Abstract
Both oestrogen deficiency and the inflammatory disease contribute to the generalized bone loss seen in postmenopausal rheumatoid arthritis (RA). Oestradiol and the selective oestrogen receptor modulator raloxifene have been shown to ameliorate the disease in collagen-induced arthritis (CIA), a well-established animal model for human RA. The aim of this study was to investigate whether raloxifene-treatment would be beneficial in long-term treatment of established CIA, both regarding anti-arthritic and anti-osteoporotic properties. Female dilute brown agouti mice were ovariectomized and CIA was induced. Raloxifene or vehicle treatment was administered 5 days per week, and the clinical arthritis score was evaluated continuously. At termination, bone mineral density was analysed, paws were collected for histological examination and sera were analysed for markers of bone and cartilage turnover, as well as antibodies to type II collagen and levels of interleukin (IL)-6. Treatment with raloxifene is beneficial in long-term treatment of established CIA. It hampers the disease severity and frequency, protects the joints from destruction and protects against the development of osteoporosis. The proinflammatory cytokine IL-6 was down-regulated in raloxifene-treated mice compared with controls. The serum levels of antibodies to collagen were not affected by raloxifene-treatment. Long-term treatment with raloxifene has both anti-arthritic and anti-osteoporotic effects in established experimental postmenopausal polyarthritis.
Keywords: BMD, CIA, mice, raloxifene
Introduction
Rheumatoid arthritis (RA) is a progressive systemic autoimmune disease, causing great morbidity. Both focal joint erosions and generalized osteoporosis result in a debilitating disease. The prevalence is 0·5–1% worldwide [1], with a female to male ratio of 3 : 1, and the prevalence of concurrent osteoporosis is 50% [2,3].
Compounds exerting their effects via activation the of oestrogen receptors have the potential to influence postmenopuasal RA in multiple ways, as the classical oestrogen receptors α and β have been found both in cells of the immune system and in bone cells. We have reported previously on the beneficial effects of hormone replacement therapy (HRT) in postmenopausal RA [4], but because of severe side effects HRT is no longer recommended [5]. Therefore, finding substances with the beneficial effects of oestrogen, but without the side effects, is of great importance. Recently, we have shown potent short-term anti-arthritic effects of raloxifene, a selective oestrogen receptor modulator approved for the treatment of postmenopausal osteoporosis, in collagen-induced arthritis (CIA) [6].
In this study, we investigate the outcome during long-term treatment of CIA with raloxifene. We used the raloxifene analogue LY117018, and treatment was started on day 26 after immunization, when 50% of animals had developed arthritis, and continued until termination of the experiment on day 70. The frequency and severity of arthritis were evaluated continuously, and after termination histological and bone mineral density (BMD) assessments were carrried out, as well as analysis of serological parameters of bone and cartilage turnover, anti-collagen II antibodies and the proinflammatory cytokine interleukin (IL)-6.
Materials and methods
Animals and experimental procedures
The ethical committee for animal experiments at Göteborg University approved this study.
Female dilute brown agouti mice were purchased from TaconicM & B A/S (Ry, Denmark). Mice were tagged electronically and five to 10 animals per cage were kept under standard environmental conditions and fed standard laboratory chow and tap water ad libitum.
The time-line in Fig. 1 illustrates the experimental protocol used. Ovariectomy was performed at 10 weeks of age. Mice were immunized at the age of 27 weeks, after bone growth finished, to avoid BMD affection by normal skeletal maturation. Twenty mice were included in the experiment. Ovaries were removed through a midline incision of the skin and flank incisions of the peritoneum. The skin incision was then closed with metallic clips. Surgery was performed after the mice were anaesthetized with ketamine (PfizerAB, Täby, Sweden) and medetomidin (OrionPharma, Espoo, Finland). Carprofen (OrionPharma) was used postoperatively.
Fig. 1.
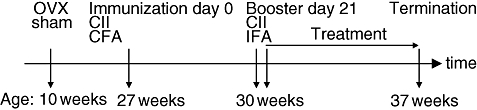
Diagram of the experimental timeline. OVX, ovariectomy; sham: sham operation; CII, collagen type II; CFA, complete Freund's adjuvant; IFA, incomplete Freund's adjuvant; w, weeks.
Treatment
Treatment was started 26 days after immunization, when mice were aged 30 weeks (Fig. 1). Mice were given subcutaneous injections 5 days per week of the raloxifene analogue LY117018 (generous gift from Eli Lilly, Indianapolis, IN, USA) (60 μg/mouse/day) dissolved in 100 μl Miglyol812 (OmyaPeralta GmbH, Hamburg, Germany) or Miglyol812 (100 μl/mouse/day). LY117018 differs from raloxifene at only one site on the molecule, with a piperidine ring on the basic side chain instead of a pyrrolidine ring. This small difference should not affect its biological properties.
The dosage of raloxifene has been shown to be equally as potent as 17β-oestradiol in preventing ovariectomy induced osteoporosis in mice. It corresponds to approximately 6 mg/m2, whereas the dosage given for osteoporosis treatment in women is approximately 35 mg/m2.
Induction and evaluation of arthritis
Seventeen weeks after ovariectomy the mice were immunized with 100 μg of chicken type II collagen (CII) (Sigma, St Louis, MO, USA) dissolved in 0·1 m acetic acid and emulsified with an equal volume of incomplete Freund's adjuvant (Sigma) supplemented with 0·5 mg/ml Mycobacterium tuberculosis (Sigma). A total volume of 100 μl was injected intradermally at the base of the tail. After 21 days, mice received a booster injection with CII emulsified in incomplete Freund's adjuvant. Arthritis developed shortly thereafter, and was evaluated continuously for frequency and severity. Scoring was performed in a blinded manner without knowledge of the treatment groups and previous scores. Severity was graded as described previously [7], scoring 1–3 in each paw (maximum of 12 points per mouse) as follows: 1, swelling or erythema in one joint; 2, swelling or erythema in two joints; 3, severe swelling of the entire paw or ankylosis.
Tissue collection and histological examination
At termination of the experiment, mice were anaesthetized for blood withdrawal, and then killed by cervical dislocation. Sera were collected individually and stored at −20°C until used. Successful removal of the ovaries was confirmed by weighing the uteri. One femur was placed in formaldehyde for analysis of BMD. Paws were placed in formaldehyde, decalcified and embedded in paraffin. Sections were stained with haematoxylin and eosin and encoded before examination. In sections from each animal, the front and back of all four paws were graded separately on a scale of 0–4, with the score then divided by 2, which yielded a maximum histological destruction score of 16 points per mouse, assessed as follows: 1 = synovial hypertrophy, 2 = pannus, erosions of cartilage, 3 = erosions of bone, 4 = complete ankylosis.
Assessment of BMD
One femur was subjected to a peripheral quantitative computed tomography (pQCT) scan with a Stratec pQCT XCT Research M, software version 5·4B (Norland, Fort Atkinson, WI, USA) at a resolution of 70 μm, as described previously [8]. Trabecular BMD was determined with a metaphyseal scan at a point 3% of the length of the femur from the growth plate. The inner 45% of the area was defined as the trabecular bone compartment. Cortical BMD was determined with a mid-diaphyseal scan, located 36% of the length of the femur from the growth plate.
Identification of serological markers of bone and cartilage remodelling
For measurement of bone resorption, serum levels of fragments of type I collagen were assessed using a RatLaps enzyme-linked immunosorbent assay (ELISA) kit (Nordic Bioscience Diagnostics A/S, Herlev, Denmark) (intra-assay coefficient of variation 5·6% and interassay 10·5%). Serum levels of osteocalcin, a marker of bone formation, were determined with a mouse osteocalcin immunoradiometric assay kit (Immutopics, Inc., San Clemente, CA, USA) (intra-assay coefficient of variation 2·8% and interassay 3·7%). As a marker of cartilage destruction, serum levels of cartilage oligomeric matrix protein (COMP) were determined with an Animal COMP® ELISA kit (AnaMar Medical AB, Uppsala, Sweden) (intra-assay coefficient of variation 9·8% and interassay 6·0%).
Serum levels of CII-specific antibodies and IL-6 bioassay
Using a previously described ELISA, serum levels of anti-CII IgG antibodies were determined [9]. A bioassay [10] with cell line B13·29, subclone B9 (which is dependent on IL-6 for growth), was used to measure serum levels of IL-6.
Statistical analysis
For statistical evaluation, the non-parametric Mann–Whitney U-test was used. A P-value ≤ 0·05 was considered significant.
Results
Arthritic score was evaluated continuously. Raloxifene treatment decreased significantly both the frequency (P < 0·001) and the severity (P < 0·01 from day 47 and <0·001 from day 68) of the disease, compared with controls (Fig. 2a and b).
Fig. 2.
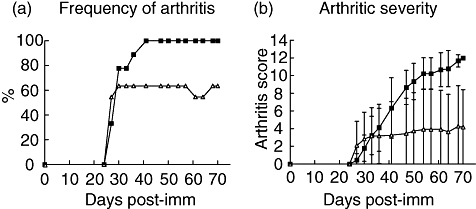
Long-term treatment with raloxifene results in decreased frequency and severity of collagen induced arthritis. Ovariectomized dilute brown agouti mice with collagen-induced arthritis were treated with raloxifene (60 μg/day; n = 11) (solid triangles) or vehicle control (Miglyol 812; n = 9) (solid squares). Treatment was administered 5 days per week from day 26 after immunization, when mice had developed arthritis, and until termination of the experiment on day 70 after immunization. Arthritis was considered present when signs of arthritis were identified in one joint for two consecutive assessments, or in >1 joint. Frequency (a) and severity (b) were evaluated every third day. Severity was graded 1–3 in each paw (maximum score of 12 per mouse), expressed as the mean ± standard deviation in each group. Raloxifene treatment decreased significantly both the frequency (P < 0·001) and the severity (P < 0·01 from day 47, and <0·001 from day 68) of the disease, compared with controls.
Histological examination of the paw sections (Fig. 3) revealed severe destruction of the joints in the vehicle-treated control group compared with very little damage in raloxifene-treated mice (P < 0·01) (median scores of 8·5 and 0 respectively).
Fig. 3.
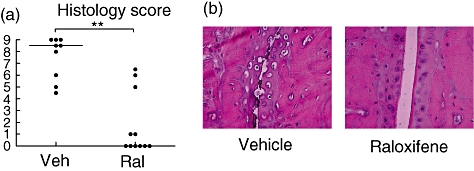
Long-term treatment with raloxifene preserves joint histology. Ovariectomized dilute brown agouti mice with collagen-induced arthritis were treated with raloxifene (60 μg/day; n = 11) or vehicle control (Miglyol 812; n = 9). Treatment was administered 5 days per week from day 26 after immunization, when mice had developed arthritis, and until termination of the experiment on day 70 after immunization. (a) Histological destruction scores of paw sections. Scores were evaluated in a blinded manner, with the front and back of each paw graded 0–4, with each score divided by 2, yielding a maximum score of 16 per mouse, as follows: 1 = synovial hypertrophy; 2 = pannus, erosions of cartilage; 3 = erosions of bone; 4 = complete ankylosis. Scatter plots show the scores of individual mice, and bars show the median in each group. **P < 0·01. (b) Representative images of paw tissue sections, revealing treatment effects on histological features in each group.
The serum levels of anti-collagen II antibodies did not differ between the raloxifene and vehicle treatment groups, whereas the levels of the proinflammatory cytokine IL-6 were significantly (P < 0·01) lowered by raloxifene (Table 1).
Table 1.
Serum levels of interleukin (IL)-6 and anti-collagen II immunoglobulin G antibodies in mice with collagen-induced arthritis treated with raloxifene or vehicle control.†
| Vehicle | Raloxifene | |
|---|---|---|
| IL-6 (pg/ml) | 175 (108–246) | 73* (68–77) |
| CIIab (absorbance) | 1·6 (1·4–1·8) | 1·5 (1·3–1·5) |
P < 0·01.
Values are the median (interquartile range) of nine to 11 animals per group.
Both trabecular and cortical BMD were higher in the raloxifene group compared with controls (P < 0·001) (Fig. 4).
Fig. 4.
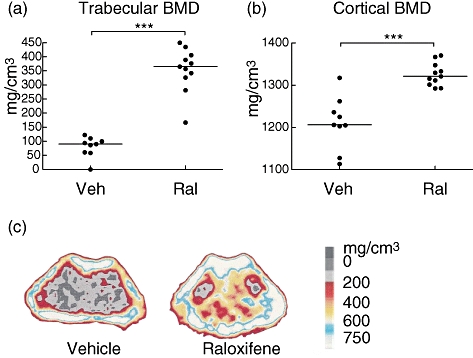
Long-term treatment with raloxifene results in preserved bone mineral density. (a, b) Scatterplots of individual data (bars indicate the median per group), showing the trabecular and cortical bone mineral density (BMD) in the femur of ovariectomized mice with collagen-induced arthritis treated with raloxifene (60 μg/day) or vehicle Miglyol (100 μl/day). ***P < 0·001. (c) Representative peripheral quantitative computer tomography images of cross-sections of the femur, showing the BMD. The scale indicates the density of the bone, from 0 (black) to >750 mg/cm3 (white).
The serum levels of RatLaps (indicating bone resorption) were lower in the raloxifene-treated group (P < 0·05) (Table 2). Bone formation, as measured by serum levels of osteocalcin, did not differ between the two groups, nor did the degree of cartilage destruction as measured by serum levels of COMP.
Table 2.
Serum levels of bone and cartilage turnover markers in mice with collagen-induced arthritis treated with raloxifene or vehicle control.†
| Vehicle | Raloxifene | |
|---|---|---|
| Bone formation | 82 | 93 |
| Osteocalcin, ng/ml | (68–94) | (88–101) |
| Bone resorption | 33 | 27* |
| RatLaps, ng/ml | (28–44) | (20–34) |
| Cartilage destruction | 3·0 | 2·0 |
| COMP, U/l | (2·6–3·8) | (1·3–3·9) |
P < 0·05.
Values are the median (interquartile range) of nine to 11 animals per group. COMP, cartilage oligomeric matrix protein.
Discussion
The present study is the first to show sustained long-term (until day 70) anti-arthritic effects of raloxifene with protection against both joint erosivity and generalized osteoporosis in established experimental postmenopausal CIA. It has been shown previously that both endogenous and exogenous oestradiol hamper CIA [11–13], and we have shown short-term (until day 43) anti-arthritic effects of raloxifene [6]. Interestingly, although 50% of the mice had already developed arthritis at the start of the treatment-period, raloxifene not only hampered the progression in the arthritic mice, but also stopped the frequency of arthritis from increasing further. Treatment was begun this late in order to mimic the clinical situation in patients with a confirmed diagnosis and established disease.
The mechanisms for protection against arthritis upon exposure to raloxifene are not understood fully. Our present and earlier data [6] demonstrate anti-inflammatory properties, indicated by the low serum levels of the inflammatory cytokine IL-6 compared with controls. It has been shown previously that raloxifene reduces the IL-6 secretion from human osteoblastic cells in culture [14], but two recent studies in postmenopausal, osteoporotic women did not show a significant decline in serum IL-6 after raloxifene treatment [15,16]. These women did not have an inflammatory disease.
Bisphosphonates are often used for treatment of osteoporosis, and intermittent exposure to the bisphosphonate etidronate was found to increase BMD as well as retard the development of erosions in RA, but in another trial radiological outcome was not influenced [17,18]. Etidronate also increased BMD in glucocorticosteroid-treated patients with RA and polymyalgia rheumatica [19]. Both alendronate and risedronate have been shown to increase BMD in RA patients on glucocorticosteroid therapy [20–22]. Risedronate also reduced the risk of vertebral fractures in a large study of patients treated with corticosteroids with different diagnoses, including RA [23]. The dual effects of raloxifene, suppressing inflammation and opposing osteoporosis, could be more beneficial in treating postmenopausal RA.
At the time of immunization, the mice had been ovariectomized for 17 weeks and they were terminated 10 weeks later. This resulted in very low BMD in vehicle-treated mice. Interestingly, raloxifene treatment increased both the trabecular and the cortical BMD in spite of the long period of sex hormone depletion, and in spite of the arthritic disease. The serum levels of RatLaps were significantly lower in the raloxifene-treated group, indicating a lower rate of bone resorption. In contrast, serum levels of osteocalcin were similar, suggesting a new steady state in bone formation. Indeed, a recent study in postmenopausal osteoporotic women also showed a significant decrease in markers of bone resorption and formation in women treated for 12 weeks with raloxifene, compared with pretreatment levels, and resulting in the same levels as non-osteoporotic controls [15].
Conclusion
The dual protective effect of raloxifene in postmenopausal polyarthritis on joint destruction and the development of osteoporosis provides a rationale for its addition to the treatment regimen of postmenopausal patients with RA.
Acknowledgments
We thank Margareta Rosenkvist, Berit Eriksson, Anette Hansevi, Malin Erlandsson and Maud Petersson for excellent technical assistance. This study was supported by grants from the Medical Faculty of Göteborg University (ALF), Göteborg Medical Society, King GustavV's 80 years' foundation, the Sahlgrenska Foundation, the NovoNordic Foundation, the Börje Dahlin foundation, the Association against Rheumatism, Reumaforskningsfond Margareta and the Swedish Research Council.
References
- 1.Doran MF, Pond GR, Crowson CS, O'Fallon WM, Gabriel SE. Trends in incidence and mortality in rheumatoid arthritis in Rochester, Minnesota, over a forty-year period. Arthritis Rheum. 2002;46:625–31. doi: 10.1002/art.509. [DOI] [PubMed] [Google Scholar]
- 2.Forsblad D'Elia H, Larsen A, Waltbrand E, et al. Radiographic joint destruction in postmenopausal rheumatoid arthritis is strongly associated with generalised osteoporosis. Ann Rheum Dis. 2003;62:617–23. doi: 10.1136/ard.62.7.617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sinigaglia L, Nervetti A, Mela Q, et al. A multicenter cross sectional study on bone mineral density in rheumatoid arthritis. Italian Study Group on Bone Mass in Rheumatoid Arthritis. J Rheumatol. 2000;27:2582–9. [PubMed] [Google Scholar]
- 4.Forsblad D'Elia H, Larsen A, Mattsson LA, et al. Influence of hormone replacement therapy on disease progression and bone mineral density in rheumatoid arthritis. J Rheumatol. 2003;30:1456–63. [PubMed] [Google Scholar]
- 5.Stefanick ML. Estrogens and progestins: background and history, trends in use, and guidelines and regimens approved by the US Food and Drug Administration. Am J Med. 2005;118:64–73. doi: 10.1016/j.amjmed.2005.09.059. [DOI] [PubMed] [Google Scholar]
- 6.Jochems C, Islander U, Kallkopf A, Lagerquist M, Ohlsson C, Carlsten H. Role of raloxifene as a potent inhibitor of experimental postmenopausal polyarthritis and osteoporosis. Arthritis Rheum. 2007;56:3261–70. doi: 10.1002/art.22873. [DOI] [PubMed] [Google Scholar]
- 7.Holmdahl R, Jansson L, Larsson E, Rubin K, Klareskog L. Homologous type II collagen induces chronic and progressive arthritis in mice. Arthritis Rheum. 1986;29:106–13. doi: 10.1002/art.1780290114. [DOI] [PubMed] [Google Scholar]
- 8.Windahl SH, Vidal O, Andersson G, Gustafsson JA, Ohlsson C. Increased cortical bone mineral content but unchanged trabecular bone mineral density in female ERbeta(–/–) mice. J Clin Invest. 1999;104:895–901. doi: 10.1172/JCI6730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Verdrengh M, Jonsson IM, Holmdahl R, Tarkowski A. Genistein as an anti-inflammatory agent. Inflamm Res. 2003;52:341–6. doi: 10.1007/s00011-003-1182-8. [DOI] [PubMed] [Google Scholar]
- 10.Helle M, Boeije L, Aarden LA. Functional discrimination between interleukin 6 and interleukin 1. Eur J Immunol. 1988;18:1535–40. doi: 10.1002/eji.1830181010. [DOI] [PubMed] [Google Scholar]
- 11.Holmdahl R, Jansson L, Andersson M. Female sex hormones suppress development of collagen-induced arthritis in mice. Arthritis Rheum. 1986;29:1501–9. doi: 10.1002/art.1780291212. [DOI] [PubMed] [Google Scholar]
- 12.Jansson L, Holmdahl R. Oestrogen induced suppression of collagen arthritis. IV. Progesterone alone does not affect the course of arthritis but enhances the oestrogen-mediated therapeutic effect. J Reprod Immunol. 1989;15:141–50. doi: 10.1016/0165-0378(89)90033-8. [DOI] [PubMed] [Google Scholar]
- 13.Yamasaki D, Enokida M, Okano T, Hagino H, Teshima R. Effects of ovariectomy and estrogen replacement therapy on arthritis and bone mineral density in rats with collagen-induced arthritis. Bone. 2001;28:634–40. doi: 10.1016/s8756-3282(01)00426-4. [DOI] [PubMed] [Google Scholar]
- 14.Cheung J, Mak YT, Papaioannou S, Evans BA, Fogelman I, Hampson G. Interleukin-6 (IL-6), IL-1, receptor activator of nuclear factor kappaB ligand (RANKL) and osteoprotegerin production by human osteoblastic cells: comparison of the effects of 17-beta oestradiol and raloxifene. J Endocrinol. 2003;177:423–33. doi: 10.1677/joe.0.1770423. [DOI] [PubMed] [Google Scholar]
- 15.Ozmen B, Kirmaz C, Aydin K, Kafesciler SO, Guclu F, Hekimsoy Z. Influence of the selective oestrogen receptor modulator (raloxifene hydrochloride) on IL-6, TNF-alpha, TGF-beta1 and bone turnover markers in the treatment of postmenopausal osteoporosis. Eur Cytokine Netw. 2007;18:148–53. doi: 10.1684/ecn.2007.0097. [DOI] [PubMed] [Google Scholar]
- 16.Messalli EM, Mainini G, Scaffa C, et al. Raloxifene therapy interacts with serum osteoprotegerin in postmenopausal women. Maturitas. 2007;56:38–44. doi: 10.1016/j.maturitas.2006.05.007. [DOI] [PubMed] [Google Scholar]
- 17.Hasegawa J, Nagashima M, Yamamoto M, Nishijima T, Katsumata S, Yoshino S. Bone resorption and inflammatory inhibition efficacy of intermittent cyclical etidronate therapy in rheumatoid arthritis. J Rheumatol. 2003;30:474–9. [PubMed] [Google Scholar]
- 18.Valleala H, Laasonen L, Koivula MK, et al. Two year randomized controlled trial of etidronate in rheumatoid arthritis: changes in serum aminoterminal telopeptides correlate with radiographic progression of disease. J Rheumatol. 2003;30:468–73. [PubMed] [Google Scholar]
- 19.Adachi JD, Bensen WG, Brown J, et al. Intermittent etidronate therapy to prevent corticosteroid-induced osteoporosis. N Engl J Med. 1997;337:382–7. doi: 10.1056/NEJM199708073370603. [DOI] [PubMed] [Google Scholar]
- 20.Yilmaz L, Ozoran K, Gunduz OH, Ucan H, Yucel M. Alendronate in rheumatoid arthritis patients treated with methotrexate and glucocorticoids. Rheumatol Int. 2001;20:65–9. doi: 10.1007/s002960000080. [DOI] [PubMed] [Google Scholar]
- 21.Saag KG, Emkey R, Schnitzer TJ, et al. Alendronate for the prevention and treatment of glucocorticoid-induced osteoporosis. Glucocorticoid-Induced Osteoporosis Intervention Study Group. N Engl J Med. 1998;339:292–9. doi: 10.1056/NEJM199807303390502. [DOI] [PubMed] [Google Scholar]
- 22.Eastell R, Devogelaer JP, Peel NF, et al. Prevention of bone loss with risedronate in glucocorticoid-treated rheumatoid arthritis patients. Osteoporos Int. 2000;11:331–7. doi: 10.1007/s001980070122. [DOI] [PubMed] [Google Scholar]
- 23.Wallach S, Cohen S, Reid DM, et al. Effects of risedronate treatment on bone density and vertebral fracture in patients on corticosteroid therapy. Calcif Tissue Int. 2000;67:277–85. doi: 10.1007/s002230001146. [DOI] [PubMed] [Google Scholar]


