Abstract
Extensive evidence indicates that ethanol (alcohol) has immunomodulatory properties. Many of its effects on innate immune response are dose-dependent, with acute or moderate use associated with attenuated inflammatory responses, and heavy ethanol consumption linked with augmentation of inflammation. Ethanol may modify innate immunity via functional alterations of the cells of the innate immune system. Mounting evidence indicates that ethanol can diversely affect antigen recognition and intracellular signaling events, which include activation of mitogen activated protein kinase (MAPK) and NFκB, mediated by Toll-like receptors (TLRs), leading to altered inflammatory responses. The mechanism(s) underlying these changes may involve dose-dependent effects of ethanol on the fluidity of cell membrane, resulting in interference with the timely assembly or disassembly of lipid rafts. Ethanol could also modify cell activation by specific interactions with cell membrane molecules.
Introduction
Extensive evidence indicates that ethanol consumption affects human health (Rehm et al., 2003). These effects are complex and depend on many factors, such as patterns of drinking (chronic or acute/binge), the amount of consumed ethanol (moderate or excessive), the body organ or system, and the sex of user. Chronic ethanol abuse, defined as 120–150 g or ≥ 8 drinks per day, has been associated with immunosuppression and increased morbidity and mortality (Nelson and Kolls, 2002). As a result, alcoholics are prone to bacterial and viral infections (Adams and Jordan, 1984; Nelson et al., 1995); (Ruiz et al., 1999). Alcoholics also show higher incidence of cardiovascular ailments such as cardiomyopathy, high blood pressure, stroke, and acute respiratory distress syndrome (ARDS) (Hanna et al., 1997; Rehm et al., 2003). Continuing ethanol abuse can lead to a development of alcoholic liver disease, which in nearly 20% of alcoholics results in liver cirrhosis (Hines and Wheeler, 2004). Furthermore, alcoholism is associated with higher rate of liver, esophageal, and pharyngeal cancers (Rehm et al., 2003). Importantly, even occasional intoxication known as binge drinking and defined as the consumption of more than 5 drinks of ethanol during a short time period is linked with increased risk of cardiovascular disease (Averina et al., 2006; Hijmering et al., 2007). In contrast, moderate ethanol exposure appears to exert positive effects on health (Power et al., 1998; Tolstrup et al., 2006). Multiple epidemiological reports demonstrate a U-shaped relationship between ethanol intake and general mortality (Djousse et al., 2002; Rimm et al., 1999). The lowest death rate correlates with low to moderate amounts of ethanol, at 15–45 g or 1 – 3 drinks per day (Gigleux et al., 2006), while abstaining from ethanol or excessive drinking are both associated with higher mortality (Power et al., 1998). An important beneficial effect of moderate ethanol consumption is the reported decrease in cardiovascular diseases (Rimm et al., 1999). This level of ethanol use has been also associated with a lower risk of dementia and Alzheimer’s disease in the elderly (Luchsinger et al., 2004). Moreover, moderate drinking may be protective against cancer-related mortality (Gun et al., 2006). Two epidemiological studies link consumption of alcohol with a lower incidence of non-Hodgkin’s lymphoma in men (Briggs et al., 2002) and women (Morton et al., 2003). Nevertheless, several studies indicate a small increase in the risk of breast cancer in women who consume low to moderate amounts of ethanol (Ellison et al., 2001; Nielsen and Gronbaek, 2008; Singletary and Gapstur, 2001). It is possible that the increased levels of estrogen and androgen in women using ethanol play a role in this effect.
Ethanol and hormesis
The epidemiological analysis of the effects of ethanol on health leads to the conclusion that one of the key factors is the dose of consumed ethanol. It is apparent that ethanol can exert opposite effects depending on the dose. This property, known as hormesis, characterizes many other substances, and has been already observed by Paracelsus in the age of alchemy (Rozman, 2005). This biphasic effect of ethanol has been reported in a wide range of studies. Studies of diverse behavioral responses in animal models and in humans indicate that the low doses of ethanol are stimulatory, while high doses have inhibitory effects (Calabrese and Baldwin, 2003). For example anxiety, and tension decrease with low level of consumption. In contrast, high dose of ethanol profoundly depresses nervous system (Williams, 1966; Ekman et al., 1964). In studies that use genetically identical mice, the low-dose stimulatory responses vary between the examined strains, suggesting the role of genetic predisposition in sensitivity to ethanol (Crabbe, 1983). It is well established that ethanol exposure is harmful to the developing fetus, however some in vitro studies in animal models show that low level (0.1%) of ethanol can promote embryonic development (Chaudhuri, 2000; Hannigan and Armant, 2000; Leach et al., 1993). Moreover, epidemiological data show that moderate drinking during pregnancy is associated with an increase in a birth weight (Primatesta et al., 1993). The study of female pregnant rats exposed to ethanol showed a biphasic effect of ethanol on birth weight in both female and male offspring. Exposure to 0.15 and 0.3 g/kg body of ethanol lead to an increase, while 3.0 g/kg to a decrease in weight of newborn rats (Abel, 1996). In addition, the opposite results of low and high levels of ethanol exposure on the function of kidney, liver, and heart, and on serum levels of testosterone and LH were also observed (Calabrese and Baldwin, 2003; Nagy, 1994; Rodrigo et al., 1998; Williams et al., 1980).
The biphasic effect of ethanol on the inflammatory response
Inflammation is a feature of innate immunity. It is the body’s response to injury and represents the first line of defense against microbial pathogens (Janeway and Medzhitov, 2002). In addition, inflammatory processes are essential in the induction of adaptive immune responses, however excessive or continuous inflammation can be harmful. For instance, inflammatory processes are believed to underlie many chronic conditions, such as atherosclerosis, multiple sclerosis, Alzheimer’s disease, and rheumatoid arthritis (Cunningham et al., 2005; Feldmann et al., 1996; Libby, 2002; Perry et al., 2007). Moreover, chronic inflammation may play a role in etiology of many cancers, including liver and pancreatic malignancies (Balkwill and Coussens, 2004; Coussens and Werb, 2002; Lawrence et al., 2007; Pikarsky et al., 2004; Shacter and Weitzman, 2002).
Long-term ethanol abuse affects both innate and adaptive immunity, however even acute ethanol intoxication, in form of binge drinking, or moderate consumption can modulate immune responses, in particular inflammatory reactions. In light of many studies, the effects of ethanol on innate immune responses may also follow a biphasic pattern. For instance, assessment of inflammatory processes shows that acute ethanol exposure is associated with a decrease in the production of inflammatory mediators (Bagby et al., 1998; Boe et al., 2003), while alcohol abusers often show elevated circulating levels of the pro-inflammatory cytokines, such as tumor necrosis factor-α (TNF-α), interleukin (IL)-1 and IL-6. (Khoruts et al., 1991; McClain et al., 1993). Also, hepatitis and pancreatitis are inflammatory diseases that significantly affect alcoholics (Hines and Wheeler, 2004; Jerrells et al., 2007). Furthermore, a recent analysis of intensive care unit patients revealed correlation between alcoholism and incidence of septic shock (O’Brien et al., 2007).
Multiple in vivo and in vitro studies show that acute ethanol treatment inhibits the production of pro-inflammatory cytokines in response to a variety of microbial compounds. For example, ethanol suppressed lipopolysaccharide (LPS)-induced synthesis of TNF-α and IL-1β by alveolar macrophages, LPS and staphylococcal enterotoxin B (SEB)- stimulated production of TNF-α and IL-1β by human blood monocytes, polyinosinic-polycytidylic acid (poly I:C)-induced IL-6 and IL-12 production by murine peritoneal macrophages, as well as LPS, peptidiglycan (PGN), and microbial unmethylated DNA (CpG)- stimulated TNF-α and IL-6 synthesis by murine splenic macrophages (Nelson et al., 1989; Verma et al., 1993; Szabo et al., 1996; Szabo, 1998); Boe et al., 2001; Pruett et al., 2004b; Goral et al., 2004; Goral and Kovacs, 2005). In the in vivo animal studies ethanol was administered by intraperitoneal (i.p.) injection or oral gavage. The inhibitory effect was consistently observed with doses of 0.8 to 6 g/kg/body weight that corresponded to moderate and binge drinking pattern, respectively. In in vitro studies the cells were exposed to 25–150 mM ethanol.The acute exposure to high levels ethanol, so called binge drinking, attenuates inflammatory responses, however this type of ethanol consumption is associated with a higher risk for pathological conditions with inflammatory etiology, such as cardiovascular disease (Averina et al., 2006; Hijmering et al., 2007). This paradoxical effect may be linked to the induction of acute phase proteins, which are markers of inflammation, by high levels of ethanol. In human population the lowest levels of acute phase proteins are measured in moderate ethanol users, while both nondrinkers and ethanol abusers have elevated levels of these markers of inflammation (Imhof et al., 2001). It was shown in a mouse model that a single high dose of 6 g/kg body weight of ethanol induced acute phase proteins: serum amyloid A and serum amyloid P, while a lower lower dose (3 g/kg) did not cause such an effect (Pruett and Pruett, 2006).
Effective detection and presentation of pathogenic antigens by the cells of innate immune system is necessary to initiate adaptive immune responses. Insufficient levels of pro-inflammatory cytokines may impair recruitment of lymphoid cells to the sites of microbial invasion. Such effect was observed in the lungs of animals treated with ethanol. It was demonstrated that acute ethanol attenuates TNFα production by alveolar macrophages stimulated with LPS (Kolls et al., 1995). Among many functions of TNFα is the induction of chemokines that attract neutrophils to the lung. In mouse models acute ethanol exposure inhibited MIP-2 and CXC chemokine CINC and reduced neutrophil influx to the lung in response to bacterial infection or intratracheal administration of LPS (Boe et al., 2003; Zhang et al., 1997). Furthermore, by negatively affecting TNFα production ethanol may impair interactions between lymphoid and endothelial cells, which could result in inadequate recruitment of lymphoid cells to the sites of inflammation. It was shown that single i.p. injection of 5 g/kg body weight of ethanol inhibited TNF-α-induced endothelial cell activation, as assessed by expression of adhesion molecules E-selectin, ICAM-1, and VCAM-1; production of chemokines IL-8, MCP-1, and RANTES, and adhesion of lymphoid cells in both carrageenan and LPS-induced air pouch model in mice (Saeed et al., 2004). In another study moderate ethanol exposure (a single dose of 0.8 g/kg body weight) increased IL-10 and reduced accessory cell function of human monocytes during a mixed lymphocyte reaction (Szabo et al., 2001).
The link between heavy ethanol consumption and lung infections is well established (Nelson and Kolls, 2002). Mechanisms that result in the increased risk of infection involve impaired function of alveolar macrophages, as well as increase in reactive oxidants due to inadequate levels of glutathione in the epithelial lining fluid (Moss et al., 2000). In addition, mucociliary apparatus of the respiratory system can be affected by ethanol (Sisson, 2007). Constant beating of cilia of the mucocialiary apparatus plays essential role in clearing bacteria and impurities from the air ways. Several studies demonstrated that ethanol exerts concentration-dependent biphasic effect on the ciliary movement. Low concentrations of ethanol (0.01 – 0.1%) were shown to increase, while higher concentration (2%) resulted in the lower beating frequency of cilia. Moderate concentrations (0.5–1%) did not cause any changes in ciliary movement (Maurer and Liebman, 1988; Wyatt et al., 2004). Therefore the exposure to moderate levels of ethanol may augment, while ethanol abuse may impair mucociliary clearance (Sisson, 2007).
Alcoholic liver disease can serve as an example of a pro-inflammatory effect of chronic ethanol exposure. Fibrosis, which is a hallmark of this disease, is thought to be a result of hepatic injury followed by chronic inflammation. Kupffer cells - the resident macrophages in the liver - are a major source of inflammatory mediators, including TNFα and reactive oxygen species (ROS). These mediators are involved in promoting cell death, inflammation, and fibrosis of the liver (Nagy, 2003), (Hines and Wheeler, 2004). It was demonstrated that depletion of Kupffer cells curbs inflammation and reduces cell death in the liver. Furthermore, destruction of Kupffer cells in animals chronically exposed to ethanol blocks the formation of ROS, implicating these cells in a production of destructive oxidants (Wheeler et al., 2001). Considerable evidence supports the hypothesis that LPS present at increased levels in the blood of alcoholics and animals chronically exposed to ethanol is involved in Kupffer cells activation (Thurman, 1998); (Hines and Wheeler, 2004). Bacteria present in the lumen of terminal ileum and colon are a potential source of circulating LPS (Wheeler et al., 2001). Under normal conditions LPS does not penetrate intestinal epithelium, however, excessive ethanol consumption disrupts epithelial barrier, allowing for increased bacterial translocation from the lumen into the underlying tissue and the blood stream (Rao et al., 2004). Once in the circulation, the bacterial products, such as LPS, affect multiple organ systems, including liver (Fukui et al., 1991). LPS-activated Kupffer cells secrete TNFα, IL-1, IL-6, and chemokines. Together these molecules increase vascular permeability of hepatic sinusoids and recruit neutrophils and other lymphoid cells to the liver (Nagy, 2003) (Hines and Wheeler, 2004). Chronic ethanol exposure further increases the sensitivity of Kupffer cells to inflammatory stimuli, which leads to additional TNFα production (Kishore et al., 2002). Moreover, chronic ethanol sensitizes hepatocytes to the damage by TNFα (Hines and Wheeler, 2004), thus the cells become more susceptible to infection. Extensive evidence shows that ethanol abuse is linked to more severe viral infections of liver. Therefore it has been proposed that viral infections could serve as cofactors in the development of liver fibrosis (Regev and Jeffers, 1999). Alcoholism has been also associated with a development of pancreatitis (Schenker and Montalvo, 1998). Similar mechanisms that result in liver inflammation and fibrosis may occur in the pancreas (Apte et al., 2000; Jerrells et al., 2007).
Natural killer (NK) cells are another important type of cells of the innate immune system. They are involved in the elimination of tumor and pathogen-infected cells (Hercend and Schmidt, 1988). Both, binge drinking and chronic ethanol exposure decrease cytotoxic activity of NK cells. In addition, chronic ethanol exposure reduces the number of NK cells (Blank et al., 1993; Wu and Pruett, 1996). Impairment of NK-mediated immune surveillance by ethanol may explain higher incidence of cancers in alcoholics (Rehm et al., 2003). It was demonstrated that diminished resistance to metastases of B16F10 melanoma cells in mice was directly linked to the negative effect of ethanol on NK cell activity (Wu and Pruett, 1999). NK cells express toll-like receptor 3 (TLR3) and can be activated by viral RNA (Schmidt et al., 2004). Synthetic poly I:C, which mimics viral exposure, induces production of multiple cytokines by NK cells. It was shown that a single dose of 4–6 g/kg of ethanol administered by oral gavage suppressed poly I:C induced mRNA for IFN-α, IFN-β, IFN-γ, IL-6, IL-9, IL-12, and IL-15 in mouse spleen tissue (Pruett et al., 2003). In addition, activated NK cells are involved in amelioration of liver fibrosis by killing activated stellate cells, that produce collagen in the liver (Radaeva et al., 2006). Thus, ethanol-induced impairment of NK cell functions may contribute to the development of liver fibrosis in alcoholics.
Ethanol and phagocytosis
Another key component of the innate immune system is the ability of professional phagocytic cells to phagocytose and present antigen to T-cells, thus linking the innate and the adaptive arms of immunity. Both acute and chronic ethanol exposure have been shown to affect the phagocytic potential and the ability of these professional antigen presenting cells to express antigen and co-stimulatory molecules on their surface (Table 1). Studies from as early as the 1960’s and 70’s have shown immunomodulatory effects of ethanol on bacterial clearance(Auerbach-Rubin and Ottolenghi-Nightingale, 1971; Laurenzi et al., 1963). Recent studies have begun to reveal a more in-depth understanding of ethanol’s effects on phagocytosis. Intratracheal endotoxin (LPS) administration typically results in recruitment and activation of neutrophils (PMN), as well as the activation of alveolar macrophages. Studies by Zhang et al. showed that acute ethanol exposure not only inhibited the recruitment of PMN, but also blocked their activation, measured by decreased CD11b/c expression. These studies also showed that acute ethanol suppressed the production of hydrogen peroxide by alveolar macrophages and PMN recruited to the lung, as well as the phagocytic activity of circulating PMN (Zhang et al., 1997). Complementary data showed that acute ethanol exposure lowered endotoxin-induced expression of phagocytic receptors CD11b/c and CD18 (Zhang et al., 1998; Zhang et al., 1999). Pretreatment with granulocyte-colony stimulating factor (G-CSF) given intraperitoneally, was shown to return surface expression of CD11b/c, as well as phagocytic activity of PMN to control levels. In contrast, acute ethanol intoxication prior to intravenous LPS stimulation resulted in enhanced phagocytic responses in liver-sequestered PMN and Kupffer cells, but not in circulating PMN (Spitzer and Zhang, 1996). Studies from the same lab show cell specific responses to ethanol. Three hours post-ethanol, f-met-leu-phe (fMLP)- induced chemotaxis and superoxide release by blood neutrophils were increased compared to the saline-treated group, and were further increased at 24 hours (Bautista and Elliott, 1994). In contrast, alcohol infusion for 3 hours attenuated fMLP-stimulated superoxide release by Kupffer cells, compared to saline- treated control rats. Additionally, Kupffer cells chemotaxis was also blunted by ethanol at 3 and 24 post-treatment (Bautista and Elliott, 1994). Because of the scarcity of data and the conflicting observations between cell types, it is difficult to accurately assess the effects of acute ethanol on superoxide production and chemotaxis of PMN and Kupffer cells in these experimental models. What we can conclude is that ethanol is having various effects on numerous cell types. By inhibiting phagocytosis or the processes that involve degradation of the pathogens, professional phagocytic cells may not adequately present antigens. This was observed by Szabo et al. who reported that monocyte derived dendritic cells isolated from healthy human subjects given a single dose of alcohol showed decreased allostimulatory potential, as well as decreased expression of CD80 and CD86 (Mandrekar et al., 2004). These data reveal that acute ethanol exposure can not only modify phagocytic capacity, but also the ability of dendritic cells to activate the adaptive arm of the immune response.
Table 1.
The effects of different patterns of ethanol exposure on phagocytosis and the molecules that regulate or facilitate phagocytic activity of macrophages and neutrophils (PMN).
| Acute Ethanol | Chronic Ethanol | |
|---|---|---|
| 1. Phagocytosis | ↓Alveolar PMN and macrophages (Zhang et al., 1997; Spitzer et al., 1996; Rimland et al., 1980)
↑Liver PMN (Spitzer et al., 1996) ↑ Kupffer cells (Zhang et al., 1998; Zhang et al.,1999) |
↓ Kupffer cells (Bautista, 2002; Messner et al., 1993; McVicker et al., 2002; Shiratori et al., 1989; Shiratori et al., 1989)
↓ Alveolar macrophages (Brown et al., 2007; Joshi et al., 2005) ↑ Splenic macrophages (Shiratori et al., 1989; Shiratori et al., 1989) ↓ Microglia (Aroor and Baker, 1998) |
| 2. Phagocytic receptor expression | ↓ CD11b/c and CD18 in circulating PMN (Zhang et al., 1998; Zhang et al.,1999) | ↓ β2 integrin in hepatic macrophages(Morio et al., 2000) |
| 3. Lysosomal vesicle conformation | ↓ H2O2 in alveolar PMN and macrophages(Zhang et al., 1997)
↓ Superoxide in Kupffer cells (Bautista and Elliott, 1994) ↑ Superoxide in blood PMN (Bautista and Elliott, 1994) |
↑ H2O2 in Kupffer cells (Bautista, 2002)
↑GSH in alveolar macrophages (Brown et al., 2007) ↑ NO in CD11b+ splenocytes (Zhu et al., 2004) ↓ Superoxide in alveolar macrophages (Morio et al., 2000) |
| 4. Allostimulatory potential | ↓ CD80 and CD86 in monocyte derived DCs(Mandrekar et al., 2004) | ↑ CD80 and CD86 in CD11b+ splenocytes (Zhu et al., 2004) |
Substantial evidence shows that chronic ethanol exposure affects phagocytic function of immune cells. It was demonstrated that chronic ethanol ingestion decreases Kupffer cell-facilitated phagocytosis and chemotaxis (Bautista, 2002). Chemotaxis, E.coli phagocytosis, and fMLP -induced superoxide anion production by Kupffer cells were downregulated in the ethanol-fed group. Interestingly these studies revealed that chronic alcohol intoxication was also associated with increased basal hydrogen peroxide formation, enhanced nuclear translocation and binding of NF-κB, AP-1 and MNP-1 in Kupffer cells. As mentioned above, these transcription factors may play a role in the proinflammatory phenotype observed in chronic alcoholics. Complement receptor-mediated phagocytosis has specifically been shown to be decreased by chronic ethanol exposure. Using a murine model, ingestion of chronic ethanol was shown to be associated with a decrease in complement-mediated clearance of opsonized erythrocytes (Messner et al., 1993). In addition, uptake of apoptotic cells, via the asialoglycoprotein receptor, was impaired in cells obtained from ethanol-fed animals, indicating that ethanol may decrease the ability to clear apoptotic cells in the liver (McVicker et al., 2002). Earlier studies have shown that Kupffer cells exposed to chronic ethanol in vivo had decreased ability to phagocytose latex beads, however splenic macrophages showed an increase in phagocytosis (Shiratori et al., 1989a; Shiratori et al., 1989b). It was also shown that chronic ingestion of ethanol decreased both the viability of alveolar macrophages and their ability to phagocytose microorganisms. This attenuated phagocytosis of alveolar macrophages may lead to an increased risk of pneumonia (Brown et al., 2004). These studies showed that the addition of glutathione precursors to the diet restored cellular function and viability of macrophages as well as decreased sensitivity to endotoxemia-induced acute lung injury. Another report showed that oxidative stress in the alveolar macrophages was potentiated via an increase of the GSH/GSSG (reduced/oxidized glutathione) redox potential in the ethanol treated group (Brown et al., 2007). Other studies showed that a decrease in alveolar macrophage phagocytosis after chronic ethanol exposure is due to attenuation in membrane expression of the GM-CSF receptor. In addition, ethanol ingestion reduced cellular expression and nuclear binding of PU.1, the master transcription factor that activates GM-CSF-dependent macrophage functions (Joshi et al., 2005). Furthermore, treatment of ethanol-fed rats with recombinant GM-CSF restored GM-CSF receptor membrane expression, as well as PU.1 protein expression and nuclear binding in alveolar macrophages.
Functions of immune cells in other compartments, such as the central nervous system (CNS), are also affected by ethanol exposure. Chronic ethanol exposure decreased phagocytosis of opsonized Escherichia coli by microglia. In the absence of stimulation, ethanol did not alter synthesis of superoxide anion by microglia, however, ethanol treatment did suppress phorbol-12 myristate-13 acetate-stimulated microglial superoxide anion production (Aroor and Baker, 1998). The consensus of data reported above gives evidence that phagocytosis is impaired in chronic ethanol treated animal models. It is apparent that ethanol is having effects on multiple signaling pathways in these experimental models. It is also interesting to note that the dichotomy between acute and chronic ethanol exposure and the attenuated versus the augmented pro-inflammatory response, respectively, is not observed with phagocytosis. Both acute and chronic ethanol exposures typically decrease phagocytosis. The current task is to elucidate the mechanisms by which the alcohol is having its effects.
Ethanol and TLRs expression
Microbial pathogens are recognized by the cells of innate immune system via pattern recognition receptors (PRRs) (Janeway and Medzhitov, 2002). One of the extensively studied families of PRRs is that of Toll-like Receptors (TLRs), which demonstrate specificity for microbial structures. Examples include TLR4, which recognizes LPS (in the context of CD14 and MD-2) from Gram negative bacteria; TLR3, which is activated by viral RNA; or TLR9, which is stimulated by unmethylated bacterial DNA (Takeda et al., 2003).Upon sensing microbial structures, TLRs initiate complex immune responses directed against invading pathogens.
It is possible that ethanol modifies TLR expression and/or TLR-mediated responses. It was reported that a single dose of ethanol (5 g/kg body weight administered intragastrically) reduced the expression of TLR4 mRNA in mouse liver 2 to 6h after ethanol treatment (Nishiyama et al., 2002). In contrast, 3 and 4 week long, chronic administration of ethanol led to higher levels of TLR4 in Kupffer cells, as shown by mRNA and fluorescent microscopy (Zuo et al., 2003). In another study, an increase in mRNA expression of TLR1, 2, 4, 6, 7, 8, and 9 was detected in liver tissue from mice fed an ethanol-containing diet (on average 139.1 mg/dL blood ethanol level was measured) for 10 days (Gustot et al., 2006). Furthermore, ethanol sensitized these mice to liver inflammation, as indicated by an increase in TNF-α mRNA expression induced by intraperitoneal injection of TLR1/TLR2, TLR2/TLR6, TLR4, TLR7, and TLR9 ligands. The administration of antibiotics did not prevent the up-regulation in TLR mRNA expression, however, the inhibition of reactive oxygen species (ROS) production by an inhibitor of NADPH oxidase, diphenyleneiodonium sulfate (DPI), resulted in diminished mRNA expression of TLR2, 4, 7, and 9. Therefore, the authors postulated that the up-regulated expression of multiple TLRs was more likely caused by ROS, than directly by intestinal bacteria.
In summary, observations of the opposite effects of acute and chronic exposure to ethanol on the expression of TLRs (down- versus up-regulation, respectively) further support the concept of the biphasic nature of ethanol effects.
Ethanol and TLR-mediated signaling
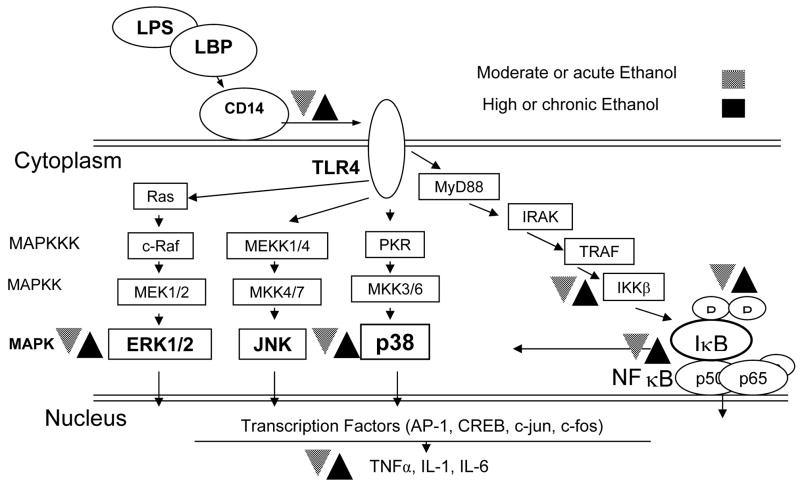
TLRs are signaling receptors. TLRs initiate inflammatory responses via intracellular signal transduction cascades, which include activation of mitogen-activated protein kinases (MAPKs) and NFκB (Takeda et al., 2003; Kawai and Akira, 2006). Multiple reports indicate that acute ethanol exposure has an inhibitory effect on activation of MAPKs and NFκB in the cells involved in inflammatory responses (Figure 1). Ethanol down-regulated LPS-induced phosphorylation of p38 in human peripheral blood leukocytes, and attenuated activation of NFκB in human monocytes (Arbabi et al., 1999; Mandrekar et al., 1999). In addition, acute ethanol inhibited poly (I:C)-induced activation of p38 and NFκB in murine peritoneal macrophages (Pruett et al., 2004a). Furthermore, ethanol diminished p38 and ERK1/2 phosphorylation in murine macrophages stimulated by PGN, LPS, and CpG (TLR2, 4, and 9 ligands, respectively) (Goral and Kovacs, 2005). It was also proposed that suppression of NFκB activation by ethanol was responsible for decreased TNF-α-induced activation of endothelial cells, which are crucial in recruitment of lymphoid cells to the sites of inflammation (Saeed et al., 2004).
Figure 1.
The effect of moderate or acute (
 ) and high or chronic (■) ethanol exposure on intracellular signaling pathways initiated by LPS. Both the MAPK and NFκB pathways mediate inflammatory responses. Different patterns of ethanol exposure have different effects on molecules involved in inflammation. LPS-lipopolysaccharide, LBP-LPS binding protein, TLR-toll like receptor, MAPK(K)(KK)-mitogen activated protein kinase (kinase) (kinase kinase), TNF-tumor necrosis factor, IL- interleukin, CREB- cAMP response element binding, JNK- Jun N-terminal kinase.
) and high or chronic (■) ethanol exposure on intracellular signaling pathways initiated by LPS. Both the MAPK and NFκB pathways mediate inflammatory responses. Different patterns of ethanol exposure have different effects on molecules involved in inflammation. LPS-lipopolysaccharide, LBP-LPS binding protein, TLR-toll like receptor, MAPK(K)(KK)-mitogen activated protein kinase (kinase) (kinase kinase), TNF-tumor necrosis factor, IL- interleukin, CREB- cAMP response element binding, JNK- Jun N-terminal kinase.
In contrast, chronic ethanol exposure increased Kupffer cell production of TNF-α in response to LPS stimulation (Nagy, 2003; Hines and Wheeler, 2004). This enhancement was accompanied by up regulation of p38 and ERK1/2 activity, and was blocked by p38 and ERK inhibitors (Kishore et al., 2001; Kishore et al., 2002; Cao et al., 2002). Studies of the mechanism of MAPK involvement in TNF-α synthesis indicate a link between activation of p38 and ERK1/2, and TNF-α mRNA stabilization. Therefore, the ethanol-induced increase in p38 and ERK1/2 activation may contribute to the excessive sensitivity of Kupffer cells to LPS and augmented TNF-α production after chronic ethanol exposure (Yao et al., 1997; Winzen et al., 1999; Nagy, 2003).
Ethanol and lipid rafts
The role of TLRs in intracellular signaling is well established, however, the mechanisms responsible for TLR recognition of pathogen-associated-molecular patterns (PAMPs), is still unclear. With regards to TLR4, the receptor does not bind its ligand LPS directly; instead, LPS binds to a receptor complex made of CD14, MD-2, and TLR4 (Fitzgerald et al., 2004). Moreover, known ligands of TLR4, which also include Taxol and respiratory syncytial virus coat protein, are molecules that do not share structural similarities (Takeda et al., 2003; Sabroe et al., 2003). Therefore, it was proposed that TLRs may not directly bind PAMPs, but rather, function as a part of a signaling complex that forms upon stimulation with appropriate microbial compounds. Since TLRs have intracellular signaling domains, they may act more as integrators of cellular signaling, than as PAMP-binding receptors (Sabroe et al., 2003). If this is the case, then something similar to the immunological synapse in the process of antigen recognition by T lymphocytes could function in the events leading to activation of the cells of innate immune system (Triantafilou et al., 2002; He et al., 2005). This notion of cooperation between different membrane receptors can be supported by observations of the role of TLRs in phagocytosis (Weck et al., 2007). TLRs are not phagocytic receptors; however, they are recruited to phagosomes, where they can sample microbial structures and can modulate phagocytosis mediated by specialized receptors (Underhill and Gantner, 2004; Blander, 2007). The realization that interactions between multiple, often closely associated, membrane molecules is crucial for the function of the cell leads to the concept of lipid rafts made of sphinglolipids and cholesterol (Hancock, 2006; Pike, 2003). This hypothesis implies that, depending on the state of activation, cell membrane proteins will freely diffuse within the plasma membrane, or will form interactive assemblies through lipid rafts. It was shown that translocation of TLRs to lipid rafts may be associated with the recognition of PAMPs by macrophages (Triantafilou and Triantafilou, 2004).
Earlier studies designed to determine the mechanisms by which ethanol affects immune function, have focused on the cell membrane. One of the consequences of ethanol exposure is an increase in membrane fluidity (Gutierrez-Ruiz et al., 1995). Importantly, chronic alcohol exposure leads to the compensatory changes in the membrane makeup with the higher content of cholesterol, which could stiffen the membranes (Peters and Preedy, 1998). If this is the case, then the explanation for the different actions of acute and chronic ethanol exposure on inflammatory responses could be at hand. With increased fluidity caused by short term exposure to ethanol, the formation of the signaling complex within the lipid raft could be impaired, yielding diminished activation of intracellular signaling and, in turn, decreased production of inflammatory mediators. In contrast, more rigid membranes of the cells chronically exposed to ethanol would delay timely dissociation of the rafts, and could result in prolonged signaling, leading to the upregulation in the production of proinflammatory mediators. The increase in cell membrane fluidity by ethanol is well documented, however, its physiological significance remains controversial. One has to realize that an increase in membrane fluidity at a lethal concentration of ethanol is comparable to the effect of an elevation in temperature by only 1° C (Rottenberg et al., 1981). Moreover, it is important to remember that an increase in temperature manifested as fever is beneficial in fighting infections and is a part of normal immune response. Besides, ethanol ingestion is linked to a decrease in body temperature in humans (Yap et al., 1993). Therefore, it is possible that the net effect of acute ethanol exposure on membrane fluidity in vivo is zero (i.e. the increase in fluidity due to the presence of ethanol is eliminated by a drop of body temperature). Furthermore, the anti-inflammatory effect of acute ethanol exposure could be a consequence of a drop in body temperature and the absence of fever.
The possible link between an ethanol-induced increase in membrane fluidity, which could alter lipid raft formation, and its anti-inflammatory effect was recently investigated in the Chinese hamster ovary cells transfected with human CD14, TLR2, or TLR4 (Dolganiuc et al., 2006). In this report, acute ethanol exposure and treatment with peptidoglycan or LPS (TLR2 and TLR4 ligands, respectively) did not disrupt lipid raft formation as examined by a distribution of a lipid raft marker, flotillin. However, ethanol impaired LPS-stimulated TLR4 recruitment to the rafts and modified CD14 membrane distribution. In another study, acute ethanol treatment of murine macrophage cell line RAW264.7 modified LPS-induced membrane distribution of CD14 (Dai et al., 2005). These results indicate that acute ethanol exposure may dampen inflammatory responses by modifying the membrane-anchored protein complexes that are formed upon the encounter with microbial compounds.
The hypothesis that the ethanol-induced changes in membrane fluidity are the mechanism by which it exhibits its effect on the cells of innate immune system could be further disputed in light of the study revealing that acute ethanol differently modified inflammatory responses, depending whether TLR4 or both TLR2 and TLR4 mediate inflammatory signals (Oak et al., 2006). The authors conclude that acute ethanol may decrease or augment inflammation depending on the complexity of signals mediated by TLRs. If the ethanol-induced increase in membrane fluidity were responsible for the altered TLR signaling, the similar results would be expected regardless of the type and/or the number of engaged TLRs. Thus, the observed differences suggest another or an additional mechanism/s of ethanol’s action. In another study the effect of ethanol on LPS-induced receptor clustering was studied by confocal microscopy (Dai and Pruett, 2006). The formation of TLR4 and CD14 receptor patches on activated macrophages was accompanied by reorganization of actin cytoskeleton and this process was impaired by ethanol.
In an effort to summarize recent observations on ethanol effects on cellular signaling it was proposed that mechanism of ethanol’s action may involve direct protein targeting, as well as nonspecific interactions with membrane lipids and membrane proteins, including membrane receptors and cytoskeletal elements. As a result ethanol would disrupt lipid protein interactions and modify protein conformation (Szabo et al., 2007).
Does ethanol have a target molecule?
In search of a still elusive mechanism of ethanol effects on immune function, concepts of nonspecific effects versus specific targets should be considered. Changes in membrane fluidity would obviously be a part of the nonspecific theory. However, a number of recent studies demonstrate that ethanol may singularly target the large conductance calcium-activated potassium (BK) channels, which are active in a variety of neuronal and non-neuronal cells (Brodie et al., 2007). Kuhlmann et al. (Kuhlmann et al., 2004) reported that ethanol directly modified BK channels in human endothelial cells. Specifically, low concentrations of ethanol (10 and 50 mM) significantly increased, while higher concentration (100 and 150 mM) significantly reduced synthesis of nitric oxide by these cells. Importantly, these results show that ethanol affects BK channels in a dose-dependent fashion, further supporting a concept of biphasic action of ethanol. It was also demonstrated that BK channels function in TLR- and IL-1 receptor-mediated responses of human macrophages (Scheel et al., 2006). In this study BK channels were activated by LPS, peptidoglycan, and IL-1. Therefore it is possible that ethanol-dependent modifications of innate immune responses could be linked to a single target cell membrane molecule. Alternatively, ethanol immunomodulatory action could result from its both nonspecific and specific effects.
Conclusions
The substantial body of evidence implicates ethanol as a significant immunomodulatory factor. However, its actions are complex and the mechanism(s) responsible for ethanol’s effect on immune responses remain elusive. In further attempts to divulge these mechanism(s), the inclusion of microscopy and imaging techniques that can explore molecular interactions in the intact cells over short distances and small time scales seems imperative. Direct observation of cells would allow determination of whether ethanol-induced changes in cell membrane fluidity affect the interactions of membrane proteins involved in the innate immune responses.
Acknowledgments
We would like to thank Vanessa Nomellini for critical review of the manuscript. This work is supported by NIH R01 AA012034 (E.J.K.), NIH T32 AA013527 (E.J.K.), NIH F31 AA017027 (JK), an Illinois Excellence in Academic Medicine grant (E.J.K.), and the Ralph and Marion C. Falk Medical Research Trust (E.J.K.)
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Abel EL. Effects of prenatal alcohol exposure on birth weight in rats: is there an inverted U-shaped function? Alcohol. 1996;13:99–102. doi: 10.1016/0741-8329(95)02020-9. [DOI] [PubMed] [Google Scholar]
- Adams HG, Jordan C. Infections in the alcoholic. Med Clin North Am. 1984;68:179–200. doi: 10.1016/s0025-7125(16)31249-4. [DOI] [PubMed] [Google Scholar]
- Apte MV, Phillips PA, Fahmy RG, Darby SJ, Rodgers SC, McCaughan GW, Korsten MA, Pirola RC, Naidoo D, Wilson JS. Does alcohol directly stimulate pancreatic fibrogenesis? Studies with rat pancreatic stellate cells. Gastroenterology. 2000;118:780–794. doi: 10.1016/s0016-5085(00)70148-x. [DOI] [PubMed] [Google Scholar]
- Arbabi S, Garcia I, Bauer GJ, Maier RV. Alcohol (ethanol) inhibits IL-8 and TNF: role of the p38 pathway. J Immunol. 1999;162:7441–7445. [PubMed] [Google Scholar]
- Aroor AR, Baker RC. Ethanol inhibition of phagocytosis and superoxide anion production by microglia. Alcohol. 1998;15:277–280. doi: 10.1016/s0741-8329(97)00129-8. [DOI] [PubMed] [Google Scholar]
- Auerbach-Rubin F, Ottolenghi-Nightingale E. Effect of Ethanol on the Clearance of Airborne Pneumococci and the Rate of Pneumococcal Transformations in the Lung. Infect Immun. 1971;3:688–693. doi: 10.1128/iai.3.5.688-693.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Averina M, Nilssen O, Arkhipovsky VL, Kalinin AG, Brox J. C-reactive protein and alcohol consumption: Is there a U-shaped association? Results from a population-based study in Russia The Arkhangelsk study. Atherosclerosis. 2006;188:309–315. doi: 10.1016/j.atherosclerosis.2005.11.007. [DOI] [PubMed] [Google Scholar]
- Bagby GJ, Zhang P, Stoltz DA, Nelson S. Suppression of the granulocyte colony-stimulating factor response to Escherichia coli challenge by alcohol intoxication. Alcohol Clin Exp Res. 1998;22:1740–1745. [PubMed] [Google Scholar]
- Balkwill F, Coussens LM. Cancer: an inflammatory link. Nature. 2004;431:405–406. doi: 10.1038/431405a. [DOI] [PubMed] [Google Scholar]
- Bautista AP. Chronic alcohol intoxication primes Kupffer cells and endothelial cells for enhanced CC-chemokine production and concomitantly suppresses phagocytosis and chemotaxis. Front Biosci. 2002;7:a117–125. doi: 10.2741/a746. [DOI] [PubMed] [Google Scholar]
- Bautista AP, Elliott KE. Acute ethanol intoxication regulates f-met-leu-phe-induced chemotaxis and superoxide release by neutrophils and Kupffer cells through modulation of the formyl peptide receptor in the rat. Life Sci. 1994;54:721–730. doi: 10.1016/0024-3205(94)90161-9. [DOI] [PubMed] [Google Scholar]
- Blander JM. Coupling Toll-like receptor signaling with phagocytosis: potentiation of antigen presentation. Trends Immunol. 2007;28:19–25. doi: 10.1016/j.it.2006.11.001. [DOI] [PubMed] [Google Scholar]
- Blank SE, Pfister LJ, Gallucci RM, Meadows GG. Ethanol-induced changes in peripheral blood and splenic natural killer cells. Alcohol Clin Exp Res. 1993;17:561–565. doi: 10.1111/j.1530-0277.1993.tb00800.x. [DOI] [PubMed] [Google Scholar]
- Boe DM, Nelson S, Zhang P, Bagby GJ. Acute ethanol intoxication suppresses lung chemokine production following infection with Streptococcus pneumoniae. J Infect Dis. 2001;184:1134–1142. doi: 10.1086/323661. [DOI] [PubMed] [Google Scholar]
- Boe DM, Nelson S, Zhang P, Quinton L, Bagby GJ. Alcohol-induced suppression of lung chemokine production and the host defense response to Streptococcus pneumoniae. Alcohol Clin Exp Res. 2003;27:1838–1845. doi: 10.1097/01.ALC.0000095634.82310.53. [DOI] [PubMed] [Google Scholar]
- Briggs NC, Levine RS, Bobo LD, Haliburton WP, Brann EA, Hennekens CH. Wine drinking and risk of non-Hodgkin’s lymphoma among men in the United States: a population-based case-control study. Am J Epidemiol. 2002;156:454–462. doi: 10.1093/aje/kwf058. [DOI] [PubMed] [Google Scholar]
- Brodie MS, Scholz A, Weiger TM, Dopico AM. Ethanol interactions with calcium-dependent potassium channels. Alcohol Clin Exp Res. 2007;31:1625–1632. doi: 10.1111/j.1530-0277.2007.00469.x. [DOI] [PubMed] [Google Scholar]
- Brown LA, Harris FL, Ping XD, Gauthier TW. Chronic ethanol ingestion and the risk of acute lung injury: a role for glutathione availability? Alcohol. 2004;33:191–197. doi: 10.1016/j.alcohol.2004.08.002. [DOI] [PubMed] [Google Scholar]
- Brown LA, Ping XD, Harris FL, Gauthier TW. Glutathione availability modulates alveolar macrophage function in the chronic ethanol-fed rat. Am J Physiol Lung Cell Mol Physiol. 2007;292:L824–832. doi: 10.1152/ajplung.00346.2006. [DOI] [PubMed] [Google Scholar]
- Calabrese EJ, Baldwin LA. Ethanol and hormesis. Crit Rev Toxicol. 2003;33:407–424. doi: 10.1080/713611043. [DOI] [PubMed] [Google Scholar]
- Cao Q, Mak KM, Lieber CS. Dilinoleoylphosphatidylcholine decreases LPS-induced TNF-alpha generation in Kupffer cells of ethanol-fed rats: respective roles of MAPKs and NF-kappaB. Biochem Biophys Res Commun. 2002;294:849–853. doi: 10.1016/S0006-291X(02)00586-7. [DOI] [PubMed] [Google Scholar]
- Chaudhuri JD. Alcohol and the developing fetus--a review. Med Sci Monit. 2000;6:1031–1041. [PubMed] [Google Scholar]
- Coussens LM, Werb Z. Inflammation and cancer. Nature. 2002;420:860–867. doi: 10.1038/nature01322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crabbe JC. Sensitivity to ethanol in inbred mice: genotypic correlations among several behavioral responses. Behav Neurosci. 1983;97:280–289. doi: 10.1037//0735-7044.97.2.280. [DOI] [PubMed] [Google Scholar]
- Cunningham C, Wilcockson DC, Campion S, Lunnon K, Perry VH. Central and systemic endotoxin challenges exacerbate the local inflammatory response and increase neuronal death during chronic neurodegeneration. J Neurosci. 2005;25:9275–9284. doi: 10.1523/JNEUROSCI.2614-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dai Q, Pruett SB. Ethanol suppresses LPS-induced Toll-like receptor 4 clustering, reorganization of the actin cytoskeleton, and associated TNF-alpha production. Alcohol Clin Exp Res. 2006;30:1436–1444. doi: 10.1111/j.1530-0277.2006.00172.x. [DOI] [PubMed] [Google Scholar]
- Dai Q, Zhang J, Pruett SB. Ethanol alters cellular activation and CD14 partitioning in lipid rafts. Biochem Biophys Res Commun. 2005;332:37–42. doi: 10.1016/j.bbrc.2005.04.088. [DOI] [PubMed] [Google Scholar]
- Djousse L, Ellison RC, Beiser A, Scaramucci A, D’Agostino RB, Wolf PA. Alcohol consumption and risk of ischemic stroke: The Framingham Study. Stroke. 2002;33:907–912. doi: 10.1161/hs0402.105245. [DOI] [PubMed] [Google Scholar]
- Dolganiuc A, Bakis G, Kodys K, Mandrekar P, Szabo G. Acute ethanol treatment modulates Toll-like receptor-4 association with lipid rafts. Alcohol Clin Exp Res. 2006;30:76–85. doi: 10.1111/j.1530-0277.2006.00003.x. [DOI] [PubMed] [Google Scholar]
- Ekman G, Frankenhaeuser M, Goldberg L, Hagdahl R, Myrsten AL. Subjective and objective effects of alcohol as functions of dosage and time. Psychopharmacologia. 1964;6:399–409. doi: 10.1007/BF00429567. [DOI] [PubMed] [Google Scholar]
- Ellison RC, Zhang Y, McLennan CE, Rothman KJ. Exploring the relation of alcohol consumption to risk of breast cancer. Am J Epidemiol. 2001;154:740–747. doi: 10.1093/aje/154.8.740. [DOI] [PubMed] [Google Scholar]
- Feldmann M, Brennan FM, Maini RN. Role of cytokines in rheumatoid arthritis. Annu Rev Immunol. 1996;14:397–440. doi: 10.1146/annurev.immunol.14.1.397. [DOI] [PubMed] [Google Scholar]
- Fitzgerald KA, Rowe DC, Golenbock DT. Endotoxin recognition and signal transduction by the TLR4/MD2-complex. Microbes Infect. 2004;6:1361–1367. doi: 10.1016/j.micinf.2004.08.015. [DOI] [PubMed] [Google Scholar]
- Fukui H, Brauner B, Bode JC, Bode C. Plasma endotoxin concentrations in patients with alcoholic and non-alcoholic liver disease: reevaluation with an improved chromogenic assay. J Hepatol. 1991;12:162–169. doi: 10.1016/0168-8278(91)90933-3. [DOI] [PubMed] [Google Scholar]
- Gigleux I, Gagnon J, St-Pierre A, Cantin B, Dagenais GR, Meyer F, Despres JP, Lamarche B. Moderate alcohol consumption is more cardioprotective in men with the metabolic syndrome. J Nutr. 2006;136:3027–3032. doi: 10.1093/jn/136.12.3027. [DOI] [PubMed] [Google Scholar]
- Goral J, Choudhry MA, Kovacs EJ. Acute ethanol exposure inhibits macrophage IL-6 production: role of p38 and ERK1/2 MAPK. J Leukoc Biol. 2004;75:553–559. doi: 10.1189/jlb.0703350. [DOI] [PubMed] [Google Scholar]
- Goral J, Kovacs EJ. In vivo ethanol exposure down-regulates TLR2-, TLR4-, and TLR9-mediated macrophage inflammatory response by limiting p38 and ERK1/2 activation. J Immunol. 2005;174:456–463. doi: 10.4049/jimmunol.174.1.456. [DOI] [PubMed] [Google Scholar]
- Gun RT, Pratt N, Ryan P, Gordon I, Roder D. Tobacco and alcohol-related mortality in men: estimates from the Australian cohort of petroleum industry workers. Aust N Z J Public Health. 2006;30:318–324. doi: 10.1111/j.1467-842x.2006.tb00842.x. [DOI] [PubMed] [Google Scholar]
- Gustot T, Lemmers A, Moreno C, Nagy N, Quertinmont E, Nicaise C, Franchimont D, Louis H, Deviere J, Le Moine O. Differential liver sensitization to toll-like receptor pathways in mice with alcoholic fatty liver. Hepatology. 2006;43:989–1000. doi: 10.1002/hep.21138. [DOI] [PubMed] [Google Scholar]
- Gutierrez-Ruiz MC, Gomez JL, Souza V, Bucio L. Chronic and acute ethanol treatment modifies fluidity and composition in plasma membranes of a human hepatic cell line (WRL-68) Cell Biol Toxicol. 1995;11:69–78. doi: 10.1007/BF00767492. [DOI] [PubMed] [Google Scholar]
- Hancock JF. Lipid rafts: contentious only from simplistic standpoints. Nat Rev Mol Cell Biol. 2006;7:456–462. doi: 10.1038/nrm1925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanna EZ, Chou SP, Grant BF. The relationship between drinking and heart disease morbidity in the United States: results from the National Health Interview Survey. Alcohol Clin Exp Res. 1997;21:111–118. [PubMed] [Google Scholar]
- Hannigan JH, Armant DR. Alcohol in pregnancy and neonatal outcome. Semin Neonatol. 2000;5:243–254. doi: 10.1053/siny.2000.0027. [DOI] [PubMed] [Google Scholar]
- He HT, Lellouch A, Marguet D. Lipid rafts and the initiation of T cell receptor signaling. Semin Immunol. 2005;17:23–33. doi: 10.1016/j.smim.2004.09.001. [DOI] [PubMed] [Google Scholar]
- Hercend T, Schmidt RE. Characteristics and uses of natural killer cells. Immunol Today. 1988;9:291–293. doi: 10.1016/0167-5699(88)91317-5. [DOI] [PubMed] [Google Scholar]
- Hijmering ML, de Lange DW, Lorsheyd A, Kraaijenhagen RJ, van de Wiel A. Binge drinking causes endothelial dysfunction, which is not prevented by wine polyphenols: a small trial in healthy volunteers. Neth J Med. 2007;65:29–35. [PubMed] [Google Scholar]
- Hines IN, Wheeler MD. Recent advances in alcoholic liver disease III. Role of the innate immune response in alcoholic hepatitis. Am J Physiol Gastrointest Liver Physiol. 2004;287:G310–314. doi: 10.1152/ajpgi.00094.2004. [DOI] [PubMed] [Google Scholar]
- Imhof A, Froehlich M, Brenner H, Boeing H, Pepys MB, Koenig W. Effect of alcohol consumption on systemic markers of inflammation. Lancet. 2001;357:763–767. doi: 10.1016/S0140-6736(00)04170-2. [DOI] [PubMed] [Google Scholar]
- Janeway CA, Jr, Medzhitov R. Innate immune recognition. Annu Rev Immunol. 2002;20:197–216. doi: 10.1146/annurev.immunol.20.083001.084359. [DOI] [PubMed] [Google Scholar]
- Jerrells TR, Vidlak D, Strachota JM. Alcoholic pancreatitis: mechanisms of viral infections as cofactors in the development of acute and chronic pancreatitis and fibrosis. J Leukoc Biol. 2007;81:430–439. doi: 10.1189/jlb.1004622. [DOI] [PubMed] [Google Scholar]
- Joshi PC, Applewhite L, Ritzenthaler JD, Roman J, Fernandez AL, Eaton DC, Brown LA, Guidot DM. Chronic ethanol ingestion in rats decreases granulocyte-macrophage colony-stimulating factor receptor expression and downstream signaling in the alveolar macrophage. J Immunol. 2005;175:6837–6845. doi: 10.4049/jimmunol.175.10.6837. [DOI] [PubMed] [Google Scholar]
- Kawai T, Akira S. TLR signaling. Cell Death Differ. 2006;13:816–825. doi: 10.1038/sj.cdd.4401850. [DOI] [PubMed] [Google Scholar]
- Khoruts A, Stahnke L, McClain CJ, Logan G, Allen JI. Circulating tumor necrosis factor, interleukin-1 and interleukin-6 concentrations in chronic alcoholic patients. Hepatology. 1991;13:267–276. [PubMed] [Google Scholar]
- Kishore R, Hill JR, McMullen MR, Frenkel J, Nagy LE. ERK1/2 and Egr-1 contribute to increased TNF-alpha production in rat Kupffer cells after chronic ethanol feeding. Am J Physiol Gastrointest Liver Physiol. 2002;282:G6–15. doi: 10.1152/ajpgi.00328.2001. [DOI] [PubMed] [Google Scholar]
- Kishore R, McMullen MR, Nagy LE. Stabilization of tumor necrosis factor alpha mRNA by chronic ethanol: role of A + U-rich elements and p38 mitogen-activated protein kinase signaling pathway. J Biol Chem. 2001;276:41930–41937. doi: 10.1074/jbc.M107181200. [DOI] [PubMed] [Google Scholar]
- Kolls JK, Xie J, Lei D, Greenberg S, Summer WR, Nelson S. Differential effects of in vivo ethanol on LPS-induced TNF and nitric oxide production in the lung. Am J Physiol. 1995;268:L991–998. doi: 10.1152/ajplung.1995.268.6.L991. [DOI] [PubMed] [Google Scholar]
- Kuhlmann CR, Li F, Ludders DW, Schaefer CA, Most AK, Backenkohler U, Neumann T, Tillmanns H, Waldecker B, Erdogan A, Wiecha J. Dose-dependent activation of Ca2+-activated K+ channels by ethanol contributes to improved endothelial cell functions. Alcohol Clin Exp Res. 2004;28:1005–1011. doi: 10.1097/01.alc.0000130811.92457.0d. [DOI] [PubMed] [Google Scholar]
- Laurenzi GA, Guarneri JJ, Endriga RB, Carey JP. Clearance of Bacteria by the Lower Respiratory Tract. Science. 1963;142:1572–1573. doi: 10.1126/science.142.3599.1572. [DOI] [PubMed] [Google Scholar]
- Lawrence T, Hageman T, Balkwill F. Cancer. Sex, cytokines, and cancer. Science. 2007;317:51–52. doi: 10.1126/science.1146052. [DOI] [PubMed] [Google Scholar]
- Leach RE, Stachecki JJ, Armant DR. Development of in vitro fertilized mouse embryos exposed to ethanol during the preimplantation period: accelerated embryogenesis at subtoxic levels. Teratology. 1993;47:57–64. doi: 10.1002/tera.1420470110. [DOI] [PubMed] [Google Scholar]
- Libby P. Inflammation in atherosclerosis. Nature. 2002;420:868–874. doi: 10.1038/nature01323. [DOI] [PubMed] [Google Scholar]
- Luchsinger JA, Tang MX, Siddiqui M, Shea S, Mayeux R. Alcohol intake and risk of dementia. J Am Geriatr Soc. 2004;52:540–546. doi: 10.1111/j.1532-5415.2004.52159.x. [DOI] [PubMed] [Google Scholar]
- Mandrekar P, Catalano D, Dolganiuc A, Kodys K, Szabo G. Inhibition of myeloid dendritic cell accessory cell function and induction of T cell anergy by alcohol correlates with decreased IL-12 production. J Immunol. 2004;173:3398–3407. doi: 10.4049/jimmunol.173.5.3398. [DOI] [PubMed] [Google Scholar]
- Mandrekar P, Catalano D, Szabo G. Inhibition of lipopolysaccharide-mediated NFkappaB activation by ethanol in human monocytes. Int Immunol. 1999;11:1781–1790. doi: 10.1093/intimm/11.11.1781. [DOI] [PubMed] [Google Scholar]
- Maurer DR, Liebman J. Effects of ethanol on in vitro ciliary motility. J Appl Physiol. 1988;65:1617–1620. doi: 10.1152/jappl.1988.65.4.1617. [DOI] [PubMed] [Google Scholar]
- McClain C, Hill D, Schmidt J, Diehl AM. Cytokines and alcoholic liver disease. Semin Liver Dis. 1993;13:170–182. doi: 10.1055/s-2007-1007347. [DOI] [PubMed] [Google Scholar]
- McVicker BL, Tuma DJ, Kubik JA, Hindemith AM, Baldwin CR, Casey CA. The effect of ethanol on asialoglycoprotein receptor-mediated phagocytosis of apoptotic cells by rat hepatocytes. Hepatology. 2002;36:1478–1487. doi: 10.1053/jhep.2002.37137. [DOI] [PubMed] [Google Scholar]
- Messner RP, Meryhew NL, DeMaster EG. Effect of ethanol on immune clearance in mice: biphasic alteration of complement-mediated clearance with chronic ethanol ingestion. J Lab Clin Med. 1993;122:506–517. [PubMed] [Google Scholar]
- Morton LM, Holford TR, Leaderer B, Zhang Y, Zahm SH, Boyle P, Flynn S, Tallini G, Owens PH, Zhang B, Zheng T. Alcohol use and risk of non-Hodgkin’s lymphoma among Connecticut women (United States) Cancer Causes Control. 2003;14:687–694. doi: 10.1023/a:1025626208861. [DOI] [PubMed] [Google Scholar]
- Moss M, Guidot DM, Wong-Lambertina M, Ten Hoor T, Perez RL, Brown LA. The effects of chronic alcohol abuse on pulmonary glutathione homeostasis. Am J Respir Crit Care Med. 2000;161:414–419. doi: 10.1164/ajrccm.161.2.9905002. [DOI] [PubMed] [Google Scholar]
- Nagy LE. Role of adenosine A1 receptors in inhibition of receptor-stimulated cyclic AMP production by ethanol in hepatocytes. Biochem Pharmacol. 1994;48:2091–2096. doi: 10.1016/0006-2952(94)90509-6. [DOI] [PubMed] [Google Scholar]
- Nagy LE. Recent insights into the role of the innate immune system in the development of alcoholic liver disease. Exp Biol Med (Maywood) 2003;228:882–890. doi: 10.1177/153537020322800803. [DOI] [PubMed] [Google Scholar]
- Nelson S, Bagby GJ, Bainton BG, Summer WR. The effects of acute and chronic alcoholism on tumor necrosis factor and the inflammatory response. J Infect Dis. 1989;160:422–429. doi: 10.1093/infdis/160.3.422. [DOI] [PubMed] [Google Scholar]
- Nelson S, Kolls JK. Alcohol, host defence and society. Nat Rev Immunol. 2002;2:205–209. doi: 10.1038/nri744. [DOI] [PubMed] [Google Scholar]
- Nelson S, Mason C, Bagby G, Summer W. Alcohol, tumor necrosis factor, and tuberculosis. Alcohol Clin Exp Res. 1995;19:17–24. doi: 10.1111/j.1530-0277.1995.tb01467.x. [DOI] [PubMed] [Google Scholar]
- Nielsen NR, Gronbaek M. Interactions between intakes of alcohol and postmenopausal hormones on risk of breast cancer. Int J Cancer. 2008;122:1109–1113. doi: 10.1002/ijc.23195. [DOI] [PubMed] [Google Scholar]
- Nishiyama D, Ikejima K, Honda H, Hirose M, Takei Y, Sato N. Acute ethanol administration down-regulates toll-like receptor-4 in the murine liver. Hepatol Res. 2002;23:130–137. doi: 10.1016/s1386-6346(01)00168-1. [DOI] [PubMed] [Google Scholar]
- O’Brien JM, Jr, Lu B, Ali NA, Martin GS, Aberegg SK, Marsh CB, Lemeshow S, Douglas IS. Alcohol dependence is independently associated with sepsis, septic shock, and hospital mortality among adult intensive care unit patients. Crit Care Med. 2007;35:345–350. doi: 10.1097/01.CCM.0000254340.91644.B2. [DOI] [PubMed] [Google Scholar]
- Oak S, Mandrekar P, Catalano D, Kodys K, Szabo G. TLR2- and TLR4-mediated signals determine attenuation or augmentation of inflammation by acute alcohol in monocytes. J Immunol. 2006;176:7628–7635. doi: 10.4049/jimmunol.176.12.7628. [DOI] [PubMed] [Google Scholar]
- Perry VH, Cunningham C, Holmes C. Systemic infections and inflammation affect chronic neurodegeneration. Nat Rev Immunol. 2007;7:161–167. doi: 10.1038/nri2015. [DOI] [PubMed] [Google Scholar]
- Peters TJ, Preedy VR. Metabolic consequences of alcohol ingestion. Novartis Found Symp . 1998;216:19–24. 24–34. doi: 10.1002/9780470515549.ch3. [DOI] [PubMed] [Google Scholar]
- Pikarsky E, Porat RM, Stein I, Abramovitch R, Amit S, Kasem S, Gutkovich-Pyest E, Urieli-Shoval S, Galun E, Ben-Neriah Y. NF-kappaB functions as a tumour promoter in inflammation-associated cancer. Nature. 2004;431:461–466. doi: 10.1038/nature02924. [DOI] [PubMed] [Google Scholar]
- Pike LJ. Lipid rafts: bringing order to chaos. J Lipid Res. 2003;44:655–667. doi: 10.1194/jlr.R200021-JLR200. [DOI] [PubMed] [Google Scholar]
- Power C, Rodgers B, Hope S. U-shaped relation for alcohol consumption and health in early adulthood and implications for mortality. Lancet. 1998;352:877. doi: 10.1016/S0140-6736(98)23937-7. [DOI] [PubMed] [Google Scholar]
- Primatesta P, Del Corno G, Bonazzi MC, Waters WE. Alcohol and pregnancy: an international comparison. J Public Health Med. 1993;15:69–76. doi: 10.1093/oxfordjournals.pubmed.a042822. [DOI] [PubMed] [Google Scholar]
- Pruett BS, Pruett SB. An explanation for the paradoxical induction and suppression of an acute phase response by ethanol. Alcohol. 2006;39:105–110. doi: 10.1016/j.alcohol.2006.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pruett SB, Fan R, Zheng Q. Acute ethanol administration profoundly alters poly I:C-induced cytokine expression in mice by a mechanism that is not dependent on corticosterone. Life Sci. 2003;72:1825–1839. doi: 10.1016/s0024-3205(02)02507-9. [DOI] [PubMed] [Google Scholar]
- Pruett SB, Schwab C, Zheng Q, Fan R. Suppression of innate immunity by acute ethanol administration: a global perspective and a new mechanism beginning with inhibition of signaling through TLR3. J Immunol. 2004a;173:2715–2724. doi: 10.4049/jimmunol.173.4.2715. [DOI] [PubMed] [Google Scholar]
- Pruett SB, Zheng Q, Fan R, Matthews K, Schwab C. Acute exposure to ethanol affects Toll-like receptor signaling and subsequent responses: an overview of recent studies. Alcohol. 2004b;33:235–239. doi: 10.1016/j.alcohol.2004.08.003. [DOI] [PubMed] [Google Scholar]
- Radaeva S, Sun R, Jaruga B, Nguyen VT, Tian Z, Gao B. Natural killer cells ameliorate liver fibrosis by killing activated stellate cells in NKG2D-dependent and tumor necrosis factor-related apoptosis-inducing ligand-dependent manners. Gastroenterology. 2006;130:435–452. doi: 10.1053/j.gastro.2005.10.055. [DOI] [PubMed] [Google Scholar]
- Rao RK, Seth A, Sheth P. Recent Advances in Alcoholic Liver Disease I. Role of intestinal permeability and endotoxemia in alcoholic liver disease. Am J Physiol Gastrointest Liver Physiol. 2004;286:G881–884. doi: 10.1152/ajpgi.00006.2004. [DOI] [PubMed] [Google Scholar]
- Regev A, Jeffers LJ. Hepatitis C and alcohol. Alcohol Clin Exp Res. 1999;23:1543–1551. [PubMed] [Google Scholar]
- Rehm J, Room R, Graham K, Monteiro M, Gmel G, Sempos CT. The relationship of average volume of alcohol consumption and patterns of drinking to burden of disease: an overview. Addiction. 2003;98:1209–1228. doi: 10.1046/j.1360-0443.2003.00467.x. [DOI] [PubMed] [Google Scholar]
- Rimm EB, Williams P, Fosher K, Criqui M, Stampfer MJ. Moderate alcohol intake and lower risk of coronary heart disease: meta-analysis of effects on lipids and haemostatic factors. Bmj. 1999;319:1523–1528. doi: 10.1136/bmj.319.7224.1523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodrigo R, Thielemann L, Orellana M. Acute and chronic effect of ethanol on (Na + K)-ATPase activity and cyclic AMP response to vasopressin in rat papillary collecting duct cells. Gen Pharmacol. 1998;30:663–667. doi: 10.1016/s0306-3623(97)00380-7. [DOI] [PubMed] [Google Scholar]
- Rottenberg H, Waring A, Rubin E. Tolerance and cross-tolerance in chronic alcoholics: reduced membrane binding of ethanol and other drugs. Science. 1981;213:583–585. doi: 10.1126/science.6264608. [DOI] [PubMed] [Google Scholar]
- Rozman KK. Hormesis and risk assessment. Hum Exp Toxicol. 2005;24:255–257. doi: 10.1191/0960327105ht522oa. [DOI] [PubMed] [Google Scholar]
- Ruiz M, Ewig S, Marcos MA, Martinez JA, Arancibia F, Mensa J, Torres A. Etiology of community-acquired pneumonia: impact of age, comorbidity, and severity. Am J Respir Crit Care Med. 1999;160:397–405. doi: 10.1164/ajrccm.160.2.9808045. [DOI] [PubMed] [Google Scholar]
- Sabroe I, Read RC, Whyte MK, Dockrell DH, Vogel SN, Dower SK. Toll-like receptors in health and disease: complex questions remain. J Immunol. 2003;171:1630–1635. doi: 10.4049/jimmunol.171.4.1630. [DOI] [PubMed] [Google Scholar]
- Saeed RW, Varma S, Peng T, Tracey KJ, Sherry B, Metz CN. Ethanol blocks leukocyte recruitment and endothelial cell activation in vivo and in vitro. J Immunol. 2004;173:6376–6383. doi: 10.4049/jimmunol.173.10.6376. [DOI] [PubMed] [Google Scholar]
- Scheel O, Papavlassopoulos M, Blunck R, Gebert A, Hartung T, Zahringer U, Seydel U, Schromm AB. Cell activation by ligands of the toll-like receptor and interleukin-1 receptor family depends on the function of the large-conductance potassium channel MaxiK in human macrophages. Infect Immun. 2006;74:4354–4356. doi: 10.1128/IAI.01783-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schenker S, Montalvo R. Alcohol and the pancreas. Recent Dev Alcohol. 1998;14:41–65. doi: 10.1007/0-306-47148-5_3. [DOI] [PubMed] [Google Scholar]
- Schmidt KN, Leung B, Kwong M, Zarember KA, Satyal S, Navas TA, Wang F, Godowski PJ. APC-independent activation of NK cells by the Toll-like receptor 3 agonist double-stranded RNA. J Immunol. 2004;172:138–143. doi: 10.4049/jimmunol.172.1.138. [DOI] [PubMed] [Google Scholar]
- Shacter E, Weitzman SA. Chronic inflammation and cancer. Oncology (Williston Park) . 2002;16:217–226. 229. discussion 230-212. [PubMed] [Google Scholar]
- Shiratori Y, Jin’nai H, Teraoka H, Matano S, Matsumoto K, Kamii K, Tanaka M, Okano K. Phagocytic properties of hepatic endothelial cells and splenic macrophages compensating for a decreased phagocytic function of Kupffer cells in the chronically ethanol-fed rats. Exp Cell Biol. 1989a;57:300–309. doi: 10.1159/000163542. [DOI] [PubMed] [Google Scholar]
- Shiratori Y, Teraoka H, Matano S, Matsumoto K, Kamii K, Tanaka M. Kupffer cell function in chronic ethanol-fed rats. Liver. 1989b;9:351–359. doi: 10.1111/j.1600-0676.1989.tb00423.x. [DOI] [PubMed] [Google Scholar]
- Singletary KW, Gapstur SM. Alcohol and breast cancer: review of epidemiologic and experimental evidence and potential mechanisms. Jama. 2001;286:2143–2151. doi: 10.1001/jama.286.17.2143. [DOI] [PubMed] [Google Scholar]
- Sisson JH. Alcohol and airways function in health and disease. Alcohol. 2007;41:293–307. doi: 10.1016/j.alcohol.2007.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spitzer JA, Zhang P. Gender differences in phagocytic responses in the blood and liver, and the generation of cytokine-induced neutrophil chemoattractant in the liver of acutely ethanol-intoxicated rats. Alcohol Clin Exp Res. 1996;20:914–920. doi: 10.1111/j.1530-0277.1996.tb05271.x. [DOI] [PubMed] [Google Scholar]
- Szabo G. Monocytes, alcohol use, and altered immunity. Alcohol Clin Exp Res. 1998;22:216S–219S. doi: 10.1097/00000374-199805001-00002. [DOI] [PubMed] [Google Scholar]
- Szabo G, Dolganiuc A, Dai Q, Pruett SB. TLR4, ethanol, and lipid rafts: a new mechanism of ethanol action with implications for other receptor-mediated effects. J Immunol. 2007;178:1243–1249. doi: 10.4049/jimmunol.178.3.1243. [DOI] [PubMed] [Google Scholar]
- Szabo G, Mandrekar P, Dolganiuc A, Catalano D, Kodys K. Reduced alloreactive T-cell activation after alcohol intake is due to impaired monocyte accessory cell function and correlates with elevated IL-10, IL-13, and decreased IFNgamma levels. Alcohol Clin Exp Res. 2001;25:1766–1772. [PubMed] [Google Scholar]
- Szabo G, Mandrekar P, Girouard L, Catalano D. Regulation of human monocyte functions by acute ethanol treatment: decreased tumor necrosis factor-alpha, interleukin-1 beta and elevated interleukin-10, and transforming growth factor-beta production. Alcohol Clin Exp Res. 1996;20:900–907. doi: 10.1111/j.1530-0277.1996.tb05269.x. [DOI] [PubMed] [Google Scholar]
- Takeda K, Kaisho T, Akira S. Toll-like receptors. Annu Rev Immunol. 2003;21:335–376. doi: 10.1146/annurev.immunol.21.120601.141126. [DOI] [PubMed] [Google Scholar]
- Thurman RG. II. Alcoholic liver injury involves activation of Kupffer cells by endotoxin. Am J Physiol. 1998;275:G605–611. doi: 10.1152/ajpgi.1998.275.4.G605. [DOI] [PubMed] [Google Scholar]
- Tolstrup J, Jensen MK, Tjonneland A, Overvad K, Mukamal KJ, Gronbaek M. Prospective study of alcohol drinking patterns and coronary heart disease in women and men. Bmj. 2006;332:1244–1248. doi: 10.1136/bmj.38831.503113.7C. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Triantafilou M, Miyake K, Golenbock DT, Triantafilou K. Mediators of innate immune recognition of bacteria concentrate in lipid rafts and facilitate lipopolysaccharide-induced cell activation. J Cell Sci. 2002;115:2603–2611. doi: 10.1242/jcs.115.12.2603. [DOI] [PubMed] [Google Scholar]
- Triantafilou M, Triantafilou K. Heat-shock protein 70 and heat-shock protein 90 associate with Toll-like receptor 4 in response to bacterial lipopolysaccharide. Biochem Soc Trans. 2004;32:636–639. doi: 10.1042/BST0320636. [DOI] [PubMed] [Google Scholar]
- Underhill DM, Gantner B. Integration of Toll-like receptor and phagocytic signaling for tailored immunity. Microbes Infect. 2004;6:1368–1373. doi: 10.1016/j.micinf.2004.08.016. [DOI] [PubMed] [Google Scholar]
- Verma BK, Fogarasi M, Szabo G. Down-regulation of tumor necrosis factor alpha activity by acute ethanol treatment in human peripheral blood monocytes. J Clin Immunol. 1993;13:8–22. doi: 10.1007/BF00920631. [DOI] [PubMed] [Google Scholar]
- Weck MM, Grunebach F, Werth D, Sinzger C, Bringmann A, Brossart P. TLR ligands differentially affect uptake and presentation of cellular antigens. Blood. 2007;109:3890–3894. doi: 10.1182/blood-2006-04-015719. [DOI] [PubMed] [Google Scholar]
- Wheeler MD, Kono H, Yin M, Nakagami M, Uesugi T, Arteel GE, Gabele E, Rusyn I, Yamashina S, Froh M, et al. The role of Kupffer cell oxidant production in early ethanol-induced liver disease. Free Radic Biol Med. 2001;31:1544–1549. doi: 10.1016/s0891-5849(01)00748-1. [DOI] [PubMed] [Google Scholar]
- Williams AF. Social drinking, anxiety, and depression. J Pers Soc Psychol. 1966;3:689–693. doi: 10.1037/h0023299. [DOI] [PubMed] [Google Scholar]
- Williams ES, Mirro MJ, Bailey JC. Electrophysiological effects of ethanol, acetaldehyde, and acetate on cardiac tissues from dog and guinea pig. Circ Res. 1980;47:473–478. doi: 10.1161/01.res.47.3.473. [DOI] [PubMed] [Google Scholar]
- Winzen R, Kracht M, Ritter B, Wilhelm A, Chen CY, Shyu AB, Muller M, Gaestel M, Resch K, Holtmann H. The p38 MAP kinase pathway signals for cytokine-induced mRNA stabilization via MAP kinase-activated protein kinase 2 and an AU-rich region-targeted mechanism. Embo J. 1999;18:4969–4980. doi: 10.1093/emboj/18.18.4969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu WJ, Pruett SB. Suppression of splenic natural killer cell activity in a mouse model for binge drinking. I Direct effects of ethanol and its major metabolites are not primarily responsible for decreased natural killer cell activity. J Pharmacol Exp Ther. 1996;278:1325–1330. [PubMed] [Google Scholar]
- Wu WJ, Pruett SB. Ethanol decreases host resistance to pulmonary metastases in a mouse model: role of natural killer cells and the ethanol-induced stress response. Int J Cancer. 1999;82:886–892. doi: 10.1002/(sici)1097-0215(19990909)82:6<886::aid-ijc19>3.0.co;2-j. [DOI] [PubMed] [Google Scholar]
- Wyatt TA, Gentry-Nielsen MJ, Pavlik JA, Sisson JH. Desensitization of PKA-stimulated ciliary beat frequency in an ethanol-fed rat model of cigarette smoke exposure. Alcohol Clin Exp Res. 2004;28:998–1004. doi: 10.1097/01.ALC.0000130805.75641.F4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yao J, Mackman N, Edgington TS, Fan ST. Lipopolysaccharide induction of the tumor necrosis factor-alpha promoter in human monocytic cells. Regulation by Egr-1, c-Jun, and NF-kappaB transcription factors. J Biol Chem. 1997;272:17795–17801. doi: 10.1074/jbc.272.28.17795. [DOI] [PubMed] [Google Scholar]
- Yap M, Mascord DJ, Starmer GA, Whitfield JB. Studies on the chronopharmacology of ethanol. Alcohol Alcohol. 1993;28:17–24. [PubMed] [Google Scholar]
- Zhang P, Bagby GJ, Xie M, Stoltz DA, Summer WR, Nelson S. Acute ethanol intoxication inhibits neutrophil beta2-integrin expression in rats during endotoxemia. Alcohol Clin Exp Res. 1998;22:135–141. [PubMed] [Google Scholar]
- Zhang P, Nelson S, Summer WR, Spitzer JA. Acute ethanol intoxication suppresses the pulmonary inflammatory response in rats challenged with intrapulmonary endotoxin. Alcohol Clin Exp Res. 1997;21:773–778. [PubMed] [Google Scholar]
- Zhang Y, Kreger BE, Dorgan JF, Splansky GL, Cupples LA, Ellison RC. Alcohol consumption and risk of breast cancer: the Framingham Study revisited. Am J Epidemiol. 1999;149:93–101. doi: 10.1093/oxfordjournals.aje.a009791. [DOI] [PubMed] [Google Scholar]
- Zuo G, Gong J, Liu C, Wu C, Li S, Dai L. Synthesis of Toll-like receptor 4 in Kupffer cells and its role in alcohol-induced liver disease. Chin Med J (Engl) 2003;116:297–300. [PubMed] [Google Scholar]