Abstract
Benzodiazepine-type drugs (benzodiazepines and the newer non-benzodiazepines) are similar to older sedative/hypnotic drugs, such as the barbiturates, in that they act at the GABAA receptor (9, 188). Unfortunately, benzodiazepine-type drugs also retain the liability for abuse and dependence associated with the earlier anxiolytics (133, 208). Action at GABAA receptors likely plays a key role in both the therapeutic as well as abuse-related effects of this important class of drugs. While the extent to which therapeutic efficacy and abuse potential can be dissociated is not yet understood fully, the biochemical processes underlying these behavioral effects are even less understood. A more comprehensive understanding of the etiology of benzodiazepine-type drug-induced abuse and dependence is likely to provide information that can inform drug development strategies to help design anxiolytics and hypnotics that have maximum clinical benefit with reduced abuse potential. Thus, this review will explore issues related to the abuse and dependence potential of benzodiazepine-type drugs and the role that GABAA receptors play in this phenomenon. Further, this review will discuss putative intracellular events that may occur as a result of the interaction between benzodiazepine-type drugs and GABAA receptors, and how those events may ultimately give rise to the abuse-related behaviors associated with these drugs.
GABAA RECEPTOR MODULATORS
Sedative/hypnotic drugs include those that are typically considered to be tranquilizers such as the barbiturates, benzodiazepines, and newer non-benzodiazepines. Clinically, these drugs are prescribed as anxiolytics, sedatives, anticonvulsants, and muscle relaxants, and share in common an ability to interact with the GABAA receptor (14, 165). Barbiturates and benzodiazepine-type drugs are positive allosteric modulators of the receptor complex. They each bind to a distinct site on the GABAA receptor and increase the affinity of the receptor by favoring an open state, thereby increasing chloride conductance (27,183). Many studies over the past decades have revealed the existence of multiple subtypes of the GABAA receptor (e.g., 115, 143), and research with transgenic mice and subtype-selective ligands has postulated that the diverse behavioral effects of benzodiazepine-type drugs in particular may reflect action at different subtypes of GABAA receptors (110, 113, 139, 153, 156).
The GABAA receptors in the central nervous system are pentamers composed of subunits from at least five different families of distinct proteins (for review, see 156). While the majority of GABAA receptors consist of α, β, and γ subunits, classical benzodiazepines bind predominantly to a site on the native GABAA receptor that occurs at the interface between the γ2 subunit and either an α1, α2, α3, or α5 subunit (114, 145, 178, 205). In contrast, these drugs are inactive at corresponding α4- and α6-subunit containing receptors (144).
More than 90% of the GABAA receptors in the brain contain α1, α2, and α3 subunits (116), and despite the existence of other subunits within the receptor, benzodiazepine action appears to be determined by the presence of particular α subunits (115,143, 156). GABAA receptors containing α1 subunits (α1GABAA receptors) recently have been implicated in the sedative effects of benzodiazepine-type drugs (113, 139, 155), whereas GABAA receptors containing α2 and α3 subunits (α2GABAA and α3GABAA receptors) have been implicated in the anxiolytic effects of benzodiazepine-type drugs (110, 113). Receptors containing α5 subunits (α5GABAA receptors), while being a relatively minor population of GABAA receptors, may play a role in memory processes, but likely not anxiolysis or motor effects (33, 39).
To the extent that the different behavioral effects of benzodiazepines are attributable to different receptor subtypes, it is feasible that a subset of receptors is responsible for the abuse-related effects of these drugs. Consequently, the heterogeneity of GABAA receptors raises the possibility that compounds lacking abuse liability can be found. However, as will be discussed later, a complex picture is emerging with respect to abuse of benzodiazepine-type drugs and the role of different GABAA receptor subtypes.
BEHAVIORAL EFFECTS OF BENZODIAZEPINE-TYPE DRUGS
Benzodiazepines were developed in the 1960s in response to a need for safe and effective anxiolytics. Barbiturates had lost favor as anxiolytics and anticonvulsants due to their low therapeutic index and high abuse potential (126). The successor to the barbiturates, meprobamate, met a similar demise as reports of overuse and illicit diversion gradually negated its clinical usefulness and popularity (107). The introduction of meprobamate, however, was the beginning of modern psychopharmacology, and led to an intense interest in the development of novel anxiolytic drugs with reduced side effects. The interest in that endeavor continues to this day (188).
Therapeutic Efficacy
Chlordiazepoxide (Librium) and diazepam (Valium) were among the earliest benzodiazepine anxiolytics to be developed. Diazepam in particular was extremely popular, and became the most widely prescribed drug in the United States and Europe between 1968 and 1987 (176). Within the past decade diazepam has maintained its popularity and along with alprazolam (Xanax), clonazepam (Klonopin), and lorazepam (Ativan), has appeared on a list of the top 100 most commonly prescribed medications (3). Among the advantages of prescribing benzodiazepines as broad-spectrum anxiolytics and hypnotics is that in addition to how well-tolerated they are they exhibit rapid onset of action and variable, yet predictable, half-lives (65).
While the hallmark of their therapeutic efficacy is their ability to reduce anxiety and seizure activity acutely as well as to induce sleep, benzodiazepines are useful for treating a variety of specific conditions (80, 140). Most notably, with respect to anxiety disorders, this group of drugs has been demonstrated empirically to treat the somatic symptoms associated with generalized anxiety disorder (e.g., 58), panic disorder (e.g., 49), and obsessive compulsive disorder (e.g., 78, 79). Status epilepticus, either as a result of neurological illness or as a precursor to epilepsy, also has been shown to benefit from treatment with benzodiazepines (123). Not only are benzodiazepines the traditional prescription for treating insomnia (92), but their amnestic properties make them invaluable when used during pre-surgical and dental sedation (49). This broad range of clinical uses signifies that benzodiazepines are some of the most important psychoactive drugs developed over the past century.
The 1980s brought reports of the new “Z-drug” hypnotics. These drugs have rapid onset and short duration of action (4), thus making them attractive non-benzodiazepine alternatives for the short-term treatment of insomnia. Although they are structurally distinct from benzodiazepines, zolpidem, zaleplon, and zopiclone (and more recently, its active enantiomer eszopiclone), all act at the benzodiazepine recognition site on the GABAA receptor. However, zolpidem and zaleplon are selective for those receptors containing an α1 subunit (16, 163), while zopiclone appears to be less specific (34, 47). Interestingly, they are also structurally unrelated to one another; zolpidem is an imidazopyridine, zaleplon is a pyrazolopyrimidine, and both zopiclone and eszopiclone are cyclopyrrolones.
Of the three hypnotics, zolpidem (Ambien) is probably the most frequently prescribed non-benzodiazepine hypnotic in the United States (127), and the most potent. Its potency has been demonstrated in vitro using oocytes expressing recombinant α1GABAA receptors. The potentiation of GABA-evoked chloride currents was measured, showing that zolpidem potentiated these currents with an EC50=78 (163), zopiclone had an EC50=107 (149), and zaleplon had an EC50=169 (163). In vivo, zolpidem can be distinguished from conventional benzodiazepines (e.g., 4, 42,161,162) such that its predominant behavioral effect is sedation despite its ability to engender anxiolytic-like, anticonvulsant, and myorelaxant effects in rodents. Moreover, sedation was observed at much lower doses than those required to engender the other effects (42, 160). Clinically, zolpidem demonstrated hypnotic efficacy in people with sleep disturbances comparable to the benzodiazepines, but without the disruption of sleep architecture (20, 22, 100, 147, 185).
Zaleplon (Sonata) has been shown to have similar preclinical (159) and clinical (see review by 48) behavioral pharmacological profiles to zolpidem. However, at therapeutic doses the agonist effects of zolpidem are greater than those of zaleplon (64). Eszopiclone (Lunesta) and zopiclone (Imovane) are similar to zolpidem and zaleplon such that they also induce anxiolytic, anticonvulsant, myorelaxant, and sedative effects in rodents (30). Clinically, eszopiclone appears to be comparable to the other non-benzodiazepine hypnotics with respect to pharmacokinetics and ability to induce and maintain sleep (see review by 130), but it is unique in that it retains its safety and efficacy for 6–12 months (97, 151). All together, the “Z-drugs” have become the first-line medication treatment for insomnia (52, 132)
Abuse Liability
Despite the usefulness of benzodiazepine-type drugs across many clinical indications, their myriad behavioral effects may sometimes be perceived as side effects, thus limiting their utility. Among those effects are daytime sedation, motor incoordination, and memory impairment (56, 194,204). In contrast, effects such as abuse and dependence serve no clinical purpose, and are always perceived as undesirable (71, 96).
The abuse potential of benzodiazepines was recognized as early as 1967, as reports in the popular media were warning of their illicit and non-medical use particularly by youth and the counter-culture (176,188). In fact, benzodiazepines had entered the popular culture. For example, the Rolling Stones’ song “Mother’s Little Helper” referred to a street name associated with the perceived widespread use of diazepam by middle-class housewives (although it is not entirely clear the extent to which this street name refers to benzodiazepines only). Another example is the prominent role played by Valium in Jacqueline Susann’s 1966 novel “Valley of the Dolls”. The story revolves around ambitious young women who medicate themselves with Valium in order to cope with the pressures they face in their personal lives and careers. During these years, doctors were generous with prescriptions prompting Valium to become a coping tool for everyone from overworked business executives to frazzled housewives. In 1975, the United States Drug Enforcement Agency (DEA) began regulating valium and several other benzodiazepines as Schedule IV drugs, and by 1979 the government used congressional hearings on the “Valium scare” (191) to urge more judicious prescribing practices.
While the notion that benzodiazepine-type drugs have the potential to be abused is not new, recent epidemiological findings suggest that their abuse may be on the rise. One prominent example comes from recent reports prepared by the Drug Abuse Warning Network (DAWN), in which yearly estimates of drug abuse-related emergency department visits from a large network of hospitals in the United States are compiled. According to the most recent data available, the number of emergency room visits associated with the use of sedative/hypnotics in 2005 was 34% of the total visits involving non-medical use of prescription drugs (182; see Figure 1). More strikingly, the number of benzodiazepine-related emergency department visits were not only comparable to those involving misuse of prescription opiates (approximately 29% of sedative/hypnotic visits), but they had increased 19% since 2004. These statistics are in agreement with current reports based on substance abuse treatment admissions. Based on findings from the Treatment Episode Data Set (TEDS), an annual compilation of patient characteristics in substance abuse treatment facilities in the United States, admissions due to “primary tranquilizer” use (including, but not limited to, benzodiazepine-type drugs) increased 79% from 1992 to 2002 (186). Thus, the DAWN and TEDS data sets demonstrate clearly that the misuse of these sedative/hypnotics is on the rise, and cause for concern.
FIG. 1.

Recent emergency department visits involving the non-medical use of prescription drugs, adapted from the Drug Abuse Warning Network report (182). Percentages are approximate.
Within the general population there are certain sub-populations who are at greater risk for inappropriate benzodiazepine taking. These groups include polydrug abusers, patients with histories of alcohol abuse, and the elderly (70, 207). With respect to polydrug abuse, benzodiazepine-type drugs are often co-abused with opiates and alcohol (37). Upwards of one-third of opiate-addicted individuals have reported taking benzodiazepines in combination with opioid drugs, particularly with methadone (40, 41, 50, 59, 86, 117,142, 166, 180). Clinical and preclinical evidence suggests that benzodiazepines enhance the abuse-related effects of opiates or “boost” their high. In that respect, opiate users report enhanced subjective effects with the combination relative to either drug alone (105,142), while otherwise ineffective doses of alprazolam and heroin engendered a significant place preference in rodents when tested in combination (197, 198, 199). Similarly, people with a history of moderate-to-heavy alcohol use tend to have a higher degree of long-term benzodiazepine use (often without a prescription) and appear more sensitive to the effects of these drugs (32, 43, 54). And while the elderly likely do not engage in recreational abuse, prevalence of use is typically higher than in the general population (70, 207).
There also are other unique instances of susceptibility to the abuse of benzodiazepine-type drugs. For example, iatrogenic factors have been shown to contribute to dependence, particularly when benzodiazepine-type drugs are used in the comfort and care of the critically ill. Intensive care units utilize benzodiazepines in high volumes, and patients often undergo withdrawal upon discontinuation despite the use of standard tapering management protocols (see review by 184). This is a pathway to dependence that is often overlooked in both adults (26, 46) and children (28, 60,192). Similarly, treatment of medication-induced insomnia also has the potential to lead to dependence on benzodiazepine-type drugs. This can be a problem particularly in the elderly for whom there is an increased likelihood of polypharmacy (94, 158), or in those individuals being treated with other medications such as antidepressants (91,108). Overall, it can be concluded that benzodiazepine-type drugs have serious abuse and dependence liability, even in seemingly innocuous medical situations.
BEHAVIORAL DETERMINANTS OF ABUSE AND DEPENDENCE LIABILITY
Drug seeking and drug taking behavior together is a complex phenomenon comprised of discrete behavioral components. The most likely property of a compound that predicts inappropriate use is the degree to which the compound has reinforcing effects. A drug is said to have reinforcing effects if its presentation increases the probability of subsequent responses to produce it. The study of the reinforcing effects of drugs has been an important emphasis of drug abuse research for decades, and the demonstration of a drug’s reinforcing effects in the laboratory forms a key component of abuse liability assessment required by worldwide regulatory agencies (8, 10,66).
Another major determinant of the extent to which a drug has abuse liability is the occurrence of physical dependence with repeated administration. Physical dependence is characterized by the emergence of a withdrawal syndrome upon cessation of chronic drug treatment. Tolerance to some or all of the effects of a drug often accompanies the development of physical dependence. It is important to note that abuse can occur in the absence of physical dependence—thus dependence is a predictor of abuse potential, but it is not a necessary condition. As with reinforcing effects, regulatory agencies also consider the extent to which a compound induces physical dependence following chronic treatment as part of scheduling decisions (10).
A final property often considered to be a key component of a drug’s abuse liability is the subjective, or interoceptive effect produced by it. These effects often are assessed with drug discrimination procedures in which subjects typically are trained to distinguish the presence and absence of a drug, i.e. a response is correct or incorrect based on whether drug or placebo is administered. In their most basic form, these procedures determine the extent to which one drug shares discriminative stimulus effects with another drug—if the latter is an abused drug of a particular class, then the likelihood that the compound of interest has subjective effects in common with the drug of abuse is high (8, 101).
Of the three properties of drugs that are considered for determination of abuse liability, the following sections will focus on the reinforcing effects and propensity to induce physical dependence of benzodiazepine-type drugs. The discriminative stimulus effects of benzodiazepine-type drugs have been reviewed extensively elsewhere (e.g., 8, 101) and will not be discussed in detail further.
Self-Administration of Benzodiazepine-Type Drugs
A consistent finding in human laboratory studies is that benzodiazepine-type drugs have reinforcing effects primarily in subjects with histories of drug or alcohol abuse, in anxious subjects, and patients with sleep disorders (70, 207). However, unlike other abused drugs, benzodiazepine-type drugs do not function as reinforcers consistently if subjects lack these characteristics. While it is unclear why the reinforcing effects should depend on subject characteristics and/or histories, it can be hypothesized that individuals who suffer from some type of anxiety self-administer benzodiazepine-type drugs because of their therapeutic efficacy; i.e., in order to alleviate anxiety (70, 75). In fact, it is completely plausible that highly anxious individuals find benzodiazepine-type drugs very reinforcing. Polydrug abusers and alcoholics likely self-administer these compounds due to some interaction that exists between the therapeutic effects of benzodiazepines and the reinforcing effects that are subsequent to prior exposure to abused substances. Although some reports have demonstrated evidence that this population may use benzodiazepine-type drugs to self-medicate “emotional disturbances” or insomnia (e.g., 62, 135), and others have observed that benzodiazepines are co-administered with other substances primarily to boost a drug “high” (e.g., 40, 86), one study has found a combination of these effects. Among a population of patients maintained on methadone for treatment of opioid dependence, relatively large proportions of the subjects self-administered benzodiazepines for either recreational purposes or treating “emotional problems”, however, approximately one-third reported taking benzodiazepines for both reasons (62).
With respect to self-administration in laboratory animals, it is predicted that if benzodiazepine-type drugs have reinforcing effects, then these compounds should be effective in models of self-administration in controlled laboratory settings. Indeed, this prediction does hold, as benzodiazepine-type compounds show reinforcing effects under a variety of experimental conditions (e.g., 8, 17, 23, 68, 153). These studies employed i.v. self-administration procedures, in which subjects are trained to press a lever in order to receive an i.v. drug injection via a chronic venous catheter. Reinforcing effects of the drug are affirmed if it maintains a higher degree of self-administration compared to that observed under conditions of vehicle availability.
Although benzodiazepines do produce self-administration behavior above levels maintained by vehicle, they might be relatively weak reinforcers in general (Weerts et al. 1998), and especially compared to other drugs of abuse. For instance, the peak levels of self-administration maintained by diazepam were below the peak levels maintained by the training drug methohexital, a short-acting barbiturate (206). This observation could be explained by a difference in pharmacokinetics between these drugs. Shorter-acting compounds have a tendency to maintain higher levels of self-administration compared to compounds with a longer duration of action (e.g., 68).
Alternatively, other drugs of abuse may indeed be more reinforcing compared to benzodiazepines. In recent years, we have evaluated self-administration of benzodiazepine-type drugs and other types of drugs of abuse using progressive-ratio schedules of intravenous drug injection in monkeys. In this procedure, the response requirement increases across a session until responding stops, permitting the determination of “break point”, which is defined as the last response requirement completed in a session. In our studies, drugs of abuse such as cocaine and opioid receptor agonists are typically studied at higher response requirements than benzodiazepine-type drugs. For example, the initial response requirement (IRR) of a progressive-ratio sequence used to study cocaine’s reinforcing effects is 100 (e.g., 154), whereas IRRs of 40 were used to evaluate benzodiazepine-type drugs (e.g., 153). Recently, we have evaluated self-administration of zolpidem and the short-acting benzodiazepine midazolam under a relatively wide range of IRRs (152), allowing us to make comparisons of the relative reinforcing strength of these drugs with stimulants and opioids under similar experimental conditions. As shown in Figure 2, break points maintained by both cocaine and alfentanil, a selective mu opioid receptor agonist, were markedly higher than the break points maintained by zolpidem and midazolam. These data provide clear support for benzodiazepine-type drugs being weaker reinforcers than other drugs of abuse (cf. 207).
FIG. 2.
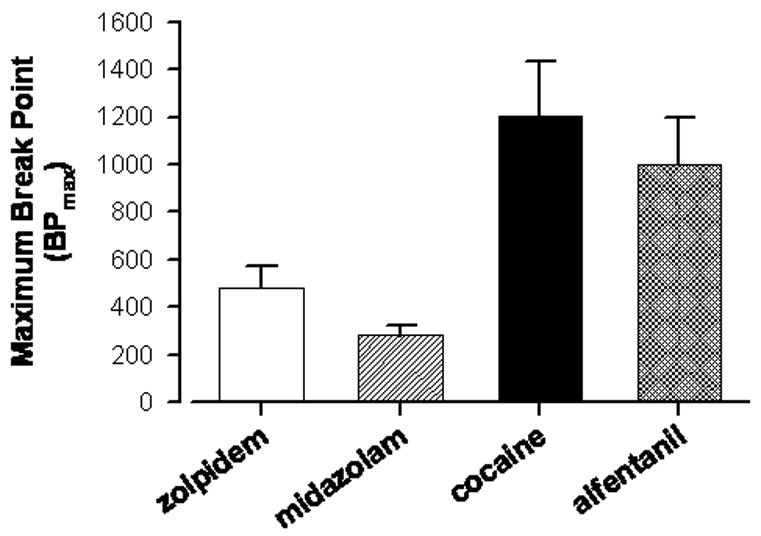
Break points maintained by zolpidem, midazolam, cocaine, or alfentanil, in rhesus monkeys trained under a progressive-ratio schedule of intravenous drug delivery. Break point was defined as the maximum response requirement obtained in a session, and the data represent the maximum break points irrespective of dose tested (referred to as “BP max”). Data are means ± SEM for N = 4 monkeys for each drug, and were obtained from Rowlett et al. (153, 154) and Rowlett and Lelas (152).
Physical Dependence Following Chronic Treatment with Benzodiazepine-Type Drugs
Prolonged use of benzodiazepine-type drugs can lead to physical dependence, which in turn may contribute to the abuse liability of these drugs (5, 137). For example, abrupt cessation of benzodiazepine use after prolonged treatment at a therapeutic dose can result in a withdrawal syndrome (for review, see 70, 208). Benzodiazepine withdrawal is characterized by many signs that are opposite to the therapeutic effects of benzodiazepines (e.g. anxiety, insomnia) and, in more severe cases, patients may experience seizures (70,120, 134). Comprehensive reviews discussing the evidence of physical dependence to benzodiazepines, as well as the factors that may influence the development of physical dependence to chronic benzodiazepine treatment can be found elsewhere (70, 207,208).
Physical dependence to a benzodiazepine-type drug is often measured in the laboratory as the emergence of characteristic withdrawal signs upon cessation of the drug that is reversed with subsequent drug administration (spontaneous withdrawal) or precipitated by administration of an antagonist, such as flumazenil (precipitated withdrawal; 207). Controlled studies examining patients who use low therapeutic doses of benzodiazepines chronically have demonstrated that flumazenil can precipitate withdrawal symptoms (18, 19, 74, 121). Likewise, precipitated withdrawal also has been observed in healthy human volunteers following daily exposure to a relatively high therapeutic dose of a benzodiazepine (120). In preclinical studies, the severity of withdrawal has been shown to be dose-dependent in non-human primates (111) and dogs (170).
Duration of treatment may also contribute to the severity of the withdrawal, although the empirical data are mixed. A study in healthy human volunteers demonstrated precipitated withdrawal as soon as 7 days after daily exposure to diazepam, but withdrawal severity did not increase with increased exposure (i.e., withdrawal symptoms were similar on days 7, 14, and 28; 120). In contrast, a study undertaken in baboons concluded that the severity of withdrawal increased with the duration of treatment (111).
Precipitated or spontaneous withdrawal from benzodiazepines in laboratory animals can be used to detect a negative affective or subjective state induced by withdrawal. For example, flumazenil administration conditioned a place aversion following chronic treatment with diazepam in rats (1). Similarly, spontaneous withdrawal from diazepam increased the amount of time spent in the drug-paired context of a conditioned place preference paradigm, and literally drove the animals away from the withdrawal-associated context (174). It is not clear if these observations are manifestations of withdrawal-induced anhedonia or anxiety-like behavior, both of which have been implicated in the discontinuation of drug use (e.g., 99, 169), but one study has demonstrated the ability of antidepressants to reverse the escape deficit induced by diazepam withdrawal in a shock avoidance task (98).
The concerns about dependence following long-term treatment are becoming more prominent as the popularity of the newer benzodiazepine-type hypnotics is on the rise. Most of the newer hypnotic benzodiazepine-type drugs are relatively short-acting, raising concerns over the possibility of more severe withdrawal after chronic treatment (134, 208). However, little evidence exists for a more severe withdrawal syndrome engendered by short-acting drugs. For example, short-acting benzodiazepines, such as midazolam, produced physical dependence similar in magnitude to longer-acting drugs such as chlordiazepoxide (207). Similarly, a review of hypnotic abuse liability led to the conclusion that the withdrawal observed after therapeutic doses of zolpidem (no information was available for zaleplon) was rated as intermediate, i.e. similar to conventional benzodiazepines (67). Importantly though, clinical studies find consistently that not all patients develop physical dependence to benzodiazepine-type drugs (207).
Tolerance Following Chronic Treatment
In addition to the development of physical dependence, chronic benzodiazepine treatment can result in tolerance to some behavioral effects. It is important to note that the development of physical dependence does not require the development of tolerance, and that tolerance can occur in the absence of physical dependence (207). Moreover, the time course for the development of tolerance varies for different behavioral effects of benzodiazepine-type drugs. In humans, for example, tolerance develops rapidly to sedative effects and motor coordination deficits; whereas tolerance does not always develop to the anxiolytic or memory impairing effects of benzodiazepine-type drugs after long periods of use (36, 70,181). A clear gap in our knowledge about tolerance development is the extent to which the reinforcing effects of benzodiazepine-type drugs change over time, i.e. whether or not tolerance to the reinforcing effects of benzodiazepines develops after chronic exposure. Based on available information, tolerance to reinforcing effects of benzodiazepine-type drugs appears unlikely, since self-administration of midazolam or zolpidem was shown to be stable over relatively long durations of exposure (202, 203). Moreover, indirect evidence that tolerance to the reinforcing effects of benzodiazepines does not occur comes from the observation that long-term use by humans is not associated with escalation in the ingested dose of drug across time (70, 207).
GABAA RECEPTOR CONTRIBUTION TO ABUSE AND DEPENDENCE LIABILITY
Recently, selective pharmacological tools have been developed that allow investigators to probe the GABAA receptor mechanisms underlying behaviors engendered by benzodiazepine-type drugs. For example, although the hypnotic benzodiazepine-type drugs zolpidem and zaleplon interact with the benzodiazepine binding site on the GABAA receptor, they enhance GABA-mediated chloride currents in recombinant GABAA receptors containing α1 subunits more selectively than those containing α2 orα3 subunits (72, 163). These findings in addition to other accumulating behavioral data led investigators to formulate the hypothesis that α1GABAA receptors are critical mediators of the sedative effects of benzodiazepine-type drugs (113, 139,155). In contrast, ligands such as L-838,417 (113) and TPA023 (6), lack intrinsic efficacy at α1GABAA receptors, and have been helpful in understanding the relationship of sedative vs. anxiolytic, myorelaxant, and anticonvulsant activity of these compounds (see Table 1). The following sections will review how pharmacological tools such as these have contributed to the current state of knowledge about the role of GABAA receptors in mediating behavior associated with the abuse liability of benzodiazepine-type drugs.
Table 1.
Non-selective and selective benzodiazepine-type drugs: Relationship of receptor binding, intrinsic efficacy and relative reinforcing effectiveness
| Selective Efficacya |
|||||||
|---|---|---|---|---|---|---|---|
| Selective Affinitya | α1 | α2 | α3 | α5 | Baboonb | Rhesus Monkeyc | |
| Diazepam | None | 1.0 | 1.0 | 1.0 | 1.0 | low-intermediate | intermediate |
| Triazolam | None | 1.7 | 1.2 | 1.3 | 1.4 | intermediate | intermediate |
| Zolpidem | α1>α2=α3≫α5 | 1.6 | 1.3 | 1.2 | -- | high | high |
| TPA023 | None | 0.01 | 0.15 | 0.38 | 0.11 | 0 | NAd |
| L-828,417 | None | 0.02 | 0.53 | 0.48 | 0.68 | NAd | low |
Binding and efficacy data are from cloned human receptors (6, 114, 171). Efficacy represents % potentiation of Cl- currents at an EC20 concentration of GABA, divided by the values obtained for diazepam (α1= 71%, α2= 81%, α3= 88%, α5= 57%).
Relative reinforcing effectiveness, i.v. self-administration in baboons (8, 68, 69): 0= not different from vehicle; low= below a mean of 4 injections/session; intermediate= 4–6 injections/session; high= 6–8 injections/session.
Relative reinforcing effectiveness using a scale adapted from baboon studies (see 8); i.v. self-administration in rhesus monkeys (153; except for triazolam, which is unpublished data from N= 4 monkeys): 0= mean 0–4 injections/session (not different from vehicle); low= 5–8 injections/session; intermediate= 9–12 injections/session; high= 13–20 injections/session.
NA: not available.
GABAA Receptor Subtypes and the Reinforcing Effects of Benzodiazepine-Type Drugs
Although benzodiazepine-type drugs generally have relatively modest reinforcing effects, notable exceptions have been observed with the hypnotics zolpidem and zaleplon. In non-human primates, zolpidem self-administration was not only greater than conventional benzodiazepines, but it was comparable to behavior maintained by barbiturates (69, 153; see also Figure 2 for comparison of break points maintained by zolpidem vs. midazolam). Similarly, another study demonstrated that zaleplon was self-administered to the same extent as zolpidem (7). Both drugs display selectivity for the α1GABAA receptor, raising the possibility that this receptor subtype may be an important substrate for self-administration of benzodiazepine-type drugs (8, 69,153).
Further support for a critical role for α1GABAA receptors in the reinforcing effects of benzodiazepine-type drugs was observed in studies involving benzodiazepine-type compounds with efficacy at specific GABAA receptor subtypes (8). TPA123 is a partial benzodiazepine binding site agonist that exhibits low intrinsic efficacy in vitro at α1GABAA receptors, while TPA023 is similar in that it is also a partial agonist, but it lacks efficacy at α1GABAA receptors (i.e. it is essentially an antagonist in vitro at α1GABAA receptors) and exhibits very low efficacy at α2GABAA receptors (12% potentiation of GABA-mediated currents vs. 81% for diazepam; see Table 1 as well as 6). In those studies, TPA123 functioned as a reinforcer in baboons trained to self-administer intravenous injections of cocaine, whereas TPA023 was ineffective (8). Together with the findings obtained with zolpidem and zaleplon, these results raise the possibility that a benzodiazepine-type compound’s potential for abuse may be directly related to its efficacy in vitro at α1GABAA receptors.
Although the findings of Ator (8) and our laboratory are seemingly contradictory, it may simply be the case that not enough data are available to make firm conclusions. Table 1 compares published in vitro receptor activity and self-administration results for TPA023 and L-838,417, zolpidem, and two non-selective benzodiazepines (diazepam and triazolam). In order to compare these results across studies using different methodologies and species, we developed a scale of zero, low, intermediate, and high degrees of self-administration based on previous work by Griffiths et al. (68, 69) and Ator (8). As can be seen in Table 1, for these particular compounds, the available results are concordant across the two laboratories, and with only minor differences (e.g., Griffiths, Ator and colleagues showed greater reinforcing effectiveness of triazolam vs. diazepam, whereas we have not observed this difference consistently). Importantly, critical information is missing, including tests of TPA023 self-administration in rhesus monkeys as well as tests of L-838,417 self-administration in baboons.
Regardless of our gaps in knowledge concerning self-administration of subtype-selective compounds, the comparisons in Table 1 provide information to draw preliminary conclusions and formulate hypotheses. First, no clear relationship between a compound’s affinity or in vitro intrinsic efficacy at α5GABAA receptors and its subsequent relative reinforcing effectiveness was observed. For example, while both zolpidem and TPA023 lack activity at the α5GABAA receptor, zolpidem was self-administered robustly whereas TPA023 lacked reinforcing effects. Second, it appears that action at α1GABAA receptors is not necessary for reinforcing effects. The primary evidence for this hypothesis is the results with L-838,417, which has no efficacy at α1GABAA receptors and did function as a reinforcer. Because TPA023 also exhibits no efficacy at α1GABAA receptors and was not self-administered, more conclusive studies need to be undertaken in which compounds with different degrees of activity at α1GABAA receptors are evaluated. Finally, intrinsic efficacy may play a key role in the differences in reinforcing effectiveness among the compounds. This hypothesis is supported by the observation that low intrinsic efficacy in general appears to be predict lower reinforcing efficacy, irrespective of action at the different receptor subtypes. Moreover, based on comparisons between the findings with TPA023 and L-838,417, it appears that a compound may require a degree of efficacy at α2GABAA receptors greater than ~10%, and/or at least ~40% efficacy at α3GABAA receptors, in order to have reinforcing effects. This latter idea assumes that action at α1GABAA receptors is not necessary for reinforcing effects, as described above.
Although differences in binding selectivity and intrinsic efficacy at GABAA receptors provides intriguing hypotheses for the observed differences in self-administration compiled in Table 1, some methodological factors must also be considered. For example, the baboons in the Ator (8) studies were trained to self-administer under a cocaine baseline, whereas the rhesus monkeys in Rowlett et al. (153) were trained to self-administer intravenous injections of the short-acting barbiturate, methohexital. Moreover, self-administration by the baboons employed a fixed-ratio schedule of intravenous drug delivery, contrasting with the progressive-ratio used in the rhesus monkey report (see 68, 153 for comparisons of the procedures). As discussed above, the history of drug use by human subjects in a major determinant of the reinforcing effects of benzodiazepines. Some evidence exists for a similar phenomenon in the animal literature. In this regard, a previous study has shown that the number of rhesus monkeys that self-administered diazepam was significantly lower when self-administration was trained with cocaine compared to pentobarbital (17). The extent to which differences in baseline training conditions influenced the findings in Table 1 is unknown, and underscores the need for more research on not only pharmacological, but behavioral factors underlying benzodiazepine self-administration.
Another key factor that deserves consideration in explanations of the differences in reinforcing effectiveness among the compounds in Table 1 is pharmacokinetics. While little has been published regarding the pharmacokinetic parameters of TPA023 and L-838,417 following intravenous administration in monkeys, L-838,417 is purported to have a relatively short half-life similar to that of midazolam (153; J.R. Atack, personal communication). In contrast, TPA023’s duration of receptor occupancy in rodents suggests that this compound may be relatively long-acting (6). These findings suggest that TPA023 might not maintain self-administration behavior due to its long duration of action. However, other compounds with a long duration of action (e.g. diazepam) clearly are self-administered under the procedures used by both Ator (8) and Rowlett et al. (153). In fact, onset of action may be the most important pharmacokinetic factor that determines the degree of reinforcing effects of abused drugs (70), but empirical information regarding the onset of action for TPA023 and L-838,417 is not yet available.
GABAA Receptor Subtypes and Physical Dependence on Benzodiazepine-Type Drugs
Withdrawal from benzodiazepine-type drugs has been characterized extensively in both humans and non-human animals, but the underlying mechanisms of benzodiazepine physical dependence have not been determined (136, 196). A study using a drug discrimination model of withdrawal in rhesus monkeys has provided preliminary evidence that the acute effects and withdrawal-associated effects of benzodiazepines might be mediated via different mechanisms (116). In this drug discrimination model of withdrawal, monkeys were treated chronically with diazepam and trained to discriminate flumazenil from vehicle injections (presumably a discrimination based on interoceptive cues associated with precipitated withdrawal). These authors demonstrated that the potencies of a series of benzodiazepines and related compounds to attenuate the withdrawal-inducing effects of flumazenil did not correlate with the potencies of these drugs to engender benzodiazepine-like discriminative stimulus effects in non-dependent monkeys. Thus, these findings suggest that distinct receptor mechanisms underlie physical dependence compared to benzodiazepine-related interoceptive effects in non-dependent subjects (116).
As with reinforcing effects, the α1GABAA-selective agonist zolpidem provides a unique opportunity to probe the contribution of α1GABAA receptors to the physical dependence on benzodiazepine-type drugs. However, it has been unclear the extent to which chronic treatment with this selective compound induces physical dependence. Studies examining chronic treatment with zolpidem in mice (51, 136, 195), as well as survey and epidemiological data of patients who had used zolpidem (90, 175), have suggested a reduced propensity to induce physical dependence compared with classical benzodiazepines. Empirical studies in non-human primates, however, have found that zolpidem can engender a withdrawal syndrome that is quite similar to that observed after chronic treatment with benzodiazepines (69, 201, 202). In fact, this finding is consistent with human case reports (see review by 73, 103, 146), and suggests that α1GABAA receptors do indeed play a role in the development of physical dependence. Lending further support for this hypothesis, another α1GABAA-selective agonist, zaleplon, engendered a withdrawal syndrome similar to zolpidem in baboons (11).
With respect to the α2GABAA, α3GABAA, and/or α5GABAA receptors, compounds with selective efficacy at these subtypes have provided the opportunity to evaluate their roles in physical dependence induced by benzodiazepine-type drugs. Using compounds that vary in both selectivity and efficacy at GABAA receptor subtypes, a recent study evaluated the degree to which chronic treatment engendered seizures in mice following administration of the inverse agonist FG-7142 (122). Chronic treatment with zolpidem, as well as the selective compounds L-838,417 (partial agonist at α 2GABAA, α3GABAA, and α5GABAA receptors) and SL651498 (full agonist at α2GABAA and α3GABAA receptors, partial agonist at α1GABAA and α5GABAA receptors), did not result in seizures following FG-7142 administration. Similarly, chronic treatment with TPA023 (partial agonist at α2GABAA, α3GABAA, and α5GABAA receptors) also did not result in FG-7142-induced seizures in mice (6). Together, these findings suggest that physical dependence does not occur with subtype-selective compounds. Rather, these data suggest an interaction with all GABAA receptor subtypes is required for physical dependence to develop, at least as measured by inverse agonist-induced seizures. This is not an unlikely hypothesis, given that physical dependence is associated with a plethora of behavioral effects. Of note, chronic treatment with non-selective partial agonists did not result in FG-7142-induced seizures, suggesting that relatively high efficacy also might be a requirement for the development of physical dependence (122).
NEUROADAPTATIONS FOLLOWING BENZODIAZEPINE ADMINISTRATION: WHAT IS THE BIOCHEMICAL BASIS OF ABUSE-RELATED EFFECTS?
Recent research efforts have been aimed at delineating the GABAA receptor mechanisms that underlie benzodiazepine-type drug-induced behavior, but relatively little is known about the downstream events that occur between allosteric modulation of the receptor by these drugs and the subsequent behavioral outcome. With respect to their abuse potential, the neurochemical, cellular, and molecular sequelae of events that occur following administration of benzodiazepine-type drugs are largely and surprisingly ignored in the vast literature aimed at understanding the neuroadaptations associated with addiction-like behavior. Instead, the preponderance of data surrounding the rewarding properties of drugs of abuse has focused on stimulants and opioids (e.g. for review, see 93). The following sections will discuss briefly the neuroadaptive changes that occur following the interaction between benzodiazepine-type drugs and GABAA receptors, and how those changes may be related to the observable behavior associated with their abuse potential, namely tolerance and dependence.
GABAA Receptor Regulation Following Benzodiazepine Administration
Many studies have demonstrated GABAA receptor down regulation following chronic exposure to benzodiazepine agonists (e.g., see review by 95). Although the number of receptors at the cell surface may not change (168), their ability to bind benzodiazepines (119, 167) and enhance GABA neurotransmission (61, 84,150, 209) becomes compromised. For instance, a 40–80% decrease in allosteric binding site coupling has been demonstrated within days of drug exposure in neuronal cultures (84, 150), and over the course of several weeks in brain homogenates prepared from animals exposed chronically (61, 77). Similarly, chronic benzodiazepine treatment leads to a decrease in postsynaptic GABA sensitivity as measured by iontophoretic application of GABA in cell preparations (38, 61). Moreover, these changes in receptor function are benzodiazepine-specific, as administration of the benzodiazepine antagonist flumazenil was able to block the uncoupling and reverse the sub-sensitivity (61, 150). Together, these findings indicate that chronic treatment with benzodiazepines reduces the function of GABAA receptors, in turn, requiring more agonist to achieve the desired result. Thus, these adaptations appear to be reasonable neuronal correlates of tolerance.
Prolonged exposure to benzodiazepines also may result in tolerance and/or dependence as a function of use-dependent changes in receptor subunit composition. Modifications in the expression of genes encoding various subunits of the GABAA receptor have been demonstrated in a number of studies. The most consistent changes that have been reported to date include down regulation of the α1, α5, and γ2 subunit mRNAs by approximately 30–50% (57, 76, 81, 83, 87, 109, 210). While these studies either did not measure (57, 76, 83, 109) or did not observe (81, 87, 210) any benzodiazepine-induced changes in α2 or α3 subunit transcripts (or β subunits for that matter), most studies examined cortical areas which are typically more enriched with the α1GABAA receptor subtype (60% vs. 10–20%; for review, see 125). The one exception was reported by Holt et al. (81), demonstrating a decrease in α3 subunit transcripts following 2 weeks of diazepam treatment.
Discontinuation of long-term treatment with diazepam resulted in a flumazenil-sensitive increase in both mRNA and protein levels of the α4 subunit (57). Despite their lack of affinity for benzodiazepines and low expression levels throughout the brain (138), these are significant findings in that the concomitant change in protein levels reflects de novo synthesis of α4GABAA receptors (57), supporting the hypothesis that benzodiazepines induce a shift in GABAA receptor composition. Further, since these alterations often involve the α subunits which are presumed to be responsible for conferring different benzodiazepine sensitivity and pharmacological effects (115, 143,156), this GABAA receptor regulation could have a significant impact on behavior. Although the behavioral consequences of these alterations remain to be elucidated, especially in light of the differences observed across brain regions and with different treatment regimens (e.g., 148), what has become apparent is that chronic treatment with and subsequent withdrawal from benzodiazepines produces not only different constellations of behaviors from one another, but also a different pattern of changes among the GABAA receptor subunits (e.g., 118).
Benzodiazepine Effects on Neurotransmission Within the Reward Circuitry
As a result of a large body of research undertaken over the past 50 years, much has been learned about the brain regions, connectivity, and neurochemistry involved in mediating the rewarding or pleasurable effects of drugs of abuse. The most critical component of the reward circuitry traditionally has been the mesolimbic dopamine system, which is comprised of cell bodies originating in the ventral tegmental area and projecting to and terminating in the nucleus accumbens and extended amygdala. However, plenty of evidence has suggested prominent roles for the ventral pallidum, hippocampus, hypothalamus, pedunculopontine nucleus, and prefrontal cortex in mediating the reinforcing effects of drugs of abuse (see reviews by 13, 93, 102).
Sufficient evidence has been provided here to assert that benzodiazepines are drugs of abuse. However, unlike most other drugs of abuse (e.g., 45) benzodiazepine-type drugs do not simply increase extracelluar dopamine levels in the nucleus accumbens. Instead, benzodiazepine-site compounds have effects on accumbal dopamine that differ markedly depending on their intrinsic efficacy. For instance, extracellular dopamine levels are decreased by administration of the full benzodiazepine agonists diazepam, midazolam, or flurazepam (55, 88, 129, 212), as well as by the partial agonist imidazenil (128). In contrast, extracellular levels of dopamine are increased by administration of inverse agonists of the benzodiazepine-binding site on the GABAA receptor such as the anxiogenic β-carboline derivatives FG 7142 and β-CCE (112, 129). These effects have been blocked by pretreatment with the benzodiazepine-binding site antagonist flumazenil (129), indicating that GABAA receptors contribute to this particular modulation of mesolimbic dopaminergic neurotransmission.
Based on the findings that both natural rewards and most drugs of abuse stimulate activity within the nucleus accumbens (see review by 29; but also see 157), it can be hypothesized that drugs of abuse must be biochemical homologues of some critical aspect of naturally rewarding stimuli. However, as the work with benzodiazepine-site agonists has demonstrated, stimulation of mesolimbic dopamine pathways cannot be the only factor that determines abuse and dependence liability. Inverse agonists especially are not known to be rewarding, but appear to be anxiogenic (187), and have been proposed to model core components of schizophrenia (164) as well as stress (128). Therefore, the abuse potential of benzodiazepine-type drugs must be a function of something other than stimulating dopamine release directly (44, 55,189). This idea is supported by a compilation of studies suggesting that various drugs of abuse may activate the reward pathways differentially (13). For example, although heroin is most certainly a drug of abuse, it appears to mediate its rewarding effects via a neural system separate from that of cocaine (53).
Currently it is not clear if activation of the different anatomical structures and neurotransmitter systems ultimately converge on one output system to mediate the reinforcing effects of various drugs of abuse (13). Specifically, it is unknown how these interactions engender benzodiazepine-induced abuse-related behaviors. Indeed, many of the neuroadaptations that contribute to the addictive processes following administration of drugs of abuse in general have been shown to occur in meso-cortico-limbic circuits involving not only dopamine, but GABA and glutamate (12, 93). As discussed previously, there are a number of adaptations that occur at the level of the GABAA receptor (i.e., downregulation, allosteric uncoupling, subsensitivity, etc.) following administration of benzodiazepines. However, they do not appear to make an impact significant enough to account entirely for such complex behaviors as those associated with abuse potential (141). Instead, non-GABAergic mechanisms must also contribute to the abuse and dependence liability of benzodiazepine-type drugs; accordingly, glutamatergic mechanisms are involved. For instance, the acquisition of a diazepam-induced conditioned place preference was attenuated by pretreatment with a glutamate receptor antagonist (63), suggesting that glutamate contributes to the rewarding or reinforcing effects of benzodiazepines.
With respect to tolerance and dependence, glutamate has been implicated in the hypothesis that in order to compensate for benzodiazepine-induced enhancement of inhibition, excitatory mechanisms become more sensitive. This sensitivity is manifested as over-activity upon withdrawal (106, 177). Further support for glutamatergic mechanisms in these behaviors has been demonstrated by the disruption of the development of tolerance and dependence (179) as well as the effects of withdrawal (174) following administration of glutamate receptor antagonists. Moreover, both NMDA and AMPA receptors have been shown to be regulated following chronic benzodiazepine treatment. Specifically, cortical levels of the NR1 and NR2B, but not NR2A, subunits of the NMDA receptor (190) and the GluR1 subunit of the AMPA receptor were increased in diazepam-withdrawn rats compared to controls (89). Similarly, in rats withdrawn from flurazepam, AMPA receptor-mediated miniature excitatory postsynaptic current amplitude was increased in hippocampal CA1 neurons (193, 211). A 50% enhancement in AMPA receptor function was attributed to an increase in GluR1 protein trafficking from the endoplasmic reticulum and subsequent incorporation into membranes (173), while NMDA receptor-mediated currents were reduced in this brain region (193, 211). In contrast to those studies, expression of the AMPA receptor subunits was decreased in the amygdala (GluR1 and GluR2) and limbic regions (GluR1; 2). Interestingly, the contribution of AMPA and NMDA receptor mechanisms may be regulated temporally such that each is involved at specific time points during the expression of withdrawal and development of tolerance, respectively (89). Similar findings have been observed in long-term potentiation and kindling, which like the neuroadaptive processes associated with the consumption of drugs of abuse, are forms of synaptic plasticity (15). However, whether or not the involvement of glutamate in the abuse and dependence liability of benzodiazepine-type drugs is similar to that observed with other drugs of abuse (e.g. psychostimulants), remains relatively unexplored.
Intracellular Signaling Molecule Adaptations Following Benzodiazepine Administration
In addition to benzodiazepine-induced receptor neuroadaptations, a recent study implemented microarray analysis to evaluate systematically the downstream signaling events following acute exposure to diazepam (85). Results demonstrated that in wild-type mice, diazepam reduced the transcripts of genes involved in regulating synaptic functions and plasticity, such as calcium/calmodulin-dependent kinase IIα (CaMKIIα; for review, see 172) and brain derived neurotrophic factor (BDNF; for review, see 21). Activation of CaMKIIα has been shown previously to be involved in the phosphorylation of the α1 subunit of the GABAA receptor, which subsequently regulated the binding of allosteric modulators to the receptor (31), and enhanced the inhibitory synaptic potential (200). Down regulation of CaMKIIα following exposure to diazepam, therefore, may contribute to the overall down regulation of the GABAA receptor and GABA sensitivity observed following prolonged exposure to benzodiazepines. Similarly, since BDNF has been shown to regulate the expression of cell surface GABAA receptors (24,124), down regulation of BDNF may reduce GABAA receptor turnover. Although this study examined only an acute dose of diazepam (85), other evidence exists demonstrating that a single exposure to diazepam can have significant effects on GABAA receptor function (82).
Interestingly, the transcriptional regulation of those genes, as well as approximately 50 others, appears to be mediated by an α1GABAA receptor-dependent mechanism (85). Compared to wild-type mice, the observed changes in transcript levels following administration of diazepam were not exhibited in mice that were mutated in order to render the α1GABAA receptor insensitive to diazepam (155). These findings may have implications for the signaling events associated with the sedative actions of benzodiazepine-type drugs, since there is a body of evidence suggesting that α1GABAA receptors are responsible for mediating these effects (113, 139,155). Similarly, these signaling cascades may be involved in the abuse-related effects of benzodiazepines since α1GABAA receptors appear to be intricately involved in their reinforcing effects (8, 69, 152,153). Indeed, both CamKIIα (e.g., 104, 131) and BDNF (e.g., 25, 35) have been demonstrated to play prominent roles in the plasticity believed to underlie the addictive potential of drugs of abuse. Together, these results are just some examples of how intracellular events may function as the liaison between allosteric modulation of GABAA receptors by benzodiazepines and behavior—again, a relatively unexplored area of research.
SUMMARY AND CONCLUSIONS
Of the diverse types of ligands that act at the GABAA receptor, the benzodiazepines and related drugs are unique in having widespread clinical use and the liability for abuse and dependence. Laboratory findings suggest that benzodiazepine-type drugs have reinforcing effects both in human and non-human subjects, and recent epidemiological data suggests that abuse of benzodiazepine-type drugs may be on the rise.
Recent research has begun to explore the role of GABAA receptor subtypes in the reinforcing effects of benzodiazepine-type drugs, and unlike other behavioral effects (e.g. motor coordination deficits) reinforcing effects are not easily attributed to a single receptor subtype. Perhaps the most firm conclusion at this point is that α1GABAA receptors are not necessary for self-administration of benzodiazepine-type compounds, although they might be sufficient. Research with more selective compounds that are full agonists for different subtypes clearly is needed to resolve some of the issues with our understanding of the reinforcing effects of benzodiazepine-type drugs.
In addition to reinforcing effects, it is well-documented that chronic exposure to benzodiazepines results in physical dependence, characterized by a withdrawal syndrome. Regarding receptor mechanisms, initial studies suggested that α1GABAA selective agonists are devoid of physical dependence liability, whereas the most recent findings in humans and non-human primates indicate that long-term use of these compounds can be associated with physical dependence. Moreover, studies examining benzodiazepine-induced changes in receptor composition primarily have demonstrated alterations in the α1 subunit. Accordingly, preliminary results suggest that compounds with selectivity for α2GABAA, α3GABAA, and/or α5GABAA receptors do not induce physical dependence, although these findings are complicated by the relatively low intrinsic efficacy of these ligands. As with reinforcing effects, systematic studies with selective compounds having relatively high intrinsic efficacy at particular subtypes should shed light on these important mechanistic issues.
In conclusion, the literature reviewed suggests that the abuse potential of benzodiazepine-type drugs is becoming an increasingly important issue to address on many levels. In the future, the epidemiology of benzodiazepine-type drug abuse should encourage empirical investigations regarding the behavioral phenomena associated with abuse potential, i.e. reinforcing effects, manifestations of tolerance and dependence. The development of new ligands should facilitate a better understanding of the GABAA receptor mechanisms underlying these behavioral effects. As new compounds become available, issues of cross-tolerance also need to be investigated. For example, it is not known the extent to which there is cross-tolerance between the new subtype-selective benzodiazepine ligands and conventional benzodiazepines (or alcohol for that matter) with respect to either the therapeutic or limiting effects of these drugs. Further, these pharmacological tools should be used to probe more comprehensively the cellular and molecular events that accompany the abuse-related effects associated with the administration of benzodiazepine-type drugs. Together, these investigations will help elucidate how benzodiazepine-type drugs exert their abuse and dependence liability, thus informing drug design strategies in order to develop safer and more effective anxiolytics and sleep-aids.
Acknowledgments
Preparation of this manuscript was supported by U.S.P.H.S. grants DA023659, DA11792, and RR00168.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Allison C, Claase LA, Pratt JA. Diazepam withdrawal-induced anxiety and place aversion in the rat: differential effects of two chronic diazepam treatment regimes. Behav Pharmacol. 2002;13:417–25. doi: 10.1097/00008877-200209000-00015. [DOI] [PubMed] [Google Scholar]
- 2.Allison C, Pratt JA. Differential effects of two chronic diazepam treatment regimes on withdrawal anxiety and AMPA receptor characteristics. Neuropsychopharmacology. 2006;31:602–19. doi: 10.1038/sj.npp.1300800. [DOI] [PubMed] [Google Scholar]
- 3.American Druggist. Top 200 drugs of 1995. New York: Hearst Corp.; 1996. [Google Scholar]
- 4.Arbilla S, Depoortere H, George P, Langer SZ. Pharmacological profile of the imidazopyridine zolpidem at benzodiazepine receptors and electrocorticogram in rats. Naunyn Schmiedebergs Arch Pharmacol. 1985;330:248–51. doi: 10.1007/BF00572441. [DOI] [PubMed] [Google Scholar]
- 5.Ashton H. Protracted withdrawal syndromes from benzodiazepines. J Subst Abuse Treat. 1991;8:19–28. doi: 10.1016/0740-5472(91)90023-4. [DOI] [PubMed] [Google Scholar]
- 6.Atack JR, Wafford KA, Tye SJ, Cook SM, Sohal B, Pike A, et al. TPA023 [7- (1,1-dimethylethyl)-6-(2-ethyl-2H-1,2,4-triazol-3-ylmethoxyl)-3-(2-fluorophenyl)-1,2,4- triazolo[4,3-b]pyridzine],an agonist selective for alpha2- and alpha3-containing GABAA receptors, is a nonsedating anxiolytic in rodents and primates. J Pharmacol Exp Ther. 2006;316:410– 22. doi: 10.1124/jpet.105.089920. [DOI] [PubMed] [Google Scholar]
- 7.Ator NA. Zaleplon and triazolam: drug discrimination, plasma levels, and self- administration in baboons. Drug Alcohol Depend. 2000;24:55–68. doi: 10.1016/s0376-8716(00)00123-x. [DOI] [PubMed] [Google Scholar]
- 8.Ator NA. Contributions of GABAA receptor subtype selectivity to abuse liability and dependence potential of pharmacological treatments for anxiety and sleep disorders. CNS Spectr. 2005;10:31–9. doi: 10.1017/s1092852900009883. [DOI] [PubMed] [Google Scholar]
- 9.Ator NA, Griffiths RR. Self-administration of barbiturates and benzodiazepines: A review. Pharmacol Biochem Behav. 1987;27:391–8. doi: 10.1016/0091-3057(87)90588-0. [DOI] [PubMed] [Google Scholar]
- 10.Ator NA, Griffiths RR. Principles of drug abuse liability assessment in laboratory animals. Drug Alcohol Depend. 2003;70:S55–S72. doi: 10.1016/s0376-8716(03)00099-1. [DOI] [PubMed] [Google Scholar]
- 11.Ator NA, Weerts EM, Kaminski BJ, Kautz MA, Griffiths RR. Zaleplon and triazolam physical dependence assessed across increasing doses under a once-daily dosing regimen in baboons. Drug Alcohol Depend. 2000;61:69–84. doi: 10.1016/s0376-8716(00)00122-8. [DOI] [PubMed] [Google Scholar]
- 12.Baler RD, Volkow ND. Drug addiction: the neurobiology of disrupted self-control. Trends Mol Med. 2006;12:559–66. doi: 10.1016/j.molmed.2006.10.005. [DOI] [PubMed] [Google Scholar]
- 13.Bardo MT. Neuropharmacological mechanisms of drug reward: beyond dopamine in the nucleus accumbens. Crit Rev Neurobiol. 1998;12:37–67. doi: 10.1615/critrevneurobiol.v12.i1-2.30. [DOI] [PubMed] [Google Scholar]
- 14.Bateson AN. The benzodiazepine site of the GABAA receptor: an old target with new potential? Sleep Med. 2004;5 (Suppl 1):S9–S15. doi: 10.1016/s1389-9457(04)90002-0. [DOI] [PubMed] [Google Scholar]
- 15.Baudry M. Long-term potentiation and kindling: similar biochemical mechanisms? Adv Neurol. 1986;44:401–10. [PubMed] [Google Scholar]
- 16.Benavides J, Peny B, Dubois A, Perrault G, Morel E, Zivkovic B, et al. In vivo interaction of zolpidem with central benzodiazepine (BZD) binding sites (as labeled by [3H]Ro 15-1788) in the mouse brain. Preferential affinity of zolpidem for the omega 1 (BZD1) subtype. J Pharmacol Exp Ther. 1988;245:1033–41. [PubMed] [Google Scholar]
- 17.Bergman J, Johanson CE. The reinforcing properties of diazepam under several conditions in the rhesus monkey. Psychopharmacology. 1985;86:108–13. doi: 10.1007/BF00431693. [DOI] [PubMed] [Google Scholar]
- 18.Bernik MA, Gorenstein C, Gentil V. Flumazenil-precipitated withdrawal symptoms in chronic users of therapeutic doses of diazepam. J Psychopharmacol. 1991;5:215–19. doi: 10.1177/026988119100500306. [DOI] [PubMed] [Google Scholar]
- 19.Bernik MA, Gorenstein C, Vieira Filho AHG. Stressful reactions and panic attacks induced by flumazenil in chronic benzodiazepine users. J Psychopharmacol. 1998;12:146–50. doi: 10.1177/026988119801200205. [DOI] [PubMed] [Google Scholar]
- 20.Besset A, Tafti M, Villemin E, Borderies P, Billiard M. Effects of zolpidem on the architecture and cyclical structure of sleep in poor sleepers. Drugs Exp Clin Res. 1995;21:161–9. [PubMed] [Google Scholar]
- 21.Binder DK, Scarfman HE. Brain-derived neurotrophic factor. Growth Factors. 2004;22:123–31. doi: 10.1080/08977190410001723308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Blois R, Gaillard JM, Attali P, Coquelin JP. Effect of zolpidem on sleep in healthy subjects: a placebo-controlled trial with polysomnographic recordings. Clin Ther. 1993;15:797–809. [PubMed] [Google Scholar]
- 23.Broadbear JH, Winger G, Woods JH. Self-administration of methohexital, midazolam and ethanol: effects on the pituitary-adrenal axis in rhesus monkeys. Psychopharmacology. 2005;178:83–91. doi: 10.1007/s00213-004-1986-4. [DOI] [PubMed] [Google Scholar]
- 24.Brünig I, Penschuck S, Berninger B, Benson J, Fritschy JM. BDNF reduces miniature inhibitory postsynaptic currents by rapid downregulation of GABA(A) receptor surface expression. Eur J Neurosci. 2001;13:1320–28. doi: 10.1046/j.0953-816x.2001.01506.x. [DOI] [PubMed] [Google Scholar]
- 25.Butovsky E, Juknat A, Goncharov I, Elbaz J, Eilam R, Zangen A. In vivo up- regulation of brain-derived neurotrophic factor in specific brain areas by chronic exposure to Delta-tetrahydrocannabinol. J Neurochem. 2005;93:802–11. doi: 10.1111/j.1471-4159.2005.03074.x. [DOI] [PubMed] [Google Scholar]
- 26.Cammarano WB, Pittet JF, Weitz S, Schlobohm RM, Marks JD. Acute withdrawal syndrome related to the administration of analgesic and sedative medications in adult intensive care unit patients. Crit Care Med. 1998;26:676–84. doi: 10.1097/00003246-199804000-00015. [DOI] [PubMed] [Google Scholar]
- 27.Campo-Soria C, Chang Y, Weiss DS. Mechanism of action of benzodiazepines on GABAA receptors. Br J Pharmacol. 2006;148:984–90. doi: 10.1038/sj.bjp.0706796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Carnevale FA, Ducharme C. Adverse reactions to the withdrawal of opioids and benzodiazepines in paediatric intensive care. Intensive Crit Care Nurs. 1997;13:181–8. doi: 10.1016/s0964-3397(97)80012-2. [DOI] [PubMed] [Google Scholar]
- 29.Carelli RM. The nucleus accumbens and reward: neurophysiological investigations in behaving animals. Behav Cogn Neurosci Rev. 2002;1:281–96. doi: 10.1177/1534582302238338. [DOI] [PubMed] [Google Scholar]
- 30.Carlson JN, Haskew R, Wacker J, Maisonneuve IM, Glick SD, Jerussi TP. Sedative and anxiolytic effects of zopiclone’s enantiomers and metabolite. Eur J Pharmacol. 2001;415:181–9. doi: 10.1016/s0014-2999(01)00851-2. [DOI] [PubMed] [Google Scholar]
- 31.Churn SB, Rana A, Lee K, Parsons JT, De Blas A, Delorenzo RJ. Calcium/calmodulin-dependent kinase II phosphorylation of the GABAA receptor alpha1 subunit modulates benzodiazepine binding. J Neurochem. 2002;82:1065–76. doi: 10.1046/j.1471-4159.2002.01032.x. [DOI] [PubMed] [Google Scholar]
- 32.Ciraulo DA, Sands BF, Shader RI. Critical review of liability for benzodiazepine abuse among alcoholics. Am J Psychiatry. 1988;145:1501–06. doi: 10.1176/ajp.145.12.1501. [DOI] [PubMed] [Google Scholar]
- 33.Collinson N, Kuenzi FM, Jarolimek W, Maubach KA, Cothliff R, Sur C, et al. Enhanced learning and memory altered GABAergic synaptic transmission in mice lacking the α5 subunit of the GABAA receptor. J Neurosci. 2002;22:5572–80. doi: 10.1523/JNEUROSCI.22-13-05572.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Concas A, Serra M, Santoro G, Maciocco E, Cuccheddu T, Biggio G. The effect of cyclopyrrolones on GABAA receptor function is different from that of benzodiazepines. Naunyn Schmiedebergs Arch Pharmacol. 1994;350:294–300. doi: 10.1007/BF00175035. [DOI] [PubMed] [Google Scholar]
- 35.Corominas M, Roncero C, Ribases M, Castells X, Casas M. Brain-derived neurotrophic factor and its intracellular signaling pathways in cocaine addiction. Neuropsychobiology. 2007;55:2–13. doi: 10.1159/000103570. [DOI] [PubMed] [Google Scholar]
- 36.Cowley DS, Roy-Byrne PP, Radant A, Ritchie JC, Greenblatt DJ, Nemeroff CB, et al. Benzodiazepine sensitivity in panic disorder: effects of chronic alprazolam treatment. Neuropsychopharmacology. 1995;12:147–57. doi: 10.1016/0893-133X(94)00074-A. [DOI] [PubMed] [Google Scholar]
- 37.Crane EH, Nemanski N. Demographic characteristics of benzodiazepine-involved ED visits. The DAWN Report, Office of Applied Studies, US Substance Abuse and Mental Health Services Administration; 2004. [Google Scholar]
- 38.Crawley JN, Marangos PJ, Stivers J, Goodwin FK. Chronic clonazepam administration induces benzodiazepine receptor subsensitivity. Neuropharmacology. 1982;21:85–9. doi: 10.1016/0028-3908(82)90216-7. [DOI] [PubMed] [Google Scholar]
- 39.Crestani F, Keist R, Fritschy J-M, Benke D, Vogt K, Prut L, et al. Trace fear conditioning involves hippocampal α5 GABAA receptors. Proc Natl Acad Sci USA. 2002;99:8980–5. doi: 10.1073/pnas.142288699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Darke SG, Ross JE, Hall WD. Benzodiazepine use among injecting heroin users. Med J Aust. 1995;162:645–7. doi: 10.5694/j.1326-5377.1995.tb126051.x. [DOI] [PubMed] [Google Scholar]
- 41.Darke SG, Swift W, Hall W, Ross M. Drug use, HIV risk-taking and psychosocial correlates of benzodiazepine use among methadone maintenance clients. Drug Alcohol Depend. 1994;34:67–70. doi: 10.1016/0376-8716(93)90047-t. [DOI] [PubMed] [Google Scholar]
- 41a.Dawson GR, Collinson N, Atack JR. Development of subtype selective GABAA modulators. CNS Spectr. 2005;10:21–7. doi: 10.1017/s1092852900009871. [DOI] [PubMed] [Google Scholar]
- 42.Depoortere H, Zivkovic B, Llyod KG, Sanger DJ, Perrault G, Langer SZ, et al. Zolpidem, a novel nonbenzodiazepine hypnotic. I. Neuropharmacological and behavioral effects. J Pharmacol Exp Ther. 1986;237:649–58. [PubMed] [Google Scholar]
- 43.de Wit H, Doty P. Preference for ethanol and diazepam in light and moderate social drinkers: a within-subjects study. Psychopharmacology. 1993;115:529–38. doi: 10.1007/BF02245577. [DOI] [PubMed] [Google Scholar]
- 44.Di Chiara G, Acquas E, Tanda G, Cadoni C. Drugs of abuse: biochemical surrogates of specific aspects of natural reward? Biochem Soc Symp. 1993;59:65–81. [PubMed] [Google Scholar]
- 45.Di Chiara G, Imperato A. Drugs abused by humans preferentially increase synaptic dopamine concentrations in the mesolimbic dopamine system of freely moving rats. Proc Natl Acad Sci USA. 1988;85:5274–78. doi: 10.1073/pnas.85.14.5274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Diehl JL, Guillibert E, Guerot E, Kimounn E, Labrousse J. Acute benzodiazepine withdrawal delirium after a short course of flunitrazepam in an intensive care patient. Ann Med Interne (Paris) 2000;151 (Suppl A):A44–A46. [PubMed] [Google Scholar]
- 47.Doble A. New insights into the mechanism of action of hypnotics. J Psychopharmacol. 1999;13 (4 Suppl 1):S11–S20. doi: 10.1177/026988119901304S03. [DOI] [PubMed] [Google Scholar]
- 48.Dooley M, Plosker GL. Zaleplon: a review of its use in the treatment of insomnia. Drugs. 2000;60:413–45. doi: 10.2165/00003495-200060020-00014. [DOI] [PubMed] [Google Scholar]
- 49.Dundee JW, Halliday NJ, Harper KW, Brogden RN. Midazolam: A review of its pharmacological properties and therapeutic use. Drugs. 1984;28:519–43. doi: 10.2165/00003495-198428060-00002. [DOI] [PubMed] [Google Scholar]
- 49a.Dunner DL, Ishiki D, Avery DH, Wilson LG, Hyde TS. Effect of alprazolam and diazepam on anxiety and panic attacks in panic disorder: a controlled study. J Clin Psychiatry. 1986;47:458–60. [PubMed] [Google Scholar]
- 50.Du Pont RL. Abuse of benzodiazepines: the problems and the solutions. Am J Drug Alcohol Abuse. 1988;14 (suppl 1):1–69. [PubMed] [Google Scholar]
- 51.Elliott EE, White JM. Precipitated and spontaneous withdrawal following administration of lorazepam but not zolpidem. Pharmacol Biochem Behav. 2000;66:361–9. doi: 10.1016/s0091-3057(00)00176-3. [DOI] [PubMed] [Google Scholar]
- 52.Erman MK. Therapeutic options in the treatment of insomnia. J Clin Psychiatry. 2005;66 (Suppl 9):18–23. [PubMed] [Google Scholar]
- 53.Ettenberg A, Pettit HO, Bloom FE, Koob GF. Heroin and cocaine intravenous self-administration in rats: mediation by separate neural systems. Psychopharmacology. 1982;78:204–9. doi: 10.1007/BF00428151. [DOI] [PubMed] [Google Scholar]
- 54.Evans SM, Griffiths RR, de Wit H. Preference for diazepam, but not buspirone, in moderate drinkers. Psychopharmacology. 1996;123:154–63. doi: 10.1007/BF02246172. [DOI] [PubMed] [Google Scholar]
- 55.Finlay JM, Damsma G, Fibiger HC. Benzodiazepine-induced decreases in extracellular concentrations of dopamine in the nucleus accumbens after acute and repeated administration. Psychopharmacology. 1992;106:202–8. doi: 10.1007/BF02801973. [DOI] [PubMed] [Google Scholar]
- 56.Fisch HU, Baktir G, Karlaganis G, Minder C, Bircher J. Excessive motor impairment two hours after triazolam in the elderly. Eur J Clin Pharmacol. 1990;38:229–32. doi: 10.1007/BF00315021. [DOI] [PubMed] [Google Scholar]
- 57.Follesa P, Cagetti E, Mancuso L, Biggio F, Manca A, Maciocco E, et al. Increase in expression of the GABAA receptor α4 subunit gene induced by withdrawal of, but not long-term treatment with, benzodiazepine full or partial agonists. Mol Brain Res. 2001;92:138–48. doi: 10.1016/s0169-328x(01)00164-4. [DOI] [PubMed] [Google Scholar]
- 58.Fontaine R, Mercier P, Beaudry P, Annable L, Chouinard G. Bromazepam and lorazepam in generalized anxiety: a placebo-controlled study with measurement of drug plasma concentrations. Acta Psychiatr Scand. 1986;74:451–58. doi: 10.1111/j.1600-0447.1986.tb06268.x. [DOI] [PubMed] [Google Scholar]
- 59.Forsyth AJM, Farquhar D, Gemmell M, Shewan D, Davies JB. The dual use of opioids and temazepam by drug injectors in Glasgow (Scotland) Drug Alcohol Depend. 1993;32:277–80. doi: 10.1016/0376-8716(93)90092-5. [DOI] [PubMed] [Google Scholar]
- 60.Franck LS, Naughton I, Winter I. Opioid and benzodiazepine withdrawal symptoms in paediatric intensive care patients. Intensive Crit Care Nurs. 2004;20:344–51. doi: 10.1016/j.iccn.2004.07.008. [DOI] [PubMed] [Google Scholar]
- 61.Gallager DW, Lakoski JM, Gonsalves SF, Rauch SL. Chronic benzodiazepine treatment decreases postsynaptic GABA sensitivity. Nature. 1984;308:74–7. doi: 10.1038/308074a0. [DOI] [PubMed] [Google Scholar]
- 62.Gelkopf M, Bleich A, Hayward R, Bodner G, Adelson M. Characteristics of benzodiazepine abuse in methadone maintenance treatment patients: a 1 year prospective study in an Israeli clinic. Drug Alcohol Depend. 1999;55:63–8. doi: 10.1016/s0376-8716(98)00175-6. [DOI] [PubMed] [Google Scholar]
- 63.Gray A, Allison C, Pratt JA. A role for AMPA/kainite receptors in conditioned place preferences induced by diazepam in the rat. Neurosci Lett. 1999;268:127–30. doi: 10.1016/s0304-3940(99)00371-7. [DOI] [PubMed] [Google Scholar]
- 64.Greenblatt DJ, Harmatz JS, von Moltke LL, Ehrenberg BL, Harrel L, Corbett K, et al. Comparative kinetics and dynamics of zaleplon, zolpidem, and placebo. Clin Pharmacol Ther. 1998;64:553–61. doi: 10.1016/S0009-9236(98)90139-4. [DOI] [PubMed] [Google Scholar]
- 65.Greenblatt DJ, Shader RI, Divoll M, Harmatz JS. Benzodiazepines: a summary of pharmacokinetic properties. Br J Clin Pharmacol. 1981;11 (Suppl 1):11S–16S. doi: 10.1111/j.1365-2125.1981.tb01833.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Griffiths RR, Bigelow GE, Ator NA. Principles of initial experimental drug abuse liability assessment in humans. Drug Alcohol Depend. 2003;70:S41–S54. doi: 10.1016/s0376-8716(03)00098-x. [DOI] [PubMed] [Google Scholar]
- 67.Griffiths RR, Johnson MW. Relative abuse liability of hypnotic drugs: a conceptual framework and algorithm for differentiating among compounds. J Clin Psychiatry. 2005;66 (Suppl 9):31–41. [PubMed] [Google Scholar]
- 68.Griffiths RR, Lamb RJ, Sannerud CA, Ator NA, Brady JV. Self-injection of barbiturates, benzodiazepines and other sedative-anxiolytics in baboons. Psychopharmacology. 1991;103:154–61. doi: 10.1007/BF02244196. [DOI] [PubMed] [Google Scholar]
- 69.Griffiths RR, Sannerud CA, Ator NA, Brady JV. Zolpidem behavioral pharmacology in baboons: self-injection, discrimination, tolerance and withdrawal. J Pharmacol Exp Ther. 1992;260:1199–1208. [PubMed] [Google Scholar]
- 70.Griffiths RR, Weerts EM. Benzodiazepine self-administration in humans and laboratory animals-implications for problems of long-term use and abuse. Psychopharmacology. 1997;134:1–37. doi: 10.1007/s002130050422. [DOI] [PubMed] [Google Scholar]
- 71.Griffiths RR, Wolf B. Relative abuse liability of different benzodiazepines in drug abusers. J Clin Psychopharmacol. 1990;10:237–43. [PubMed] [Google Scholar]
- 72.Hadingham KL, Wingrove P, Le Bourdelles B, Palmer KJ, Ragan CI, Whiting PJ. Cloning of cDNA sequences encoding human α2 and α3 γ-aminobutyric acidA receptor subunits and characterization of the benzodiazepine pharmacology of recombinant α1-, α2-, α3-, and α5-containing human γ-aminobutyric acidA receptors. Mol Pharmacol. 1993;43:970–5. [PubMed] [Google Scholar]
- 73.Hajak G, Muller WE, Wittchen HU, Pittrow D, Kirch W. Abuse and dependence potential for the non-benzodiazepine hypnotics zolpidem and zopiclone: a review of case reports and epidemiological data. Addiction. 2003;98:1371–8. doi: 10.1046/j.1360-0443.2003.00491.x. [DOI] [PubMed] [Google Scholar]
- 74.Harrison-Read PE, Tyrer P, Lawson C, Lack S, Fernandes C, File SE. Flumazenil-precipitated panic and dysphoria in patients dependent on benzodiazepines: a possible aid to abstinence. J Psychopharmacol. 1996;10:89–97. doi: 10.1177/026988119601000201. [DOI] [PubMed] [Google Scholar]
- 75.Helmus TC, Tancer M, Johanson CE. Reinforcing effects of diazepam under anxiogenic conditions in individuals with social anxiety. Exp Clin Psychopharmacol. 2005;13:348–56. doi: 10.1037/1064-1297.13.4.348. [DOI] [PubMed] [Google Scholar]
- 76.Heninger C, Saito N, Talleman JF, Garrett KM, Vitek MP, Duman RS, et al. Effect of continuous diazepam administration on GABAA subunit mRNA in rat brain. J Mol Neurosci. 1990;2:101–7. doi: 10.1007/BF02876917. [DOI] [PubMed] [Google Scholar]
- 77.Hernandez TD, Heninger C, Wilson MA, Gallager DW. Relationship of agonist efficacy to changes in GABA sensitivity and anticonvulsant tolerance following chronic benzodiazepine ligand exposure. Eur J Pharmacol. 1989;170:145–55. doi: 10.1016/0014-2999(89)90535-9. [DOI] [PubMed] [Google Scholar]
- 78.Hewlett WA, Vinogradov S, Agras WS. Clonazepam treatment of obsessions and compulsions. J Clin Psychiatry. 1990;51:158–61. [PubMed] [Google Scholar]
- 79.Hewlett WA, Vinogradov S, Agras WS. Clomipramine, clonazepam, and clonidine treatment of obsessive-compulsive disorder. J Clin Psychopharmacol. 1992;12:420–30. [PubMed] [Google Scholar]
- 80.Hollister L, Muller-Oerlinghausen B, Rickels K, Shader R. Clinical uses of benzodiazepines. J Clin Psychpharmacol. 1993;13 (Suppl 1):1–169. [PubMed] [Google Scholar]
- 81.Holt RA, Bateson AN, Martin IL. Chronic treatment with diazepam or abecarnil differently affects the expression of GABAA receptor subunit mRNAs in the rat cortex. Neuropharmacology. 1996;35:1457–63. doi: 10.1016/s0028-3908(96)00064-0. [DOI] [PubMed] [Google Scholar]
- 82.Holt RA, Bateson AN, Martin IL. Decreased GABA enhancement of benzodiazepine binding after a single dose of diazepam. J Neurochem. 1999;72:2219–22. doi: 10.1046/j.1471-4159.1999.0722219.x. [DOI] [PubMed] [Google Scholar]
- 83.Holt RA, Martin IL, Bateson AN. Chronic diazepam exposure decreases transcription of the rat GABAA receptor γ2-subunit gene. Mol Brain Res. 1997;48:164–6. doi: 10.1016/s0169-328x(97)00129-0. [DOI] [PubMed] [Google Scholar]
- 84.Hu XJ, Ticku MJ. Chronic benzodiazepine agonist treatment produces functional uncoupling of the γ-aminobutyric acid-benzodiazepine receptor ionophore complex in cortical neurons. Mol Pharmacol. 1994;45:618–25. [PubMed] [Google Scholar]
- 85.Huopaniemi L, Keist R, Randolph A, Certa U, Rudoph U. Diazepam-induced adaptive plasticity revealed by α1 GABAA receptor-specific expression profiling. J Neurochem. 2004;88:1059–67. doi: 10.1046/j.1471-4159.2003.02216.x. [DOI] [PubMed] [Google Scholar]
- 86.Iguchi MY, Handelsman L, Bickel WK, Griffiths RR. Benzodiazepine and sedative use/abuse by methadone maintenance clients. Drug Alcohol Depend. 1993;32:257–66. doi: 10.1016/0376-8716(93)90090-d. [DOI] [PubMed] [Google Scholar]
- 87.Impagnatiello F, Pesold C, Longone P, Caruncho H, Fritschy JM, Costa E, et al. Modifications of γ-aminobutyric acid receptor subunit expression in rat neocortex during tolerance to diazepam. Mol Pharmacol. 1996;49:822–31. [PubMed] [Google Scholar]
- 88.Invernizzi R, Pozzi L, Samanin R. Release of dopamine is reduced by diazepam more in the nucleus accumbens than in the caudate nucleus of conscious rats. Neuropharmacology. 1991;30:575–8. doi: 10.1016/0028-3908(91)90075-m. [DOI] [PubMed] [Google Scholar]
- 89.Izzo E, Auta J, Impagnatiello F, Pesold C, Guidotti A, Costa E. Glutamic acid decarboxylase and glutamate receptor changes during tolerance and dependence to benzodiazepines. Proc Natl Acad Sci USA. 2001;98:3483–8. doi: 10.1073/pnas.051628698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Jaffe JH, Bloor R, Crome I, Carr M, Alam F, Simmons A, et al. A postmarketing study of relative abuse liability of hypnotic sedative drugs. Addiction. 2004;99:165–73. doi: 10.1111/j.1360-0443.2003.00631.x. [DOI] [PubMed] [Google Scholar]
- 91.Jindal RD, Thase ME. Treatment of insomnia associated with clinical depression. Sleep Med Rev. 2004;8:19–30. doi: 10.1016/S1087-0792(03)00025-X. [DOI] [PubMed] [Google Scholar]
- 92.Kales A, Kales JD. Sleep laboratory studies of hypnotic drugs: efficacy and withdrawal effects. J Clin Psychopharmacol. 1983;3:140–50. doi: 10.1097/00004714-198304000-00038. [DOI] [PubMed] [Google Scholar]
- 93.Kalivas PW, Volkow ND. The neural basis of addiction: a pathology of motivation and choice. Am J Psychiatry. 2005;162:1403–13. doi: 10.1176/appi.ajp.162.8.1403. [DOI] [PubMed] [Google Scholar]
- 94.Kamel NS, Gammack JK. Insomnia in the elderly: cause, approach, and treatment. Am J Med. 2006;119:463–9. doi: 10.1016/j.amjmed.2005.10.051. [DOI] [PubMed] [Google Scholar]
- 95.Klein RL, Harris PA. Regulation of GABAA receptor structure and function by chronic drug treatments in vivo and with stably transfected cells. Jpn J Pharmacol. 1996;70:1–15. doi: 10.1254/jjp.70.1. [DOI] [PubMed] [Google Scholar]
- 96.Korpi ER, Mattila MJ, Wisden W, Lüddens H. GABAA-receptor subtypes: clinical efficacy and selectivity of benzodiazepine-site ligands. Ann Med. 1997;29:275–82. doi: 10.3109/07853899708999348. [DOI] [PubMed] [Google Scholar]
- 97.Krystal AD, Walsh JK, Laska E, Caron J, Amato DA, Wessel TC, et al. Sustained efficacy of eszopiclone over 6 months of nightly treatment: results of a randomized, double-blind, placebo-controlled study in adults with chronic insomnia. Sleep. 2003;26:793–9. doi: 10.1093/sleep/26.7.793. [DOI] [PubMed] [Google Scholar]
- 98.Lacerra C, Martijena ID, Bustos SG, Molina VA. Benzodiazepine withdrawal facilitates the subsequent onset of escape failures and anhedonia: influence of different antidepressant drugs. Brain Res. 1999;819:40–7. doi: 10.1016/s0006-8993(98)01341-9. [DOI] [PubMed] [Google Scholar]
- 99.Lago JA, Kosten TR. Stimulant withdrawal. Addiction. 1994;89:1477–81. doi: 10.1111/j.1360-0443.1994.tb03746.x. [DOI] [PubMed] [Google Scholar]
- 100.Lavoisy J, Zivkovic B, Benavides J, Perrault GH, Rober P. Contribution of zolpidem in the management of sleep disorders. Encephale. 1992;18:379–82. [PubMed] [Google Scholar]
- 101.Lelas S, Spealman RD, Rowlett JK. Using behavior to elucidate receptor mechanisms: A review of the discriminative stimulus effects of benzodiazepines. Exp Clin Psychopharmacol. 2000;8:294–311. doi: 10.1037//1064-1297.8.3.294. [DOI] [PubMed] [Google Scholar]
- 102.Leshner AI, Koob GF. Drugs of abuse and the brain. Proc Assoc Am Physicians. 1999;111:99–108. doi: 10.1046/j.1525-1381.1999.09218.x. [DOI] [PubMed] [Google Scholar]
- 103.Liappas IA, Malitas PN, Dimopoulos NP, Gitsa OE, Liappas AI Nikolau ChK, et al. Zolpidem dependence case series: possible neurobiological mechanisms and clinical management. J Psychopharmacol. 2003;17:131–5. doi: 10.1177/0269881103017001723. [DOI] [PubMed] [Google Scholar]
- 104.Licata SC, Schmidt HD, Pierce RC. Suppressing calcium/calmodulin-dependent kinase II activity in the ventral tegmental area enhances the acute behavioural response to cocaine but attenuates the initiation of cocaine- induced behavioural sensitization in rats. Eur J Neurosci. 2004;19:405–14. doi: 10.1111/j.0953-816x.2003.03110.x. [DOI] [PubMed] [Google Scholar]
- 105.Lintzeris N, Mitchell TB, Bond AJ, Nestor L, Strang J. Pharmacodynamics of diazepam co-administered with methadone or buprenorphine under high dose conditions in opioid dependent patients. Drug Alcohol Depend. 2007;91:187–94. doi: 10.1016/j.drugalcdep.2007.05.019. [DOI] [PubMed] [Google Scholar]
- 106.Little HJ, Gale R, Sellars N, Nutt DJ, Taylor SC. Chronic benzodiazepine treatment increases the effects of the inverse agonist FG 7142. Neuropharmacology. 1988;27:383–9. doi: 10.1016/0028-3908(88)90147-5. [DOI] [PubMed] [Google Scholar]
- 107.Littrell RA, Hayes LR, Stillner V. Carisoprodol (Soma): a new and cautious perspective on an old agent. South Med J. 1993;86:753–6. doi: 10.1097/00007611-199307000-00006. [DOI] [PubMed] [Google Scholar]
- 108.Londborg PD, Smith WT, Glaudin V, Painter JR. Short-term cotherapy with clonazepam and fluoxetine: anxiety, sleep disturbance and core symptoms of depression. J Affect Disord. 2000;61:73–9. doi: 10.1016/s0165-0327(99)00195-0. [DOI] [PubMed] [Google Scholar]
- 109.Longone P, Impagnatiello F, Guidotti A, Costa E. Reversible modification of GABAA receptor subunit mRNA expression during tolerance to diazepam-induced cognition dysfunction. Neuropharmacology. 1996;35:1465–73. doi: 10.1016/s0028-3908(96)00071-8. [DOI] [PubMed] [Google Scholar]
- 110.Löw K, Crestani F, Keist R, Benke D, Brunig I, Benson JA, et al. Molecular and neuronal substrate for the selective attenuation of anxiety. Science. 2000;290:131–4. doi: 10.1126/science.290.5489.131. [DOI] [PubMed] [Google Scholar]
- 111.Lukas SE, Griffiths RR. Precipitated withdrawal in baboons: effects of dose and duration of diazepam exposure. Eur J Pharmacol. 1984;100:163–71. doi: 10.1016/0014-2999(84)90218-8. [DOI] [PubMed] [Google Scholar]
- 112.McCullough LD, Salamone JD. Anxiogenic drugs beta-CCE and FG 7142 increase extracellular dopamine levels in nucleus accumbens. Psychopharmacology. 1992;109:379–82. doi: 10.1007/BF02245888. [DOI] [PubMed] [Google Scholar]
- 113.McKernan RM, Rosahl TW, Reynolds DS, Sur C, Wafford KA, Atack JR, et al. Sedative but not anxiolytic properties of benzodiazepines are mediated by the GABAA receptor alpha1 subtype. Nat Neurosci. 2000;3:587–92. doi: 10.1038/75761. [DOI] [PubMed] [Google Scholar]
- 114.McKernan RM, Wafford K, Quirk K, Hadingham KL, Harley EA, Ragan CI, et al. The pharmacology of the benzodiazepine site of the GABA-A receptor is dependent upon the type of γ-subunit present. J Recept Signal Transduct Res. 1995;15:173–83. doi: 10.3109/10799899509045215. [DOI] [PubMed] [Google Scholar]
- 115.McKernan RM, Whiting PJ. Which GABAA-receptor subtypes really occur in the brain? Trends Pharmacol Sci. 1996;19:139–43. doi: 10.1016/s0166-2236(96)80023-3. [DOI] [PubMed] [Google Scholar]
- 116.McMahon LR, Gerak LR, France CP. Potency of positive γ-aminobutyric acidA modulators to substitute for a midazolam discriminative stimulus in untreated monkeys does not predict potency to attenuate a flumazenil discriminative stimulus in diazepam-treated monkeys. J Pharmacol Exp Ther. 2001;298:1227–35. [PubMed] [Google Scholar]
- 117.Metzger D, Woody G, De Philippis D, McLellan AT, O’Brien CP, Platt JJ. Risk factors for needle sharing among methadone-treated patients. Am J Psychiatry. 1991;148:636–40. doi: 10.1176/ajp.148.5.636. [DOI] [PubMed] [Google Scholar]
- 118.Miller LG. Chronic benzodiazepine administration: from the patient to the gene. J Clin Pharmacol. 1991;31:492–5. doi: 10.1002/j.1552-4604.1991.tb03725.x. [DOI] [PubMed] [Google Scholar]
- 119.Miller LG, Roy RB, Weill CL. Chronic clonazepam administration decreases γ-aminobutryic acidA receptor function in cultured cortical neurons. Mol Pharmacol. 1989;36:796–802. [PubMed] [Google Scholar]
- 120.Mintzer MZ, Griffiths RR. Flumazenil-precipitated withdrawal in healthy volunteers following repeated diazepam exposure. Psychopharmacology. 2005;178:259–67. doi: 10.1007/s00213-004-2009-1. [DOI] [PubMed] [Google Scholar]
- 121.Mintzer MZ, Stoller KB, Griffiths RR. A controlled study of flumazenil-precipitated withdrawal in chronic low-dose benzodiazepine users. Psychopharmacology. 1999;147:200–9. doi: 10.1007/s002130051161. [DOI] [PubMed] [Google Scholar]
- 122.Mirza NR, Nielsen EØ. Do subtype-selective GABAA receptor modulators have a reduced propensity to induce physical dependence in mice? J Pharmacol Exp Ther. 2006;316:1378–85. doi: 10.1124/jpet.105.094474. [DOI] [PubMed] [Google Scholar]
- 123.Mitchell WG. Status epilepticus and acute repetitive seizures in children, adolescents, and young adults: etiology, outcome, and treatment. Epilepsia. 1996;37 (Suppl 1):S74–S80. doi: 10.1111/j.1528-1157.1996.tb06025.x. [DOI] [PubMed] [Google Scholar]
- 124.Mizoguchi Y, Kanematsu T, Hirata M, Nabekura J. A rapid increase in the total number of cell surface functional GABAA receptors induced by brain- derived neurotrophic factor in rat visual cortex. J Biol Chem. 2003;278:44097–102. doi: 10.1074/jbc.M305872200. [DOI] [PubMed] [Google Scholar]
- 125.Möhler H. GABAA receptor diversity and pharmacology. Cell Tissue Res. 2006;326:505–16. doi: 10.1007/s00441-006-0284-3. [DOI] [PubMed] [Google Scholar]
- 126.Morgan WW. Abuse liability of barbiturates and other sedative-hypnotics. Adv Alcohol Subst Abuse. 1990;9:67–82. doi: 10.1300/J251v09n01_05. [DOI] [PubMed] [Google Scholar]
- 127.Morlock RJ, Tan M, Mitchell DY. Patient characteristics and patterns of drug use for sleep complaints in the United States: analysis of National Ambulatory Medical Survey Data, 1997–2002. Clin Ther. 2006;28:1044–53. doi: 10.1016/j.clinthera.2006.07.008. [DOI] [PubMed] [Google Scholar]
- 128.Motzo C, Porceddu ML, Dazzi L, Sanna A, Serra M, Biggio G. Enhancement by flumazenil of dopamine release in the nucleus accumbens of rats repeatedly exposed to diazepam or imidazenil. Psychopharmacology. 1997;131:34–9. doi: 10.1007/s002130050262. [DOI] [PubMed] [Google Scholar]
- 129.Murai T, Koshikawa N, Kanayama T, Takada K, Tomiyama K, Kobayashi M. Opposite effects of midazolam and beta-carboline-3-carboxylate ethyl ester on the release of dopamine from rat nucleus accumbens measured by in vivo microdialysis. Eur J Pharmacol. 1994;261:65–71. doi: 10.1016/0014-2999(94)90301-8. [DOI] [PubMed] [Google Scholar]
- 130.Najib J. Eszopiclone, a nonbenzodiazepine sedative-hypnotic agent for the treatment of transient and chronic insomnia. Clin Ther. 2006;28:491–516. doi: 10.1016/j.clinthera.2006.04.014. [DOI] [PubMed] [Google Scholar]
- 131.Narita M, Matsumura Y, Ozaki S, Ise Y, Yajima Y, Suzuki T. Role of the calcium/calmodulin-dependent protein kinase ii (CaMKII) in the morphine- induced pharmacological effects in the mouse. Neuroscience. 2004;126:415–21. doi: 10.1016/j.neuroscience.2004.03.006. [DOI] [PubMed] [Google Scholar]
- 132.Neubauer DN. New approaches in managing chronic insomnia. CNS Spectr. 11 (8 Suppl 8):1–13. [Google Scholar]
- 133.Nutt DJ. Overview of diagnosis and drug treatments of anxiety disorders. CNS Spectr. 2005;10:49–56. doi: 10.1017/s1092852900009901. [DOI] [PubMed] [Google Scholar]
- 134.O’Brien CP. Benzodiazepine use, abuse, and dependence. J Clin Psychiatry. 2005;66:28–33. [PubMed] [Google Scholar]
- 135.Perera KM, Tulley M, Jenner FA. The use of benzodiazepines among drug addicts. Br J Addict. 1987;82:511–15. doi: 10.1111/j.1360-0443.1987.tb01508.x. [DOI] [PubMed] [Google Scholar]
- 136.Perrault G, Morel E, Sanger DJ, Zivkovic B. Lack of tolerance and physical dependence upon repeated treatment with the novel hypnotic zolpidem. J Pharmacol Exp Ther. 1992;263:298–303. [PubMed] [Google Scholar]
- 137.Petursson H. The benzodiazepine withdrawal syndrome. Addiction. 1994;89:1455–59. doi: 10.1111/j.1360-0443.1994.tb03743.x. [DOI] [PubMed] [Google Scholar]
- 138.Pirker S, Schwarzer C, Wieselthaler A, Sieghart W, Sperk G. GABAA receptors: immunocytochemical distribution of 13 subunits in the adult rat brain. Neuroscience. 2000;101:815–30. doi: 10.1016/s0306-4522(00)00442-5. [DOI] [PubMed] [Google Scholar]
- 139.Platt DM, Rowlett JK, Spealman RD, Cook J, Ma C. Selective antagonism of the ataxic effects of zolpidem and triazolam by the GABAA/α1-preferring antagonistβ–CCT in squirrel monkeys. Psychopharmacology. 2002;164:151–9. doi: 10.1007/s00213-002-1189-9. [DOI] [PubMed] [Google Scholar]
- 140.Pollack MH. Innovative uses of benzodiazepines in psychiatry. Can J Psychiatry. 1993;38 (Suppl 4):S122–26. [PubMed] [Google Scholar]
- 141.Pratt JA, Brett RR, Laurie DJ. Benzodiazepine dependence: from neural circuits to gene expression. Pharmaol Biochem Behav. 1998;59:925–34. doi: 10.1016/s0091-3057(97)00539-x. [DOI] [PubMed] [Google Scholar]
- 142.Preston KL, Griffiths RR, Stitzer ML, Bigelow GE, Liebson IA. Diazepam and methadone interactions in methadone maintenance. Clin Pharmacol Ther. 1984;36:534–41. doi: 10.1038/clpt.1984.215. [DOI] [PubMed] [Google Scholar]
- 143.Pritchett DB, Lüddens H, Seeburg PH. Type I and type II GABAA-benzodiazepine receptors produced in transfected cells. Science. 1989;245:1389–92. doi: 10.1126/science.2551039. [DOI] [PubMed] [Google Scholar]
- 144.Pritchett DB, Seeburg PH. Gamma-aminobutyric acidA receptor alpha 5- subunit creates novel type II benzodiazepine receptor pharmacology. J Neurochem. 1990;54:1802–4. doi: 10.1111/j.1471-4159.1990.tb01237.x. [DOI] [PubMed] [Google Scholar]
- 145.Pritchett DB, Seeburg PH. gamma-aminobutyric acid type A receptor point mutation increases the affinity of compounds for the benzodiazepine site. Proc Natl Acad Sci USA. 1991;88:1421–5. doi: 10.1073/pnas.88.4.1421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Quaglio G, Lugoboni F, Fornasiero A, Lechi A, Gerra G, Mezzelani P. Dependence on zolpidem: two case reports of detoxification with flumazenil infusion. Int Clin Psychopharmacol. 2005;20:285–7. doi: 10.1097/01.yic.0000166404.41850.b4. [DOI] [PubMed] [Google Scholar]
- 147.Quera-Salva MA, McCann C, Boudet J, Frisk M, Borderies P, Meyer P. Effects of zolpidem on sleep architecture, night time ventilation, daytime vigilance and performance in heavy snorers. Br J Clin Pharmacol. 1994;37:539–43. doi: 10.1111/j.1365-2125.1994.tb04301.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Ramsey-Williams VA, Carter DB. Chronic triazolam and its withdrawal alters GABAA receptor subunit mRNA levels: an in situ hybridization study. Mol Brain Res. 1996;43:132–40. doi: 10.1016/s0169-328x(96)00166-0. [DOI] [PubMed] [Google Scholar]
- 149.Reynolds JN, Maitra R. Propofol and flurazepam act synergistically to potentiate GABAA receptor activation in human recombinant receptors. Eur J Pharmacol. 1996;314:151–6. doi: 10.1016/s0014-2999(96)00527-4. [DOI] [PubMed] [Google Scholar]
- 150.Roca DJ, Schiller GD, Friedman L, Rozenberg I, Gibbs TT, Farb DH. γ-aminobutyric acidA receptor regulation in culture: altered allosteric interactions following prolonged exposure to benzodiazepines, barbiturates, and methylxanthines. Mol Pharmacol. 1990;37:710–19. [PubMed] [Google Scholar]
- 151.Roth T, Walsh JK, Krystal A, Wessel T, Roehrs TA. An evaluation of the efficacy and safety of eszopiclone over 12 months in patients with chronic primary insomnia. Sleep Med. 2005;6:487–95. doi: 10.1016/j.sleep.2005.06.004. [DOI] [PubMed] [Google Scholar]
- 152.Rowlett JK, Lelas S. Comparison of zolpidem and midazolam self-administration under progressive-ratio schedules: consumer demand and labor supply analyses. Exp Clin Psychopharmacol. 2007;15:328–37. doi: 10.1037/1064-1297.15.4.328. [DOI] [PubMed] [Google Scholar]
- 153.Rowlett JK, Platt DM, Lelas S, Atack JR, Dawson GR. Different GABAA receptor subtypes mediate the anxiolytic, abuse-related, and motor effects of benzodiazepine-like drugs in primates. Proc Natl Acad Sci USA. 2005;102:915–20. doi: 10.1073/pnas.0405621102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Rowlett JK, Rodefer JS, Spealman RD. Self-administration of cocaine, alfentanil, and nalbuphine under progressive-ratio schedules: Consumer demand and labor supply analysis of relative reinforcing effectiveness. Exp Clin Psychopharmacol. 2002;10:367–75. doi: 10.1037//1064-1297.10.4.367. [DOI] [PubMed] [Google Scholar]
- 155.Rudolph U, Crestani F, Benke D, Brunig I, Benson JA, Fritschy JM, et al. Benzodiazepine actions mediated by specific gamma-aminobutyric acid (A) receptor subtypes. Nature. 1999;401:796–800. doi: 10.1038/44579. [DOI] [PubMed] [Google Scholar]
- 156.Rudolph U, Crestani F, Möhler H. GABAA receptor subtypes: dissecting their pharmacological functions. Trends Pharmacol Sci. 2001;22:188–94. doi: 10.1016/s0165-6147(00)01646-1. [DOI] [PubMed] [Google Scholar]
- 157.Salamone JD, Correa M, Farrar A, Mingote SM. Effort-related functions of nucleus accumbens dopamine and associated forebrain circuits. Psychopharmacology. 2007;191:461–82. doi: 10.1007/s00213-006-0668-9. [DOI] [PubMed] [Google Scholar]
- 158.Salzman C. Geriatric psychopharmacology. Annu Rev Med. 1985;36:217–28. doi: 10.1146/annurev.me.36.020185.001245. [DOI] [PubMed] [Google Scholar]
- 159.Sanger DJ, Morel E, Perrault G. Comparison of the pharmacological profiles of the hypnotics drugs, zaleplon and zolpidem. Eur J Pharmacol. 1996;313:35–42. doi: 10.1016/0014-2999(96)00510-9. [DOI] [PubMed] [Google Scholar]
- 160.Sanger DJ, Perrault G, Morel E, Joly D, Zivkovic B. The behavioral profile of zolpidem, a novel hypnotic drug of imidazopyridine structure. Physiol Behav. 1987;41:235–40. doi: 10.1016/0031-9384(87)90359-3. [DOI] [PubMed] [Google Scholar]
- 161.Sanger DJ, Zivkovic B. The discriminative stimulus properties of zolpidem, a novel imdiazopyridine hypnotic. Psychopharmacology. 1986;89:317–22. doi: 10.1007/BF00174367. [DOI] [PubMed] [Google Scholar]
- 162.Sanger DJ, Zivkovic B. Investigation of the development of tolerance to the actions of zolpidem and midazolam. Neuropharmacology. 1987;26:1513–18. doi: 10.1016/0028-3908(87)90172-9. [DOI] [PubMed] [Google Scholar]
- 163.Sanna E, Busonero F, Talani G, Carta M, Massa F, Peis M, et al. Comparison of the effects of zaleplon, zolpidem, and triazolam at various GABA(A) receptor subtypes. Eur J Pharmacol. 2002;451:103–10. doi: 10.1016/s0014-2999(02)02191-x. [DOI] [PubMed] [Google Scholar]
- 164.Sarter M, Bruno JP, Berntson GG. Psychotogenic properties of benzodiazepine receptor inverse agonists. Psychopharmacology. 2001;156:1–13. doi: 10.1007/s002130100756. [DOI] [PubMed] [Google Scholar]
- 165.Saunders PA, Ho IK. Barbiturates and the GABAA receptor complex. Prog Drug Res. 1990;34:261–86. doi: 10.1007/978-3-0348-7128-0_7. [DOI] [PubMed] [Google Scholar]
- 166.Segura M, Barbosa J, Torrens M, Farré M, Castillo C, Segura J, et al. Analytical methodology for the detection of benzodiazepine consumption in opioid- dependent subjects. J Anal Toxicol. 2001;25:130–6. doi: 10.1093/jat/25.2.130. [DOI] [PubMed] [Google Scholar]
- 167.Sher PK, Study RE, Mazzetta J, Barker JL, Nelson PG. Depression of benzodiazepine binding and diazepam potentiation of GABA-mediated inhibition after chronic exposure of spinal cord cultures to diazepam. Brain Res. 1983;268:171–6. doi: 10.1016/0006-8993(83)90404-3. [DOI] [PubMed] [Google Scholar]
- 168.Shibla DB, Gardell MA, Neale JH. The insensitivity of developing benzodiazepine receptors to chronic treatment with diazepam. Brain Res. 1981;210:471–4. doi: 10.1016/0006-8993(81)90929-x. [DOI] [PubMed] [Google Scholar]
- 169.Shippenberg TS, Zapata A, Chefer VI. Dynorphin and the pathophysiology of drug addiction. Pharmacol Ther. 2007;116:306–21. doi: 10.1016/j.pharmthera.2007.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Sloan JW, Martin WR, Wala E. Effect of the chronic dose of diazepam on the intensity and characteristic of the precipitated abstinence syndrome in the dog. J Pharmacol Exp Ther. 1993;265:1152–62. [PubMed] [Google Scholar]
- 171.Smith AJ, Alder L, Silk J, Adkins C, Fletcher AE, Scales T, et al. Effect of a subunit on allosteric modulation of ion channel function in stably expressed human recombinant γ-aminobutyric acidA receptors determined using 36Cl ion flux. Mol Pharmacol. 2001;59:1108–18. doi: 10.1124/mol.59.5.1108. [DOI] [PubMed] [Google Scholar]
- 172.Soderling TR, Chang B, Brickey D. Cellular signaling through multifunctional Ca2+/calmodulin-dependent protein kinase II. J Biol Chem. 2001;276:3719–22. doi: 10.1074/jbc.R000013200. [DOI] [PubMed] [Google Scholar]
- 173.Song J, Shen G, Greenfield LJ, Jr, Tietz EI. Benzodiazepine withdrawal-induced glutamatergic plasticity involves up-regulation of GluR1-containing alpha- amino-3-hydroxy-5-methylisoxazole-4-propionic acid receptors in Hippocampal CA1 neurons. J Pharmacol Exp Ther. 2007;322:569–81. doi: 10.1124/jpet.107.121798. [DOI] [PubMed] [Google Scholar]
- 174.Souza-Pinto LF, Castilho VM, Brandão ML, Nobre MJ. The blockade of AMPA- kainate and NMDA receptors in the dorsal periaqueductal gray reduces the effects of diazepam withdrawal in rats. Pharmacol Biochem Behav. 2007;87:250–7. doi: 10.1016/j.pbb.2007.04.021. [DOI] [PubMed] [Google Scholar]
- 175.Soyka M, Bottlender R, Moller HJ. Epidemiological evidence for a low abuse potential of zolpidem. Pharmacopsychiatry. 2000;33:138–41. doi: 10.1055/s-2000-11224. [DOI] [PubMed] [Google Scholar]
- 176.Speaker S. From “happiness pills” to “national nightmare”: changing cultural assessment of minor tranquilizers in America, 1955–1980. J Hist Med Allied Sci. 1997;52:338–76. doi: 10.1093/jhmas/52.3.338. [DOI] [PubMed] [Google Scholar]
- 177.Stephens DN. A glutamatergic hypothesis of drug dependence. Behav Pharmacol. 1995;6:425–46. [PubMed] [Google Scholar]
- 178.Stephenson FA, Duggan MJ, Pollard S. The γ2 subunit is an integral component of the γ–aminobutyric acidA receptor but the α1 polypeptide is the principle site of the agonist benzodiazepine photoaffinity labeling reaction. J Biol Chem. 1990;265:21160–5. [PubMed] [Google Scholar]
- 179.Steppuhn KG, Turski L. Diazepam dependence prevented by glutamate antagonists. Proc Natl Acad Sci USA. 1993;90:6889–93. doi: 10.1073/pnas.90.14.6889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Stitzer M, Griffiths RR, McLellan A, Grabowski J, Hawthorne J. Diazepam use among methadone maintenance patients: patterns and dosages. Drug Alcohol Depend. 1981;8:189–99. doi: 10.1016/0376-8716(81)90061-2. [DOI] [PubMed] [Google Scholar]
- 181.Stoops WW, Rush CR. Differential effects in humans after repeated administrations of zolpidem and triazolam. Am J Drug Alcohol Abuse. 2003;29:281–99. doi: 10.1081/ada-120020513. [DOI] [PubMed] [Google Scholar]
- 182.Substance Abuse and Mental Health Services Administration, Office of Applied Studies. Drug Abuse Warning Network, 2005: National Estimates of Drug- Related Emergency Department Visits. DAWN Series D-29, DHS Publication No. (SMA) 07-4256. Rockville MD: 2007. [Google Scholar]
- 183.Tallman JF, Thomas JW, Gallager DW. GABAergic modulation of benzodiazepine binding site sensitivity. Nature. 1978;274:383–5. doi: 10.1038/274383a0. [DOI] [PubMed] [Google Scholar]
- 184.Taylor D. Iatrogenic drug dependence-a problem in intensive care? Case study and literature review Intensive. Crit Care Nurs. 1999;15:95–100. doi: 10.1016/s0964-3397(99)80005-6. [DOI] [PubMed] [Google Scholar]
- 185.Terzano MG, Parrino L. Effect of hypnotic drugs on sleep architecture. Pol J Pharmacol. 1994;46:487–90. [PubMed] [Google Scholar]
- 186.The DASIS Report: Characteristics of primary tranquilizer admissions. Drug and Alcohol Services Information System. Office of Applied Studies, Substance Abuse and Mental Health Services Administration; 2002. 2005. [Google Scholar]
- 187.Thiébot MH, Soubrié P, Sanger D. Anxiogenic properties of beta-CCE and FG 7142: a review of promises and pitfalls. Psychopharmacology. 1988;94:452–63. doi: 10.1007/BF00212837. [DOI] [PubMed] [Google Scholar]
- 188.Tone A. Listening to the past: history, psychiatry, and anxiety. Can J Psychiatry. 2005;50:373–80. doi: 10.1177/070674370505000702. [DOI] [PubMed] [Google Scholar]
- 189.Tsankova M, Renthal W, Kumar A, Nestler EJ. Epigenetic regulation in psychiatric disorders. Nat Rev Neurosci. 2007;8:355–67. doi: 10.1038/nrn2132. [DOI] [PubMed] [Google Scholar]
- 190.Tsuda M, Chiba Y, Suzuki T, Misawa M. Upregulation of NMDA receptor subunit proteins in the cerebral cortex during diazepam withdrawal. Eur J Pharmacol. 1998;341:R1–R2. doi: 10.1016/s0014-2999(97)01501-x. [DOI] [PubMed] [Google Scholar]
- 191.U.S. Committee on Labor and Human Resources. Use and Misuse of benzodiazepines. Washington, D.C.: U.S. Government Printing Office; 1979. [Google Scholar]
- 192.van Engelen BG, Gimbrere JS, Booy LH. Benzodiazepine withdrawal reaction in two children following discontinuation of sedation with midazolam. Ann Pharmacother. 1993;27:579–81. doi: 10.1177/106002809302700509. [DOI] [PubMed] [Google Scholar]
- 193.Van Sickle BJ, Xiang K, Tietz EI. Transient plasticity of hippocampal CA1 neuron glutamate receptors contributes to benzodiazepine withdrawal-anxiety. Neuropsychopharmacology. 2004;29:1994–2006. doi: 10.1038/sj.npp.1300531. [DOI] [PubMed] [Google Scholar]
- 194.Verster JC, Volkerts ER, Verbaten MN. Effects of alprazolam on driving ability, memory functioning and psychomotor performance: a randomized, placebo-controlled study. Neuropsychopharmacology. 2002;27:260–9. doi: 10.1016/S0893-133X(02)00310-X. [DOI] [PubMed] [Google Scholar]
- 195.VonVoigtlander PF, Lewis RA. A rapid screening method for the assessment of benzodiazepine receptor-related physical dependence in mice. Evaluation of benzodiazepine-related agonists and partial agonists. J Pharmacol Methods. 1991;26:1–5. doi: 10.1016/0160-5402(91)90049-b. [DOI] [PubMed] [Google Scholar]
- 196.Wafford KA. GABAA receptor subtypes: any clues to the mechanism of benzodiazepine dependence? Curr Opin Pharmacol. 2005;5:47–52. doi: 10.1016/j.coph.2004.08.006. [DOI] [PubMed] [Google Scholar]
- 197.Walker BM, Ettenberg A. Benzodiazepine modulation of opiate reward. Exp Clin Psychopharmacol. 2001;9:191–7. doi: 10.1037//1064-1297.9.2.191. [DOI] [PubMed] [Google Scholar]
- 198.Walker BM, Ettenberg A. The effects of alprazolam on conditioned place preferences produced by intravenous heroin. Pharmacol Biochem Behav. 2003;75:75–80. doi: 10.1016/s0091-3057(03)00043-1. [DOI] [PubMed] [Google Scholar]
- 199.Walker BM, Ettenberg A. Intra-ventral tegmental area heroin-induced place preferences in rats are potentiated by peripherally administered alprazolam. Pharmacol Biochem Behav. 2005;82:470–7. doi: 10.1016/j.pbb.2005.10.002. [DOI] [PubMed] [Google Scholar]
- 200.Wang RA, Cheng G, Kolaj M, Randi ZM. Alpha-subunit of calcium/calmodulin- dependent protein kinase II enhances gamma-aminobutyric acid and inhibitory synaptic responses of rat neurons in vivo. J Neurophysiol. 1995;73:2099–2106. doi: 10.1152/jn.1995.73.5.2099. [DOI] [PubMed] [Google Scholar]
- 201.Weerts EM, Ator NA, Grech DM, Griffiths RR. Zolpidem physical dependence assessed across increasing doses under a one-daily dosing regimen in baboons. J Pharmacol Exp Ther. 1998;285:41–53. [PubMed] [Google Scholar]
- 202.Weerts EM, Griffiths RR. Zolpidem self-injection with concurrent physical dependence under conditions of long-term continuous availability in baboons. Behav Pharmacol. 1998;9:285–97. [PubMed] [Google Scholar]
- 203.Weerts EM, Kaminski BJ, Gritffiths RR. Stable low-rate midazolam self-injection with concurrent physical dependence under conditions of long-term continuous availability in baboons. Psychopharmacology. 1998;135:70–81. doi: 10.1007/s002130050487. [DOI] [PubMed] [Google Scholar]
- 204.Wesensten NJ, Balkin TJ, Belenky GL. Effects of daytime administration of zolpidem versus triazolam on memory. Eur J Clin Pharmacol. 1995;48:115–22. doi: 10.1007/BF00192735. [DOI] [PubMed] [Google Scholar]
- 205.Wieland HA, Lüddens H, Seeburg PH. A single histidine in GABAA receptors is essential for benzodiazepine agonist binding. J Biol Chem. 1992;267:1426–29. [PubMed] [Google Scholar]
- 206.Winger G, Stitzer ML, Woods JH. Barbiturate-reinforced responding in rhesus monkeys: comparisons of drugs with different durations of action. J Pharmacol Exp Ther. 1975;195:505–14. [PubMed] [Google Scholar]
- 207.Woods JH, Katz JL, Winger G. Benzodiazepines: Use, abuse, and consequences. Pharmacol Rev. 1992;44:151–347. [PubMed] [Google Scholar]
- 208.Woods JH, Winger G. Current benzodiazepine issues. Psychopharmacology. 1995;118:107–15. doi: 10.1007/BF02245824. [DOI] [PubMed] [Google Scholar]
- 209.Wu Y, Rosenberg HC, Chiu TH, Ramsey-Williams V. Regional changes in [3H]zolpidem binding to brain benzodiazepine receptors in flurazepam tolerant rat: comparison with changes in [3H]flunitrazepam binding. J Pharmacol Exp Ther. 1994a;268:675–82. [PubMed] [Google Scholar]
- 210.Wu Y, Rosenberg HC, Chiu TH, Zhao T. Subunit- and brain region-specific reduction of GABAA receptor subunit mRNAs during chronic treatment of rats with diazepam. J Mol Neurosci. 1994b;5:105–20. doi: 10.1007/BF02736752. [DOI] [PubMed] [Google Scholar]
- 211.Xiang K, Tietz EI. Benzodiazepine-induced hippocampal CA1 neuron alpha- amino-3-hydroxy-5-methylisoxasole-4-propionic acid (AMPA) receptor plasticity linked to severity of withdrawal anxiety: differential role of voltage-gated calcium channels and N-methyl-D-aspartic acid receptors. Behav Pharmacol. 2007;18:447–60. doi: 10.1097/FBP.0b013e3282d28f2b. [DOI] [PubMed] [Google Scholar]
- 212.Zetterström T, Fillenz M. Local administration of flurazepam has different effects on dopamine release in striatum and nucleus accumbens: a microdialysis study. Neuropharmacology. 1990;29:129–34. doi: 10.1016/0028-3908(90)90052-s. [DOI] [PubMed] [Google Scholar]


