Abstract
Cytotoxic T lymphocytes (CTL) and natural killer (NK) cells have a key role in host defence against infectious pathogens, but their response to bacteria is not well characterized. Non-typeable Haemophilus influenzae is a major cause of respiratory tract infection including otitis media, sinusitis, tonsillitis and chronic bronchitis (especially in chronic obstructive pulmonary disease and bronchiectasis). This bacterium is also present in the pharynx of most healthy adults. The primary factor that may determine whether clinical disease occurs or not is the nature of the lymphocyte response. Here we examined the CTL cell and NK cell responses to nontypeable H. influenzae in healthy control subjects and in subjects who had bronchiectasis and recurrent bronchial infection with this bacterium. Cells were stimulated with live H. influenzae and intracellular cytokine production and release of cytotoxic granules measured. Control subjects had significantly higher levels of interferon gamma production by both CTL and NK cells, while levels of cytotoxic granule release were similar in both groups. The main lymphocyte subsets that proliferated in response to H. influenzae stimulation were the CTL and NK cells. The results suggest that CTL and NK cell responses may be important in preventing disease from nontypeable H. influenzae infection.
Keywords: bacteria, cytotoxic T cell, lung, natural killer cell
Introduction
Cytotoxic T lymphocytes (CTL) and natural killer (NK) cells are fundamental to host defence against pathogenic microorganisms. They have cytotoxic functions mediated by release of cytotoxic granules and Fas ligand and also produce cytokines. These two types of lymphocytes act against intracellular pathogens (NK cells are also involved in innate immune responses and priming adaptive immunity). Their best described effect is protection against viruses. There have only been several bacteria to which CTL and NK cell responses have been well defined; these include Salmonella typhimurium, Listeria monocytogenes and Mycobacterium tuberculosis[1–3].
Non-typeable Haemophilus influenzae (NTHi) is a fastidious Gram-negative bacterium that does not have a polysaccharide capsule, which distinguishes it from typeable forms of H. influenzae (such as type b or Hib) and is found in the nasopharynx of up to 75% of healthy adults [4]. It is also a major cause of respiratory infection which includes otitis media, sinusitis, tonsillitis, pneumonia and bronchitis [4–8]. Infection with NTHi is often chronic and this bacterium has evolved a number of mechanisms which enable it to persist in the human host [5]. Recently it has been recognized that NTHi is capable of extensive invasion of lung parenchyma [9] and intracellular survival inside monocytes/macrophages [10–13] and respiratory tract epithelial cells [8,14].
Non-typeable H. influenzae is a prevalent pathogen in chronic obstructive pulmonary disease (COPD) and bronchiectasis. COPD is one of the world's major health problems, the fourth leading cause of mortality worldwide, and its prevalence is predicted to increase significantly [15]. Bronchiectasis is a form of severe bronchitis characterized by chronic infection and bronchial widening, affects over 110 000 adults in the United States [3] and has significant overlap with COPD (up to 50% of subjects with COPD have evidence of bronchiectasis on computed tomography (CT) scanning [16,17]). The most common cause of bacterial colonization in COPD and bronchiectasis is NTHi [8,17–21]. Subjects with COPD and bronchiectasis are subject to recurrent exacerbations which often require hospitalization and have a high mortality rate. The most common pathogen isolated in exacerbations is NTHi [8,18,22].
The nature of the immune response to NTHi is not well defined. It has been shown that lymphocyte proliferation to the outermembrane protein P6 of NTHi is associated with protection from exacerbations in COPD [23]. The authors have demonstrated that a T helper (Th) cell type 1 immune response with interferon (IFN)-γ and CD40 ligand production was protective and subjects with bronchiectasis and chronic bronchial infection with NTHi did not make a protective response [24]. These results suggest that lymphocyte responses may be important in preventing clinical disease. The authors also observed that there were other classes of lymphocytes that produced IFN-γ apart from Th cells (unpublished data). As NTHi has been shown to be present intracellularly, the authors hypothesized that CTL and NK cells may be involved in the immune response to NTHi.
A cohort of healthy control subjects (who had detectable antibody to NTHi) and bronchiectasis subjects with recurrent NTHi airway infection were assessed for their CTL and NK cell response to NTHi. Peripheral blood mononuclear cells (PBMC) were incubated with live NTHi overnight, and the next day intracellular cytokine production and release of cytotoxic granules were measured (by surface expression of CD107a) using flow cytometry. The results showed that control subjects had significantly higher levels of IFN-γ production by both CTL and NK cells than bronchiectasis subjects. The expression of CD107a was similar in both groups. Lymphocyte proliferation to NTHi was assessed in control subjects by carboxyfluorescein diacetate, carboxyfluorescein succinimidyl ester (CFSE) staining and the main lymphocyte subsets that proliferated were the CTL and NK cells. These results suggest that CTL and NK cells have a role in host defence against NTHi.
Materials and methods
Subjects
Peripheral blood was obtained after informed consent from healthy control subjects [n = 15, mean age: 58·7 ± 15·0 years (standard deviation: SD)] and bronchiectasis subjects (n = 14, mean age: 58·9 ± 12·7 years). The bronchiectasis subjects had been screened for underlying causes of bronchiectasis (clinical and laboratory assessment including; cystic fibrosis mutation analysis, full blood examination, immunoglobulins, lymphocyte subsets and proliferation, complement and neutrophil function) and 13 were classified as having idiopathic bronchiectasis and one as having disease in association with COPD. Bronchiectasis subjects were tested when clinically stable (i.e. they had not had an exacerbation in the past month), and H. influenzae was the dominant bacteria isolated from their sputum with at least two isolates in the past 2 years. The bronchiectasis patients were not taking systemic immunosuppressive medication and all subjects were living independently and did not have other major illnesses. Only one subject had been a smoker. Bronchiectasis was diagnosed with high-resolution CT scanning using standard criteria [25]. The bronchiectasis subjects had moderate airflow obstruction [mean forced expiratory volume in 1 s of 65 ± 23% (SD)]. This project was approved by the Ethics Committee of Monash Medical Centre/Monash University.
All control and all bronchiectasis subjects had detectable antibody to two forms of inactivated NTHi antigen (described below) using a standard sandwich enzyme-linked immunosorbent assay (ELISA), as described previously [24]. Thus all subjects had developed an adaptive immune response to NTHi.
Antigen
Inactivated NTHi antigen was used to quantify antibody responses and live NTHi was used to measure lymphocyte responses. Two forms of NTHi antigen were used. The first of these was a pooled antigen obtained from nine separate sputum isolates that had been heat-inactivated and sonicated as described previously to give a lysate antigen [24]. The second form was outermembrane P6 (gift from Professor T. F. Murphy, Buffalo, NY, USA) that had been used previously to quantify antibody responses [26]. To measure lymphocyte responses, live isolates of NTHi were used. These live isolates had been obtained from sputum samples of patients (two from subjects with COPD and two from subjects with bronchiectasis). Isolates were shown to be non-typeable by biotyping and shown to be distinct isolates by different outermembrane protein expression [27]. Isolates were designated as MU/MMC 1–4 (or as strains 1–4 in Fig. 5). Most experiments were performed using MU/MMC 1. Isolates of NTHi were grown on chocolate agar and incubated at 37°C in 5% CO2 and replated every 2–4 days. All four strains were sensitive to gentamicin, a concentration of 100 μg/ml caused 100% killing of 108 bacteria in 1 h.
Fig. 5.
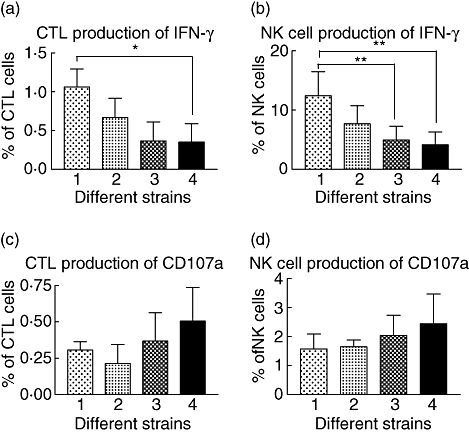
Response of cytotoxic T cells and natural killer (NK) cells to stimulation with four different isolates of non-typeable Haemophilus influenzae in controls (n = 5). All four different isolates produced measurable interferon (IFN)-γ and CD107a expression, but there were some differences between strains. (a) Cytotoxic lymphocyte (CTL) cell production of IFN-γ with strain 1 (MU/MMC 1) producing higher levels of IFN-γ than strain 4 (*P = 0·021). (b) NK cell production of IFN-γ with strain 1 producing significantly higher levels of IFN-γ than strain 3 (**P = 0·004) and strain 4 (**P = 0·003). (c) CTL production of CD107a. (d) NK cell production of CD107. Differences between different groups were analysed using analysis of variance.
Preparation of PBMC and live NTHi
Peripheral blood mononuclear cells were isolated from heparinized venous blood by Ficoll-Hypaque density gradient centrifugation (GE Healthcare, Melbourne, Australia). PBMC were resuspended in culture medium [RPMI-1640 (JRH, Lenexa, KS, USA), 10% fetal calf serum (JRH), 1% l-glutamine (Sigma, Melbourne, Australia)] and cell counts performed using a haemocytometer. Live NTHi was suspended in phosphate-buffered saline (PBS) and concentration quantified using McFarlane Standard (Bio-Merieux, Marcy-I'Etoile, France). A control sample and NTHi were set up in two tubes with the PBMC in culture medium. For the NTHi tube live bacteria were added at a concentration of 10 : 1 (to an average of 1–2 × 106 PBMC) and incubated at 37°C in 5% CO2 for 4 h to enable uptake of bacteria by antigen-presenting cells. Gentamicin was then added to kill all extracellular NTHi. The two tubes were then incubated overnight at 37°C in 5% CO2 to allow the antigen-presenting cells to stimulate the lymphocytes.
Measurement of antigen-specific CTL and NK cell responses by flow cytometry
Previously published flow cytometry techniques were modified to measure cytokine production by CTL and NK cells to stimulation with NTHi [24,28]. The next day anti-CD107a (BD, San Diego, CA, USA) was added and tubes were incubated at 37°C, 5% CO2 for 1 h and then Brefeldin A (Sigma) added at final concentration of 10 μg/ml and incubated at 37°C, 5% CO2 for 5 h. Cells were then washed once with 1% bovine serum albumin/PBS, resuspended in 2% paraformaldehyde (Sigma) and incubated on ice for 30 min. Cells were then resuspended in 0·2% saponin/PBS (Sigma) and incubated on ice for 5 min. Cells were then spun and resuspended in 0·2% saponin/PBS for antibody staining. Various combinations of antibodies [CD107, IFN-γ, CD56, interleukin (IL)-2, IL-4, IL-13 (BD), CD3 (Beckman Coulter, Fullerton, CA, USA), CD8, CD4 (Caltag, Burlingame, CA, USA) and CD19 (Dako, Carpinteria, CA, USA)], were added. Samples were then vortexed and incubated on ice for 30 min. Finally, samples were washed with 1 ml 0·2% saponin/PBS and resuspended in 400 μl of fluorescence activated cell sorter (FACS) wash and analysed using five colours on a Mo-Flo (Cytomation, Boulder, CO, USA) flow cytometer with Summit software.
Some modifications were made for specific measurements. To assess the effect of blockage of the type I major histocompatibility complex (MHC) on CTL cytokine production, a MHC-I blocking antibody [HLA class 1 W632 (Sapphire Bioscience, Melbourn, Australia)] was added when the NTHi was incubated with the PBMC. The superantigen Staphylococcus enterotoxin-B (SEB) (Sigma) was used as a positive control for some experiments. Two different time-points were also used for the addition of CD107a.
Cytotoxic T cells were designated as CD3+ CD8+, NK cells as CD3- CD56+ and Th cells as CD3+ CD4+. For analysis of CTL and Th cell responses at least 100 000 live events were collected for analysis and for NK cell responses at least 20 000 live events. Results were calculated by subtracting cytokine production from cells incubated with a control antibody from those stimulated with NTHi.
Carboxyfluorescein succinimdyl ester proliferation assay
Mononuclear cells were isolated as described previously and a published method was used to measure CFSE proliferation [29]. For CFSE labelling of mononuclear cells a working dilution of CFSE (Molecular Probes, San Diego, CA, USA) was prepared just prior to staining: a final working concentration of 7·5 μM CFSE was used to label cells incubated for 10 min in a 37°C water-bath before quenching the staining with ice-cold sRPMI (RPMI + 10% FCS + 1% l-glutamine). Cells were washed with ice-cold sRPMI and resuspended in warmed sRPMI.
To stimulate CFSE-labelled cells, control, NTHi and SEB samples (NTHi 10:1, SEB 1 μg/ml) were incubated at 37°C, 5% CO2 and cells were resuspended in FACSWash and labelled with appropriate antibodies for 20 min at room temperature and then washed, resuspended and run on the Mo-Flo flow cytometer.
Statistics
The data were analysed using Prism software (version 2·0, GraphPad software, San Diego, CA, USA) using Student's unpaired t-test or paired t-test as appropriate. Analysis of variance was used to analyse difference between strains. Differences with a P-value of less than 0·05 were considered significant. Results are expressed as mean ± standard error of the mean.
Results
Cytokine production by lymphocyte subsets
We measured cytokine production of the different lymphocyte subsets (cytotoxic T cells, NK cells and Th cells) in the control and bronchiectasis groups. To assess this response a variety of different forms of NTHi antigen were used in preliminary experiments. Initially, the lysate antigen that had been used previously to assess Th cell responses was used. However, with this antigen there was no measurable response made by CTL or NK cells. NTHi outer-membrane protein P6 and a peptide form of P6 [peptide antigens have been used previously to measure responses to pathogens including cytomegalovirus [30]] were tried, but again there was no detectable response. Finally, live NTHi was used and this produced a strong response; therefore, live NTHi was used to measure lymphocyte cytokine responses. For most experiments the strain MU/MMC1 was used.
Interferon-γ was the dominant cytokine produced by cytotoxic T cells after being stimulated with live NTHi in control subjects. A significant difference was observed in levels of CTL production of IFN-γ that was more than fivefold higher in the control group. In contrast, production of other cytokines, IL-2, IL-4 and IL-13, were similar in both groups (Fig. 1a). Similarly, measurement of cytokine responses by NK cells demonstrated that levels of IFN-γ were more than twofold higher in controls (P = 0·005), while levels of IL-4 were not significantly different between the two groups (Fig. 1b).
Fig. 1.
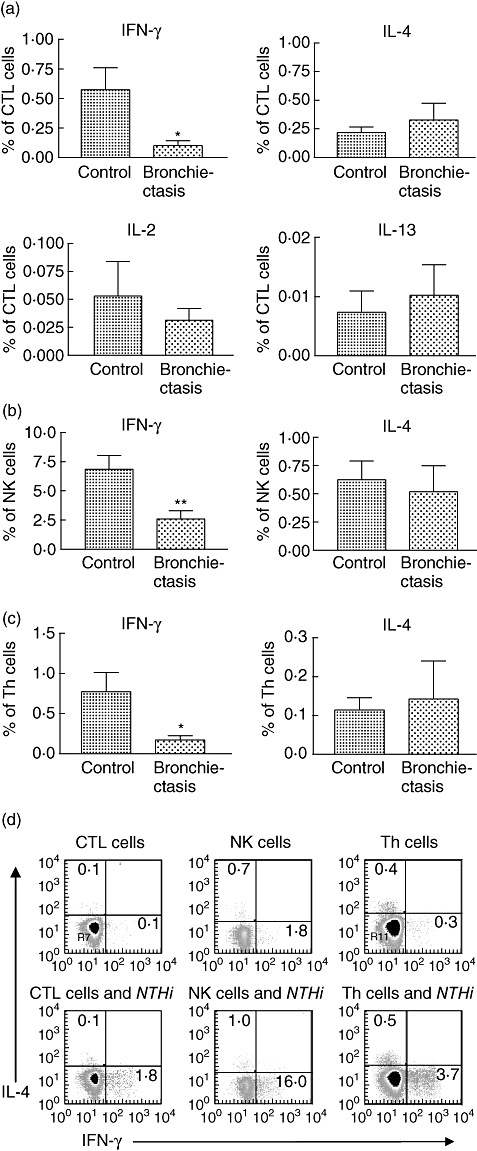
Cytokine production of lymphocyte subsets stimulated using live non-typeable Haemophilus influenzae in control (n = 15) and bronchiectasis (n = 14) subjects. Responses were measured using intracellular cytokine staining and flow cytometry. (a) Cytokine production by cytotoxic T cells. Levels of interferon (IFN)-γ were significantly higher in the control group (*P = 0·023), but levels of other cytokines interleukin (IL)-2, IL-4 and IL-13 were similar in both groups. (b) Cytokine production by natural killer (NK) cells. Levels of IFN-γ were significantly higher in the control group (**P = 0·005), but levels of IL-4 were similar in both groups. (c) Cytokine production by T helper cells. As with the cytotoxic lymphocyte (CTL) and NK cells, the levels of IFN-γ were significantly higher in the control group (*P = 0·025). (d) Example of cytokine staining for IFN-γ and IL-4 in a control subject.
When live NTHi was used to test Th cell responses, control subjects had fourfold higher levels of IFN-γ production than bronchiectasis subjects (Fig. 1c), similar to the result obtained using the lysate antigen. The main difference between the two different forms of antigen was that live NTHi produced almost 10 times the level of cytokine production. Cytokine staining in one control subject for the different lymphocyte subsets is demonstrated in Fig. 1d.
CD107a expression by CTL and NK cells
We adopted a recently described assay using CD107a expression to measure cytotoxic granule release [28]. Cytotoxic granules are contained inside vesicles. When triggered by antigen presentation the vesicles move to the surface of the CTL (and NK cell) to release granules; when this occurs the inside surface of the vesicle is exposed for a period before being reincorporated into the cell. CD107a is expressed on the inner surface of the vesicle and monoclonal antibodies have been developed which bind to it. By measuring CD107a expression it is possible to directly quantify the release of cytotoxic granules and correlate this with the release of other mediators, such as IFN-γ.
To validate the method, preliminary experiments were performed in which cells were stimulated with SEB. This produced a prominent up-regulation of CD107a production by CTL cells in control subjects. An example is demonstrated in Fig. 2c.
Fig. 2.
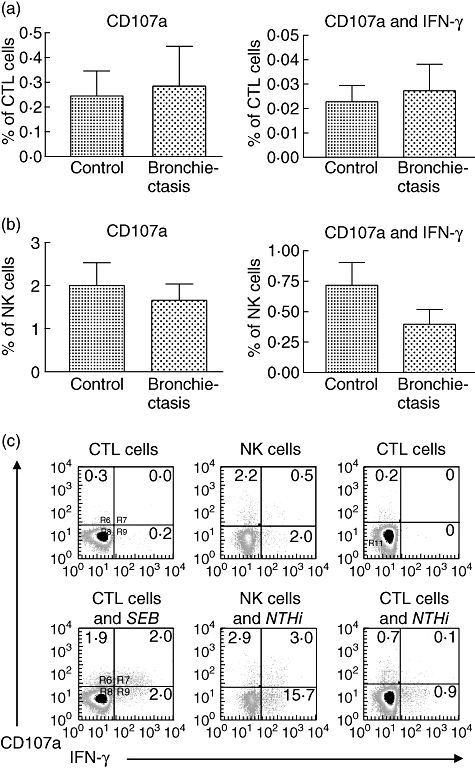
Cytotoxic granule release by lymphocytes stimulated using live non-typeable Haemophilus influenzae (NTHi) in control (n = 15) and bronchiectasis (n = 14) subjects. CD107a expression was measured using flow cytometry. (a) Production of CD107 and interferon (IFN)-γ by cytotoxic T cells. Levels of CD107a expression were similar in both groups. Only a small proportion (< 10%) of cytotoxic lymphocyte (CTL) cells stained for both CD107a and IFN-γ. (b) Production of CD107a and IFN-γ by natural killer (NK) cells. Levels of CD107a and CD107a/IFN-γ production were similar in both groups. (c) Shows an example of CD107a production by CTL cells in a control subject in response to stimulation with Staphylococcus enterotoxin-B and to NTHi and NK cell response to NTHi.
Stimulation with live NTHi produced up-regulation of CD107a expression in CTL cells. Levels of CD107a expression were similar in both control and bronchiectasis groups (Fig. 2a). Only a small proportion of cells expressing CD107a also stained for IFN-γ (about 10% in both groups). NK cell stimulation resulted in up-regulation of CD107a expression and levels were similar in both groups. Approximately 30% of the NK cells expressed both CD107a and IFN-γ (Fig. 2b). The results suggest that production of cytotoxic granules and IFN-γ in response to stimulation with NTHi may be mediated through different pathways. As was the case with IFN-γ production, the percentage of NK cells expressing CD107a was 10-fold higher than that of CTL cells, suggesting that these cells are metabolically very active in response to this stimulus. Expression of CD107a and IFN-γ in a control subject is demonstrated in Fig. 2c.
Natural killer cells; CD56bright and CD56dim subset responses
Natural killer cells can be divided into two different subsets based on their cell-surface density of CD56: CD56bright and CD56dim, each with distinct properties [31]. The CD56dim NK cell subset is highly cytotoxic, expresses high levels of CD16 and comprises approximately 90% of NK cells. In contrast, the CD56bright NK cell subset produces abundant cytokines but has low cytotoxicity. Cells could be distinguished clearly on the basis of their staining for CD56 into CD56bright and CD56dim populations (Fig. 3a). Both NK subsets demonstrated up-regulation of CD107a and IFN-γ staining following stimulation with NTHi. However, the CD56bright subset produced significantly higher levels of IFN-γ than the CD56dim subset in both control and bronchiectasis subjects (Fig. 3b). In contrast, levels of CD107a production were not significantly different between the two NK subsets (Fig. 3c). As the CD56dim NK cell subset was much greater in numbers, most of the IFN-γ produced by the NK cells came from this subset. An example of a control subject is shown in Fig. 3d.
Fig. 3.
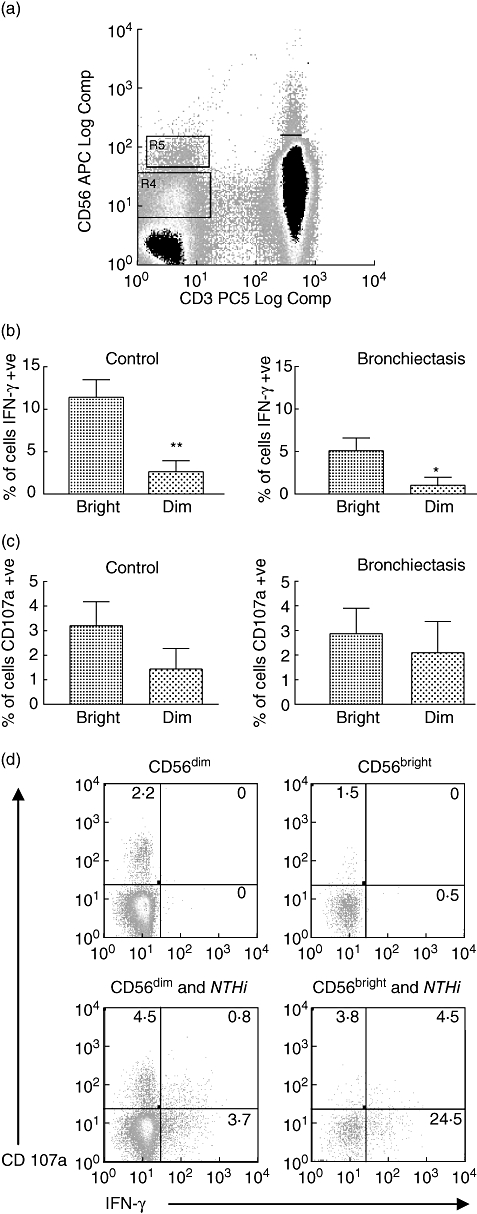
Responses of CD56bright and CD56dim natural killer (NK) cell subsets to stimulation with non-typeable Haemophilus influenzae in control (n = 15) and bronchiectasis (n = 14) subjects. (a) Dot plot of cytokine staining of lymphocytes for CD3 and CD56. NK cells were negative for CD3 and could be divided into bright (R5) and dim (R4) populations on the basis of their intensity of staining for CD56. (b) In both control (**P = 0·004) and bronchiectasis subjects (*P = 0·046) the CD56bright NK subset produced significantly higher levels of interferon (IFN)-γ than the CD56dim NK cell subset. (c) In both control and bronchiectasis subjects there was no significant difference in CD107a production between the two NK subsets. (d) Cytokine staining for CD107 and IFN-γ in CD56bright and CD56dim NK cell subsets in a control subject.
Cytotoxic T cell response: effect of blocking of MHC-I
The mechanisms of intracellular behaviour and antigen presentation of NTHi are not well defined. Cytotoxic T cells may be activated through MHC-I or through CD1 pathways [32,33]. A recent study suggested that the CD1 pathway was the primary activating pathway for human CD8+ T cell responses to live M. tuberculosis infection and was particularly important in the production of IFN-γ[34]. To assess whether MHC-I presentation was involved in activation of CTL cell responses to NTHi, a MHC-I blocking antibody was added with the bacteria and the effect was measured in five control subjects, resulting in a mean reduction of 70% of IFN-γ production by CTL cells ( Fig. 4). This result implies that MHC-I presentation is important in activating cytotoxic T cell responses to NTHi.
Fig. 4.
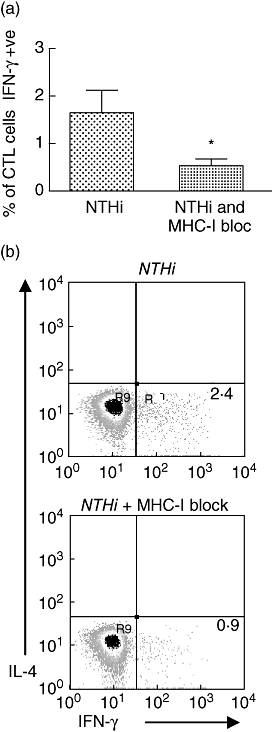
Effect of blockade of major histocompatibility complex (MHC)-I on interferon (IFN)-γ production by cytotoxic T cells; in control subjects (n = 5). (a) MHC-I blockage in controls produced a 70% reduction in expression of IFN-γ by cytotoxic lymphocyte cells that had been stimulated with non-typeable Haemophilus influenzae (NTHi) (*P = 0·033). (b) Cytokine staining for MHC-I block in subject. IL, interleukin; CTL, cytotoxic T lymphocytes.
Responses to different strains of NTHi
Non-typeable H. influenzae is a diverse pathogen with over 100 different subtypes [10]. Subjects are often infected simultaneously with multiple different subtypes [9,26]. Strain MU/MMC 1 appeared to be an appropriate choice to test immune responses, but we also wanted to assess the response to other isolates. Therefore, the response to four different strains of NTHi was tested in five control subjects. All strains produced measurable IFN-γ and CD107a and there were modest differences in responses between the different strains. No significant differences were seen with CD107a (Fig. 5). The numbers of subjects tested were small, but the results imply that MU/MMC 1 was an appropriate strain to use for the testing of immune responses and all four strains of NTHi produced a detectable response.
Proliferation of different lymphocyte subsets measured by CFSE labelling
A key feature of the adaptive immune response is the expansion of lymphocyte subsets following antigen exposure. Lymphocyte proliferation to stimulation with P6 NTHi protein measured by thymidine incorporation has been shown previously to be protective against infectious exacerbations in COPD. We were interested in assessing which subsets of lymphocytes in healthy controls would proliferate with stimulation with NTHi. To assess subset proliferation, lymphocytes from controls (n = 9) were labelled with CFSE and incubated with (i) SEB and (ii) NTHi for 7 days. Cells were then labelled with subset markers and analysed using flow cytometry. Proliferation was assessed by the total number of cells that had proliferated (in response to stimulation) over the week minus the proliferation that has occurred in unstimulated cells [29].
Stimulation with SEB produced strong T cell (Th and CTL) proliferation in all subjects, but no measurable NK or B cell proliferation. In contrast, a different pattern occurred with NTHi stimulation; seven of nine subjects had NK cell proliferation and six of nine subjects had CTL proliferation, three subjects had B cell proliferation and, surprisingly, only one had detectable Th cell proliferation. An example of a subject's response is shown in Fig. 6a. Analysis of the CD56bright NK cell subset demonstrated that these cells proliferated when unstimulated and this increased with the addition of NTHi, suggesting that these cells are very active metabolically. An example is shown in Fig. 6b.
Fig. 6.
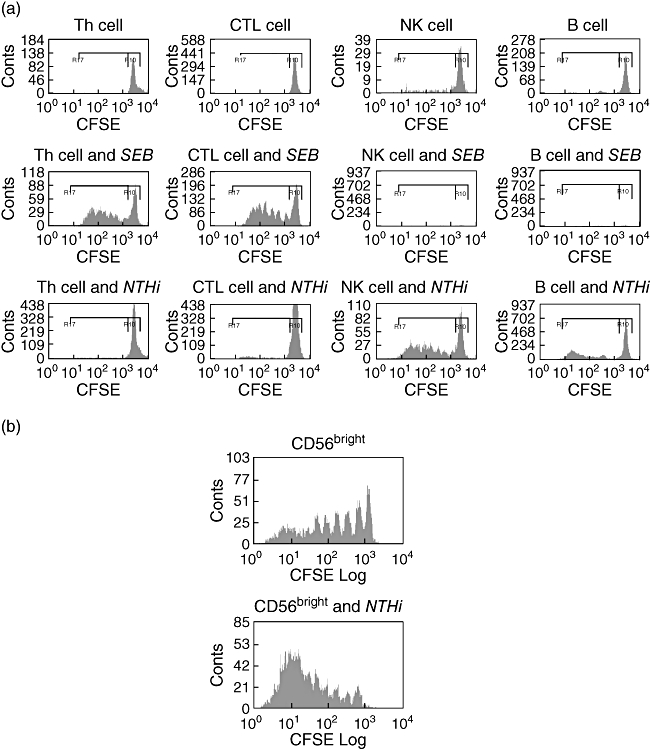
Lymphocyte subset proliferation measured by carboxyfluorescein succinimidyl ester in control subject. (a) Lymphocyte subset proliferation with control, Staphylococcus enterotoxin-B (SEB) and non-typeable Haemophilus influenzae (NTHi) in a typical patient. In the unstimulated cells there was some proliferation of the natural killer (NK) cell population. The SEB produced strong stimulation of the T cell populations, but no obvious response could be measured from the other lymphocyte subsets. The NTHi produces proliferation predominantly in the NK cells and with some proliferation in cytotoxic lymphocyte and B cell populations. (b) Example of CD56bright NK cell proliferation, showing proliferation at baseline which was increased with the addition of NTHi. CTL, cytotoxic T lymphocytes; CFSE, carboxyfluorescein succinimidyl ester.
Discussion
We and another group have reported previously that lymphocyte responses may be important in protection against infection with NTHi. In this study we show that live NTHi induces a clear response in CTL and NK cells in both controls and in subjects with chronic bronchial infection. Protective immune responses in controls were characterized by higher levels of IFN-γ production than in bronchiectasis subjects. Levels of cytotoxic granule release as assessed by CD107a expression were not different. Cytotoxic T cells and NK cells proliferated in response to NTHi. This information suggests that CTL and NK cells have an important role in host defence against NTHi.
The CTL has a key role in host defence by mediating the killing of intracellular pathogens. Most CTL responses have been described against viral pathogens. Cytotoxic T cell responses have been described against only a few bacteria, which include L. monocytogenes, M. tuberculosis and S. typhimurium[1]. Cytotoxic T cells have been recognized to be producers of large quantities of cytokines. Levels of IFN-γ were significantly higher in the control group compared with the bronchiectasis patients, and this cytokine may have an important role in preventing infection from NTHi. IFN-γ production has been shown to be essential for clearance of M. tuberculosis following lung infection with NTHi [35,36] and transferred T cells provide protection only if they produce IFN-γ[37]. Th cells are thought to be important in the initial response to tuberculosis and CTL cells are important (with Th) in controlling latent infection [1]. For infection with L. monocytogenes, IFN-γ production by CTL cells is important and this may be an innate immune response [3]. Infection with S. typhimurium produces a marked up-regulation of T cell function, with 20–30% of CD8+ T cells producing IFN-γ[38]; the actual effect of these cells is not well defined.
Cytotoxic T lymphocytes produce cytotoxic granules, granzyme and perforin which kill cells infected with intracellular pathogens. Assessment of the release of cytotoxic granules has been performed generally by measuring the death of target cells using methods such as the chromium release assay [39] or the release of fluorescent dyes from target cells [40,41]. The authors measured CD107a expression, which allows direct quantification of CTL cell function [28]. This method also has the advantage of over other techniques in being able to measure simultaneous cytokine production. The results showed that both control and bronchiectasis subjects had measurable CD107a and levels were similar between the two groups. Interestingly, there was no close association between degranulation and cytokine production, specifically IFN-γ release. While it would be expected that activated cells would produce both cytotoxic granzymes and cytokines, it has been observed in some patients and with different antigens that this may not occur [28]. The measurement of Fas ligand expression would add significantly to characterizing the behaviour of the CTL cell response, but we were not able to measure this using flow cytometry.
The intracellular behaviour and antigen presentation of NTHi is not well understood. In this study, the main pathway of cytotoxic T cell response and IFN-γ production appeared to be mediated through MHC-I presentation. This effect in the PBMC solution is likely to be mediated predominantly through monocytes. In vivo, dendritic cells and infected epithelial cells would also be expected to activate CTL cells. For dendritic cells this effect could be mediated through the CD1 pathway [34]. The involvement of the MHC-I pathway implies cytosolic presentation of NTHi antigen by monocytes/macrophages. It is possible that NTHi may behave like L. monocytogenes escaping from the phagosome into the cytoplasm of infected cells [42]. However, in M. tuberculosis infection, both cytosolic and noncytosolic pathways may be involved in access of antigens to the MHC-I processing pathway [33].
The immune response of NK cells to bacteria is still not well characterized, and bacteria which induce an NK cell response include Shigella flexneri, L. monocytogenes and M. tuberculosis[2]. In this study the response of NK cells was similar to that measured in cytotoxic T cells. The main cytokine produced was IFN-γ and levels were significantly higher in controls than bronchiectasis subjects with chronic NTHi infection. Levels of cytotoxic granule release were similar in both groups. Production of IFN-γ by NK cells is thought to have a crucial role in protection against L. monocytogenes[3]. How much of the response was directed against intracellular NTHi and how much was due to the innate immune response was not assessed. The innate NK cell response with its production of IFN-γ may be important in driving protective CTL and Th cell responses.
It has been shown previously that lymphocyte proliferation to NTHi is associated with protection from exacerbations in COPD [23]. The CFSE assay demonstrated that the CTL and NK cells are the main lymphocyte subtypes that proliferate in controls in response to NTHi. The CD56bright NK cells appeared to be active in response to NTHi and these cells may be important in priming a protective immune response by the production of IFN-γ. Sarcoidosis is thought to be a Th1-driven immune disorder, and the dominant bronchial NK cell subset in this condition has been shown to be the CD56bright NK cells [43]. It would be of interest to characterize the lymphocyte subset responses further by studying the bronchiectasis patients and also by measuring mediator release from the proliferating cells.
This study and previous work by the investigators have shown that both control and bronchiectasis subjects all have detectable antibody to NTHi that is of similar titre. It has been demonstrated previously that immunoglobulin with complement is bactericidal for NTHi and this would be predicted to be important in defence against extracellular bacteria. In controls, IFN-γ production by Th cells, cytotoxic T cells and NK cells was associated with protective immunity and subjects with bronchiectasis and chronic NTHi infection had significantly lower levels of IFN-γ production. These results imply that the subjects with chronic NTHi infection may make a non-clearing immune response against intracellular NTHi, and this would explain the persistence of infection and clinical disease. However, it is possible that other mechanisms may be involved. To establish further the significance of these findings will require a clearer understanding of both the intracellular behaviour of NTHi and the nature of the host defence to this bacterium, particularly the mucosal immune response.
Enhancing the immune response to NTHi may have therapeutic implications. IFN-γ, which has been used to treat subjects with chronic granulomatous disease and reduces infections by 70% [44], could potentially have a role against NTHi. Other cytokines, such as IL-2 which enhances NK cell function (especially the CD56bright cells) [31,45] and IL-12 which enhances Th1 responses, may also be useful. A vaccine against NTHi would have enormous potential. Vaccine developments against NTHi have concentrated generally on inactivated vaccines to induce humoral immune responses. However, as subjects with chronic NTHi infection have antibodies that do not seem to protect against respiratory infection, this approach may have limited effectiveness. Live-attenuated vaccines may have the potential to induce more effective T cell immunity. Defining the nature of the protective immune response to NTHi may provide important information for vaccine development.
Non-typeable H. influenzae is a major cause of chronic respiratory infection particularly chronic bronchitis. It is the most common bacterial pathogen isolated in both bronchiectasis and COPD. The findings from this study emphasize the importance of cellular immune responses in host defence against NTHi. Protective immunity is characterized by IFN-γ production by cytotoxic T cells and NK cells. Subjects with bronchiectasis and chronic NTHi infection fail to make a protective response. Further defining the immune response to NTHi may help to clarify the pathogenesis and treatment of chronic bronchitis.
Acknowledgments
We would like to thank the staff of the Clinical Immunology laboratory of Monash Medical Centre for their help, particularly Dilini Gunawardena for performing the ELISA. We would also like to thank Professor T. F. Murphy (State University of New York, Buffalo) for providing the P6 antigen. None of the authors have any conflict of interest in the publication of this work.
References
- 1.Wong P, Pamer EG. CD8 T cell responses to infectious pathogens. Annu Rev Immunol. 2003;21:29–70. doi: 10.1146/annurev.immunol.21.120601.141114. [DOI] [PubMed] [Google Scholar]
- 2.Lodoen MB, Lanier LL. Natural killer cells as an initial defense against pathogens. Curr Opin Immunol. 2006;18:391–8. doi: 10.1016/j.coi.2006.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Weycker D, Edelsberg J, Oster G, Tino G. Prevalence and economic burden of bronchiectasis. Clin Pulm Med. 2005;4:205–9. [Google Scholar]
- 4.Murphy TF. Haemophilus infections. In: Braunwald E, Fauci A, Kasper D, Hauser S, Longo D, Jameson J, editors. Harrison's principles of internal medicine. 15. New York: McGraw-Hill; 2001. pp. 939–42. [Google Scholar]
- 5.St Geme JW., III Insights into the mechanism of respiratory tract colonization by nontypable Haemophilus influenzae. Pediatr Infect Dis J. 1997;16:931–5. doi: 10.1097/00006454-199710000-00005. [DOI] [PubMed] [Google Scholar]
- 6.Lindroos R. Bacteriology of the tonsil core in recurrent tonsillitis and tonsillar hyperplasia − a short review. Acta Otolaryngol Suppl. 2000;543:206–8. doi: 10.1080/000164800454404. [DOI] [PubMed] [Google Scholar]
- 7.Moxon ER, Wilson R. The role of Haemophilus influenzae in the pathogenesis of pneumonia. Rev Infect Dis. 1991;13(Suppl. 6):S518–27. doi: 10.1093/clinids/13.supplement_6.s518. [DOI] [PubMed] [Google Scholar]
- 8.Bandi V, Apicella MA, Mason E, et al. Nontypeable Haemophilus influenzae in the lower respiratory tract of patients with chronic bronchitis. Am J Respir Crit Care Med. 2001;164:2114–19. doi: 10.1164/ajrccm.164.11.2104093. [DOI] [PubMed] [Google Scholar]
- 9.Moller LV, Timens W, van der Bij W, et al. Haemophilus influenzae in lung explants of patients with end-stage pulmonary disease. Am J Respir Crit Care Med. 1998;157(Part 1):950–6. doi: 10.1164/ajrccm.157.3.9707010. [DOI] [PubMed] [Google Scholar]
- 10.Forsgren J, Samuelson A, Ahlin A, Jonasson J, Rynnel-Dagoo B, Lindberg A. Haemophilus influenzae resides and multiplies intracellularly in human adenoid tissue as demonstrated by in situ hybridization and bacterial viability assay. Infect Immun. 1994;62:673–9. doi: 10.1128/iai.62.2.673-679.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ahren IL, Janson H, Forsgren A, Riesbeck K. Protein D expression promotes the adherence and internalization of non-typeable Haemophilus influenzae into human monocytic cells. Microb Pathog. 2001;31:151–8. doi: 10.1006/mpat.2001.0456. [DOI] [PubMed] [Google Scholar]
- 12.Craig JE, Cliffe A, Garnett K, High NJ. Survival of nontypeable Haemophilus influenzae in macrophages. FEMS Microbiol Lett. 2001;203:55–61. doi: 10.1111/j.1574-6968.2001.tb10820.x. [DOI] [PubMed] [Google Scholar]
- 13.Craig JE, Nobbs A, High NJ. The extracytoplasmic sigma factor, final sigma(E), is required for intracellular survival of nontypeable Haemophilus influenzae in J774 macrophages. Infect Immun. 2002;70:708–15. doi: 10.1128/IAI.70.2.708-715.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Swords WE, Ketterer MR, Shao J, Campbell CA, Weiser JN, Apicella MA. Binding of the non-typeable Haemophilus influenzae lipooligosaccharide to the PAF receptor initiates host cell signalling. Cell Microbiol. 2001;3:525–36. doi: 10.1046/j.1462-5822.2001.00132.x. [DOI] [PubMed] [Google Scholar]
- 15.Barnes PJ. Chronic obstructive pulmonary disease. N Engl J Med. 2000;343:269–80. doi: 10.1056/NEJM200007273430407. [DOI] [PubMed] [Google Scholar]
- 16.O'Brien C, Guest PJ, Hill SL, Stockley RA. Physiological and radiological characterisation of patients diagnosed with chronic obstructive pulmonary disease in primary care. Thorax. 2000;55:635–42. doi: 10.1136/thorax.55.8.635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Patel IS, Seemungal TA, Wilks M, Lloyd-Owen SJ, Donaldson GC, Wedzicha JA. Relationship between bacterial colonisation and the frequency, character, and severity of COPD exacerbations. Thorax. 2002;57:759–64. doi: 10.1136/thorax.57.9.759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rosell A, Monso E, Soler N, et al. Microbiologic determinants of exacerbation in chronic obstructive pulmonary disease. Arch Intern Med. 2005;165:891–7. doi: 10.1001/archinte.165.8.891. [DOI] [PubMed] [Google Scholar]
- 19.Hill AT, Campbell EJ, Hill SL, Bayley DL, Stockley RA. Association between airway bacterial load and markers of airway inflammation in patients with stable chronic bronchitis. Am J Med. 2000;109:288–95. doi: 10.1016/s0002-9343(00)00507-6. [DOI] [PubMed] [Google Scholar]
- 20.Urban BC, Ferguson DJ, Pain A, et al. Plasmodium falciparum-infected erythrocytes modulate the maturation of dendritic cells. Nature. 1999;400:73–7. doi: 10.1038/21900. [DOI] [PubMed] [Google Scholar]
- 21.Angrill J, Agusti C, de Celis R, et al. Bacterial colonisation in patients with bronchiectasis: microbiological pattern and risk factors. Thorax. 2002;57:15–19. doi: 10.1136/thorax.57.1.15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Groenewegen KH, Wouters EF. Bacterial infections in patients requiring admission for an acute exacerbation of COPD; a 1-year prospective study. Respir Med. 2003;97:770–7. doi: 10.1016/s0954-6111(03)00026-x. [DOI] [PubMed] [Google Scholar]
- 23.Abe Y, Murphy TF, Sethi S, et al. Lymphocyte proliferative response to P6 of Haemophilus influenzae is associated with relative protection from exacerbations of chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2002;165:967–71. doi: 10.1164/ajrccm.165.7.2109009. [DOI] [PubMed] [Google Scholar]
- 24.King PT, Hutchinson PE, Johnson PD, Holmes PW, Freezer NJ, Holdsworth SR. Adaptive immunity to nontypeable Haemophilus influenzae. Am J Respir Crit Care Med. 2003;167:587–92. doi: 10.1164/rccm.200207-728OC. [DOI] [PubMed] [Google Scholar]
- 25.McGuinness G, Naidich DP. CT of airways disease and bronchiectasis. Radiol Clin North Am. 2002;40:1–19. doi: 10.1016/s0033-8389(03)00105-2. [DOI] [PubMed] [Google Scholar]
- 26.Badr WH, Loghmanee D, Karalus RJ, Murphy TF, Thanavala Y. Immunization of mice with P6 of nontypeable Haemophilus influenzae: kinetics of the antibody response and IgG subclasses. Vaccine. 1999;18:29–37. doi: 10.1016/s0264-410x(99)00166-8. [DOI] [PubMed] [Google Scholar]
- 27.Barenkamp SJ, Munson RS, Granoff DM. Subtyping isolates of Haemophilus influenzae type b by outer-membrane protein profiles. J Infect Dis. 1981;143:668–76. doi: 10.1093/infdis/143.5.668. [DOI] [PubMed] [Google Scholar]
- 28.Betts MR, Brenchley JM, Price DA, et al. Sensitive and viable identification of antigen-specific CD8+ T cells by a flow cytometric assay for degranulation. J Immunol Methods. 2003;281:65–78. doi: 10.1016/s0022-1759(03)00265-5. [DOI] [PubMed] [Google Scholar]
- 29.Lyons AB. Analysing cell division in vivo and in vitro using flow cytometric measurement of CFSE dye dilution. J Immunol Methods. 2000;243:147–54. doi: 10.1016/s0022-1759(00)00231-3. [DOI] [PubMed] [Google Scholar]
- 30.Maecker HT, Dunn HS, Suni MA, et al. Use of overlapping peptide mixtures as antigens for cytokine flow cytometry. J Immunol Methods. 2001;255:27–40. doi: 10.1016/s0022-1759(01)00416-1. [DOI] [PubMed] [Google Scholar]
- 31.Cooper MA, Fehniger TA, Caligiuri MA. The biology of human natural killer-cell subsets. Trends Immunol. 2001;22:633–40. doi: 10.1016/s1471-4906(01)02060-9. [DOI] [PubMed] [Google Scholar]
- 32.Schaible UE, Winau F, Sieling PA, et al. Apoptosis facilitates antigen presentation to T lymphocytes through MHC-I and CD1 in tuberculosis. Nat Med. 2003;9:1039–46. doi: 10.1038/nm906. [DOI] [PubMed] [Google Scholar]
- 33.Grotzke JE, Lewinsohn DM. Role of CD8+ T lymphocytes in control of Mycobacterium tuberculosis infection. Microbes Infect. 2005;7:776–88. doi: 10.1016/j.micinf.2005.03.001. [DOI] [PubMed] [Google Scholar]
- 34.Kawashima T, Norose Y, Watanabe Y, et al. Cutting edge: major CD8 T cell response to live bacillus Calmette–Guerin is mediated by CD1 molecules. J Immunol. 2003;170:5345–8. doi: 10.4049/jimmunol.170.11.5345. [DOI] [PubMed] [Google Scholar]
- 35.Caruso AM, Serbina N, Klein E, Triebold K, Bloom BR, Flynn JL. Mice deficient in CD4 T cells have only transiently diminished levels of IFN-gamma, yet succumb to tuberculosis. J Immunol. 1999;162:5407–16. [PubMed] [Google Scholar]
- 36.van Pinxteren LA, Cassidy JP, Smedegaard BH, Agger EM, Andersen P. Control of latent Mycobacterium tuberculosis infection is dependent on CD8 T cells. Eur J Immunol. 2000;30:3689–98. doi: 10.1002/1521-4141(200012)30:12<3689::AID-IMMU3689>3.0.CO;2-4. [DOI] [PubMed] [Google Scholar]
- 37.Tascon RE, Stavropoulos E, Lukacs KV, Colston MJ. Protection against Mycobacterium tuberculosis infection by CD8+ T cells requires the production of gamma interferon. Infect Immun. 1998;66:830–4. doi: 10.1128/iai.66.2.830-834.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Mittrucker HW, Kohler A, Kaufmann SH. Characterization of the murine T-lymphocyte response to Salmonella enterica serovar Typhimurium infection. Infect Immun. 2002;70:199–203. doi: 10.1128/IAI.70.1.199-203.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Brunner KT, Mauel J, Cerottini JC, Chapuis B. Quantitative assay of the lytic action of immune lymphoid cells on 51-Cr-labelled allogeneic target cells in vitro; inhibition by isoantibody and by drugs. Immunology. 1968;14:181–96. [PMC free article] [PubMed] [Google Scholar]
- 40.Sheehy ME, McDermott AB, Furlan SN, Klenerman P, Nixon DF. A novel technique for the fluorometric assessment of T lymphocyte antigen specific lysis. J Immunol Methods. 2001;249:99–110. doi: 10.1016/s0022-1759(00)00329-x. [DOI] [PubMed] [Google Scholar]
- 41.Liu L, Chahroudi A, Silvestri G, et al. Visualization and quantification of T cell-mediated cytotoxicity using cell-permeable fluorogenic caspase substrates. Nat Med. 2002;8:185–9. doi: 10.1038/nm0202-185. [DOI] [PubMed] [Google Scholar]
- 42.Goebel W, Kuhn M. Bacterial replication in the host cell cytosol. Curr Opin Microbiol. 2000;3:49–53. doi: 10.1016/s1369-5274(99)00050-8. [DOI] [PubMed] [Google Scholar]
- 43.Katchar K, Soderstrom K, Wahlstrom J, Eklund A, Grunewald J. Characterisation of natural killer cells and CD56+ T-cells in sarcoidosis patients. Eur Respir J. 2005;26:77–85. doi: 10.1183/09031936.05.00030805. [DOI] [PubMed] [Google Scholar]
- 44.International Chronic Granulomatous Disease Cooperative Study Group. A controlled trial of interferon gamma to prevent infection in chronic granulomatous disease. N Engl J Med. 1991;324:509–16. doi: 10.1056/NEJM199102213240801. [DOI] [PubMed] [Google Scholar]
- 45.Shah MH, Baiocchi RA, Fehniger TA, et al. Cytokine replacement in patients with HIV-1 non-Hodgkin's lymphoma: the rationale for low-dose interleukin-2 therapy. Cancer J Sci Am. 2000;6(Suppl. 1):S45–51. [PubMed] [Google Scholar]


