Abstract
‘Good-genes’ models of sexual selection predict significant additive genetic variation for fitness-correlated traits within populations to be revealed by phenotypic traits. To test this prediction, we sampled brown trout (Salmo trutta) from their natural spawning place, analysed their carotenoid-based red and melanin-based dark skin colours and tested whether these colours can be used to predict offspring viability. We produced half-sib families by in vitro fertilization, reared the resulting embryos under standardized conditions, released the hatchlings into a streamlet and identified the surviving juveniles 20 months later with microsatellite markers. Embryo viability was revealed by the sires' dark pigmentation: darker males sired more viable offspring. However, the sires' red coloration correlated negatively with embryo survival. Our study demonstrates that genetic variation for fitness-correlated traits is revealed by male colour traits in our study population, but contrary to predictions from other studies, intense red colours do not signal good genes.
Keywords: salmonid, genetic load, good-genes sexual selection, offspring survival, heritability of colour traits
1. Introduction
Some ‘good-genes’ models of sexual selection predict that attractive males or males that are superior competitors in intrasexual selection are of high genetic quality and hence offer indirect genetic benefits to females (Neff & Pitcher 2005). Empirical tests of such models need to control for potentially confounding effects of, for example, differential female investment into embryos and juveniles (Parker 2003). Therefore, the ideal species to test for genetic effects of sexual selection are those with no parental care, external fertilization and large family size, i.e. some frogs (Welch et al. 1998) and some fish species (e.g. Wedekind et al. 2001, 2004; Rudolfsen et al. 2005; Pitcher & Neff 2006). However, it is still unclear whether colours can be linked to genetic quality in such species.
Animals often use colours to advertise their quality and to attract mates. Two main groups have been discussed as candidates in this context (McGraw 2005; Griffith et al. 2006): carotenoid-based yellow or red colours and melanin-based brown, black and grey colours. The reliability of the signals they produce is assumed to be given by the costs of colour production and/or maintenance (Grafen 1990). Various non-exclusive types of costs have been discussed (Hadfield & Owens 2006) as follows: (i) the pigments are costly to acquire, e.g. because they are rare or owing to costly physiological handling (metabolism or transport), (ii) the pigments are required by other physiological processes, e.g. for immune functioning, or (iii) the pigments are toxic or the colours are dangerous, e.g. because they enhance conspicuousness to predators or they signal a social status that may need to be defended.
In vertebrates, carotenoids that are responsible for yellow and red colours have to be obtained through the diet. They may therefore reliably signal foraging quality (Olson & Owens 1998) or foraging strategy (Bakker et al. 1997). Such colours have often been investigated in sexual selection studies because they are usually conspicuous and based on pigments that also have antioxidant and immunoregulatory properties (McGraw 2005; Peters 2007). Hence, by using these pigments, the signaller may reveal its superior health and vigour to potential mates (McGraw & Ardia 2003). In the three-spined stickleback (Gasterosteus aculeatus), for example, males use conspicuous red colours to attract females (Milinski & Bakker 1990; Bakker & Mundwiler 1994). These colours are based on at least three different carotenoids (Wedekind et al. 1998) that are known to be important in immune function (Krinsky 1993). Accordingly, red colours in these fish reveal good condition (Frischknecht 1993; Barber et al. 2000) and high resistance against parasite infection (Milinski & Bakker 1990; Barber et al. 2001), and females benefit from preferring red males especially when males are energy constrained during their paternal care (Candolin 2000).
In species without paternal care, i.e. where males only provide genes to their offspring, the role of carotenoid-based colours in signalling and mate choice is less clear. In Arctic charr (Salvelinus alpinus), for example, first observations found a link between red colours and immune function (Skarstein & Folstad 1996), but these colours do not seem to be useful indicators of health and vigour or resistance against infection: Skarstein et al. (2005) even found a positive correlation between red coloration and parasite intensity, and Masvaer et al. (2004) reported a positive link between red coloration and milt characteristics that indicate low dominance in male–male interaction at the spawning place (Rudolfsen et al. 2006). In guppies (Poecilia reticulata), another fish with no paternal care, variation in carotenoid-based colour traits can be linked to infections and reactions of the immune system (Grether et al. 2004; Kolluru et al. 2006), and females have been found to prefer males with larger amounts of carotenoids in their orange spots (Grether 2000). However, female responsiveness to male colours varies with carotenoid availability (Grether et al. 2005) and age (Kodric-Brown & Nicoletto 2001), and females are also reported to prefer larger males to brightly coloured ones (Houde 1997).
In contrast to carotenoid-based colours, melanin-based colours can be synthesized by the animal itself (e.g. in specialized organelles called melanosomes; Sugimoto 2002) and the synthesis is under strong genetic control (Majerus & Mundy 2003). Melanin-based colours may have therefore been less obvious candidates for reliable signals of genetic quality, but it has recently been recognized that significant relationships exist between melanin-based pigmentation and overall health and vigour (Roulin 2004a; McGraw 2005). In birds, melanin-based traits are frequently linked to fitness traits (reviewed by Roulin (2004b); recent examples include Bize et al. (2006), Fargallo et al. (2007) and Roulin (2007)). These links could be due to the antioxidant activity of melanin (McGraw 2005) or its involvement in calcium, zinc and iron metabolism and in the maintenance of body structures (Niecke et al. 2003; Roulin et al. 2006; McGraw 2007). In addition, melanin plays important roles in the developmental pathways of various functions and may therefore be an indicator of homeostasis (Badyaev & Young 2004; Roulin 2004a). Finally, although melanin-based colours often function as camouflage, some melanin-based signals may make the signaller more conspicuous in some environments (Smith et al. 2004), and only animals in good health and vigour may be able to pay the costs of being conspicuous (Kodric-Brown 1998).
The brown trout is a salmonid with external fertilization and no paternal care, and with migratory and resident forms. The resident form (Salmo trutta resident morph) develops brown, black and red spots on the body sides and the adipose fin. The red spots on the skin and the adipose fin contain large quantities of different carotenoids (xanthophylls and astacene esters) and much more so than any other tissue studied (Steven 1948). The migratory forms of brown trout are usually larger and therefore expected to be more dominant at the spawning place (Jacob et al. 2007); they have dark spots but usually no red spots when they arrive at the spawning place. We chose a resident population that has not been supplemented by hatchery fish since at least 10 years. We tested whether males differ in their genetic quality, determined as their offspring viability, and if so, whether male colour traits reveal genetic quality. We also tested for heritabilities of colour traits by comparing 2-year-old juveniles with their fathers.
2. Material and methods
Brown trout were collected from their natural spawning place in River Enziwigger (near Willisau, Switzerland) in November by electrofishing. Fifteen mature males were narcotized, measured for size and weight and their milt stripped for the in vitro fertilization of the eggs of one large female of the same population. The eggs of this one female were about equally distributed to 15 Petri dishes and 20 μl of milt were added each and activated with sand-filtered lake water (8°C), following the methods of Wedekind & Müller (2004). The resulting embryos were reared in four separate Petri dishes per sibship in 50 ml sand-filtered lake water at 4.7°C (mean number of eggs per Petri dish: 22.3±12.0 s.d.; three unfertilized eggs were earlier discarded).
The males were individually marked and kept together in a large tank at approximately 8°C for another breeding experiment 14 days later (Jacob et al. 2007). They were then killed with a sharp blow on their head and photographed from both sides under standardized conditions with a digital camera (Nikon E995, 2048×1536 pixels; figure 1a,b). Scale samples were taken from below the adipose fin near the lateral line to estimate the age of the fish from yearly grow rings (Rifflart et al. 2006).
Figure 1.
Size-standardized photos of (a) the male that received the highest values for red coloration but whose embryos had the lowest survival and (b) the male with the highest embryo survival. (c) Standard-distance photo of a typical juvenile that was caught back after 20 months in the wild. The reference colour patches are taken from Kodak Color Separation Guide and Grey Scale.
From day 60 after fertilization onwards, non-viable embryos and hatched larvae were recorded and carefully removed from the Petri dishes with a plastic spoon (in regular intervals of approx. 10 days each). Alevins were kept in darkness in running water at 7–8°C until all embryos had hatched and most alevins had nearly used up their yolk sac, i.e. until day 131 after fertilization. We then released all fish plus some additional ones from two other studies (Jacob et al. 2007; Evanno et al. submitted) into a 600 m long semi-natural, structured streamlet near Willisau that is typical for the streamlets in the region and that is confined by physical barriers. The streamlet has a width of up to 0.5 m and an average depth of approximately 10 cm. We removed all present trout by electrofishing and released our fish by carefully distributing them over the full length of the streamlet during a period when water discharge was low and not obviously affecting the larvae. We caught the fish back 20 months later again by electrofishing, measured their weight and length and took standard photos from both sides (Olympus C77OUZ, 2288×1712 pixels; figure 1c). DNA was extracted from fin clips, and eight microsatellite markers (Mst85, Mst543AE, BS131, T3-13, AETG1, Sssosl417, Ssa171 and Str58) were used for parental assignment following the procedure described by Jacob et al. (2007). Paternity was established by exclusion using the Cervus program (Marshall et al. 1998). All inferred parent–offspring pairs were checked for the absence of mismatching loci.
The photos of the parental and juvenile fish were used to count the number of red and brown or black spots. Further colour analyses were done on TIFF files in the open-access software ImageJ (http://rsb.info.nih.gov/ij/). We used a mode that defines hue, saturation and brightness for every pixel of the image (the ‘HSB mode’). Hue is given as an angle on a continuous circular scale from 0° to 360°, with 0° for red, 60° for yellow, 120° for green, 180° for cyan, 240° for blue and 300° for magenta. Saturation is the purity of the colour from 0% (grey) to 100% (fully saturated colour), and brightness is the relative lightness or darkness of the colour from 0% (black) to 100% (white). In ImageJ, all three values are converted to a scale from 0 to 255, and thus every pixel is defined by three values within this range. To obtain the measurements of the red coloration of our fish, we defined a priori a range of reddish hue values (225°–255° and 0°–20°). We then counted all the pixels within this range to determine a fish's overall redness as the total red area on the body sides and the adipose fin divided by the total area of the body sides and the adipose fin. The average modal hue and saturation of the red coloration, i.e. the most frequent hue and saturation values within the area that was perceived as red, were used as a qualitative measure of the red pigmentation. We also determined the average modal grey value on both body sides, i.e. the most frequent grey value as a measure of overall dark pigmentation. Our methods did not, however, allow us to measure colours in the ultraviolet spectrum. See Stevens et al. (2007) for a discussion of colour measurements from digital photos. Figure 1 indicates the range of observed male phenotypes. The analogous measures were taken from the standard photos of the 2-year-old offspring.
Statistical analyses were done in JMP v. 6.0 (www.jmp.com) or the open-access software R (www.r-project.org; see also Bates 2005). Pearson's correlation coefficients r are used when graphical inspection of the data suggested that the model assumptions of this statistics were not notably violated, otherwise Spearman's rank-order correlation coefficients rs are used. Mixed-effects logistic models were used to test for sire effects on offspring viability. To avoid potentially confounding Petri-dish effects, we excluded all Petri dishes that could be considered as outliers, i.e. they were linked to embryo mortalities that were 1.5 times larger than the interquartile of the respective half-sib family (4 out of 60 Petri dishes). The sire effect was then assessed in two tests: first, a log-likelihood ratio test between a reference model (including both Petri dish and sire effect) and a reduced model (with only one of the two parameters); second, the generation of a log-likelihood distribution of the model under the null hypothesis by random permutation (n=1000) and a rank comparison of the observed likelihood with this distribution.
3. Results
Fertilization success was close to 100% for all males (only three out of in total 1454 eggs did not seem to contain an embryo). Embryos were clearly visible on day 60 after fertilization, with no apparent mortality until then. Total embryo survival until hatching was 86.9% (median 90%, range 20–100% per Petri dish). Males differed in the viability of their embryos (permutation test, male effect on embryo mortality: p=0.002; table 1).
Table 1.
Sire effects on embryo survival. (Three mixed-effects logistic models to test whether sire effects explain a significant part of the variance in embryo survival (a binary response variable; egg number n=1290). Model parameters are random effects. The goodness of fit is given by the logarithm of the approximated likelihood (log L) and the Akaike information criterion (AIC). A measure to compare the quality of fit between two models is the difference in AICs between two models. Likelihood ratio tests between the reference model and the other models are used to test which parameter significantly improves the goodness of fit.)
| likelihood ratio test against the reference model | |||||||
|---|---|---|---|---|---|---|---|
| model | effect tested | model parameters | log L | AIC | Χ2 | d.f. | p |
| reference model | Petri dish, sire | −383.79 | 773.58 | ||||
| environmental model | sire | Petri dish | −387.15 | 778.31 | 6.73 | 1 | 0.0095 |
| sire model | Petri dish | sire | −383.79 | 771.58 | <0.01 | 1 | 1 |
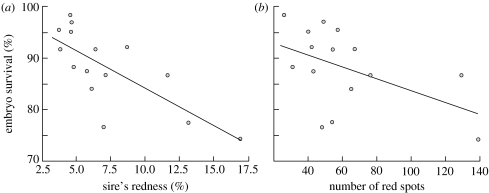
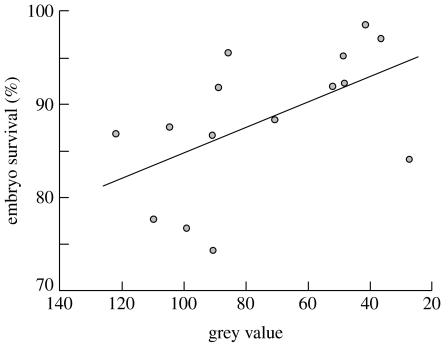
All red coloration measures seemed to be independent of male size and age (the sires' overall redness, the number of red spots or red hue and saturation versus age or size measures: −0.39<rs<0.11, n=15, always p>0.05). Embryo viability was negatively correlated with the sires' overall redness and the number of red spots (figure 2). Embryo survival increased with increasing dark pigmentation of their fathers as revealed by low modal grey values (figure 3). Increasing dark pigmentation was also linked to male age (rs=−0.53, p=0.04) and male weight (rs=−0.51, p=0.05), but embryo survival could not significantly be predicted by male age or size (0.002<rs<0.18, always p>0.05).
Figure 2.
Male red colour characteristics versus embryo survival. Total embryo survival until hatching versus (a) overall redness, calculated as the relative red area on the body sides and the adipose fin (rs=−0.76, p=0.0009), and (b) total number of red spots on both the body sides (rs=−0.52, p=0.048). The lines give the regressions.
Figure 3.
Modal grey value (most frequently occurring grey value, average of two body sides) versus embryo survival (regression line; rs=−0.60, p=0.018). Low modal grey values indicate dark overall pigmentation.
Out of 1261 hatchlings that were released into the wild, 10 juveniles (0.8%) could be caught back 20 months later. They were sired by eight males that did not differ significantly from the other seven males in age or in any of the colour or size measurements (Kruskal–Wallis, always p>0.05). There was also no significant correlation between juveniles' size or condition factor and the sires' age, size or colour traits (Spearman's rank-order correlations, always p>0.05). The low recapture rate remains unexplained but could be linked to an extraordinary high water that occurred few months before recapture and that caused strong currents at the study site.
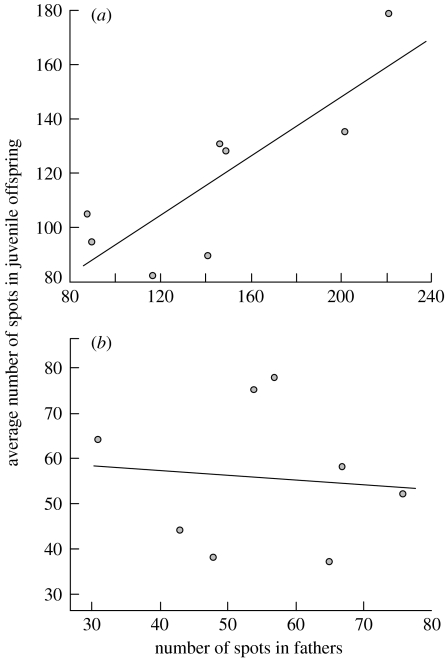
Spottiness, i.e. the total number of black, brown and red spots on both body sides, appeared to be heritable (figure 4a), while no evidence for a heritability of other pigmentation traits was found (correlation between sire and offspring, overall dark pigmentation: r=−0.28, p>0.05; overall redness: r=−0.23, n=8, p>0.05; red spots, figure 4b). We found no variation in the hue value of the red colour patches among the juveniles that could be recaptured.
Figure 4.
Regression of sires' spottiness versus average spottiness of their juvenile offspring. Total number of (a) spots on both body sides (r=0.83, p=0.01) and (b) red spots only (r=−0.09, p>0.05).
4. Discussion
In fishes, nuptial coloration can function both in inter- and intrasexual selection (Kodric-Brown 1998). In the case of the brown trout, the relative importance of agonistic interactions between males and courtship to females is still unclear. It is even unclear whether there is any female mate choice in the brown trout (Petersson et al. 1999; Jacob et al. 2007). However, generalizations about a species' breeding system are difficult to draw because fishes often respond opportunistically to temporal or spatial variation in sex ratio, population density, habitat structure and other ecological factors (Kodric-Brown 1998). Our results suggest that if females choose and base their mate choice on colours, they should prefer darker males in order to increase the viability of their embryos. Contrary to what could be found in many other species (see §1 and below), they should not prefer red males. In fact, they should even avoid mating with bright and red males owing to the low viability of the embryos these males sire. However, we could not detect any positive or negative links between sire colour characteristics and the viability of their juvenile offspring. This could be due to either low statistical power or the different nature of embryo and juvenile mortality (Heath et al. 1999).
In salmonids, females are usually restricted in their choice of mates. They often defend spawning territories where they spawn their eggs in several batches, and males compete for access to these females (Fleming & Reynolds 2004; Quinn 2005). Large males are more successful in such interactions, and females seem to prefer larger males as they deposit more eggs with larger males (Foote 1989), and they are more aggressive towards small males than towards large males (Allen et al. 2007) or may delay spawning when courted by non-preferred males (Esteve 2005). Some Oncorhynchus species, especially the anadromous and non-anadromous forms of Oncorhynchus nerka (sockeye salmon and kokanee, respectively) or Oncorhynchus mykiss (steelhead and rainbow trout, respectively), develop intense red colours during the spawning season. These colours are carotenoid based (Bjerkeng et al. 1992; Craig et al. 2005), and, as in the three-spined stickleback (see §1), the red breeding colour of O. nerka has been found to positively influence mate choice (Craig & Foote 2001; Foote et al. 2004).
Animals often announce their aggressive stage and their fighting ability in colours and other characteristics (Huntingford & Turner 1987; Roulin 2004b), and fighting ability could be correlated with overall health and vigour and hence with good genes. However, it is not yet clear whether darker skin colours signal dominance or subordination in brown trout. Among juvenile fishes, social subordination has been linked to darker skin colours in rainbow trout (O. mykiss; Abbott et al. 1985), Atlantic salmon (Salmo salar; O'Connor et al. 1999) and Arctic charr (S. alpinus; Hoglund et al. 2000). Among spawners, however, the situation could be very different (Fleming & Reynolds 2004; Esteve 2005). A change to dark colours that may signal dominance or attractiveness during the spawning season is often seen in freshwater fishes (Kodric-Brown 1998).
There are different types of fish chromatophores, including melanophores that contain melanized organelles and that produce dark colours on the skin (Fujii 1993; Sugimoto 2002). Some chromatophores show very motile responses, for example, in response to their surroundings (some fishes are dark on a black background and pale on a white background; Sugimoto 2002). The variation in colours that we observed is, however, unlikely to reflect responses of the fish to their light environment. We controlled for such environmental variation by keeping the males for 14 days in one large unstructured tank before they were photographed.
Carotenoid-based colours can have a genetic basis (Craig et al. 2005), but the within-population variation is often due to diet and factors that influence carotenoid uptake and storage (Craig et al. 2005; Hadfield & Owens 2006). Darker skin patterns are usually due to higher melanin content (Hoglund et al. 2000; Pavlidis et al. 2006). Compared with carotenoid-based colours, melanin-based colours seem to have a stronger genetic basis (Hudon 1994; Hadfield & Owens 2006), but environmental influences are nevertheless possible (Hoglund et al. 2000; Fargallo et al. 2007). We found a significant father–offspring correlation in the total number of spots, i.e. this aspect of the skin coloration appears to be heritable. An analogous heritability has been observed in rainbow trout (Kause et al. 2003). We found no significant evidence for any heritability of red colour traits, but since our heritability analyses are based on a rather small sample size, such non-significant findings cannot be taken as evidence for a lack of heritability.
In a recent study on brown trout, Forsberg et al. (2007, p. 1863) found ‘… no apparent reproductive skew that would have indicated a strong ‘good genes’ effect’. However, they also found that their most successful males had a higher ‘microsatellite score’, i.e. these males were more different in their average genetics from the rest of the sample than males with lower reproductive success. It remains unclear whether this microsatellite score is linked to variation in genetic load or to the origin of the breeders (the authors used a mixture of hatchery- and wild-born males). In the former case, within-population variation in mating success may be caused by variation in genetic load. Our findings would then suggest that the genetic load is revealed by melanin-based colour traits. However, contrary to other species, a mate preference for red colours would not promote indirect genetic benefits in the case of the brown trout.
Acknowledgments
The study was done with permission of the Fischerei- und Jagdverwaltung des Kanton Luzern and conforms to Swiss laws.
We thank P. Amrein for providing the fish; P. Amrein, B. von Siebenthal, D. Urbach and K. von Wattenwyl for their discussion and assistance in the field; and S. Cotton, A. Roulin and the anonymous reviewers for their constructive comments on the manuscript. The project was supported by the Swiss National Science Foundation.
References
- Abbott J.C, Dunbrack R.L, Orr C.D. The interaction of size and experience in dominance relationships of juvenile steelhead trout (Salmo gairdneri) Behaviour. 1985;92:241–253. [Google Scholar]
- Allen C.S, Rich H.B, Jr, Quinn T.P. Condition-dependent reproductive tactics by large and small anadromous male sockey salmon Oncorhynchus nerka. J. Fish Biol. 2007;70:1302–1307. doi:10.1111/j.1095-8649.2007.01391.x [Google Scholar]
- Badyaev A.V, Young R.L. Complexity and integration in sexual ornamentation: an example with carotenoid and melanin plumage pigmentation. J. Evol. Biol. 2004;17:1317–1327. doi: 10.1111/j.1420-9101.2004.00781.x. doi:10.1111/j.1420-9101.2004.00781.x [DOI] [PubMed] [Google Scholar]
- Bakker T.C.M, Mundwiler B. Female mate choice and male red coloration in a natural 3-spined stickleback (Gasterosteus aculeatus) population. Behav. Ecol. 1994;5:74–80. doi:10.1093/beheco/5.1.74 [Google Scholar]
- Bakker T.C.M, Mazzi D, Zala S. Parasite-induced changes in behavior and color make Gammarus pulex more prone to fish predation. Ecology. 1997;78:1098–1104. [Google Scholar]
- Barber I, Arnott S.A, Braithwaite V.A, Andrew J, Mullen W, Huntingford F.A. Carotenoid-based sexual coloration and body condition in nesting male sticklebacks. J. Fish Biol. 2000;57:777–790. doi:10.1111/j.1095-8649.2000.tb00274.x [Google Scholar]
- Barber I, Arnott S.A, Braithwaite V.A, Andrew J, Huntingford F.A. Indirect fitness consequences of mate choice in sticklebacks: offspring of brighter males grow slowly but resist parasitic infections. Proc. R. Soc. B. 2001;268:71–76. doi: 10.1098/rspb.2000.1331. doi:10.1098/rspb.2000.1331 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bates D.M. Fitting linear mixed models in R. R News. 2005;5:27–30. [Google Scholar]
- Bize P, Gasparini J, Klopfenstein A, Altwegg R, Roulin A. Melanin-based coloration is a nondirectionally selected sex-specific signal of offspring development in the Alpine swift. Evolution. 2006;60:2370–2380. [PubMed] [Google Scholar]
- Bjerkeng B, Storebakken T, Liaaenjensen S. Pigmentation of rainbow trout from start feeding to sexual maturation. Aquaculture. 1992;108:333–346. doi:10.1016/0044-8486(92)90117-4 [Google Scholar]
- Candolin U. Changes in expression and honesty of sexual signalling over the reproductive lifetime of sticklebacks. Proc. R. Soc. B. 2000;267:2425–2430. doi: 10.1098/rspb.2000.1301. doi:10.1098/rspb.2000.1301 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Craig J.K, Foote C.J. Countergradient variation and secondary sexual color: phenotypic convergence promotes genetic divergence in carotenoid use between sympatric anadromous and nonanadromous morphs of sockeye salmon (Oncorhynchus nerka) Evolution. 2001;55:380–391. doi: 10.1111/j.0014-3820.2001.tb01301.x. doi:10.1111/j.0014-3820.2001.tb01301.x [DOI] [PubMed] [Google Scholar]
- Craig J.K, Foote C.J, Wood C.C. Countergradient variation in carotenoid use between sympatric morphs of sockeye salmon (Oncorhynchus nerka) exposes nonanadromous hybrids in the wild by their mismatched spawning colour. Biol. J. Linn. Soc. 2005;84:287–305. doi:10.1111/j.1095-8312.2005.00430.x [Google Scholar]
- Esteve M. Observations of spawning behaviour in Salmoninae: Salmo, Oncorhynchus and Salvelinus. Rev. Fish Biol. Fish. 2005;15:1–21. doi:10.1007/s11160-005-7434-7 [Google Scholar]
- Evanno, G., Müller, R. & Wedekind, C. Submitted. Differential influence of maternal and paternal effects on embryo and juvenile survival in a salmonid.
- Fargallo J.A, Laaksonen T, Korpimaki E, Wakamatsu K. A melanin-based trait reflects environmental growth conditions of nestling male Eurasian kestrels. Evol. Ecol. 2007;21:157–171. doi:10.1007/s10682-006-0020-1 [Google Scholar]
- Fleming I.A, Reynolds J.D. Salmonid breeding system. In: Hendry A.P, Stearns S.C, editors. Evolution illuminated: salmon and their relatives. Oxford University Press; Oxford, UK: 2004. pp. 264–294. [Google Scholar]
- Foote C.J. Female mate preference in Pacific salmon. Anim. Behav. 1989;38:721–723. doi:10.1016/S0003-3472(89)80022-3 [Google Scholar]
- Foote C.J, Brown G.S, Hawryshyn C.W. Female colour and male choice in sockeye salmon: implications for the phenotypic convergence of anadromous and nonanadromous morphs. Anim. Behav. 2004;67:69–83. doi:10.1016/j.anbehav.2003.02.004 [Google Scholar]
- Forsberg L.A, Dannewitz J, Petersson E, Grahn M. Influence of genetic dissimilarity in the reproductive success and mate choice of brown trout—female fishing for optimal MHC dissimilarity. J. Evol. Biol. 2007;20:1859–1869. doi: 10.1111/j.1420-9101.2007.01380.x. doi:10.1111/j.1420-9101.2007.01380.x [DOI] [PubMed] [Google Scholar]
- Frischknecht M. The breeding coloration of male 3-spined sticklebacks (Gasterosteus aculeatus) as an indicator of energy investment in vigor. Evol. Ecol. 1993;7:439–450. doi:10.1007/BF01237640 [Google Scholar]
- Fujii R. Coloration and chromatophores. In: Evans D.H, editor. The physiology of fishes. CRC Press; Boca Raton, FL: 1993. pp. 535–562. [Google Scholar]
- Grafen A. Biological signals as handicaps. J. Theor. Biol. 1990;144:517–546. doi: 10.1016/s0022-5193(05)80088-8. [DOI] [PubMed] [Google Scholar]
- Grether G.F. Carotenoid limitation and mate preference evolution: a test of the indicator hypothesis in guppies (Poecilia reticulata) Evolution. 2000;54:1712–1724. doi: 10.1111/j.0014-3820.2000.tb00715.x. doi:10.1111/j.0014-3820.2000.tb00715.x [DOI] [PubMed] [Google Scholar]
- Grether G.F, Kasahara S, Kolluru G.R, Cooper E.L. Sex-specific effects of carotenoid intake on the immunological response to allografts in guppies (Poecilia reticulata) Proc. R. Soc. B. 2004;271:45–49. doi: 10.1098/rspb.2003.2526. doi:10.1098/rspb.2003.2526 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grether G.F, Kolluru G.R, Rodd F.H, de la Cerda J, Shimazaki K. Carotenoid availability affects the development of a colour-based mate preference and the sensory bias to which it is genetically linked. Proc. R. Soc. B. 2005;272:2181–2188. doi: 10.1098/rspb.2005.3197. doi:10.1098/rspb.2005.3197 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griffith S.C, Parker T.H, Olson V.A. Melanin-versus carotenoid-based sexual signals: is the difference really so black and red? Anim. Behav. 2006;71:749–763. doi:10.1016/j.anbehav.2005.07.016 [Google Scholar]
- Hadfield J.D, Owens I.P.F. Strong environmental determination of a carotenoid-based plumage trait is not mediated by carotenoid availability. J. Evol. Biol. 2006;19:1104–1114. doi: 10.1111/j.1420-9101.2006.01095.x. doi:10.1111/j.1420-9101.2006.01095.x [DOI] [PubMed] [Google Scholar]
- Heath D.D, Fox C.W, Heath J.W. Maternal effects on offspring size: variation through early development of Chinook salmon. Evolution. 1999;53:1605–1611. doi: 10.1111/j.1558-5646.1999.tb05424.x. doi:10.2307/2640906 [DOI] [PubMed] [Google Scholar]
- Hoglund E, Balm P.H.M, Winberg S. Skin darkening, a potential social signal in subordinate Arctic charr (Salvelinus alpinus): the regulatory role of brain monoamines and pro-opiomelanocortin-derived peptides. J. Exp. Biol. 2000;203:1711–1721. doi: 10.1242/jeb.203.11.1711. [DOI] [PubMed] [Google Scholar]
- Houde A.E. Princeton University Press; Princeton, NJ: 1997. Sex, color and mate choice in guppies. [Google Scholar]
- Hudon J. Biotechnological applications of research on animal pigmentation. Biotechnol. Adv. 1994;12:49–69. doi: 10.1016/0734-9750(94)90290-9. doi:10.1016/0734-9750(94)90290-9 [DOI] [PubMed] [Google Scholar]
- Huntingford F.A, Turner A.M. Chapman & Hall; London, UK: 1987. Animal conflict. [Google Scholar]
- Jacob A, Nusslé S, Britschgi A, Müller R, Wedekind C. Male dominance linked to size and age, but not to ‘genetic quality’ in brown trout (Salmo trutta) BMC Evol. Biol. 2007;71:207. doi: 10.1186/1471-2148-7-207. doi:10.1186/1471-2148-7-207 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kause A, Ritola O, Paananen T, Eskelinen U, Mantysaari E. Big and beautiful? Quantitative genetic parameters for appearance of large rainbow trout. J. Fish Biol. 2003;62:610–622. doi:10.1046/j.1095-8649.2003.00051.x [Google Scholar]
- Kodric-Brown A. Sexual dichromatism and temporary color changes in the reproduction of fishes. Am. Zool. 1998;38:70–81. doi:10.1093/icb/38.1.70 [Google Scholar]
- Kodric-Brown A, Nicoletto P.F. Age and experience affect female choice in the guppy (Poecilia reticulata) Am. Nat. 2001;157:316–323. doi: 10.1086/319191. doi:10.1086/319191 [DOI] [PubMed] [Google Scholar]
- Kolluru G.R, Grether G.F, South S.H, Dunlop E, Cardinali A, Liu L, Carapiet A. The effects of carotenoid and food availability on resistance to a naturally occurring parasite (Gyrodactylus turnbulli) in guppies (Poecilia reticulata) Biol. J. Linn. Soc. 2006;89:301–309. doi:10.1111/j.1095-8312.2006.00675.x [Google Scholar]
- Krinsky N.I. Actions of carotenoids in biological systems. Annu. Rev. Nutr. 1993;13:561–587. doi: 10.1146/annurev.nu.13.070193.003021. doi:10.1146/annurev.nu.13.070193.003021 [DOI] [PubMed] [Google Scholar]
- Majerus M.E.N, Mundy N.I. Mammalian melanism: natural selection in black and white. Trends Genet. 2003;19:585–588. doi: 10.1016/j.tig.2003.09.003. doi:10.1016/j.tig.2003.09.003 [DOI] [PubMed] [Google Scholar]
- Marshall T.C, Slate J, Kruuk L.E.B, Pemberton J.M. Statistical confidence for likelihood-based paternity inference in natural populations. Mol. Ecol. 1998;7:639–655. doi: 10.1046/j.1365-294x.1998.00374.x. doi:10.1046/j.1365-294x.1998.00374.x [DOI] [PubMed] [Google Scholar]
- Masvaer M, Liljedal S, Folstad I. Are secondary sex traits, parasites and immunity related to variation in primary sex traits in the Arctic charr? Proc. R. Soc. B. 2004;271:S40–S42. doi: 10.1098/rsbl.2003.0090. doi:10.1098/rsbl.2003.0090 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGraw K.J. The antioxidant function of many animal pigments: are there consistent health benefits of sexually selected colourants? Anim. Behav. 2005;69:757–764. doi:10.1016/j.anbehav.2004.06.022 [Google Scholar]
- McGraw K.J. Dietary mineral content influences the expression of melanin-based ornamental coloration. Behav. Ecol. 2007;18:137–142. doi:10.1093/beheco/arl059 [Google Scholar]
- McGraw K.J, Ardia D.R. Carotenoids, immunocompetence, and the information content of sexual colors: an experimental test. Am. Nat. 2003;162:704–712. doi: 10.1086/378904. doi:10.1086/378904 [DOI] [PubMed] [Google Scholar]
- Milinski M, Bakker T.C.M. Female sticklebacks use male coloration in mate choice and hence avoid parasitized males. Nature. 1990;344:330–333. doi:10.1038/344330a0 [Google Scholar]
- Neff B.D, Pitcher T.E. Genetic quality and sexual selection: an integrated framework for good genes and compatible genes. Mol. Ecol. 2005;14:19–38. doi: 10.1111/j.1365-294X.2004.02395.x. doi:10.1111/j.1365-294X.2004.02395.x [DOI] [PubMed] [Google Scholar]
- Niecke M, Rothlaender S, Roulin A. Why do melanin ornaments signal individual quality? Insights from metal element analysis of barn owl feathers. Oecologia. 2003;137:153–158. doi: 10.1007/s00442-003-1307-3. doi:10.1007/s00442-003-1307-3 [DOI] [PubMed] [Google Scholar]
- O'Connor K.I, Metcalfe N.B, Taylor A.C. Does darkening signal submission in territorial contests between juvenile Atlantic salmon, Salmo salar? Anim. Behav. 1999;58:1269–1276. doi: 10.1006/anbe.1999.1260. doi:10.1006/anbe.1999.1260 [DOI] [PubMed] [Google Scholar]
- Olson V.A, Owens I.P.F. Costly sexual signals: are carotenoids rare, risky or required? Trends Ecol. Evol. 1998;13:510–514. doi: 10.1016/s0169-5347(98)01484-0. doi:10.1016/S0169-5347(98)01484-0 [DOI] [PubMed] [Google Scholar]
- Parker T.H. Genetic benefits of mate choice separated from differential maternal investment in red junglefowl (Gallus gallus) Evolution. 2003;57:2157–2165. doi: 10.1111/j.0014-3820.2003.tb00393.x. doi:10.1111/j.0014-3820.2003.tb00393.x [DOI] [PubMed] [Google Scholar]
- Pavlidis M, Papandroulakis N, Divanach P. A method for the comparison of chromaticity parameters in fish skin: preliminary results for coloration pattern of red skin Sparidae. Aquaculture. 2006;258:211–219. doi:10.1016/j.aquaculture.2006.05.028 [Google Scholar]
- Peters A. Testosterone and carotenoids: an integrated view of trade-offs between immunity and sexual signalling. Bioessays. 2007;29:427–430. doi: 10.1002/bies.20563. doi:10.1002/bies.20563 [DOI] [PubMed] [Google Scholar]
- Petersson E, Järvi T, Olsen H, Mayer I, Hedenskog M. Male–male competition and female choice in brown trout. Anim. Behav. 1999;57:777–783. doi: 10.1006/anbe.1998.1043. doi:10.1006/anbe.1998.1043 [DOI] [PubMed] [Google Scholar]
- Pitcher T.E, Neff B.D. MHC class IIB alleles contribute to both additive and nonadditive genetic effects on survival in Chinook salmon. Mol. Ecol. 2006;15:2357–2365. doi: 10.1111/j.1365-294X.2006.02942.x. doi:10.1111/j.1365-294X.2006.02942.x [DOI] [PubMed] [Google Scholar]
- Quinn T.P. University of Washington Press; Seattle, WA: 2005. The behavior and ecology of Pacific salmon and trout. [Google Scholar]
- Rifflart R, Marchand F, Rivot E, Bagliniere J.L. Scale reading validation for estimating age from tagged fish recapture in a brown trout (Salmo trutta) population. Fish. Res. 2006;78:380–384. doi:10.1016/j.fishres.2005.11.018 [Google Scholar]
- Roulin A. Proximate basis of the covariation between a melanin-based female ornament and offspring quality. Oecologia. 2004a;140:668–675. doi: 10.1007/s00442-004-1636-x. doi:10.1007/s00442-004-1636-x [DOI] [PubMed] [Google Scholar]
- Roulin A. The evolution, maintenance and adaptive function of genetic colour polymorphism in bird. Biol. Rev. 2004b;79:1–34. doi: 10.1017/s1464793104006487. doi:10.1017/S1464793104006487 [DOI] [PubMed] [Google Scholar]
- Roulin A. Melanin pigmentation negatively correlates with plumage preening effort in barn owls. Funct. Ecol. 2007;21:264–271. doi:10.1111/j.1365-2435.2006.01229.x [Google Scholar]
- Roulin A, Dauwe T, Blust R, Eens M, Beaud M. A link between eumelanism and calcium physiology in the barn owl. Naturwissenschaften. 2006;93:426–430. doi: 10.1007/s00114-006-0128-8. doi:10.1007/s00114-006-0128-8 [DOI] [PubMed] [Google Scholar]
- Rudolfsen G, Figenschou L, Folstad I, Nordeide J.T, Soreng E. Potential fitness benefits from mate selection in the Atlantic cod (Gadus morhua) J. Evol. Biol. 2005;18:172–179. doi: 10.1111/j.1420-9101.2004.00778.x. doi:10.1111/j.1420-9101.2004.00778.x [DOI] [PubMed] [Google Scholar]
- Rudolfsen G, Figenschou L, Folstad I, Tveiten H, Figenschou M. Rapid adjustments of sperm characteristics in relation to social status. Proc. R. Soc. B. 2006;273:325–332. doi: 10.1098/rspb.2005.3305. doi:10.1098/rspb.2005.3305 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skarstein F, Folstad I. Sexual dichromatism and the immunocompetence handicap: an observational approach using Arctic charr. Oikos. 1996;76:359–367. doi:10.2307/3546208 [Google Scholar]
- Skarstein F, Folstad I, Ronning H.P. Spawning colouration, parasites and habitat selection in Salvelinus alpinus: initiating speciation by sexual selection? J. Fish Biol. 2005;67:969–980. doi:10.1111/j.0022-1112.2005.00796.x [Google Scholar]
- Smith C, Barber I, Wootton R.J, Chittka L. A receiver bias in the origin of three-spined stickleback mate choice. Proc. R. Soc. B. 2004;271:949–955. doi: 10.1098/rspb.2004.2690. doi:10.1098/rspb.2004.2690 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steven D.M. Studies on animal carotenoids. 1. Carotenoids of the brown trout (Salmo trutta Linnaeus) J. Exp. Biol. 1948;25:369–387. doi: 10.1242/jeb.25.4.369. [DOI] [PubMed] [Google Scholar]
- Stevens M, Parraga C.A, Cuthill I.C, Partridge J.C, Troscianko T.S. Using digital photography to study animal coloration. Biol. J. Linn. Soc. 2007;90:211–237. doi:10.1111/j.1095-8312.2007.00725.x [Google Scholar]
- Sugimoto M. Morphological color changes in fish: regulation of pigment cell density and morphology. Microsc. Res. Tech. 2002;58:496–503. doi: 10.1002/jemt.10168. doi:10.1002/jemt.10168 [DOI] [PubMed] [Google Scholar]
- Wedekind C, Müller R. The experimental rearing of large salmonid eggs in Petri dishes. Funct. Ecol. 2004;18:138–140. doi:10.1111/j.1365-2435.2004.00822.x [Google Scholar]
- Wedekind C, Meyer P, Frischknecht M, Niggli U.A, Pfander H. Different carotenoids and potential information content of red coloration of male three-spined stickleback. J. Chem. Ecol. 1998;24:787–801. doi:10.1023/A:1022365315836 [Google Scholar]
- Wedekind C, Müller R, Spicher H. Potential genetic benefits of mate selection in whitefish. J. Evol. Biol. 2001;14:980–986. doi:10.1046/j.1420-9101.2001.00349.x [Google Scholar]
- Wedekind C, Walker M, Portmann J, Cenni B, Müller R, Binz T. MHC-linked susceptibility to a bacterial infection, but no MHC-linked cryptic female choice in whitefish. J. Evol. Biol. 2004;17:11–18. doi: 10.1046/j.1420-9101.2004.00669.x. doi:10.1046/j.1420-9101.2004.00669.x [DOI] [PubMed] [Google Scholar]
- Welch A.M, Semlitsch R.D, Gerhardt H.C. Call duration as an indicator of genetic quality in male gray tree frogs. Science. 1998;280:1928–1930. doi: 10.1126/science.280.5371.1928. doi:10.1126/science.280.5371.1928 [DOI] [PubMed] [Google Scholar]