Abstract
The current study investigated whether, for spatial reference memory, age impacts (1) sensitivity to surgical ovarian hormone loss (Ovx), (2) response to estradiol treatment (ET), and (3) the relation between circulating estradiol levels and memory scores in ovary-intact sham and Ovx plus ET rats. Young, middle-aged and aged Fisher-344 rats received sham, Ovx or Ovx plus ET treatments, and were then tested on the Morris maze. After the last test trial, a probe trial was given whereby the platform was removed. Circulating estradiol levels were then determined and correlated with performance. In Study 1, Ovx facilitated learning on day one, but impaired performance after day one, in young rats. Ovx did not influence performance in middle-aged rats. In young and middle-aged Ovx rats, ET enhanced performance with higher exogenous estradiol levels correlating with better performance during testing and the probe trial. There was no relationship between endogenous estradiol levels and performance in sham young or middle-aged rats. Study 2 showed that, like middle-aged rats, aged rats were not impacted by Ovx. Further, for aged Ovx rats, the ET regimen that was beneficial at earlier ages was no longer effective during test trials, and had only minor benefits for platform localization as assessed by the probe trial. Collectively, the findings suggest that the effects of Ovx as well as responsivity to the currently utilized ET regimen changes with age. Further, there appears to be a distinction between sensitivity to Ovx and responsiveness to ET after Ovx for spatial reference memory performance.
Keywords: estrogen, ovariectomy, spatial memory, water maze, aging
Introduction
There is abundant clinical and basic science evidence that estrogens impact cognitive function (Dohanich, 2002). Since the first controlled clinical evaluation showing that estrogen injections given to 75 year-old women enhanced memory (Caldwell and Watson, 1952), there have been numerous studies showing cognitive decline after ovarian hormone loss, and enhancement after estrogen replacement, in menopausal women (Sherwin, 2006). However, several newer clinical studies, including the large, placebo-controlled, multi-center Women’s Health Initiative Memory Study, have indicated that certain regimens of hormone treatment, e.g., use of conjugated equine estrogens with or without medroxyprogesterone, can have null or detrimental effects on cognition and dementia risk in women (Mulnard et al., 2000; Rapp et al., 2003; Shumaker et al., 2003, 2004; Wang et al., 2000).
The majority of the animal studies evaluating the activational effects of estrogens on learning and memory have been performed in young rodents (Bimonte and Denenberg, 1999; Daniel et al., 1997, 1999; Dohanich et al., 1994; Galea et al., 2001; Holmes et al., 2002; Luine et al., 1998, 2003; Marriott and Korol, 2003; Packard and Teather, 1997; Sandstrom and Williams, 2001; Singh et al., 1994). While there has been a recent increase in the number of studies evaluating estrogen effects in middle-aged or older female rodents, most using the most potent estrogen, estradiol (Bimonte-Nelson et al., 2006; Foster et al., 2003; Frick et al., 2002; Gibbs, 2000; Luine and Rodriguez, 1994; Markham et al., 2002; Markowska and Savonenko, 2002a; Savonenko and Markowska, 2003; Ziegler and Gallagher, 2005), there is only limited work comparing estrogen replacement effects in ovariectomized (Ovx) animals at different ages within the same study (Foster et al., 2003). Hence, it is still a question whether age changes responsiveness to ovarian hormone replacement. This is a clinically important question, as some menopausal women begin hormone therapy at a younger age, while some start later in life. Women in the Women’s Health Initiative Memory Study were 65 years of age and over, which may have played a critical role in the lack of efficacy of estrogen treatment since age may impact responsiveness (see Craig et al., 2005).
Animal work that has been done testing estradiol replacement at multiple ages showed that administered estradiol dose impacted spatial memory retention, and that these effects interacted with age in Ovx rodents (Foster et al., 2003). We and others have also noted divergent cognitive effects depending on administered estradiol dose in mice and rats (Bimonte and Denenberg, 1999; Bimonte-Nelson et al., 2006; El-Bakri et al., 2004; Frick et al., 2002; Gresack and Frick, 2004; Holmes et al., 2002; Packard and Teather, 1997; Rissanen et al., 1999). In these studies, however, serum hormone levels were estimated based on prior studies or manufacturer reports (Bimonte and Denenberg, 1999; Bimonte-Nelson et al., 2006; El-Bakri et al., 2004; Frick et al., 2002; Gresack and Frick, 2004; Holmes et al., 2002; Packard and Teather, 1997; Rissanen et al., 1999), or circulating estradiol levels were determined but statistical correlations with performance were not reported (Foster et al., 2003). In fact, in no animal study testing estradiol replacement have serum levels within individual animals been correlated with memory scores. There may be a positive correlation between circulating estradiol level and memory, as seen with endogenous levels in healthy menopausal women (Phillips and Sherwin, 1992; Wolf and Kirschbaum, 2002), and after exogenous estradiol administration to postmenopausal women with probable mild to moderate Alzheimer’s disease (Asthana et al., 1999). Alternatively, the relation between serum estradiol level and memory could hold to an inverted U-shaped function, whereby very low or very high values result in the poorest cognitive function. Many biological systems fit this quadratic function (Bimonte et al., 2002), and low, but not high, estradiol levels have been associated with better cognitive performance in older women (Barrett-Connor and Goodman-Gruen, 1999). Hence, while some studies suggest that estradiol treatment influences cognition in women and the rodent model, the relation between circulating level of estradiol and memory performance is unclear.
We previously showed that Ovx did not influence spatial reference memory performance in middle-aged female rats (Bimonte-Nelson et al., 2006). Yet, in this age group estradiol replacement resulted in marked enhancements in spatial reference memory maze performance (Bimonte-Nelson et al., 2006). These data suggest that (1) naturally circulating, endogenous estradiol in an ovary-intact individual may relate to cognition in a different way than exogenously administered estradiol due to replacement after Ovx, and (2) sensitivity to ovarian hormone loss does not predict sensitivity to estradiol replacement for spatial reference memory. We and others have shown, in young rats, working memory deficiencies after Ovx and enhancements after estradiol treatment (Bimonte and Denenberg, 1999; Daniel et al., 1997, 1999; Holmes et al., 2002; Sandstrom and Williams, 2001). Additionally, in middle-aged rats no reference memory deficiencies were seen after Ovx, but benefits of estradiol replacement were observed (Bimonte-Nelson et al., 2006). While suggestive of differences in response to ovarian hormone loss and replacement depending on age, these separate studies could have been due to memory type and not age.
The current studies examined the spatial reference memory effects of Ovx and estradiol replacement in young, middle-aged and aged rats. We and others have found significant variability in circulating estradiol levels after treatment with the same manufacture-labeled dose of estradiol pellets from Innovative Research of America (unpublished observations; Diel et al., 2005). Here we capitalized on this range of serum estradiol levels to investigate relation with memory scores. Circulating estradiol levels were determined in all animals with the intention of correlating these values with maze performance. We performed correlation analyses in animals that were ovary-intact to determine associations between endogenous estradiol levels and performance, and within animals that had received Ovx plus estradiol replacement to determine relations between exogenous estradiol levels and performance. The relation between circulating estradiol and cognition may be affected by whether the estradiol is endogenous (via an intact ovary) or exogenous (experimentally administered) due to presence of progesterone in ovary-intact animals (Bimonte-Nelson et al., 2004a; 2004b, 2006; Woolley and McEwen, 1992; Johansson et al., 2002; Nilsen and Brinton, 2002a,b), as well as the cyclicity of estradiol exposure (e.g. cyclic from the ovary vs. a tonic regimen of replacement; Bimonte-Nelson et al., 2004a, 2006; Gibbs, 2000; Woolley and McEwen, 1992; Johansson et al., 2002; Markowska and Savonenko, 2002a; Nilsen and Brinton, 2002a,b).
Materials and Methods
Subjects and Treatment Procedures
For Study 1, subjects were 34 young (4 months old at test) and 33 middle-aged (16 months old at test) Fischer-344 female rats born and raised at the National Institute on Aging at Harlan Laboratories (Indianapolis, IN). After arrival, animals were pair housed, had exposure to food and water ad lib, and were maintained on a 12-h light/dark cycle. All procedures were approved by IACUC and adhered to NIH standards. Each young and middle-aged group contained the following treatment groups: ovary-intact sham (Sham), Ovx with no hormone treatment (Ovx), Ovx plus a 0.25 mg/60 day release estradiol pellet and Ovx plus a 0.50 mg/60 day release estradiol pellet. Thus, there were a total of eight groups: Young-Sham (n=8), Young-Ovx (n=9), Young-Ovx + 0.25 estradiol pellet (n=8), Young Ovx + 0.50 estradiol pellet (n=9), Middle-aged-Sham (n=8), Middle-aged-Ovx (n=9), Middle-aged Ovx + 0.25 estradiol pellet (n=8), and Middle-aged Ovx + 0.50 estradiol pellet (n=8). Ovx surgery was performed two months before testing at 2 and 14 months of age for young and middle-aged groups, respectively. Rats were anesthetized with an intraperitoneal injection of 70 mg/kg ketamine (Fort Dodge Animal Health, Fort Dodge, IA, USA)/and 6 mg/kg xylazine (Lloyd Laboratories, Shenandoah, IA, USA). For Ovx, bilateral dorsolateral incisions were made in the skin and peritoneum, and the ovaries and tips of uterine horns were ligatured and removed. The muscle was then sutured and the skin stapled. Sham surgery consisted of skin incision and staple in the same fashion.
Estradiol replacement via pellets (Innovative Research of America, Sarasota, Florida) was administered one month after Ovx, one month before testing ensued, at 3 months old for young rats, and 15 months old for middle-aged rats. Since pellets released hormone for 60 days, animals with the pellets received estradiol for the entire study, including during behavioral testing and through sacrifice. Under Ketamine/Xylazine anesthesia, a small incision was made in the scruff of the neck, and a subcutaneous pocket was created. For animals receiving estradiol, one pellet of the appropriate dose was inserted and the skin stapled. The groups not receiving estradiol replacement (Sham and Ovx groups) received a sham pellet surgery, which included identical procedures except the pocket was left empty.
For Study 2, subjects were 6 young (4 months old at test) and 21 aged (24 months old at test) Fischer-344 female rats born and raised at the aging colony of the National Institute on Aging at Harlan Laboratories (Indianapolis, IN). There were a total of four groups in Study 2: Young-Sham (n=6), Aged-Sham (n=6), Aged-Ovx (n=7), and Aged-Ovx-E (n=7). Just one type of estradiol pellet was chosen for this study (0.25 mg pellet) since the variability and mean levels were comparable between the 0.25 and 0.50 doses in Study 1. The timeframes and surgical procedures of Study 2 were identical to those of Study 1.
Assessment of serum hormone levels
For both studies, after behavioral testing rats were anesthetized with Halothane anesthesia and decapitated. Blood was collected from the trunk (Vacutainer 367986, Becton Dickinson and Company, Franklin Lakes, NJ), was allowed to clot at 4º C and serum was collected after centrifugation (3220xG, 20 min). Serum was stored at −20º C until estradiol assays were performed by the Core Endocrinology Laboratory at Pennsylvania State University College of Medicine using a kit from Diagnostic Products Corporation, Los Angeles, CA (Coat-A-Count estradiol kit, product number TKE21). Estradiol was determined in serum by a solid-phase radioimmunoassay based on estradiol-specific antibodies that are immobilized to the wall of polypropylene tubes and 125I -labeled estradiol as the tracer following extraction with diethyl ether. Serum (2.4. mls) were extracted and the ether portion collected and evaporated to dryness. The sample was reconstituted in assay buffer and a competitive radioimmunoassay was performed using 125I estradiol with high specific activity and a high affinity, highly specific antibody. Separation of bound from free was achieved with activated charcoal and the data reduction was performed with the use of a five point standard curve and purified estradiol standards. The functional sensitivity of the assay was 5 pg/ml. The interassay precision at a concentration of 35 pg/ml was 8%.
Spatial reference memory Morris maze testing
Rats in both studies received identical testing on the Morris maze, which consisted of a round water-filled tub with a hidden platform. The rat was placed in the maze from any of four locations (North, South, East, or West) and had 60s to locate the platform which remained in a fixed location throughout testing. After 15s on the platform, the rat was removed from the maze and placed into its heated cage until the next trial. Rats were given 3 trials a day for 5 days. After the last trial on Day 5, each rat was given a 60s probe trial whereby the platform was removed. A video camera suspended on the ceiling above the maze tracked the rat’s path and a tracking system (SMART system, San Diego Instruments) was used to analyze each rat’s tracing.
Dependent variables and statistical analyses
Analyses for Study 1
Performance was assessed by swim path distance (centimeters) to the platform. We also analyzed swim speed (distance/time) since it can be affected by age, cognitive demand and estradiol replacement; this measure may aid interpretation of our findings (Bimonte-Nelson et al., 2006; Foster et al., 2003; Markowska and Savonenko, 2002a and b). Studies 1 and 2 were analyzed separately, as were Distance and Speed measures. For Study 1, the estradiol groups (Ovx + 0.25 pellet and Ovx + 0.50 pellet) were combined into one group (Ovx-E; Table I). To analyze Study 1, we first performed an omnibus 3 × 5 × 3 mixed model ANOVA within each age with Treatment (Sham, Ovx, Ovx-E) as the between-subjects factor, and Days (1–5) and Trials (1–3) as repeated measures. Specific a priori, planned contrasts were run to further evaluate significant omnibus ANOVA main effects or interactions with Treatment. Unless otherwise specified, these analyses were run as described above but with one between-subjects factor (Treatment) comparing two groups (Sham vs. Ovx to determine Ovx effects, and Ovx vs. Ovx-E to ascertain estradiol treatment effects). Post hoc comparisons across ages were used selectively to aid interpretation of estradiol-related effects. To evaluate effects of age, we used the same mixed model ANOVA, except Age (young vs. middle-aged) was the between-subject variable and the comparison was limited to the Sham groups.
Table I.
Mean±SE, median and range of estradiol levels for each group in Study 1 and Study 2.
| Group | Mean estradiol level ±SE | Range of estradiol levels | Median estradiol level |
|---|---|---|---|
| STUDY 1 | |||
| Young Sham | 13.50 ± 4.11 | Undetectable-26 | 17.5 |
| Young Ovx | 3.78 ± 1.91 | Undetectable-13 | 0 |
| Young Ovx + 0.25 E | 65.88 ± 10.75 | 56–98 | 77 |
| Young Ovx + 0.50 E | 61.75 ± 12.30 | 22–114 | 49.5 |
| Middle-aged Sham | 21.88 ± 1.89 | 13–29 | 23 |
| Middle-aged Ovx | 5.11 ± 2.70 | Undetectable-21 | 0 |
| Middle-aged Ovx + 0.25 E | 54.80 ± 6.19 | 32–68 | 57 |
| Middle-aged Ovx + 0.50 E | 53.38 ± 13.24 | 15–121 | 44 |
| STUDY 2 | |||
| Young Sham | 16.83±4.56 | Undetectable-34 | 18.5 |
| Aged Sham | 19.20 ±2.06 | 12–24 | 20 |
| Aged Ovx | 11.00±2.15 | Undetectable-19 | 11 |
| Aged Ovx + 0.25 E | 112.50 ±20.79 | 55–180 | 104 |
To assess whether rats localized the platform to the spatial location and to confirm that animals were not utilizing a motoric strategy to solve the task, the platform was removed after all testing trials were completed and animals’ swim distance was quantified (day 5, trial 4 was the probe trial). The maze was virtually divided into four equal quadrants (Bimonte-Nelson et al., 2006). Percent of total distance in the platform quadrant (NE, or target) was compared to the quadrant diagonally opposite (SW). Rats that learned the platform location and were not using a motoric strategy were expected to spend the greatest percent of total distance in the quadrant that had contained the platform. Probe trial data were first analyzed using repeated measures ANOVA within each age with Treatment as the between-subject variable and maze Quadrant as the within variable (Bimonte-Nelson et al., 2006). Unless otherwise specified, follow-up two group comparisons and age effects were run as above, except Treatment or Age was the between variable. Estradiol levels were run similar to the above procedures; post-hoc evaluations were run using t-tests.
To evaluate potential relations between maze scores and estradiol levels, multiple regression analyses and Pearson correlations were run to analyze estradiol’s association with five performance variables: distance score on the final test trial (day 5, trial 3), distance score averaged across all five days, percent of total distance in the target (NE) quadrant on the probe trial, speed on the final test trial, and speed averaged across all five testing days. We also evaluated the correlation between speed and distance, and speed and percent distance swum for the target (NE) quadrant on the probe trial. Each of these was run as follows: average of days 1–5 distance was correlated with the average of days 1–5 speed, distance on the final test trial was correlated with speed on the final test trial, and the average of days 1–5 speed was correlated with percent distance in the target (NE) quadrant for the probe trial. Unless otherwise specified, multiple regression analysis and Pearson correlations were run within ovary-intact groups, within Ovx groups and within estradiol replacement groups collapsed across young and middle-aged animals. Next, Pearson r correlations within each young and each middle-aged group were run (thus, ages run separately), for these groups.
Analyses for Study 2
For Study 2, for distance scores, speed and probe trial data, an omnibus ANOVA was run within the three Aged groups (Aged-Sham, Aged-Ovx and Aged-Ovx-E) and repeated measures as described above. For estradiol levels, an omnibus ANOVA was run within the three Aged groups. Age effects and follow up two-group comparisons were run as for Study 1. Multiple regression analyses and correlations including estradiol were not run for Study 2 since two serum samples from the estradiol group were of low quality and could not be assayed for estradiol, limiting the sample size to five for the estradiol replacement group. For Study 2, we evaluated the correlation between speed and distance, and speed and percent distance swum for the target (NE) quadrant on the probe trial as described for Study 1 above. Correlations were run first with all subjects included, then within ovary-intact groups collapsed across young and aged groups, and then within all aged groups combined.
Results
Study 1: Effects of Ovx and estradiol replacement in young and middle-aged animals
Study 1: Serum estradiol levels
The top half of Table I shows the mean±SE, range and median serum levels of estradiol for each group of young and middle-aged rats. An omnibus one-way ANOVA (with the two dosage levels combined for one estradiol replacement group) for young animals revealed a Treatment main effect [F(2,29)=35.17; p < .0001], as seen for middle-aged animals also [F(2,27)=16.13; p < .0001]. As expected, Ovx animals exhibited lower estradiol levels than Sham animals for young [t(15)=2.23; p < .05] and middle-aged [t(15)=4.97; p < .0002] groups, and Ovx-E animals had higher levels than Ovx animals for young [t(22)=6.83; p < .0001] and middle-aged groups [t(20)=3.81; p < .001]. Of importance, there was great variability within the estradiol-treated rats, as anticipated and detailed in Table I. There were no mean differences in estradiol level between any of the estradiol-treated groups, within and across ages (ps > .36).
Study 1: Maze performance
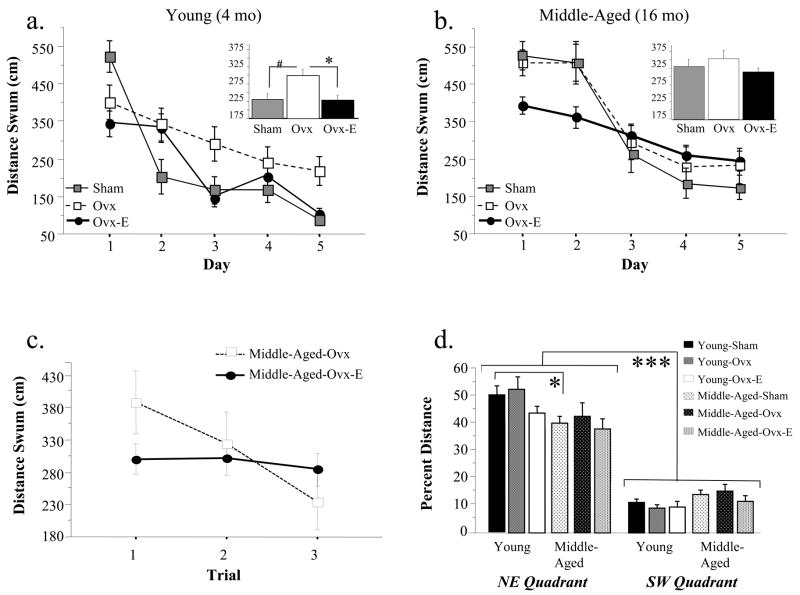
Figure 1 depicts the learning curves across days for each treatment group of (a) young 4 month olds and (b) middle-aged 16 month olds, and the inset graphs show the mean ± SE distance scores for each group collapsed across all testing days. The omnibus mixed model ANOVA in young animals revealed a main effect of Treatment [F(2,29)=3.91; p < .05], and a Day x Treatment interaction [F(8,116)=3.74l; p < .001]; there was no main effects of interaction with Trial. Distance scores of Young-Ovx rats were higher than Young-Sham rats, although this main effect only approached significance [F(1,15)=4.276; p = .06]. For this analysis, there was a significant Day x Ovx interaction [F(4,12)=3.137; p < .05], with Young-Ovx rats swimming a shorter distance to the platform on day 1, but a greater distance on days 2–5 as compared to Young-Sham rats (Figure 1a). A post-hoc test comparing these two groups on day 1 showed that Ovx did not influence performance on this first testing day. However, a post-hoc repeated measures ANOVA revealed that Young-Ovx animals swam a greater distance than Young-Sham animals for days 2–5 [F(1,15)=7.83; P < .05]. Young-Ovx-E animals showed lower distance scores than Young-Ovx animals across all testing days [Estradiol Treatment main effect: F(1,22)=6.39; p < .05; Figure 1a].
Figure 1.
For Study 1, mean± SE distance scores (cm) across days of testing on the Morris maze for (a) Young Sham, Ovx and Ovx-E groups and (b) Middle-Aged Sham, Ovx and Ovx-E groups; the inset graphs represent the data collapsed across days. For Young animals, Ovx impaired performance on the latter portion of testing, as represented by the Ovx x Day interaction. There was no effect of Ovx in middle-aged animals. Estradiol treatment enhanced performance in both age groups. Thus, there was a dissociation between sensitivity to Ovx and responsiveness to estradiol replacement. (c) Mean±SE distance scores for each trial for days 2–5 for Middle-Aged-Ovx and Middle-Aged-Ovx-E groups for Study 1. There was a significant Trial x Estradiol Treatment interaction, indicating that estradiol aided in remembering the platform location overnight in middle-aged rats. (d) Mean percent distance±SE spent in the target (NE) and opposite (SW) quadrant for each treatment group within each age for Study 1. Neither Ovx nor estradiol replacement influenced performance on the probe trial. All groups localized to the platform location, spending a greater percent distance in the target versus the opposite quadrant. * p < .05, # p = .06, *** p < .0001
For the omnibus ANOVA with middle-aged animals, there was a Day x Treatment interaction [F(8,120)=2.41; p < .05], and a marginal Trial x Treatment interaction [F(4,60)=2.34; p = .06], in the absence of a Treatment main effect. Distance scores of Middle-Aged-Sham and Middle-Aged-Ovx rats did not differ, nor did Trial or Days interact with Ovx. Middle-Aged-Ovx rats showed enhancements due to estradiol replacement, exhibiting faster learning of the platform location [Day x Estradiol Treatment interaction: F(4,92) = 2.66 ; p < .05; Figure 1b]; a post-hoc assessment confirmed that estradiol treatment enhanced performance on the initial two testing days in middle-aged animals [Estradiol Treatment main effect for days 1 and 2: F(1,23)=16.45; p < .001].
There was a Trial x Estradiol Treatment interaction for middle-aged animals only, with estradiol-treated rats exhibiting lower distance scores on trial 1 as compared to Ovx rats [F(2,46)=3.64; p < .05; Figure 1c]. Given previous findings that estradiol aids overnight retention of the platform location in middle-aged rats (Bimonte-Nelson et al., 2006; Markham et al., 2002) we further evaluated this effect by removing day 1 since there was no prior information about this maze to the animal on trial 1, day 1. Estradiol aided in remembering the platform location overnight in middle-aged rats, as shown by the Trial x Estradiol Treatment interaction for days 2–5 [F(2,46)=3.21; p < .05]. Estradiol did not influence overnight forgetting in young animals, as determined by the same analysis in young animals (Trial x Estradiol Treatment interaction, p > .86), nor did Ovx influence overnight forgetting in young or middle-aged animals (separate repeated measures ANOVAs within each age, Trial x Ovx interaction ps > .89).
There was a main effect of Age for Distance scores. Young-Sham rats exhibited better performance than Middle-Aged-Sham rats [F(1,14)=14.50; p < .005]. Post-hoc comparisons determined that Ovx in young rats impaired performance to the extent that the Young-Ovx group did not differ from the Middle-Aged-Ovx group nor the Middle-Aged-Sham group for overall performance across the five testing days. However, Middle-Aged-Ovx-E animals exhibited poorer overall performance than the Young-Sham rats [F(1,22)=6.48; p < .05], indicating that estrogen treatment did not reverse age-related performance deficiencies.
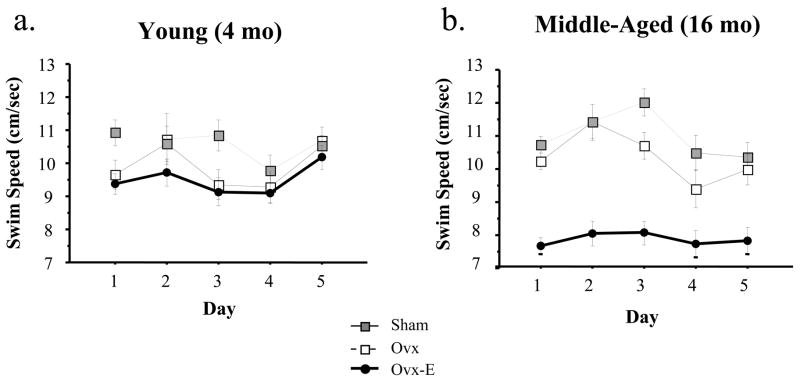
Figure 2 shows the mean swim speed±SE for each treatment group for a) young animals and b) middle-aged animals. While the omnibus ANOVA for speed for young animals was not significant, for middle-aged animals there was a main effect of Treatment [F(2,30)=18.88; p < .0001]. In middle-aged animals Ovx did not affect swim speed and estradiol replacement decreased swim speed [F(1,23)=16.56; p = .0005]. Age did not influence swim speed [F(1,14)=0.68; p = .42].
Figure 2.
Mean±SE swim speed for each treatment group for (a) young animals and (b) middle-aged animals for Study 1. While there was no effect of swim speed in young animals, middle-aged animals showed a main effect of Treatment. Follow-up analyses showed that Ovx did not influence swim speed in middle-aged animals, and estradiol replacement decreased swim speed.
Figure 1d depicts the mean percent distance±SE spent in the target and opposite quadrants for the probe trial for each treatment group within each age. All groups localized to the platform location. As revealed by the omnibus ANOVA, all young animals spent a greater percent distance in the target versus the opposite quadrant [Quadrant main effect: F(1,28)=156.16; p < .0001], regardless of treatment [Treatment x Quadrant interaction not significant]. Identical findings were shown for middle-aged animals [Quadrant main effect: F(1,29)=62.38; p < .0001; Treatment x Quadrant interaction not significant]. For the analysis of Age, there was a Quadrant main effect [F(1,14)=132.78; p < .0001] with a higher percent distance in the target quadrant, and a Treatment x Quadrant interaction [F(1,14)=4.89; p < .05]. A post-hoc t-test determined that Young-Shams spent a greater percent distance in the target quadrant than Middle-Aged-Shams [t(29)=7.90; p < .05].
Study 1: Multiple Regression and correlations
We conducted two primary regression analyses relating serum estradiol levels to performance measures in the young and middle-aged Ovx rats with estradiol replacement.
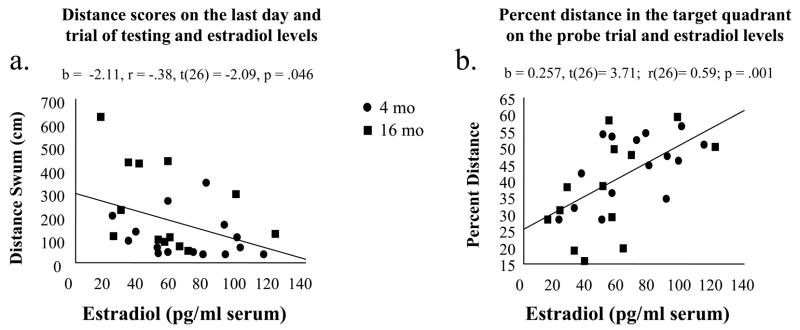
Serum estradiol level was related to maze performance on the last trial of testing [b = −2.11, r = −.38, t(26)= −2.09, p = .046; Figure 3a] indicating that for each pg/ml increase in serum estradiol level, there was a 2.09 cm decrease in swimming distance. We used several procedures described in Cook and Weisberg (1999) to probe for possible curvilinear effects; none approached statistical significance. The Cook and Weisberg (1983) test could not reject the null hypothesis of constant variance and the results of an alternative regression analysis that used the log of swimming distance as the dependent variable to stabilize the variance did not differ from the first [t(26) =−2.09, p = .046]. Finally, we found no difference in the slopes of the young and middle-aged Ovx rats. These results show that the relationship approximates linearity over the range of serum estradiol studied, which ranged from low physiological to supraphysiological (Butcher et al., 1975).
Figure 3.
For Study 1, scatterplots for serum estradiol levels and (a) the last trial on the last day of day of testing with the platform in the maze, and (b) percent distance in the previously platformed quadrant on the probe trial. Higher levels of estradiol replacement, as determined by serum estradiol levels, correlated with better performance (lower distance) on the last test trial as well as a higher percent distance in the previously platformed quadrant, representing superior platform localization.
Serum estradiol level was also related to better spatial localization of the platform location during the probe trial in the young and middle-aged Ovx rats. Higher serum estradiol levels were related to greater percent distance in the target quadrant [b = 0.257, t(26)= 3.71; r(26)= 0.59; p = .001; Figure 3b]. Once again, we detected no evidence of curvilinearity, nonconstant variance, or differences in the slopes of Young-Ovx-E and Middle-Aged-Ovx-E groups. There were no significant estradiol-percent distance relationships in the ovary-intact Young-Sham or Middle-Aged-Sham analyses when groups were analyzed together or separately.
Speed did not significantly correlate with estradiol levels for any assessment when young and middle-aged animals were analyzed together, nor when they were analyzed separately. Moreover, speed did not significantly correlate with distance scores during testing, nor with percent distance in the target quadrant on the probe trial, for Sham, Ovx or Ovx-E animals. This was true when young and middle-aged animals were analyzed together, as well as separately.
Study 2: Effects of ovariectomy and estradiol replacement in aged animals
Study 2 was performed to assess whether Ovx had an impact on performance in aged 24 month old animals, and whether this estradiol treatment regimen was still effective in aged animals. Young 4 month old ovary-intact sham animals were also evaluated to confirm an age-related performance deterioration, and as a relative performance assessment for the estradiol-treated group.
Study 2: Serum estradiol levels
The bottom half of Table I shows the mean±SE, range and median serum levels of estradiol for young and each group of aged rats in Study 2. The omnibus ANOVA with all aged groups revealed a main effect of Treatment [F(2,15) = 21.91; p < .0001]. Aged-Ovx animals exhibited lower estradiol levels than Aged-Sham rats [t(10)=2.65; p < .05], and Aged-Ovx-E rats had higher levels than Aged-Ovx rats [t(11)=5.28; p < .0005]. Age did not influence estradiol levels (p = .67).
Study 2: Maze performance
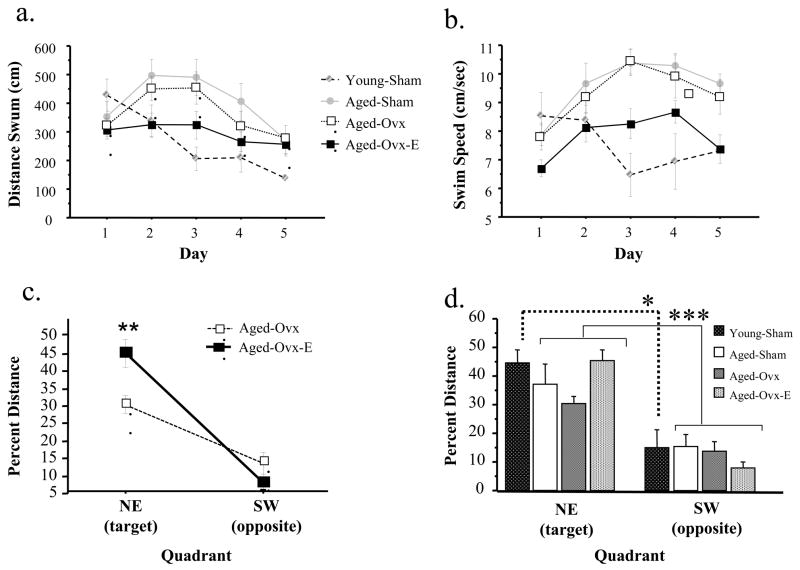
Figure 4a depicts the learning curves across days for each treatment group of aged of 21 month olds. Once again, an omnibus mixed model ANOVA including all three aged groups revealed no main effect or Day or Trial interactions with Treatment for distance scores. It was noted, however, that overall performance collapsed across all five testing days of the Aged-Ovx-E group appeared better than the Aged-Sham and Aged-Ovx groups (Figure 4a). As a post-hoc assessment, repeated measures ANOVAs were used to test two-group comparisons. These analyses confirmed that the Aged-Ovx-E group did not significantly differ from the Aged-Ovx nor the Aged-Sham group across the five testing days; this was true for both main effects and interactions with Treatment (all ps > .12).
Figure 4.
(a) Mean±SE distance scores (cm) across day of testing on the Morris maze for Aged-Sham, Aged-Ovx, Aged-Ovx-E and Young groups for Study 2. There were no significant main effects or interactions for distance scores due to Ovx or estradiol replacement in aged animals. (b) mean±SE swim speed for each aged group for each test day. The omnibus ANOVA revealed that Treatment affected swim speed as days progressed, with a significant Day x Treatment interaction. As observed in middle-aged animals, Ovx did not influence swim speed and estradiol replacement decreased swim speed. (c) Mean±SE percent distance in the target (NE) and opposite (SW) quadrants on the probe trial for Aged-Ovx-E and Aged-Ovx groups for Study 2. There was a Quadrant x Treatment interaction, and a post-hoc t-test showed that aged Ovx animals receiving estradiol spent a higher percent distance in the target quadrant as compared to aged Ovx animals that did not, suggesting better platform acquisition in aged Ovx animals given estradiol treatment. (d) Mean±SE percent distance in the target (NE) and opposite (SW) quadrants on the probe trial for Aged-Ovx and Aged-Ovx-E groups for Study 2. There was a Quadrant x Treatment interaction, and a post-hoc t-test showed that aged Ovx animals receiving estradiol spent a higher percent distance in the target quadrant as compared to aged Ovx animals that did not, suggesting better platform acquisition in aged Ovx animals given estradiol treatment. ** p < .01 * p < .05, *** p < .0001
Age influenced performance; Young-Sham rats exhibited lower distance scores collapsed across all days as compared to Aged-Sham rats [Age main effect: F(1,10)=4.96; p = .05]. There was also a Day x Age interaction [F(4,40)=3.75; p < .05], with distance scores comparable on day 1 but markedly lower for days 2 through 5 for the Young-Sham group (Figure 4a).
Figure 4b shows the mean swim speed±SE for each aged group for each test day. The omnibus ANOVA for all aged groups revealed that treatment affected swim speed as days progressed [Day x Treatment interaction: F(4,12)=2.38; p < .05]. There was no Ovx effect on swim speed. Estradiol replacement decreased swim speed [Treatment main effect: F(1,13)=6.56; p < .05]. Age influenced swim speed as days progressed, with Young-Sham animals swimming slower than Aged-Sham animals toward the end of testing [Day x Age interaction: F(4,40)=4.16; p < .05; Figure 4b]. A post-hoc repeated measures ANOVA (Day and Trial as repeated measures) for each sham group alone revealed that Young-Sham animals did not change swim speed across days (p = .31), while Aged-Sham animals increased speed in the initial portion of testing [F(4,20)= −6.50; p < .05; Figure 4b].
All aged rats localized to the target location. For the ANOVA with all aged groups included, there was a main effect of Quadrant, with rats spending a greater percent distance in the target versus the opposite quadrant suggesting platform localization [F(1,17)=39.62; p < .0001; Figure 4d]. The null Group x Quadrant interaction indicated that all groups showed this pattern of spending a greater percent distance in the target versus opposite quadrant. To further detail potential estradiol replacement effects in aged animals, we performed a post-hoc analysis comparing the Aged-Ovx-E and Aged-Ovx groups on the probe trial. The repeated measures ANOVA [with Estradiol Treatment as the between variable and Quadrant as the repeated measures] revealed a Treatment x Quadrant interaction [F(1,12)= 59.61; p < .05; Figure 4c]. A post-hoc t-test showed that aged Ovx animals receiving estradiol spent a higher percent distance in the target quadrant as compared to aged Ovx animals that did not [t(12)= 3.11; p < .01]. Young rats localized to the platform location, as shown on the probe trial with a greater percent distance spent in the target quadrant [main effect of Quadrant: F(1,5)=7.50; p <.05; Figure 4d].
Study 2: Correlations
Speed did not significantly correlate with distance scores during testing, nor percent distance in the target quadrant on the probe trial, for any evaluation.
Discussion
The current findings show for the first time that higher serum levels of estradiol replacement correlate with better maze performance in young and middle-aged Ovx animals. We found that higher exogenous estradiol replacement levels were related to better spatial reference memory performance after surgical ovarian hormone loss, while endogenous estradiol levels in ovary-intact animals did not relate to spatial reference memory performance in either age (Study 1). This report replicates our previous findings for spatial reference memory in middle-aged female rats, whereby Ovx does not affect performance, yet estradiol replacement enhances performance after Ovx (Bimonte-Nelson et al., 2006). We now extend these findings, demonstrating that in contrast to a null Ovx effect in middle-aged females, in young females Ovx impacts spatial reference memory performance. Young animals were responsive to both ovarian hormone removal and replacement, and middle-aged animals were not responsive to ovarian hormone removal but were responsive to estrogen replacement. Furthermore, at the current timing of ovarian hormone loss and regimen of estradiol replacement, aged animals were not responsive to ovarian hormone removal or replacement for the test trials on the spatial reference memory Morris maze (Study 2).
While Ovx did not influence spatial reference memory performance in middle-aged animals, in young animals Ovx compromised performance relative to young ovary-intact sham animals on days 2–5, the latter portion of testing. Ovx in young animals impaired performance to the point that this group did not differ from middle-aged Ovx or Sham animals. This negative effect of Ovx corresponds with findings of others that Ovx in young rats impairs Morris maze performance, specifically, at the end of a five-day testing period (Feng et al., 2004). Whether Ovx animals would have eventually attained performance comparable to that of young ovary-intact sham animals had testing continued remains to be determined. It is noteworthy that by the last trial on the last day of testing, however, Young-Ovx animals did learn the platform location. This is revealed by the probe trial data showing that all groups spent a greater percent distance in the platform versus the opposite quadrant. These findings, combined with the lack of a Quadrant x Treatment interaction, suggests that regardless of ovarian hormone status all young and middle-aged groups learned the platform location by the end of testing.
Our observation that estradiol enhances spatial reference memory on the Morris maze in young and middle-aged female rats is consistent with other studies using young to middle-aged rodents tested on the Morris maze (Markham et al., 2002; Feng et al, 2004; El-Bakri et al., 2004). It is noteworthy that we found estradiol-induced enhancements on the first two days of testing in middle-aged animals, indicating faster platform acquisition, yet the middle-aged Ovx group given estradiol still performed worse than the young sham group. These findings indicate that while estradiol does enhance learning of spatial reference memory on the Morris maze, it does not completely reverse age-related performance deficiencies.
In contrast to the current findings that estradiol did not improve spatial learning in aged Ovx rats, some studies have shown that aged female rodents exhibit cognitive enhancements in response to estradiol treatment. For example, estradiol injections enhanced spatial reference memory in 27–28 month old ovary-intact mice (Frick et al., 2002). The difference in results may relate to the type of estradiol administration as cyclic versus tonic; cyclic administration via daily injection was used in the Frick study (Frick et al., 2002) described above, while tonic administration was used in the current studies. Providing further support for an influence of administration method on cognitive effectiveness in aged rodents, priming with cyclic estradiol enhanced responsiveness to tonic estradiol in older Ovx rats (Markowska and Savonenko, 2002a). Whether estradiol replacement improves performance of aged animals may also relate to memory type. Working memory enhancements due to tonic estradiol treatment have been reported in aged Ovx rats (Gibbs, 2000; Luine and Rodriguez, 1994). We and others have shown estradiol-induced working memory improvements in young Ovx rats as well, an effect reported to depend on administered estradiol dose, although dose-dependent differences in circulating estradiol levels were not confirmed (Bimonte and Denenberg, 1999; Daniel et al., 1997; Holmes et al., 2002; Sandstrom and Williams, 2001). Accordingly, in young rats high physiological estradiol levels have been shown to enhance learning a place strategy and impair learning a response strategy on a land plus maze (Korol and Kolo, 2002). Further, while Foster et al. (2003) found that tonic estradiol treatment had no influence on acquisition of the Morris maze in 6, 12 or 17 month old Ovx rats, physiological-dose estradiol treatment influenced 24 hour retention in the 12 month old rats only, and the supraphysiological dose influenced retention in the 17 month old rats only. Thus, a higher supraphysiological estradiol dose may be necessary to enhance spatial reference memory retention in rats approaching old age.
Age impacted whether swim speed changed across days, an effect seen in Study 2 comparing Young-Sham and Aged-Sham animals (Figure 4b). Young-Sham animals showed no change in swim speed across days, and Aged-Sham animals increased swim speed across the initial testing sessions and stabilized by the end of testing. This increase in swim speed after day 2 in aged animals may be influenced by maze acclimation after an initial stress response due to the swimming component of the task, an effect which may not be seen in younger animals (Kramer and Bodnar, 1986). Swim speed in both middle-aged and aged animals decreased with estradiol treatment (Studies 1 and 2), an effect observed previously in our and other laboratories (Bimonte-Nelson et al., 2006; Foster et al., 2003; Markowska and Savonenko, 2002a). Middle-aged and aged animals may be particularly sensitive to the motor effects of estradiol treatment. Indeed, estradiol treatment did not influence swim speed in young animals in our study. Interestingly, swim speed did not correlate with distance to the escape platform nor probe trial performance, indicating that swim speed does not relate to a more- or less- direct search path to the platform. This was true when correlations were run within and across ages, and within and across treatment groups for Studies 1 and 2. Distance to the platform and probe trial measures have been asserted to be most reflective of task acquisition (e.g. Foster et al., 2003). The currently reported lack of correlation between distance and probe trial measures with speed, as well as other research showing that decreased motor function with age does not correlate with Morris maze performance (Shukitt-Hale et al., 1998), support the tenet that there is a disconnect between cognitive status and sensory/motor performance variables such as latency and speed on this test. Yet, it is also tempting to speculate that a slower swim speed may be reflective of solving strategy wherein the animal is encoding or attending to spatial stimuli, with this process not necessarily related to final performance scores as operationally defined by distance swum. Further evaluations are necessary to decipher the complex age and estradiol interactive effects on swim speed, as well as whether swim speed relates to encoding or utilization of spatial stimuli.
It is noteworthy that the range of estradiol levels using the pellets from Innovative Research of America was quite large. This has been discussed in prior work from other laboratories, and we have noted this in prior studies within our laboratory as well (unpublished observations; Diel et al., 2005). While for the current experiments we capitalized on this variability to address our question of relationships between estradiol replacement levels and memory scores within individuals, the lack of difference in the mean serum estradiol level for the 0.25 mg and 0.50 mg pellets is disconcerting, as is the similarity in range and median values. Importantly, sacrifice and blood collection for estradiol assays occurred within the 60 day timeframe of pellet release. Collectively, these findings suggest that serum estradiol levels should be determined after using these pellets, and potentially other forms of estradiol treatment as well, to ascertain an accurate estimate of estradiol replacement and allow appropriate interpretation of findings.
Performance on the hidden platform version of the Morris maze, as used in the herein report, is known to be influenced by integrity of the hippocampus (e.g. Morris et al., 1982; Schenk and Morris, 1985). The estradiol-induced spatial memory enhancement seen in the current and other reports coincides with evidence that estradiol influences the physiology, connectivity, and function of the hippocampus, including increases in dendritic spine density and reductions in GABA neurotransmission (Bimonte-Nelson et al., 2006; Gould et al., 1990; Murphy et al., 1998; Woolley and McEwen, 1992). While it is not currently known why variables such as specific estradiol regimen and age may influence efficacy of estradiol treatment for cognition, data from neurobiological experiments yield insight and suggest that such interactions are biologically plausible. It has been well-documented that age-related changes occur in neural systems known to influence learning and memory, and many of these neural systems are also influenced by estradiol. For example, short-term estradiol treatment via injection enhanced place-learning-induced hippocampal acetylcholine release (Marriott and Korol, 2003). There are interactions between the cholinergic system, estrogen treatment and age; susceptibility to cholinergic challenge differed in middle-aged versus aged rodents given estradiol, with middle-aged, but not aged, animals showing protection due to estradiol treatment (Markowska and Savonenko, 2002a; Savonenko and Markowska, 2003). In addition, there are age-related alterations in the number and activity of estrogen receptors which could also influence responsivity as aging ensues (Chakraborty and Gore, 2004). Newer data suggest an estrogen-receptor dependent mechanism of estradiol-induced benefits on spatial memory, as the benefits of systemic estradiol treatment on place learning were reversed with hippocampal estrogen-receptor antagonism using ICI 182,780 (Zurkovsky et al., 2006). Thus, changes in estrogen receptors may relate to age-related responsiveness to estrogen for spatial memory.
In summary, the current report indicates that for spatial reference memory, the effects of surgical ovarian hormone loss and estradiol replacement change with age, at least for the temporal and dose parameters utilized in our studies. The data indicate that young and middle-aged animals that have undergone Ovx benefit from estradiol treatment, and that higher exogenous estradiol levels relate to better spatial reference memory in both ages. Indeed, higher levels of estradiol replacement correlated with better maze performance during testing and enhanced platform localization as demonstrated during the probe trial. No relationships between endogenous estradiol level and maze scores were seen in young and middle-aged sham animals that were ovary-intact. By old age, the estradiol replacement regimen beneficial at earlier ages was no longer effective during testing, and had only minor benefits for platform localization as assessed on the probe trial. Our findings demonstrate a distinction between sensitivity to surgical ovarian hormone loss and responsiveness to estradiol replacement after surgical ovarian hormone loss.
Acknowledgments
This research was funded by grants awarded to HAB-N [National Institute on Aging (AG023925-01), Institute for Mental Health Research, Alzheimer’s Disease Core Center Pilot Program, the state of Arizona and a Specialized Center of Research Pilot Research Grant co-funded by the Office of Research on Women’s Health and NIDA (P50 DA16511)].
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
This PDF receipt will only be used as the basis for generating PubMed Central (PMC) documents. PMC documents will be made available for review after conversion (approx. 2-3 weeks time). Any corrections that need to be made will be done at that time. No materials will be released to PMC without the approval of an author. Only the PMC documents will appear on PubMed Central -- this PDF Receipt will not appear on PubMed Central.
References
- Armstrong DM, Sheffield R, Buzsaki G, Chen KS, Hersh LB, Nearing B, Gage FH. Morphologic alterations of choline acetyltransferase-positive neurons in the basal forebrain of aged behaviorally characterized Fischer 344 rats. Neurobiology of Aging. 1993;14:457–470. doi: 10.1016/0197-4580(93)90104-j. [DOI] [PubMed] [Google Scholar]
- Asthana S, Craft S, Baker LD, Raskind MA, Birnbaum RS, Lofgreen CP, Veith RC. Cognitive and neuroendocrine response to transdermal estrogen in postmenopausal women with Alzheimer’s disease: results of a placebo-controlled, double-blind, pilot study. Psychoneuroendocrinology. 1999;24(6):657–77. doi: 10.1016/s0306-4530(99)00020-7. [DOI] [PubMed] [Google Scholar]
- Backman C, Rose GM, Hoffer BJ, Henry MA, Bartus RT, Friden P, Granholm A-Ch. Systemic administration of nerve growth factor conjugate reverses age-related cognitive dysfuction and prevents cholinergic neuron atrophy. Journal of Neuroscience. 1996;16(17):5437–5442. doi: 10.1523/JNEUROSCI.16-17-05437.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barrett-Connor E, Goodman-Gruen D. Cognitive function and endogenous sex hormones in older women. Journal of the American Geriatrics. 1999;47(11):1289–93. doi: 10.1111/j.1532-5415.1999.tb07427.x. [DOI] [PubMed] [Google Scholar]
- Becker WJ. Use of oral contraceptives in patients with migraine. Neurology. 1999;53(4 Suppl 1):S19–25. [PubMed] [Google Scholar]
- Bimonte HA, Denenberg VH. Estradiol facilitates performance as working memory load increases. Psychoneuroendocrinology. 1999;24:161–173. doi: 10.1016/s0306-4530(98)00068-7. [DOI] [PubMed] [Google Scholar]
- Bimonte-Nelson HA, Francis KR, Umphlet CD, Granholm A–C. Progesterone reverses the spatial memory enhancements of tonic and cyclic estrogen therapy in middle-aged female rats. European Journal of Neuroscience. 2006;24:229–242. doi: 10.1111/j.1460-9568.2006.04867.x. [DOI] [PubMed] [Google Scholar]
- Bimonte H, Hunter C, Nelson M, Granholm AC. Frontal cortex BDNF levels correlate with working memory in an animal model of Down Syndrome. Behavior and Brain Research. 2002;139(1–2):47–57. doi: 10.1016/s0166-4328(02)00082-7. [DOI] [PubMed] [Google Scholar]
- Bimonte-Nelson HA, Singleton RS, Williams BJ, Granholm AC. Ovarian hormones and cognition in the aged female rat: II. Progesterone supplementation reverses the cognitive enhancing effects of ovariectomy. Behavioral Neuroscience. 2004a;118:707–714. doi: 10.1037/0735-7044.118.4.707. [DOI] [PubMed] [Google Scholar]
- Bimonte-Nelson HA, Nelson ME, Granholm AC. Progesterone counteracts estrogen-induced increases in neurotrophins in the aged female rat brain. Neuroreport. 2004b;15:2659–2663. doi: 10.1097/00001756-200412030-00021. [DOI] [PubMed] [Google Scholar]
- Butcher RL, Collins WE, Fugo NW. Plasma concentration of LH, FSH, prolactin, progesterone and estradiol-17beta throughout the 4-day estrous cycle of the rat. Endocrinology. 1974;94(6):1704–1708. doi: 10.1210/endo-94-6-1704. [DOI] [PubMed] [Google Scholar]
- Caldwell B, Watson R. An evaluation of psychologic effects of sex hormone administration in aged women. I. Results of therapy after six months. Journal of Gerontology. 1952;7(2):228–44. doi: 10.1093/geronj/7.2.228. [DOI] [PubMed] [Google Scholar]
- Chakraborty TR, Gore AC. Aging-related changes in ovarian hormones, their receptors, and neuroendocrine function. Experimental Biology and Medicine. 2004;229(10):977–87. doi: 10.1177/153537020422901001. [DOI] [PubMed] [Google Scholar]
- Cook RD, Weisberg S. Diagnostics for heteroscedasticity in regression. Biometrika. 1983;70:1–10. [Google Scholar]
- Cook RD, Weisberg S. Applied regression including computing and graphics. New York, NY: Wiley; 1999. [Google Scholar]
- Craig MC, Maki PM, Murphy DG. The Women’s Health Initiative Memory Study: findings and implications for treatment. Lancet neurology. 2005;4(3):190–4. doi: 10.1016/S1474-4422(05)01016-1. [DOI] [PubMed] [Google Scholar]
- Croll SD, Ip NY, Lindsay RM, Wiegand SJ. Expression of BDNF and trkB as a function of age and cognitive performance. Brain Research. 1998;812:200–208. doi: 10.1016/s0006-8993(98)00993-7. [DOI] [PubMed] [Google Scholar]
- Daniel J, Fader A, Spencer A, Dohanich G. Estrogen enhances performance of female rats during acquisition of a radial arm maze. Hormones and Behavior. 1997;32:217–225. doi: 10.1006/hbeh.1997.1433. [DOI] [PubMed] [Google Scholar]
- Daniel JM, Roberts SL, Dohanich GP. Effects of ovarian hormones and environment on radial maze and water maze performance of female rats. Physiology and Behavior. 1999;66(1):11–20. doi: 10.1016/s0031-9384(98)00272-8. [DOI] [PubMed] [Google Scholar]
- Diana G, Domenici MR, Scotti de Caroli A, Loizzo A, Sagratella S. Reduced hippocampal CA1 Ca2+-induced long-term potentiation is associated with age-dependent impairment of spatial learning. Brain Research. 1995;686:107–110. doi: 10.1016/0006-8993(95)00440-2. [DOI] [PubMed] [Google Scholar]
- Díaz-Véliz G, Benavides MS, Butrón S, Dussaubat N, Mora S. Behavioral effects of dopamine agonists and antagonists: influence of estrous cycle, ovariectomy, and estrogen replacement in rats 1. Pharmacology, Biochemistry and Behavior. 1999;62(1):21–29. doi: 10.1016/s0091-3057(98)00097-5. [DOI] [PubMed] [Google Scholar]
- Diel P, Laudenbach-Leschowsky U, Friedel A, Voss A, Roussel J. Pulsed estradiol exposure has a limited ability to induce uterine proliferation in ovariectomised female Wistar rats. Molecular and Cell Endocrinology. 2005;230(1–2):7–15. doi: 10.1016/j.mce.2004.11.007. [DOI] [PubMed] [Google Scholar]
- Dohanich G. Gonadal steroids, learning and memory. In: Pfaff DW, Arnold AP, Etgen AM, Farbach SE, Rubin RT, editors. Hormones, brain and behavior. Vol. 1. San Diego, CA: Academic Press; 2002. pp. 265–327. [Google Scholar]
- Dohanich GP, Fader AJ, Javorsky DJ. Estrogen and estrogen-progesterone treatments counteract the effect of scopolamine on reinforced T-maze alternation in female rats. Behavioral Neuroscience. 1994;108(5):988–92. doi: 10.1037//0735-7044.108.5.988. [DOI] [PubMed] [Google Scholar]
- El-Bakri NK, Islam A, Zhu S, Elhassan A, Mohammed A, Winblad B, Adem A. Effects of estrogen and progesterone treatment on rat hippocampal NMDA receptors: relationship to Morris water maze performance. Journal of Cell and Molecular Medicine. 2004;8(4):537–44. doi: 10.1111/j.1582-4934.2004.tb00478.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Estrada-Camarena E, Fernández-Guasti A, López-Rubalcava C. Antidepressant-like effect of different estrogenic compounds in the forced swimming test. Neuropsychopharmacology. 2003;28(5):830–8. doi: 10.1038/sj.npp.1300097. [DOI] [PubMed] [Google Scholar]
- Feng Z, Cheng Y, Zhang JT. Long-term effects of melatonin or 17 beta-estradiol on improving spatial memory performance in cognitively impaired, ovariectomized adult rats. Journal of Pineal Research. 2004;37(3):198–206. doi: 10.1111/j.1600-079X.2004.00158.x. [DOI] [PubMed] [Google Scholar]
- Fischer W, Bjorklund A, Chen K, Gage FH. NGF improves spatial memory in aged rodents as a function of age. Journal of Neuroscience. 1991;11(7):1889–1906. doi: 10.1523/JNEUROSCI.11-07-01889.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foster TC, Sharrow KM, Kumar A, Masse J. Interaction of age and chronic estradiol replacement on memory and markers of brain aging. Neurobiology Aging. 2003;24(6):839–52. doi: 10.1016/s0197-4580(03)00014-9. [DOI] [PubMed] [Google Scholar]
- Frick KM, Fernandez SM, Bulinski SC. Estrogen replacement improves spatial reference memory and increases hippocampal synaptophysin in aged female mice. Neuroscience. 2002;115:547–58. doi: 10.1016/s0306-4522(02)00377-9. [DOI] [PubMed] [Google Scholar]
- Galea LA, Wide JK, Paine TA, Holmes MM, Ormerod BK, Floresco SB. High levels of estradiol disrupt conditioned place preference learning, stimulus response learning and reference memory but have limited effects on working memory. Behavioral Brain Research. 2001;126(1–2):115–26. doi: 10.1016/s0166-4328(01)00255-8. [DOI] [PubMed] [Google Scholar]
- Gallagher M, Burwell R, Burchinal M. Severity of spatial learning impairment in aging: development of a learning index for performance in the Morris water maze. Behavioral Neuroscience. 1993;107(4):618–626. doi: 10.1037//0735-7044.107.4.618. [DOI] [PubMed] [Google Scholar]
- Gibbs R. Long-term treatment with estrogen and progesterone enhances acquisition of a spatial memory task by ovariectomized aged rats. Neurobiology of Aging. 2000;21:107–116. doi: 10.1016/s0197-4580(00)00103-2. [DOI] [PubMed] [Google Scholar]
- Gould E, Woolley C, Frankfurt M, McEwen B. Gonadal steroids regulate dendritic spine density in hippocampal pyramidal cells in adulthood. Journal of Neuroscience. 1990;10:1286–1291. doi: 10.1523/JNEUROSCI.10-04-01286.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gresack JE, Frick KM. Environmental enrichment reduces the mnemonic and neural benefits of estrogen. Neuroscience. 2004;128(3):459–71. doi: 10.1016/j.neuroscience.2004.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hasenohrl RU, Soderstrom S, Mohammed AH, Eberdal T, Huston JP. Reciprocal changes in expression of mRNA for nerve growth factor and its receptors TrkA and LNGFR in brain of aged rats in relation to maze learning deficits. Experimental Brain Research. 1997;114:205–213. doi: 10.1007/pl00005629. [DOI] [PubMed] [Google Scholar]
- Holmes M, Wide J, Galea L. Low levels of estradiol facilitate, whereas high levels of estradiol impair, working memory performance on the radial arm maze. Behavioral Neuroscience. 2002;116:928–934. doi: 10.1037//0735-7044.116.5.928. [DOI] [PubMed] [Google Scholar]
- Johansson IM, Birzniece V, Lindblad C, Olsson T, Backstrom T. Allopregnanolone inhibits learning in the Morris water maze. Brain Research. 2002;934:125–31. doi: 10.1016/s0006-8993(02)02414-9. [DOI] [PubMed] [Google Scholar]
- Korol DL, Kolo LL. Estrogen-induced changes in place and response learning in young adult female rats. Behavioral Neuroscience. 2002;116:411–420. doi: 10.1037//0735-7044.116.3.411. [DOI] [PubMed] [Google Scholar]
- Kramer E, Bodnar RJ. Age-related decrements in the analgesic response to cold-water swims. Physiology and Behavior. 1986;36:875–80. doi: 10.1016/0031-9384(86)90446-4. [DOI] [PubMed] [Google Scholar]
- Lindner MD, Kearns CE, Winn SR, Frydel B, Emerich DF. Effects in intraventricular encapsulated hNGF-secreting fibroblasts in aged rats. Cell Transplantation. 1996;5(2):205–223. doi: 10.1177/096368979600500210. [DOI] [PubMed] [Google Scholar]
- Luine VN, Jacome LF, Maclusky NJ. Rapid enhancement of visual and place memory by estrogens in rats. Endocrinology. 2003;144(7):2836–44. doi: 10.1210/en.2003-0004. [DOI] [PubMed] [Google Scholar]
- Luine VN, Richards ST, Wu VY, Beck KD. Estradiol enhances learning and memory in a spatial memory task and effects levels of monoaminergic neurotransmitters. Hormones and Behavior. 1998;34(2):149–62. doi: 10.1006/hbeh.1998.1473. [DOI] [PubMed] [Google Scholar]
- Luine V, Rodriguez M. Effects of estradiol on radial arm maze performance of young and aged rats. Behavioral and Neural Biology. 1994;62:230–236. doi: 10.1016/s0163-1047(05)80021-4. [DOI] [PubMed] [Google Scholar]
- Marriott LK, Korol DL. Short-term estrogen treatment in ovariectomized rats augments hippocampal acetylcholine release during place learning. Neurobiology of Learning Memory. 2003;80:315–22. doi: 10.1016/j.nlm.2003.08.003. [DOI] [PubMed] [Google Scholar]
- Markham JA, Pych JC, Juraska JM. Ovarian hormone replacement to aged ovariectomized female rats benefits acquisition of the Morris water maze. Hormones and Behavior. 2002;42:284–293. doi: 10.1006/hbeh.2002.1819. [DOI] [PubMed] [Google Scholar]
- Markowska AL, Savonenko AV. Effectiveness of estrogen replacement in restoration of cognitive function after long-term estrogen withdrawal in aging rats. Journal of Neuroscience. 2002a;22:10985–95. doi: 10.1523/JNEUROSCI.22-24-10985.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Markowska AL, Savonenko AV. Protective effect of practice on cognition during aging: implications for predictive characteristics of performance and efficacy of practice. Neurobiology of Learning and Memory. 2002b;78(2):294–320. doi: 10.1006/nlme.2002.4064. [DOI] [PubMed] [Google Scholar]
- Morris RG, Garrud P, Rawlins JN, O’Keefe J. Place navigation impaired in rats with hippocampal lesions. Nature. 1982;297:681–3. doi: 10.1038/297681a0. [DOI] [PubMed] [Google Scholar]
- Mulnard R, Cotman C, Kawas C, van Dyck C, Sana M, Doody R, Koss E, Pfeiffer E, Jin S, Gamst A, Grundman M, Thomas R, Thal L for the Alzheimer’s Cooperative Study. Estrogen replacement therapy for treatment of mild to moderate Alzheimer’s disease. Journal of the American Medical Association. 2000;283:1007–1015. doi: 10.1001/jama.283.8.1007. [DOI] [PubMed] [Google Scholar]
- Murphy DD, Cole NB, Greenberger V, Segal M. Estradiol increases dendritic spine density by reducing GABA neurotransmission in hippocampal neurons. Journal of Neuroscience. 1998;18(7):2550–9. doi: 10.1523/JNEUROSCI.18-07-02550.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nilsen J, Brinton R. Impact of progestins and estrogen-induced neuroprotection: synergy by progesterone and 19-norprogesterone and antagonism by medroxyprogesterone acetate. Endocrinology. 2002a;143:205–212. doi: 10.1210/endo.143.1.8582. [DOI] [PubMed] [Google Scholar]
- Nilsen J, Brinton R. Impact of progestins on estradiol potentiation of the glutamate calcium response. Neuroreport. 2002b;13(6):825–830. doi: 10.1097/00001756-200205070-00018. [DOI] [PubMed] [Google Scholar]
- Nilsson OG, Gage FH. Anticholinergic sensitivity in the aging rat septohippocampal system as assessed in a spatial memory task. Neurobiology of Aging. 1993;14:487–497. doi: 10.1016/0197-4580(93)90107-m. [DOI] [PubMed] [Google Scholar]
- Packard MG, Teather LA. Posttraining estradiol injections enhance memory in ovariectomized rats: cholinergic blockade and synergism. Neurobiology of Learning and Memory. 1997;68(2):172–88. doi: 10.1006/nlme.1997.3785. [DOI] [PubMed] [Google Scholar]
- Phillips SM, Sherwin BB. Variations in memory function and sex steroid hormones across the menstrual cycle. Psychoneuroendocrinology. 1992;17(5):497–506. doi: 10.1016/0306-4530(92)90008-u. [DOI] [PubMed] [Google Scholar]
- Quirion R, Wilson A, Rowe W, Aubert I, Richard J, Doods H, Parent A, White N, Meaney M. Facilitation of acetylcholine release and cognitive performance by an M(2)-muscarinic receptor antagonist in aged memory-impaired rats. Journal of Neuroscience. 1995;15:1455–1462. doi: 10.1523/JNEUROSCI.15-02-01455.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rapp PR, Gallagher M. Preserved neuron number in the hippocampus of aged rats with spatial learning deficits. Neurobiology. 1996;93:9926–9930. doi: 10.1073/pnas.93.18.9926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rapp PR, Morrison JH, Roberts JA. Cyclic estrogen replacement improves cognitive function in aged ovariectomized rhesus monkeys. Journal of Neuroscience. 2003;23(13):5708–14. doi: 10.1523/JNEUROSCI.23-13-05708.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rissanen A, Puolivali J, van Groen T, Riekkinen P., Jr In mice tonic estrogen replacement therapy improves non-spatial and spatial memory in a water maze task. Neuroreport. 1999;10(6):1369–72. doi: 10.1097/00001756-199904260-00039. [DOI] [PubMed] [Google Scholar]
- Sandstrom NJ, Williams CL. Memory retention is modulated by acute estradiol and progesterone replacement. Behavioral Neuroscience. 2001;115(2):384–93. [PubMed] [Google Scholar]
- Savonenko AV, Markowska AL. The cognitive effects of ovariectomy and estrogen replacement are modulated by aging. Neuroscience. 2003;119(3):821–30. doi: 10.1016/s0306-4522(03)00213-6. [DOI] [PubMed] [Google Scholar]
- Schenk F, Morris RG. Dissociation between components of spatial memory in rats after recovery from the effects of retrohippocampal lesions. Experimental Brain Research. 1985;58:11–28. doi: 10.1007/BF00238949. [DOI] [PubMed] [Google Scholar]
- Sherwin BB. Estrogen and cognitive aging in women. Neuroscience. 2006;138(3):1021–1026. doi: 10.1016/j.neuroscience.2005.07.051. [DOI] [PubMed] [Google Scholar]
- Singh M, Meyer EM, Millard WJ, Simpkins JW. Ovarian steroid deprivation results in a reversible learning impairment and compromised cholinergic function in female Sprague-Dawley rats. Brain Research. 1994;644(2):305–12. doi: 10.1016/0006-8993(94)91694-2. [DOI] [PubMed] [Google Scholar]
- Shumaker SA, Legault C, Thal L, Wallace RB, Ockene JK, Hendrix SL, Jones BN, 3rd, Assaf AR, Jackson RD, Kotchen JM, Wassertheil-Smoller S, Wactawski-Wende J WHIMS Investigators. Estrogen plus progestin and the incidence of dementia and mild cognitive impairment in postmenopausal women: the Women’s Health Initiative Memory Study: a randomized controlled trial. Journal of the American Medical Association. 2003;289(20):2651–62. doi: 10.1001/jama.289.20.2651. [DOI] [PubMed] [Google Scholar]
- Shumaker SA, Legault C, Kuller L, Rapp SR, Thal L, et al. Women’s Health Initiative Memory Study. (2004) Conjugated equine estrogens and incidence of probable dementia and mild cognitive impairment in postmenopausal women: Women’s Health Initiative Memory Study. JAMA. 291:2947–58. doi: 10.1001/jama.291.24.2947. [DOI] [PubMed] [Google Scholar]
- Shukitt-Hale B, Denisova NA, Strain JG, Joseph JA. Psychomotor and spatial memory performance in aging male Fischer 344 rats. Experimental Gerontology. 1998;33(6):615–624. doi: 10.1016/s0531-5565(98)00024-2. [DOI] [PubMed] [Google Scholar]
- Sugaya K, Greene R, Personett D, Robbins M, Kent C, Bryan D, Skiba E, Gallagher M, McKinney M. Septo-hippocampal cholinergic and neurotrophin markers in age-induced cognitive decline. Neurobiology of Aging. 1998;19(4):351–361. doi: 10.1016/s0197-4580(98)00072-4. [DOI] [PubMed] [Google Scholar]
- Terry D, Engler E, Millan M, Zay C, Crain I, Bimonte-Nelson H. Ovarian hormone loss and cognitive decline at multiple timepoints during aging. Society for Neuroscience Abstracts. 2006 [sufficient?] [Google Scholar]
- Wang Y, Yano T, Kikuchi A, Yano N, Matsumi H, Ando K, Kasai Y, Watanabe M, Okagaki R, Osuga Y, Taketani Y. Comparison of the effects of add-back therapy with various natural oestrogens on bone metabolism in rats administered a long-acting gonadotrophin-releasing hormone agonist. Journal of Endocrinology. 2000;165(2):467–73. doi: 10.1677/joe.0.1650467. [DOI] [PubMed] [Google Scholar]
- Wolf OT, Kirschbaum C. DHEA replacement and cognition in healthy elderly humans: A summary of results from placebo controlled experiments. In: Morfin A, editor. DHEA and Brain, S. London: Taylor & Francis; 2002. pp. 187–198. [Google Scholar]
- Woolley CS, McEwen BS. Estradiol mediates fluctuation in hippocampal synapse density during the estrous cycle in the adult rat. Journal of Neuroscience. 1992;12:2549–2554. doi: 10.1523/JNEUROSCI.12-07-02549.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ziegler DR, Gallagher M. Spatial memory in middle-aged female rats: assessment of estrogen replacement after ovariectomy. Brain Research. 2005;1052(2):163–73. doi: 10.1016/j.brainres.2005.06.006. [DOI] [PubMed] [Google Scholar]
- Zurkovsky L, Brown SL, Korol DL. Estrogen modulates place learning through estrogen receptors in the hippocampus. Neurobiology of Learning and Memory. 2006;86:336–343. doi: 10.1016/j.nlm.2006.07.008. [DOI] [PubMed] [Google Scholar]