004;104:3886–93. doi: 10.1182/blood-2004-02-0656. [DOI] [PubMed] [Google Scholar]
- 42.Mazurier F, Doedens M, Gan OI, Dick JE. Rapid myeloerythroid repopulation after intrafemoral transplantation of NOD-SCID mice reveals a new class of human stem cells. Nat Med. 2003;9:959–63. doi: 10.1038/nm886. [DOI] [PubMed] [Google Scholar]
- 43.Schoeberlein A, Schatt S, Troeger C, Surbek D, Holzgreve W, Hahn S. Engraftment kinetics of human cord blood and murine fetal liver stem cells following in utero transplantation into immunodeficient mice. Stem Cells Dev. 2004;13:677–84. doi: 10.1089/scd.2004.13.677. [DOI] [PubMed] [Google Scholar]
- 44.Shultz LD, Lang PA, Christianson SW, et al. NOD/LtSz-Rag1null mice: an immunodeficient and radioresistant model for engraftment of human hematolymphoid cells, HIV infection, and adoptive transfer of NOD mouse diabetogenic T cells. J Immunol. 2000;164:2496–507. doi: 10.4049/jimmunol.164.5.2496. [DOI] [PubMed] [Google Scholar]
- 45.Berges BK, Wheat WH, Palmer BE, Connick E, Akkina R. HIV-1 infection and CD4 T cell depletion in the humanized Rag2–/–gamma c–/– (RAG-hu) mouse model. Retrovirology. 2006;3:76. doi: 10.1186/1742-4690-3-76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Watanabe S, Terashima K, Ohta S, et al. Hematopoietic stem cell-engrafted NOD/SCID/IL2Rgamma null mice develop human lymphoid systems and induce long-lasting HIV-1 infection with specific humoral immune responses. Blood. 2007;109:212–8. doi: 10.1182/blood-2006-04-017681. [DOI] [PubMed] [Google Scholar]
- 47.Zhang L, Kovalev GI, Su L. HIV-1 infection and pathogenesis in a novel humanized mouse model. Blood. 2007;109:2978–81. doi: 10.1182/blood-2006-07-033159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Gorantla S, Sneller H, Walters L, et al. Human immunodeficiency virus type 1 pathobiology studied in humanized BALB/c-Rag2–/–gammac–/– mice. J Virol. 2007;81:2700–12. doi: 10.1128/JVI.02010-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Sun Z, Denton PW, Estes JD, et al. Intrarectal transmission, systemic infection, and CD4+ T cell depletion in humanized mice infected with HIV-1. J Exp Med. 2007;204:705–14. doi: 10.1084/jem.20062411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Bente DA, Melkus MW, Garcia JV, Rico-Hesse R. Dengue fever in humanized NOD/SCID mice. J Virol. 2005;79:13797–9. doi: 10.1128/JVI.79.21.13797-13799.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Islas-Ohlmayer M, Padgett-Thomas A, Domiati-Saad R, et al. Experimental infection of NOD/SCID mice reconstituted with human CD34+ cells with Epstein–Barr virus. J Virol. 2004;78:13891–900. doi: 10.1128/JVI.78.24.13891-13900.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Melkus MW, Estes JD, Padgett-Thomas A, et al. Humanized mice mount specific adaptive and innate immune responses to EBV and TSST-1. Nat Med. 2006;12:1316–22. doi: 10.1038/nm1431. [DOI] [PubMed] [Google Scholar]
- 53.Flynn JC, Meroueh C, Snower DP, David CS, Kong YM. Depletion of CD4+CD25+ regulatory T cells exacerbates sodium iodide-induced experimental autoimmune thyroiditis in human leucocyte antigen DR3 (DRB1*0301) transgenic class II-knock-out non-obese diabetic mice. Clin Exp Immunol. 2007;147:547–54. doi: 10.1111/j.1365-2249.2006.03303.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Kaiser S, Kagi D, Ihorst G, Kapp U. Perforin-independent rejection of transplanted human stem cells. Clin Exp Immunol. 2006;145:332–8. doi: 10.1111/j.1365-2249.2006.03128.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Vassilev T, Mihaylova N, Voynova E, Nikolova M, Kazatchkine M, Kaveri S. IgM-enriched human intravenous immunoglobulin suppresses T lymphocyte functions in vitro and delays the activation of T lymphocytes in hu-SCID mice. Clin Exp Immunol. 2006;145:108–15. doi: 10.1111/j.1365-2249.2006.03098.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Katakura T, Yoshida T, Kobayashi M, Herndon DN, Suzuki F. Immunological control of methicillin-resistant Staphylococcus aureus (MRSA) infection in an immunodeficient murine model of thermal injuries. Clin Exp Immunol. 2005;142:419–25. doi: 10.1111/j.1365-2249.2005.02944.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Maerten P, Kwon BS, Shen C, et al. Involvement of 4-1BB (CD137)-4-1BBligand interaction in the modulation of CD4 T cell-mediated inflammatory colitis. Clin Exp Immunol. 2006;143:228–36. doi: 10.1111/j.1365-2249.2005.02991.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Dayan CM, Wraith DC. Preparing for first-in-man studies: the challenges for translational immunology post-TGN1412. Clin Exp Immunol. 2008;151:231–4. doi: 10.1111/j.1365-2249.2007.03559.x. [DOI] [PMC free article] [PubMed] [Google Scholar]


