Abstract
Both aberrant meiotic recombination and an increased frequency of sperm aneuploidy have been observed in infertile men. However, this association has not been demonstrated within individual men. The purpose of this study was to determine the association between the frequency of recombination observed in pachytene spermatocytes and the frequency of aneuploidy in sperm from the same infertile men. Testicular tissue from seven men with non-obstructive azoospermia (NOA) and six men undergoing vasectomy reversal (controls) underwent meiotic analysis. Recombination sites were recorded for individual chromosomes. Testicular and ejaculated sperm from NOA patients and controls, respectively, were tested for aneuploidy frequencies for chromosomes 9, 21, X and Y. There was a significant increase in the frequency of pachytene cells with at least one achiasmate bivalent in infertile men (12.4%) compared with controls (4.2%, P = 0.02). Infertile men also had a significantly higher frequency of sperm disomy than controls for chromosomes 21 (1.0% versus 0.24%, P = 0.001), XX (0.16% versus 0.03%, P = 0.004) and YY (0.12% versus 0.03%, P = 0.04). There was a significant correlation between meiotic cells with zero MLH1 foci in the sex body and total sex chromosome disomy (XX + YY + XY) in sperm from men with NOA (r = 0.79, P = 0.036).
Keywords: azoospermia, sperm aneuploidy, ICSI, meiotic recombination, synaptonemal complex
Introduction
Meiotic recombination binds homologous chromosomes together with crossovers, thereby assisting in the proper segregation of homologs at the first meiotic division in spermatogenesis (Coop and Przeworski, 2007). Using genetic linkage analysis, aberrations in meiotic recombination, such as diminished frequency (Hassold et al., 1991; Thomas et al., 2000; Shi et al., 2001; Reish et al., 2004) and suboptimal location (Hassold et al., 1995; Lamb et al., 1997), have been suggested to impart a risk for non-disjunction and aneuploid gametes in humans as well as model organisms.
Several studies have reported that infertile men have an increased frequency of aneuploid sperm (Moosani et al., 1995; Aran et al., 1999; Pang et al., 1999; Calogero et al., 2003) and this is particularly marked for infertile men with non-obstructive azoospermia (NOA) (Bernardini et al., 2000; Palermo et al., 2002; Martin et al., 2003). Men with NOA are now able to father children with the advent of intracytoplasmic sperm injection (ICSI) techniques (Palermo et al., 1992) following testicular extraction of sperm. However, it is also recognized that ICSI carries a significantly increased risk of producing aneuploid offspring (Martin, 1996; Van Steirteghem et al., 2002). Although relatively little is known about the genetic basis of aneuploidy, meiotic studies suggest that errors in recombination are a cause of aneuploid gametes (Hassold et al., 1991, 1995; Lamb et al., 1997; Thomas et al., 2000; Shi et al., 2001; Reish et al., 2004). Immunofluorescence methods that directly visualize important meiotic proteins have made possible the close examination of recombination events during meiosis (Barlow and Hultén, 1998; Tease et al., 2002; Sun et al., 2004a,b). Antibodies against SCP1 and SCP3 [synaptonemal complex (SC) proteins] mark the transverse and lateral elements of the SC, respectively; CREST (Calcinosis, Raynaud's phenomenon, Esophageal dysfunction, Sclerodactyly, Telangiectasia) marks the centromere, and MLH1 (mut L homolog 1, a mismatch repair protein) marks the recombination foci, allowing the precise identification of recombination foci along SCs during meiotic prophase. This assay, combined with centromere-specific multicolor fluorescence in situ hybridization (cenM-FISH), allows analysis of recombination distributions of individual chromosomes in human germ cells in great detail (Nietzel et al., 2001; Oliver-Bonet et al., 2003). Indeed, with these techniques, the first recombination maps for individual human chromosomes have been reported (Sun et al., 2004b, 2006b).
Using immunofluorescence techniques to examine meiotic recombination directly, we have determined that men with NOA have a variety of meiotic defects and a dramatic decrease in the frequency of meiotic recombination (Gonsalves et al., 2004; Sun et al., 2004a, 2005). We and others have also determined that the chromosomes most commonly observed to be achiasmate (with no recombination foci) are chromosomes 21, 22 and the sex chromosomes (Codina-Pascual et al., 2006; Sun et al., 2006a,b). These same chromosomes are the ones most frequently observed to be aneuploid in sperm from both normal and infertile men (Martin et al., 1987; Spriggs et al., 1996; Shi and Martin, 2000; Hristova et al., 2002). This strongly suggests a link between lack of recombination and the generation of aneuploid sperm; but to date, meiotic and sperm studies have not been performed on the same individuals. The aim of the current study of NOA men was to investigate the association between the frequency of meiotic recombination in specific chromosomes and the frequency of sperm aneuploidy for the same chromosomes in the same individuals.
Materials and Methods
Sample collection
Testicular samples were obtained from seven patients with NOA (patient 12 had a left varicocoele) and six patients undergoing vasectomy reversal (University of California San Francisco, CA, USA) who had no history of meiotic defects or infertility (controls). Histological examination showed normal spermatogenesis in the six control donors (ages 38–54 years). Testicular tissues were kept in phosphate-buffered saline (PBS; pH 7.4) until use and were shipped on ice to Calgary by air courier where appropriate. We have previously demonstrated that cold storage of testicular tissue for 2 days has no effect on the quality of preparations, or on chromosome pairing or recombination data (Sun et al., 2004c). Testicular spermatozoa were retrieved from testicular tissue in seven NOA patients. Ejaculated semen specimens were available from six control patients 2–26 months after vasectomy reversal. Analysis of these samples has been reported previously (Sun et al., 2008). Samples were air-freighted on ice to Calgary. Informed consent was obtained from all patients, and this study received ethical approval from the institutional review boards at the University of Calgary and the University of California San Francisco.
Fluorescence immunostaining
Slides with pachytene chromosome spreads were subjected to immunofluorescence staining as described previously (Sun et al., 2004b). Primary antibodies against the following proteins were used: SCP1 (1:1000 dilution, a gift from P. Moens, York University, Toronto, Canada), SCP3 (1:250 dilution, a gift from T. Ashley, Yale University), MLH1 (1:100 dilution, Oncogene, San Diego, CA, USA) and CREST (1:100 dilution, a gift from M. Fritzler, University of Calgary, Canada). These primary antibodies were detected using a cocktail of secondary antibodies (donkey antisera) conjugated with different fluorochromes: 1-amino-4-methylcoumarin-3 acetic acid (AMCA) and Cy3 (1:100 dilution, Jackson Immunoresearch, West Grove, PA, USA), AlexaFluor 488 and AlexaFluor 555 (1:125 dilution, Molecular Probes, Eugene, OR, USA). Primary and secondary antibodies were incubated overnight, and for 90 min at 37°C, respectively. Slides were examined on a Zeiss Axiophot epifluorescence microscope equipped with rhodamine, fluorescein isothiocyanate (FITC), and 4',6-diamidino-2-phenylindole (DAPI) filters and a cooled charged coupled device camera. Three fluorescent images (red, green and blue) of the SCs, MLH1 sites and CREST locations, respectively, were captured using Applied Imaging Cytovision 3.1 software (Applied Imaging Corporation, Santa Clara, CA, USA). Spreads were localized using a gridded finder slide.
Each pachytene-stage nucleus used for analysis met the following criteria: the correct numbers of bivalents (i.e. 22 autosomes and 1 sex body) were present; the SCs were not unduly overlapped with other SCs or bent back on themselves, allowing all foci to be scored; and background was fairly low, allowing the SCs to be distinguished from background noise and from each other. MLH1 signals were scored if they were distinct and localized on an SC. SCs were classified as normally synapsed if they were completely linear, without any obvious bubbles, forks, loops or irregularities. Up to 100 pachytene-stage cells were analyzed for each man, and the number of MLH1 foci per bivalent and the total number of MLH1 foci per autosomal complement were scored.
cenM-FISH on spermatocytes
After analysis of the captured immunofluorescence images, either two-color FISH for chromosomes 9 and 21 or cenM-FISH (which allows simultaneous identification of each SC) was carried out on the already analyzed spermatocytes, to identify chromosomes 9 and 21. Chromosome 21 was chosen as it is observed as an achiasmate bivalent relatively frequently with normally only one crossover per bivalent (Sun et al., 2006a). Chromosome 9 was chosen because it has a higher frequency of recombination and heterochromatic regions (Sun et al., 2006b). Two-color FISH hybridizations were carried out with a SpectrumGreen centromeric CEP probe for chromosome 9 and a locus specific SpectrumOrange LSI probe for chromosome 21 (Vysis, Downer's Grove, IL, USA), using techniques described previously (Ko et al., 2001). Previously developed cenM-FISH techniques (Nietzel et al., 2001; Oliver-Bonet et al., 2003) were modified to make use of the microwave-decondensation/codenaturation FISH technique (Ko et al., 2001).
After FISH identification of 9 and 21 bivalents, or cenM-FISH identification of each pachytene bivalent, the images of corresponding SC spreads were analyzed for MLH1 focus distribution in SCs 9 and 21. The numbers of MLH1 foci per bivalent and per SC spread were scored in six control males and four NOA patients.
FISH on testicular and ejaculated sperm
Ejaculated sperm specimens from controls were microwave decondensed and hybridized as described previously (Ko et al., 2001). Sex chromosome hybridizations were carried out using a Fluorogreen™-labelled (Amersham, Baie d'Urfé, QC, Canada) X-specific α-satellite probe, kindly provided by E. Jabs of the Johns Hopkins University, Baltimore, MD, USA (Jabs et al., 1989), a Fluoroblue™-labelled chromosome 1-specific satellite III sequence, pUC1.77, generously provided by H.J. Cooke of Edinburgh, UK (Cooke and Hindley, 1979), and a CEP SpectrumOrange Yq probe (Vysis). Chromosome 9/21 hybridizations were carried out using a SpectrumGreen 9 CEP probe and a SpectrumOrange 21 LSI probe (Vysis). Testicular sperm from NOA patients required up to four times the usual microwave decondensation treatment before adequate decondensation was achieved, but were otherwise hybridized identically to ejaculated specimens.
Scoring of sperm nuclei
Slides were counted using a Zeiss Axiophot microscope fitted with four filter sets: FITC, rhodamine/FITC, DAPI and rhodamine/FITC/DAPI. Two same-colored signals were counted as individual signals if they were separated by at least one signal diameter (half signal diameter for the overlarge Y signal) and were of similar size, shape and intensity. The blue chromosome 1 signal in sex chromosome hybridizations was used as an internal autosomal control to distinguish between disomy and diploidy.
Statistical analysis
The mean frequency of MLH1 foci/cell and the frequency of pachytene cells with at least one bivalent with no MLH1 foci were compared in NOA patients and controls using analysis of variance, accounting for the clustering of individual cell data by donor. The frequency of sperm aneuploidy for individual chromosomes was compared in NOA patients and controls using a Z-test to compare clustered binomial proportions (Donner and Klar, 1994). The correlation between MLH1 focus frequency and sperm aneuploidy for individual chromosomes was tested using a Spearman correlation coefficient. Similarly, the correlation between bivalents with no recombination foci and sperm aneuploidy was tested with a Spearman correlation coefficient.
Results
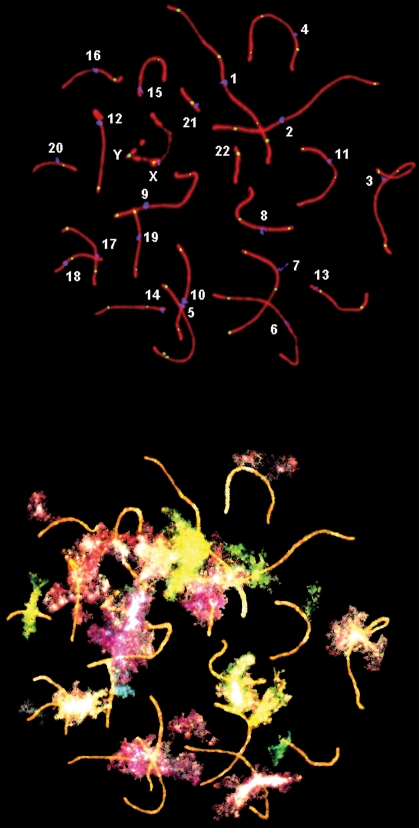
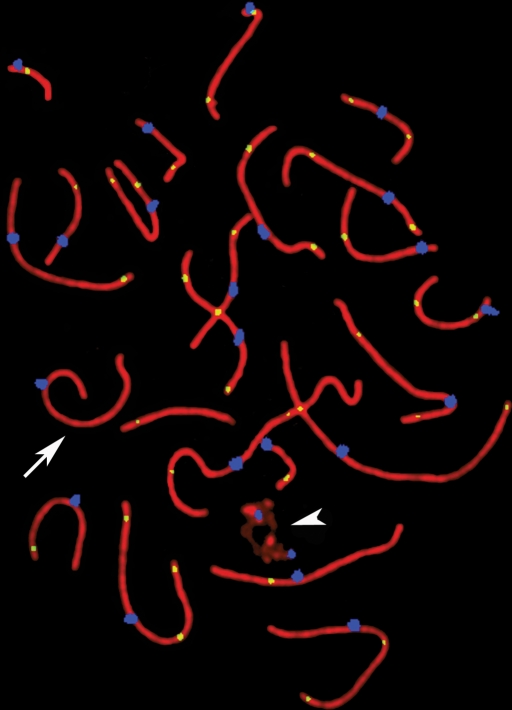
An example of pachytene SCs, with identification of individual bivalents and cenM-FISH signals in the same cell, is shown in Fig. 1. For the seven NOA patients, a total of 688 pachytene stage cells were analyzed; the overall mean frequency of autosomal MLH1 foci per cell was 48.4, with a range of 12–67 foci per cell (Table I). In all, 600 pachytene-stage spermatocytes were analyzed in controls (100 cells/donor) to determine the mean MLH1 focus frequency per cell for autosomes, with an overall mean of 50.7 foci (range: 32–63; Table I). Unlike previous studies (Sun et al., 2004a, 2005, 2007), there was no significant overall difference in autosomal recombination frequencies between NOA patients and controls (P = 0.25). However, the proportion of cells in NOA patients containing one or more autosomal SCs without an MLH1 focus (12.4%) was significantly higher than that observed in controls (4.2%; P = 0.024, Table I). An example of a pachytene cell with achiasmate bivalents is shown in Fig. 2.
Figure 1:
(Upper) Human pachytene spermatocyte with SCs shown in red, centromeres in blue and MLH1 foci in yellow. (Lower) Subsequent cenM-FISH analysis permits identification of individual chromosomes so that recombination (MLH1) foci can be analyzed for each SC.
Table I.
Analysis of MLH1 focus frequencies.
| Controls | Autosomal MLH1 foci per cell | % cells with an autosomal bivalent containing 0 MLH1 | % cells with 0 MLH1 foci in the sex body | |
|---|---|---|---|---|
| Mean | Range | |||
| 1 | 53.2 | 37–62 | 5 | 10.0 |
| 2 | 49.2 | 32–60 | 4 | 9.0 |
| 3 | 49.9 | 33–61 | 7 | 20.0 |
| 4 | 49.9 | 40–60 | 6 | 10.0 |
| 5 | 50.7 | 38–59 | 2 | 15.0 |
| 6 | 51.5 | 37–63 | 1 | 19.0 |
| Mean | 50.7 | 32–63 | 4.2 | 13.8 |
| NOA | Autosomal MLH1 foci per cell | % cells with an autosomal bivalent containing 0 MLH1 | % cells with 0 MLH1 foci in the sex body | |
| Mean | Range | |||
| 7 | 45.2 | 20–54 | 23 | 14.9 |
| 8 | 48.9 | 23–61 | 7 | 23.0 |
| 9 | 42.7 | 15–61 | 16 | 38.0 |
| 10 | 55.7 | 44–65 | 0 | 3.0 |
| 11 | 45.0 | 12–57 | 18 | 21.0 |
| 12 | 48.4 | 27–62 | 18 | 37.0 |
| 13 | 53.2 | 25–67 | 3 | 18.2 |
| Mean | 48.4 | 12–67 | 12.4* | 22.3 |
One hundred pachytene stage spermatocytes were analyzed for all control donors and for 5 NOA patients; 87 and 99 pachytene stage spermatocytes were analyzed for NOA patients 7 and 13, respectively.
*P = 0.024 compared with controls.
Figure 2:
Example of a pachytene cell with achiasmate sex body (arrow head) and bivalent (arrow).
The frequencies of achiasmate (non-crossover) bivalents for the sex chromosomes are presented in Table I.
The frequency of MLH1 foci per cell in SCs 9 and 21 and of achiasmate bivalents are presented in Table II. Individual SCs for chromosomes 9 and 21 were identified in only four NOA patients (because FISH analysis of spermatocytes failed in three men). Compared with controls, there was a significantly increased achiasmate frequency for sex bivalents (P = 0.03) (data not shown) and bivalent 9 (P = 0.001) in NOA patients.
Table II.
Analysis of MLH1 focus frequencies for chromosomes 9 and 21.
| Controls | Mean no. MLH1 foci in individual SCs (no. cells analyzed) | % cells with 0 MLH1 foci | ||
|---|---|---|---|---|
| SC9 | SC21 | SC9 | SC21 | |
| 1 | 2.44 (85) | 0.99 (85) | 0.0 | 4.7 |
| 2 | 2.23 (84) | 1.00 (87) | 0.0 | 3.4 |
| 3 | 2.39 (92) | 0.96 (92) | 0.0 | 5.4 |
| 4 | 2.35 (48) | 0.92 (48) | 0.0 | 8.3 |
| 5 | 2.24 (143) | 0.84 (143) | 0.0 | 16.8 |
| 6 | 2.39 (84) | 1.00 (83) | 0.0 | 0.0 |
| Mean | 2.34 | 0.95 | 0.0 | 7.4 |
| NOA* | Mean no. MLH1 foci in individual SCs (no. cells analyzed) | % cells with 0 MLH1 foci | ||
| SC9 | SC21 | SC9 | SC21 | |
| 7 | 2.25 (92) | 0.97 (92) | 0.0 | 3.3 |
| 9 | 1.93 (160) | 0.96 (156) | 3.1 | 9.0 |
| 12 | 2.09 (126) | 0.89 (129) | 4.8 | 11.6 |
| 13 | 2.51 (143) | 0.97 (140) | 2.8 | 4.3 |
| Mean | 2.19 | 0.94 | 2.9† | 7.3 |
*Four NOA patients had information available for chromosomes 9 and 21.
†P = 0.001 compared with controls.
Sperm aneuploidy frequencies for chromosomes 9, 21, X and Y were assessed by FISH analysis. More than 50 000 spermatozoa were scored for each group (Table III). Disomy frequencies were significantly elevated in NOA patients compared with controls for chromosome 21 (P = 0.001), XX (P = 0.004) and YY (P = 0.04).
Table III.
Aneuploidy frequency in spermatozoa for chromosomes 9, 21 and the sex body.
| Controls | No. sperm, XY hyb | No. Sperm, 1/9 hyb | % Disomy | ||||
|---|---|---|---|---|---|---|---|
| XX | YY | XY | 9 | 21 | |||
| 1 | 9990 | 9990 | 0.03 | 0.05 | 0.29 | 0.06 | 0.58 |
| 2 | 9990 | 9990 | 0.00 | 0.02 | 0.04 | 0.08 | 0.22 |
| 3 | 9990 | 9990 | 0.02 | 0.02 | 0.09 | 0.25 | 0.08 |
| 4 | 9990 | 9990 | 0.05 | 0.03 | 0.11 | 0.79 | 0.27 |
| 5 | 9990 | 9990 | 0.04 | 0.01 | 0.15 | 0.16 | 0.18 |
| 6 | 9990 | 9990 | 0.05 | 0.02 | 0.32 | 0.07 | 0.08 |
| Mean | 9990 | 9990 | 0.03 | 0.03 | 0.17 | 0.24 | 0.24 |
| NOA | No. sperm, XY hyb | No. Sperm, 1/9 hyb | % Disomy | ||||
| XX | YY | XY | 9 | 21 | |||
| 7 | 9990 | 4831 | 0.21 | 0.06 | 0.13 | 0.21 | 0.56 |
| 8 | 9990 | 6234 | 0.32 | 0.29 | 0.56 | 0.19 | 0.71 |
| 9 | 9990 | 1843 | 0.13 | 0.12 | 0.33 | 0.22 | 1.25 |
| 10 | 9990 | 3959 | 0.02 | 0.02 | 0.17 | 2.55 | 0.93 |
| 11 | 7733 | 2378 | 0.12 | 0.09 | 0.45 | 0.38 | 1.43 |
| 12 | 1717 | 714 | 0.29 | 0.41 | 1.40 | 1.40 | 1.97 |
| 13 | 2440 | 1998 | 0.12 | 0.04 | 0.24 | 0.50 | 2.05 |
| Mean | 7407 | 3137 | 0.16† | 0.12* | 0.35 | 0.71 | 1.00‡ |
*P = 0.04 compared with controls.
†P = 0.004 compared with controls.
‡P = 0.001 compared with controls.
There was a significant correlation between the frequency of pachytene cells with zero MLH1 foci in the sex body and the frequency of YY disomy (r = 0.86, P = 0.014) and the frequency of total sex chromosomal disomy (XX + YY + XY, r = 0.79, P = 0.036) in testicular sperm from NOA men.
Discussion
We and others have previously demonstrated that infertile patients with NOA have a significantly reduced frequency of recombination in pachytene spermatocytes compared with controls (Gonsalves et al., 2004; Sun et al., 2004a, 2007). However, in this study, we found no significant decrease in mean recombination frequency in NOA patients compared with controls, similar to two other recent studies (Ma et al., 2006a; Topping et al., 2006). This discrepancy is likely to be related to differences in subject populations. Indeed, NOA patients in this study were only a subset of our total NOA population and were selected for inclusion because they had sperm present in the testes to allow for comparison of meiotic recombination and sperm aneuploidy. Thus, these NOA patients likely had fewer meiotic errors than NOA patients without sperm, as they completed meiosis and developed sperm. Despite this, these NOA patients had a significantly increased frequency of pachytene cells with at least one bivalent with no recombination. These bivalents are at high risk of producing aneuploid gametes, because there is no crossover to ensure that homologous chromosomes remain tethered and correctly oriented on the metaphase plate for proper segregation.
Analysis of individual chromosomes demonstrated that NOA patients had a significant decrease in the frequency of recombination in the sex chromosomes, and an increase in the frequency of bivalents without a recombination focus for chromosome 9 and the sex chromosomes. Once again, this demonstrates the susceptibility of the sex chromosome pair to a lack of recombination.
The frequency of sperm disomy was significantly increased in NOA patients compared with controls for chromosomes 21, XX and YY. The frequency of XY disomy was 2-fold higher than in controls, but did not reach statistical significance. Other studies have also demonstrated that testicular sperm in NOA patients have a significantly increased frequency of disomy compared with controls, an effect most pronounced for the sex chromosomes (Levron et al., 2001; Burello et al., 2002; Martin et al., 2003). It is interesting that a significant increase in the frequency of XX and YY disomy was found, since these are derived from meiosis II errors, and involve the malsegregation of sister chromatids. Since recombination occurs at meiosis I, an obvious question arises: how can altered recombination be associated with meiosis II-derived disomies? One potential explanation is that aberrations in crossovers disrupt sister chromatid cohesion, which could lead to the premature separation of sister chromatids at meiosis I (Hassold and Hunt, 2001). Subsequently the two sister chromatids could travel to the same pole in anaphase, resulting in a disomic gamete (Hassold and Hunt, 2001; McDougall et al., 2005).
The most novel aspect of this study is that it is the first to demonstrate, in a population of infertile men, that a correlation exists between meiotic recombination and testicular sperm aneuploidy in the same individual. A low frequency of meiotic recombination in the sex chromosomes of NOA patients was significantly correlated with a high frequency of YY disomy and total sex chromosomal disomy in their sperm. A prior study of one individual (Ma et al., 2006b) found that an absence of recombination in the sex chromosomes was associated with an extremely high frequency of sex chromosomal aneuploidy in testicular sperm. In our prior study of fertile donors, we found no correlation between recombination and aneuploidy (Sun et al., 2008). These fertile men actually had a lower frequency of sperm aneuploidy than that observed in previous control populations. We hypothesized that these fertile men did not reach the threshold of abnormality to demonstrate a correlation. The current study demonstrates the correlation between a lack of meiotic recombination and sperm aneuploidy for sex chromosomes in infertile NOA patients. The sex chromosomes have consistently been shown to be the most susceptible to both recombination errors (Gonsalves et al., 2004; Codina-Pascual et al., 2006; Sun et al., 2006a) and sperm aneuploidy (Shi et al., 2001; Ma et al., 2006b), and we have observed this linkage within individuals for the first time.
A number of studies have shown that aberrant meiotic recombination in normal women is associated with the production of an aneuploid child (Hassold and Hunt, 2001; Thomas et al., 2001; Laurent et al., 2003). We were not able to directly demonstrate this association in normal men (Sun et al., 2008), perhaps because a stringent male pachytene checkpoint eliminates spermatocytes with meiotic abnormalities. Studies in a number of model organisms have demonstrated evidence for a pachytene checkpoint that responds to defective meiotic recombination and/or synapsis in spermatocytes in a p53-dependent or -independent manner (Odorisio et al., 1998; Cohen and Pollard, 2001; Hunt and Hassold, 2002). There is also evidence for a spindle assembly checkpoint which responds to chromosome kinetochores that fail to attach properly (Woods et al., 1999; Sluder and McCollum, 2000). Our study demonstrates that infertile men with dramatic meiotic abnormalities can succumb to the meiotic checkpoint (with loss of cells and consequence infertility), but may also escape the checkpoint, leading to an increased risk of aneuploidy.
Funding
Canada Research Chair in Genetics to R.H.M.; Canadian Institutes of Health Research (MA7961) to R.H.M.; Canadian Institutes of Health Research Strategic Training Fellowship in Genetics, Child Development and Health to F. S and M.O.B.
Acknowledgements
Thanks to Jie Lian for help with the manuscript.
References
- Aran B, Blanco J, Vidal F, Vendrell J, Egozcue S, Barri P, Egozcue J, Veiga A. Screening for abnormalities of chromosomes X, Y, and 18 and for diploidy in spermatozoa from infertile men participating in an in vitro fertilization-intracytoplasmic sperm injection program. Fertil Steril. 1999;72:696–701. doi: 10.1016/s0015-0282(99)00307-6. [DOI] [PubMed] [Google Scholar]
- Barlow AL, Hultén MA. Crossing over analysis at pachytene in man. Eur J Hum Genet. 1998;6:350–358. doi: 10.1038/sj.ejhg.5200200. [DOI] [PubMed] [Google Scholar]
- Bernardini L, Gianaroli L, Fortini D, Conte N, Magli C, Cavani S, Gaggero G, Tindiglia C, Ragni N, Venturini P. Frequency of hyper-, hypohaploidy and diploidy in ejaculate, epididymal and testicular germ cells of infertile patients. Hum Reprod. 2000;15:2165–2172. doi: 10.1093/humrep/15.10.2165. [DOI] [PubMed] [Google Scholar]
- Burello N, Calogero AE, De Palma A, Grazioso C, Torrisi C, Barone N, Pafumi C, Agata R, Vicari E. Chromosome analysis of epididymal and testicular spermatozoa in patients with azoospermia. Eur J Hum Genet. 2002;10:362–366. doi: 10.1038/sj.ejhg.5200814. [DOI] [PubMed] [Google Scholar]
- Calogero AE, Burrello N, De Palma A, Barone N, D'Agata R, Vicari E. Sperm aneuploidy in infertile men. Reprod BioMed Online. 2003;6:310–317. doi: 10.1016/s1472-6483(10)61850-0. [DOI] [PubMed] [Google Scholar]
- Codina-Pascual M, Campillo M, Kraus J, Speicher MR, Egozcue J, Navarro J, Benet J. Crossover frequency and synaptonemal complex length: their variability and effects on human male meiosis. Mol Hum Reprod. 2006;12:123–133. doi: 10.1093/molehr/gal007. [DOI] [PubMed] [Google Scholar]
- Cohen PE, Pollard JW. Regulation of meiotic recombination and prophase I progression in mammals. 2001;23:996–1009. doi: 10.1002/bies.1145. [DOI] [PubMed] [Google Scholar]
- Cooke HJ, Hindley J. Cloning of human satellite III DNA: different components are on different chromosomes. Nucleic Acids Res. 1979;6:3177–3197. doi: 10.1093/nar/6.10.3177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coop G, Przeworski M. An evolutionary view of human recombination. Nat Rev Genet. 2007;8:23–34. doi: 10.1038/nrg1947. [DOI] [PubMed] [Google Scholar]
- Donner A, Klar N. Methods for comparing event rates in intervention studies when the unit of allocation is a cluster. Am J Epidemiol. 1994;140:279–289. doi: 10.1093/oxfordjournals.aje.a117247. discussion 300–301. [DOI] [PubMed] [Google Scholar]
- Gonsalves J, Sun F, Schlegal P, Hopps C, Turek P, Greene C, Martin RH, Reijo-Pera RA. Defective recombination in infertile men. Hum Mol Genet. 2004;13:2875–2883. doi: 10.1093/hmg/ddh302. [DOI] [PubMed] [Google Scholar]
- Hassold T, Hunt P. To err (meiotically) is human: the genesis of human aneuploidy. Nat Rev Genet. 2001;2:280–291. doi: 10.1038/35066065. [DOI] [PubMed] [Google Scholar]
- Hassold T, Sherman S, Pettay D, Page D, Jacobs P. XY chromosome nondisjunction in man is associated with diminished recombination in the pseudoautosomal region. Am J Hum Genet. 1991;49:253–260. [PMC free article] [PubMed] [Google Scholar]
- Hassold T, Merrill M, Adkins K, Freeman S, Sherman SL. Recombination and maternal age-dependent nondisjunction: molecular studies of trisomy 16. Am J Hum Genet. 1995;57:867–874. [PMC free article] [PubMed] [Google Scholar]
- Hristova R, Ko E, Greene C, Rademaker A, Chernos J, Martin RH. Chromosome abnormalities in sperm from infertile men with aesthenoteratozoospermia. Biol Reprod. 2002;66:1781–1783. doi: 10.1095/biolreprod66.6.1781. [DOI] [PubMed] [Google Scholar]
- Hunt P, Hassold T. Sex matters in meiosis. Science. 2002;296:2181–2183. doi: 10.1126/science.1071907. [DOI] [PubMed] [Google Scholar]
- Jabs E, Goble CA, Cutting GR. Macromolecular organization of human centromeric regions reveals high-frequency, polymorphic macro DNA repeats. Proc Natl Acad Sci USA. 1989;86:202–206. doi: 10.1073/pnas.86.1.202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ko E, Rademaker A, Martin RH. Microwave decondensation and codenaturation: a new methodology to maximize FISH data from donors with very low concentrations of sperm. Cytogenet Cell Genet. 2001;95:143–145. doi: 10.1159/000059336. [DOI] [PubMed] [Google Scholar]
- Lamb NE, Feingold E, Savage A, Avramopoulos D, Freeman S, Gu Y, Hallberg A, Hersey J, Karadima G, Pettay D, et al. Characterization of susceptible chiasma configurations that increase the risk for maternal nondisjunction of chromosome 21. Hum Mol Genet. 1997;6:1391–1399. doi: 10.1093/hmg/6.9.1391. [DOI] [PubMed] [Google Scholar]
- Laurent A-M, Li M, Sherman S, Roizes G, Buard J. Recombination across the centromere of disjoined and non-disjoined chromosome 21. Hum Mol Genet. 2003;12:2229–2239. doi: 10.1093/hmg/ddg220. [DOI] [PubMed] [Google Scholar]
- Levron J, Aviain-Goldring A, Madgar I, Raviv G, Barkai G, Dor J. Sperm chromosome abnormalities in men with severe male factor infertility who are undergoing in vitro fertilization with intracytoplasmic sperm injection. Fertil Steril. 2001;76:479–484. doi: 10.1016/s0015-0282(01)01957-4. [DOI] [PubMed] [Google Scholar]
- Ma S, Arsovska S, Moens P, Nigro M, Chow V. Analysis of early meiotic events and aneuploidy in nonobstructive azoospermic men: a preliminary report. Fertil Steril. 2006;a 85:646–652. doi: 10.1016/j.fertnstert.2005.08.055. [DOI] [PubMed] [Google Scholar]
- Ma S, Ferguson KA, Arsovska S, Moens P, Chow V. Reduced recombination associated with the production of aneuploid sperm in an infertile man: a case report. Hum Reprod. 2006;b 21:980–985. doi: 10.1093/humrep/dei428. [DOI] [PubMed] [Google Scholar]
- Martin R. The risk of chromosomal abnormalities following ICSI. Hum Reprod. 1996;11:924–925. doi: 10.1093/oxfordjournals.humrep.a019319. [DOI] [PubMed] [Google Scholar]
- Martin RH, Rademaker AW, Hildebrand K, Long-Simpson L, Peterson D, Yamamoto J. Variation in the frequency and type of sperm chromosomal abnormalities among normal men. Hum Genet. 1987;77:108–114. doi: 10.1007/BF00272374. [DOI] [PubMed] [Google Scholar]
- Martin R, Greene C, Rademaker A, Ko E, Chernos J. Analysis of aneuploidy in spermatozoa from testicular biopsies from men with nonobstructive azoospermia. J Androl. 2003;24:100–103. [PubMed] [Google Scholar]
- McDougall A, Elliott DJ, Hunter N. Pairing, connecting, exchanging, pausing and pulling chromosomes. EMBO Rep. 2005;6:120–125. doi: 10.1038/sj.embor.7400331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moosani N, Pattinson H, Carter M, Cox D, Rademaker A, Martin R. Chromosomal analysis of sperm from men with idiopathic infertility using sperm karyotyping and fluorescence in situ hybridization. Fertil Steril. 1995;64:811–817. doi: 10.1016/s0015-0282(16)57859-5. [DOI] [PubMed] [Google Scholar]
- Nietzel A, Rocchi M, Starke H, Heller A, Fiedler W, Wlodarska I, Longarevic IF, Beensen V, Claussen U, Liehr T. A new multicolor-FISH approach for the characterization of marker chromosomes: centromere-specific multicolour-FISH (cenM-FISH) Hum Genet. 2001;108:199–204. doi: 10.1007/s004390100459. [DOI] [PubMed] [Google Scholar]
- Odorisio T, Rodriguez TA, Evans EP, Clarke AR, Burgoyne PS. The meiotic checkpoint monitoring synapsis eliminates spermatocytes via p53-independent apoptosis. Nat Genet. 1998;18:257–261. doi: 10.1038/ng0398-257. [DOI] [PubMed] [Google Scholar]
- Oliver-Bonet M, Liehr T, Nietzel A, Heller A, Starke H, Claussen U, Codina-Pascual M, Pujol A, Abad C, Egozcue J, et al. Karyotyping of human synaptonemal complexes by cenM-FISH. Eur J Hum Genet. 2003;11:879–883. doi: 10.1038/sj.ejhg.5201067. [DOI] [PubMed] [Google Scholar]
- Palermo G, Joris H, Devroey P, Van Steirteghem AC. Pregnancies after intracytoplasmic injection of single spermatozoon into an oocyte. Lancet. 1992;340:17–18. doi: 10.1016/0140-6736(92)92425-f. [DOI] [PubMed] [Google Scholar]
- Palermo G, Colombero L, Hariprashad J, Schlegel P, Rosenwaks Z. Chromosome analysis of epididymal and testicular sperm in azoospermic patients undergoing ICSI. Hum Reprod. 2002;17:570–575. doi: 10.1093/humrep/17.3.570. [DOI] [PubMed] [Google Scholar]
- Pang M, Hoegerman S, Cuticchia A, Moon S, Doncel G, Acosta A, Kearns W. Detection of aneuploidy for chromosomes 4, 6, 7, 8, 9, 10, 11, 12, 13, 17, 18, 21, X and Y by fluorescence in-situ hybridization in spermatozoa from nine patients with oligoasthenoteratozoospermia undergoing intracytoplasmic sperm injection. Hum Reprod. 1999;14:1266–1273. doi: 10.1093/humrep/14.5.1266. [DOI] [PubMed] [Google Scholar]
- Reish O, Berryman T, Cunningham TR, Sher C, Oetting WS. Reduced recombination in maternal meiosis coupled with non-disjunction at meiosis II leading to recurrent 47,XXX. Chromosome Res. 2004;12:125–132. doi: 10.1023/b:chro.0000013164.56757.bd. [DOI] [PubMed] [Google Scholar]
- Shi Q, Martin R. Spontaneous frequencies of aneuploid and diploid sperm in 10 normal Chinese men: assessed by multicolor fluorescence in situ hybridization. Cytogenet Cell Genet. 2000;90:79–83. doi: 10.1159/000015668. [DOI] [PubMed] [Google Scholar]
- Shi Q, Spriggs E, Field L, Ko E, Barclay L, Martin R. Single sperm typing demonstrates that reduced recombination is associated with the production of aneuploid 24,XY human sperm. Am J Med Genet. 2001;99:34–38. doi: 10.1002/1096-8628(20010215)99:1<34::aid-ajmg1106>3.0.co;2-d. [DOI] [PubMed] [Google Scholar]
- Sluder G, McCollum D. Molecular biology. The mad ways of meiosis. Science. 2000;289:254–255. doi: 10.1126/science.289.5477.254. [DOI] [PubMed] [Google Scholar]
- Spriggs E, Rademaker A, Martin R. Aneuploidy in human sperm: the use of multicolor FISH to test various theories of nondisjunction. Am J Hum Genet. 1996;58:356–362. [PMC free article] [PubMed] [Google Scholar]
- Sun F, Kozak G, Scott S, Trpkov K, Ko E, Mikhaail-Philips M, Bestor TH, Moens P, Martin RH. Meiotic defects in a man with non-obstructive azoospermia: case report. Hum Reprod. 2004;a 19:1770–1773. doi: 10.1093/humrep/deh335. [DOI] [PubMed] [Google Scholar]
- Sun F, Oliver-Bonet M, Liehr T, Starke H, Ko E, Rademaker A, Navarro J, Benet J, Martin RH. Human male recombination maps for individual chromosomes. Am J Hum Genet. 2004;b 74:521–531. doi: 10.1086/382138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun F, Trpkov K, Rademaker A, Ko E, Barclay L, Mikhaail-Philips M, Martin RH. The effect of cold storage on recombination frequencies in human male testicular cells. Cytogenet Genome Res. 2004;c 106:39–42. doi: 10.1159/000078558. [DOI] [PubMed] [Google Scholar]
- Sun F, Greene C, Turek PJ, Ko E, Rademaker A, Martin RH. Immunofluorescent synaptonemal complex analysis in azoospermic men. Cytogenet Genome Res. 2005;111:366–370. doi: 10.1159/000086913. [DOI] [PubMed] [Google Scholar]
- Sun F, Oliver-Bonet M, Liehr T, Starke H, Turek P, Ko E, Rademaker A, Martin R. Analysis of achiasmate bivalents in pachytene cells from 10 normal men. Hum Reprod. 2006;a 21:2335–2339. doi: 10.1093/humrep/del190. [DOI] [PubMed] [Google Scholar]
- Sun F, Oliver-Bonet M, Liehr T, Starke H, Turek P, Ko E, Rademaker A, Martin RH. Variation in MLH1 distribution in recombination maps for individual chromosomes from human males. Hum Mol Genet. 2006;b 15:2376–2391. doi: 10.1093/hmg/ddl162. [DOI] [PubMed] [Google Scholar]
- Sun F, Turek P, Greene C, Ko E, Rademaker A, Martin RH. Abnormal progression through meiosis in men with nonobstructive azoospermia. Fertil Steril. 2007;87:565–571. doi: 10.1016/j.fertnstert.2006.07.1531. [DOI] [PubMed] [Google Scholar]
- Sun F, Mikhaail-Philips M, Oliver-Bonet M, Ko E, Rademaker A, Turek P, Martin RH. The relationship between meiotic recombination in human spermatocytes and aneuploidy in sperm. Hum Reprod. 2008 doi: 10.1093/humrep/den027. doi:10.1093/humrep/den027. [DOI] [PubMed] [Google Scholar]
- Tease C, Hartshorne GM, Hultén MA. Patterns of meiotic recombination in human fetal oocytes. Am J Hum Genet. 2002;70:1469–1479. doi: 10.1086/340734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas N, Collins A, Hassold T, Jacobs P. A reinvestigation of non- disjunction resulting in 47, XXY males of paternal origin. Eur J Hum Genet. 2000;8:805–808. doi: 10.1038/sj.ejhg.5200531. [DOI] [PubMed] [Google Scholar]
- Thomas N, Ennis S, Sharp A, Durkie M, Hassold T, Collins A, Jacobs P. Maternal sex chromosome non-disjunction: evidence for X chromosome- specific risk factors. Hum Mol Gen. 2001;2001:243–250. doi: 10.1093/hmg/10.3.243. [DOI] [PubMed] [Google Scholar]
- Topping D, Brown P, Judis L, Schwartz S, Seftel A, Thomas A, Hassold TJ. Synaptic defects at meiosis I and non-obstructive azoospermia. Hum Reprod. 2006;21:3171–3177. doi: 10.1093/humrep/del281. [DOI] [PubMed] [Google Scholar]
- Van Steirteghem A, Bonduelle M, Devroey P, Liebaers I. Follow-up of children born after ICSI. Hum Reprod Update. 2002;8:111–116. doi: 10.1093/humupd/8.2.111. [DOI] [PubMed] [Google Scholar]
- Woods LM, Hodges CA, Baart E, Baker SM, Liskay M, Hunt PA. Chromosomal influence on meiotic spindle assembly: abnormal meiosis I in female Mlh1 mutant mice. J Cell Biol. 1999;145:1395–1406. doi: 10.1083/jcb.145.7.1395. [DOI] [PMC free article] [PubMed] [Google Scholar]