Abstract
Anchorage and growth factor independence are cardinal features of the transformed phenotype. Although it is logical that the two pathways must be coregulated in normal tissues to maintain homeostasis, this has not been demonstrated directly. We showed previously that down-modulation of β1-integrin signaling reverted the malignant behavior of a human breast tumor cell line (T4–2) derived from phenotypically normal cells (HMT-3522) and led to growth arrest in a three-dimensional (3D) basement membrane assay in which the cells formed tissue-like acini (14). Here, we show that there is a bidirectional cross-modulation of β1-integrin and epidermal growth factor receptor (EGFR) signaling via the mitogen-activated protein kinase (MAPK) pathway. The reciprocal modulation does not occur in monolayer (2D) cultures. Antibody-mediated inhibition of either of these receptors in the tumor cells, or inhibition of MAPK kinase, induced a concomitant down-regulation of both receptors, followed by growth-arrest and restoration of normal breast tissue morphogenesis. Cross-modulation and tissue morphogenesis were associated with attenuation of EGF-induced transient MAPK activation. To specifically test EGFR and β1-integrin interdependency, EGFR was overexpressed in nonmalignant cells, leading to disruption of morphogenesis and a compensatory up-regulation of β1-integrin expression, again only in 3D. Our results indicate that when breast cells are spatially organized as a result of contact with basement membrane, the signaling pathways become coupled and bidirectional. They further explain why breast cells fail to differentiate in monolayer cultures in which these events are mostly uncoupled. Moreover, in a subset of tumor cells in which these pathways are misregulated but functional, the cells could be “normalized” by manipulating either pathway.
The tissue microenvironment is composed of an interactive network of soluble growth factors, extracellular matrix (ECM) components, and neighboring cells. Proliferation and differentiation within a tissue are modulated by growth factors, cell–ECM interactions, and cell–cell adhesion (1–3); thus; the ultimate decision a cell makes to proliferate or differentiate must be an integrated response to its adhesive and growth factor cues within the tissue. How the integration is achieved, however, remains an open question. Cell adhesion to the ECM is mediated predominantly by integrins, a family of transmembrane proteins that link the extracellular matrix with the cytoskeleton and act as signal transducers (3, 4). Recent studies have implied that integrin- and growth factor-dependent signals cooperate functionally in a variety of biological processes (5–7). Models for this cross-talk have been depicted as linear processes in which integrins and growth factor receptors act cumulatively at different junctions of their signaling pathways (6–8). Subtle variations with respect to the duration and intensity of this synergistic signaling are predicted to influence cell growth and differentiation (9, 10). These studies almost exclusively have relied on data obtained from monolayer cultures. Whether these models also can explain the signaling coordination required for the maintenance of complex tissue organization and gene expression has not been determined.
The HMT-3522 human mammary epithelial cells (MEC) and their tumor progression series were established from a reduction mammoplasty of a woman with a nonmalignant breast lesion and were derived by continuous cell passaging in defined medium (11, 12). We have shown that the nonmalignant early passage cells (designated S1) form phenotypically normal mammary tissue structures (acini) and growth arrest in response to cues from a three-dimensional (3D) basement membrane (BM) (13). In contrast, their tumorigenic counterparts (designated T4–2), which have striking perturbations in their integrin regulation (14), form disorganized, continuously growing colonies in response to the same BM stimuli. Treatment with a β1-integrin function-blocking antibody (or its Fab fragments), but not a β1-integrin function stimulatory antibody, was sufficient to induce a phenotypic and functional normalization of the T4–2 tumor cells when they were cultivated within a 3D BM (14). The reverted acini, referred to as T4β1, reassembled a BM, reestablished E-cadherin-catenin complexes, reorganized their cytoskeletons, and ceased growth. The malignant and reverted T4–2 cells, therefore, constituted a modulatable model system in which the mechanism by which these pathways converge could be studied systematically.
The epidermal growth factor receptor (EGFR) is a transmembrane tyrosine kinase required for normal mammary development and lactation (15, 16). EGFR is expressed aberrantly in ≈40% of breast carcinomas, particularly those with a poor prognostic and an invasive phenotype, and currently is being explored as a potential target for cancer therapy (17). To determine the relationship between cell adhesion, growth, and phenotypic reversion, we examined the EGFR signaling pathway in HMT-3522 cell series by using the 3D BM assay. We show here that EGFR is overexpressed in the tumorigenic T4–2 cells but is significantly down-regulated when these cells are reverted phenotypically by the β1-integrin function-blocking antibody. Conversely, treatment of T4–2 cells with an EGFR neutralizing antibody and an EGFR-specific inhibitor also induces phenotypic reversion and down-regulation of β1-integrin. We find that the bidirectional cross-modulation of β1-integrin and EGFR pathways is induced by a 3D BM and is absent in monolayer (2D) cultures. We show further that the BM-directed cross-modulation of β1-integrin and EGFR pathways and MEC tissue morphogenesis are associated with attenuation of EGF-induced transient mitogen-activated protein kinase (MAPK) activation. These results provide a mechanistic framework for the growth-suppression observed in the tumor cells by β1-integrin inhibitory antibody treatment. They also indicate that, when breast cells are organized spatially as a result of contact with BM, the signaling pathways become coupled and bidirectional, leading to global changes in protein and gene expression. Furthermore, these results may prove useful in alternative therapeutic strategies in the early stages of breast cancer progression.
MATERIALS AND METHODS
Cell Culture.
HMT-3522 mammary epithelial cells were cultured in H14 medium (11, 12, 14) consisting of DMEM/F12 (GIBCO/BRL) with 250 ng/ml insulin, 10 μg/ml transferrin, 2.6 ng/ml sodium selenite, 10−10 M estradiol, 1.4 × 10−6 M hydrocortisone, and 5 μg/ml prolactin. The nonmalignant S1 cells were grown on plastic in the presence of 10 ng/ml EGF, and the malignant T4–2 cells were propagated on collagen type I-coated dishes (Vitrogen 100, Celtrix Laboratories, Palo Alto, CA) in the absence of EGF. Previous experiments demonstrated that the addition of EGF to T4–2 cells or the culturing of S1 cells on collagen I did not alter their behavior or the expression of various cellular proteins, including EGFR and β1-integrin. Cultures were prepared by growing S1 and T4–2 cells to confluence as monolayers, followed by trypsinization and embedding (8.5 × 105/ml) into a commercially prepared reconstituted BM from Englebreth-Holm–Swarm tumors (Matrigel, Collaborative Research) (13, 14). The human breast cancer cell lines MDA-MB-453 and MDA-MB-468 were obtained from American Type Culture Collection and were maintained in DMEM/F12 with 5% fetal bovine serum.
Blocking Antibodies and Inhibitors.
The β1-integrin function-blocking mAb AIIB2 (a gift from C. Damsky, University of California at San Francisco; ref. 18) was introduced into the cell-embedded substratum at a concentration of 100 μg/ml ascites protein (which corresponds to 4–10 μg/ml purified rat IgG1) at the time of Matrigel gelation. The human EGFR-blocking mAb 225 (Oncogene) was added at a concentration of 4 μg/ml purified mouse IgG1. C-erbB-2 neutralizing-antibody clones N12 and N29 (NeoMarkers, Fremont, CA) were used at a concentration of 10 μg/ml purified mouse IgG1 and IgG2a, respectively. Mouse and rat IgG1 was obtained from the Jackson Laboratory. Tyrphostin AG 1478 (Calbiochem) and PD 98059 (New England Biolabs) were used at a concentrations of 100 nM and 20 μM, respectively. Chelerythrine chloride and GF 109203X (Calbiochem) were used at 5 and 4 μM, respectively. All inhibitors were dissolved in dimethyl sulfoxide and were added to the medium on alternate days. Control cultures were treated with vehicle only.
Fluorescence-Activated Cell Sorter (FACS) Analysis and Radioreceptor Binding Assay.
Cells were gently trypsinized, washed, and blocked in Dulbecco’s modified PBS buffer containing 0.5% RIA grade BSA (60 minutes at 4°C). Cells (1.5 × 106) in duplicate tubes were incubated at saturation with an anti-EGFR mAb for 60 minutes at 4°C (mAb 225, Oncogene; 1:100) and were washed with ice-cold PBS. Cells then were incubated with fluorescein isothiocyanate-labeled secondary antibody (The Jackson Laboratory) for 30 minutes at 4°C, followed by two PBS washes. Flow cytometry was performed with a cytofluorimeter, EPICS Profile II (Coulter). Cells were gated by forward and side-scattered signals, and 20,000 events were recorded. Control-treated samples consisted of cells incubated with secondary antibody alone. The number of EGF binding sites was assessed by using a receptor binding assay as described (24).
BrdUrd Incorporation Index.
The proliferative rate of cells grown as monolayers or in 3D BM was measured by assaying 5-bromo-2-deoxyuridine (BrdUrd) incorporation by using a commercially available labeling and detection kit (Boehringer Mannheim). In brief, cells were pulsed with BrdUrd for 12 (monolayer) or 24 hours (3D). Essentially, labeled nuclei were detected according to manufacturer’s instructions, although cells grown in 3D were frozen and cryosectioned (5 μm) before fixation. BrdUrd-labeled indices were determined by visually scoring nuclei (200–400 cells) by DAPI and thereafter scoring BrdUrd-positive cells as a percentage of total cell number (14).
Immunoblotting and Immunoprecipitation.
Cells grown as monolayers were lysed in situ in RIPA buffer [1% Nonidet P-40, 0.5% deoxycholate, 0.2% SDS, 150 mM sodium chloride, 50 mM Tris⋅HCl (pH 7.4) containing 2 mM sodium fluoride, 1 mM sodium orthovanadate, 10 μg/ml leupeptin, 10 μg/ml pepstatin, 10 μg/ml aprotinin, 10 μg/ml E 64, and 1 mM Pefabloc] whereas cells grown in 3D BM cultures for 10 days first were isolated as colonies by using ice-cold PBS/EDTA [0.01 M sodium phosphate (pH 7.2) containing 138 mM sodium chloride and 5 mM EDTA] as described (14) and thereafter were lysed in RIPA buffer. Equal amounts of protein lysates were loaded on reducing Laemmli gels, were immunoblotted, and were detected with an ECL system (Amersham). For immunoblot analysis of EGFR, phosphorylated (activated) EGFR, E-cadherin, and focal adhesion kinase (FAK), we used antibody clones 13, 74, 36, and 77, respectively (Transduction Laboratories, Lexington, KY). For immunoblot analysis of β1-integrin, c-erbB-2, and phosphotyrosine, we used antibody clones DF5 (Chemicon), Ab-3 (Oncogene) and 4G10 (Upstate Biotechnology, Lake Placid, NY), respectively. Phosphorylated (activated) MAPK was detected with an anti-phosphorylated-MAPK antibody (New England Biolabs). The level of integrin-linked kinase (ILK) was detected with an affinity-purified polyclonal anti-ILK antibody, 91–3 [as described (19, 20)].
For immunoprecipitation of FAK, cells grown in 3D cultures were lysed directly in RIPA buffer. Equal numbers of cells were precleared with protein G-plus/protein A agarose (Oncogene) and thereafter were incubated with an anti-FAK antibody (Transduction Laboratories), and antibody-protein complexes were isolated with protein G-plus/protein A. Equal amounts of immunocomplex were loaded on reducing Laemmli gels, and phosphorylated (active) FAK was detected with anti-phosphotyrosine antibody, 4G10 (Upstate Biotechnology).
Kinase Assay.
For ILK analysis, cells cultured in 3D for 10 days first were isolated with ice-cold PBS/EDTA and thereafter were lysed in Nonidet P-40 buffer [1% Nonidet P-40, 0.5% sodium deoxycholate, 150 mM NaCl, 50 mM Hepes (pH 7.5) containing 1 mM Pefabloc, 2 mM sodium fluoride, 1 mM sodium orthovanadate, 10 μg/ml leupeptin, 10 μg/ml aprotinin, and 1 mM Pefabloc). The Nonidet P-40 lysates were immunoprecipitated with polyclonal anti-ILK antibody, 91–3 [as described (19, 20)]. Immune complex kinase assays were performed, and ILK activity was measured as the amount of 32P incorporated into myelin basic protein (19). For assay of MAPK activity, cells were propagated in either 3D or monolayers, were deprived of EGF for 48 hours, and thereafter were treated with 20 ng/ml EGF for 15 minutes and then were lysed in assay buffer [0.5% Nonidet P-40, 150 mM sodium chloride, 10 mM Tris⋅HCl (pH 7.4), and 2 mM EGTA containing 2 mM DTT, 1 mM orthovanadate, 1 mM phenylmethylsulfonyl fluoride, 10 μg/ml leupeptin, and 10 μg/ml aprotinin]. MAPK activity was assayed as the amount of 32P-ATP incorporated into substrate peptide by using the Biotraktm p42/p44 MAPK enzyme assay system (Amersham), essentially according to the manufacturer’s instructions. The effects of PD 98059 on MAPK activity were examined by treating cells with 20 ng/ml EGF for 15 minutes in the presence of 20 μM PD 98059 and thereafter were lysed in RIPA buffer. RIPA lysates were loaded on reducing Laemmli gels, and phosphorylated (activated) MAPK was detected with anti-phosphorylated-MAPK antibody (New England Biolabs). For the assay of protein kinase C (PKC) activity, cells were lysed in assay buffer [50 mM Tris⋅HCl (pH 7.5), 5 mM EDTA, and 10 mM EGTA containing 0.3% β-mercaptoethanol and 50 μg/ml phenylmethylsulfonyl fluoride). PKC activity was determined by using the Biotrak PKC enzyme assay system (Amersham) following the manufacturer’s instructions.
Infection.
S1 cells were grown to 40% confluency and were infected with an EGFR retrovirus (pLWERNL vector, a gift from Steven Wiley, University of Utah), as previously described (21). Cells were grown to confluency, and survivors were selected by using 50 μg/ml G418. Total and active EGFR protein was assayed by immunoblot analysis; cell surface EGFR expression was evaluated by using FACS analysis as described above.
RESULTS AND DISCUSSION
Down-Regulation of EGFR in EGFR-Overexpressing T4–2 Cells Treated with Blocking Antibodies to β1-Integrin in 3D Cultures.
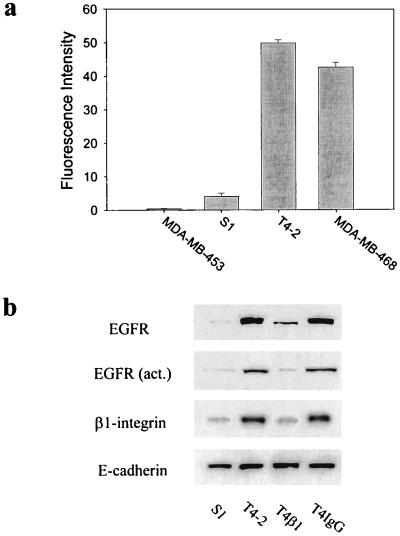
The T4–2 cells in HMT-3522 cell series were derived by EGF removal and were shown previously to have increased steady-state EGFR mRNA levels (12, 22). Using FACS analysis, we established that the T4–2 cell surface EGFR levels were elevated 12.3-fold over the S1 cells (Fig. 1a) and >126-fold over MDA-MB-453 cells (a human breast cancer cell line that lacks detectable EGFR) (23). EGF binding activity measured by a radio-receptor binding assay was, for S1, 1–2 × 105 vs., for T4–2, 1–2 × 106 sites per cell. The level of expression and activity of EGFR in the T4–2 cells was found to be similar to that expressed by MDA-MB-468 cells, a malignant breast cancer cell line used frequently as a model for EGFR-overexpressing breast tumors in vivo (24). In contrast, the protooncogenes c-erbB-2 and B3, which encode transmembrane protein tyrosine kinase homologues of the EGFR family (25), were expressed marginally in the S1 cells and were elevated no more than 2-fold in the T4–2 cells (data not shown). The high level of expression of EGFR in T4–2 cells was associated with an elevated rate of growth both in monolayer and in 3D BM cultures (data not shown); however, when treated with inhibitory β1-integrin antibody AIIB2 (used as acites or purified IgG1), which has been shown to inhibit ligand binding (18), these cells growth-arrested and formed a near normal acini whereas S1 cells underwent apoptosis (14). We reasoned that the growth-arrest should be associated with an inhibition of EGFR activity and/or expression. Immunoblot analysis of lysates from cells that had been grown within a BM for 10 days revealed that addition of AIIB2 triggered down-regulation of both total and activated EGFR protein in T4–2 cells to levels comparable to those seen in S1 cells (Fig. 1b). To determine whether this were true also for endogenous integrin expression, we assayed β1-integrin levels in the T4–2-reverted structures and found a significant reduction in the total amount expressed compared with untreated T4–2 cells (Fig. 1b). In contrast, the levels of E-cadherin as well as α- and β-catenin remained constant (Fig. 1b; ref. 14). Moreover, the level of c-erbB-2 also remained unaltered (not shown), indicating that reductions observed in β1-integrin and EGFR expression were specific and were not caused by a general reduction in protein expression.
Figure 1.
Down-regulation of total and activated EGFR in EGFR-overexpressing T4–2 cells treated with an inhibitory β1-integrin mAb, AIIB2. (a) FACS analysis of cell surface EGFR levels (using EGFR mAb 225) of S1, T4–2, MDA-MB-468, and MDA-MB-453 cells. Data are expressed as the average relative fluorescence intensity unit (±SEM) derived from three independent experiments. (b) Immunoblot analysis of total EGFR, activated EGFR, β1-integrin, and E-cadherin levels in S1, T4–2, and T4–2 cells treated with β1-integrin inhibitory mAb AIIB2 (100 μg/ml ascites protein) (T4β1) and T4–2 cells treated with an irrelevant IgG (T4IgG). Total E-cadherin levels remained constant regardless of the cell type or treatment regime and is used as a measure of loading. Cells were analyzed after 10 days of culture within the reconstituted BMs.
Phenotypic Reversion of T4–2 Cells by Blocking Antibodies to EGFR and Tyrphostin AG 1478 in 3D BM Cultures.
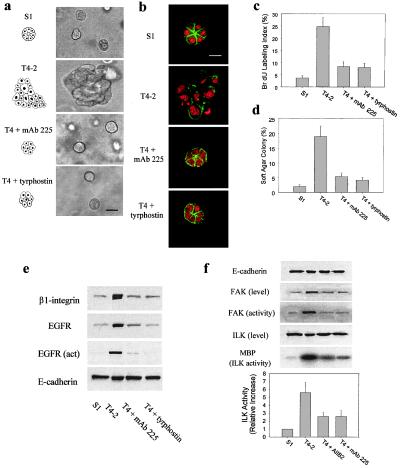
Because both EGFR and β1-integrin expression in T4–2 cells were reduced to normal levels as a result of inhibition of β1-integrin signaling, we asked whether the reverse—i.e., inhibiting EGFR activity—could be sufficient also to induce reversion as well as down-modulation of both receptors. T4–2 cells were grown within BM cultures with an EGFR-neutralizing antibody, mAb 225, used extensively to inhibit EGF-induced tyrosine protein kinase activation both in culture and in vivo (26). Although cells treated with a control IgG (Fig. 2a), c-erbB-2 neutralizing antibodies, or a non-function altering α6-integrin antibody J1B5 (data not shown) (27) were found to be unaffected, T4–2 cells treated with mAb 225 underwent a dramatic phenotypic reversion (Fig. 2a). A similar reversion was observed when T4–2 cells were treated with the EGFR-specific tyrosine kinase inhibitor, tyrphostin AG 1478 (Fig. 2a) (28). Viability and growth assays performed on cells grown as monolayers ruled out toxicity of tyrphostin (not shown). Cryosections of mAb 225 or tyrphostin-treated acini revealed polarized nuclei and well organized, cortical, filamentous actin, in contrast to untreated or IgG-treated T4–2 cells, which exhibited a grossly disorganized actin cytoskeleton (Fig. 2b). Furthermore, as shown previously, untreated T4–2 colonies exhibited inversely polarized β4-integrins, disorganized collagen IV, and laminin immunostaining (14) whereas both the mAb 225 and tyrphostin AG 1478-treated acini showed polarized β4-integrins and deposited an endogenous, basally organized BM, as revealed by collagen IV and laminin staining (not shown). Of the reverted acini, >90% were growth-arrested, as indicated by morphology and a decrease in BrdUrd incorporation into DNA (Fig. 2c). Moreover, the percent of cells forming colonies in soft agar (Fig. 2d), and the final size of the average acini, which contained 6–8 cells in a cryostained cross-section, were found to be similar to that observed in the S1 cells (data not shown). In contrast, untreated T4–2 cell colonies, which consisted of an average of 18–22 cells per 3D colony cross-section by day 10 (data not shown) (14), were found to grow continuously in BM cultures and exhibited anchorage-independent growth in the soft agar assay (Fig. 2d).
Figure 2.
Phenotypic reversion and down-regulation of total EGFR and β1-integrins and modulation of downstream signaling by EGFR mAb 225 and tyrphostin AG 1478 treatment in 3D cultures. (a) Phase contrast micrographs of S1, T4–2, and T4–2 cell colonies in the presence of EGFR mAb 225 (4 μg purified protein/ml) (T4 + mAb 225), or tyrphostin AG 1478 (100 nM) (T4 + tyrphostin), viewed directly inside BM. (Bar = 30 μm.) (b) Confocal fluorescence microscopy images of F-actin (green, fluorescein isothiocyanate) and nuclei (red, propidium iodide) in 5-μm cryosections of S1, T4–2, and T4–2 cell colonies phenotypically reverted by either the addition of EGFR mAb 225 or tyrphostin AG 1478, showing a cortical reorganization of F-actin in the reverted colonies. (Bar = 15 μm.) (c) BrdUrd labeling index from three separate experiments in S1, T4–2, and T4–2 + mAb 225 or T4–2 + tyrphostin AG 1478. 3D BM cultures described in a–c were analyzed after 10 days of culture inside BM. (d) Anchorage-independent growth of S1, T4–2, and T4–2 cells treated with EGFR mAb 225 or tyrphostin AG 1478 in soft agar. Tissue culture plates (12-well plates, Falcon) were used, and 20,000 cells were plated in 1 ml of DMEM/F12 containing 0.3% agarose overlaid with 1 ml of 1% agarose. Cultures were maintained for 15 days. Duplicate wells of colonies were scored positive when they exceeded the minimum diameter of 40 μm. (e) Immunoblot analysis of β1-integrin, total and activated EGFR, and E-cadherin proteins in S1, T4–2, and T4–2 + mAb 225 or T4–2 + tyrphostin AG 1478 in 3D BM. (f) Immunoblot analysis of ILK, total and activated FAK, and total E-cadherin proteins and a typical experiment of ILK kinase activity showing phosphorylation of myelin basic protein in cell lysates of S1, T4–2, and T4–2 + mAb AIIB2, or T4–2 + mAb 225. The numbers below are average of three independent experiments (mean ± SEM) showing the relative increase in ILK activity in the cell lysates as normalized to the value of 100% assigned to the S1 cells. Cells were analyzed after 10 days of culture inside 3D BM.
To show that the phenotypic reversion induced by mAb 225 and tyrphostin was reversible and was not caused by selection of possible contaminants or toxicity, a series of “reversion rescue” studies were undertaken as described (14). Despite reversion of T4–2 cells by mAb 225 and tyrphostin, these cells could be repropagated as monolayers, and, if cultured in a 3D BM without mAb 225 or tyrphostin, they were able to resume their tumorigenic phenotype (not shown; ref. 14).
Down-Regulation of EGFR and β1-Integrin and Modulation of Downstream Signaling by mAb 225 and Tyrphostin AG 1478 in 3D BM Cultures.
Immunoblot analysis of total protein lysates prepared from the phenotypically reverted mAb 225 and tyrphostin AG 1478 structures demonstrated a significant down-regulation of both β1-integrin and EGFR endogenous levels, as found for β1-integrin-induced reversion (Fig. 2e). The levels of E-cadherin, α-catenin, and β-catenin remained constant as described (Fig. 2e and data not shown). Time-course studies revealed that the down-regulation of β1-integrins and EGFRs by inhibitory antibody or tyrphostin treatment occurred within 72 hours of culturing within a 3D reconstituted BM (not shown) at a time when >40% of the cells still were actively growing (as indicated by a BrdUrd assay) and thus preceded the induction of growth arrest and tissue morphogenesis. The decrease in EGFR expression was accompanied by a decrease in the activity of the EGFR (Fig. 2e), implying that downstream signaling was involved in the reversion process. To examine this process in the β1-integrin pathway, we assayed the levels and the activity of both FAK and ILK. FAK is a tyrosine kinase found in focal adhesions; its activity is associated with integrin ligation (29, 30). ILK is a β1-integrin linked serine-threonine kinase (19, 20) that is activated transiently on ligand binding (31). Overexpression of FAK or ILK induces anchorage-independent growth in a soft agar assay (19, 30). The total levels of FAK were elevated in the T4–2 cells, but the ILK protein levels expressed in the S1 and T4–2 cell colonies were similar. Nevertheless, both FAK and ILK activities were increased significantly in the anchorage-independent T4–2 cell colonies (Fig. 2f). Inhibition of β1-integrin activity by AIIB2, and that of EGFR activity by mAb 225 treatments in T4–2 cells, were associated with reduced FAK and ILK activities (Fig. 2f), indicating reduced signaling through these pathways.
Cross-Modulation of β1-Integrin and EGFR in a 3D BM, and Its Absence in Monolayer Cultures.
Reciprocal down-regulation of β1-integrins and EGFR and phenotypic reversion of the T4–2 cells was associated with the presence of an exogenously supplied malleable BM, which in turn leads to formation of an endogenously synthesized BM (13, 14). Because mammary cells do not differentiate in monolayer cultures if an endogenously formed or exogenously added BM is lacking (3), we asked whether the cross-modulation was operative in 2D cultures. To test this, T4–2 cells were grown as monolayer cultures in the absence of an exogenous BM, with or without β1-integrin- and/or EGFR-inhibitory treatments, and were assayed for changes in growth, tissue organization, and β1-integrin and EGFR levels. Inhibition of either β1-integrins (by mAb AIIB2) or EGFRs (by mAb 225 or tyrphostin AG 1478) decreased the rate of T4–2 cell growth by 50% but failed to down-modulate β1-integrin or EGFR expression (Fig. 3 a, b, and c); nor did these treatments induce tissue morphogenesis. Moreover, inhibition of both the EGFR and β1-integrin pathways was required to reduce the growth of T4–2 cells below 20% of control in monolayer cultures (Fig. 3 a and b) yet did not induce down-regulation of EGFR or β1-integrin (Fig. 3c). In contrast, any one of the inhibitory treatments alone was sufficient to induce complete growth-arrest, phenotypic reversion, and down-regulation of β1-integrin and EGFR when cells were placed in 3D BM as shown previously and above (Fig. 2e; ref. 14). In addition to underscoring the importance of cell–ECM interactions and tissue structure in normal epithelial function, these results also indicate that reducing growth per se is insufficient for phenotypic reversion or cross-signaling between the EGFR and β1-integrin pathways.
Figure 3.
β1-integrin and EGFR are cross-modulated in a 3D BM but not in monolayer cultures. (a) Effect of β1-integrin mAb AIIB2 and EGFR mAb 225 alone or in combination on T4–2 cells grown in monolayer. T4–2 cells were plated at 25,000 cells/cm2 in 12-well tissue culture plates (Falcon) and were treated with either vehicle (T4–2), 100 μg/ml β1-integrin AIIB2 mAb ascites protein (T4 + AIIB2), or 4 μg/ml purified EGFR mAb 225 (T4 + mAb 225) alone or in combination (T4 + AIIB2 + mAb 225). Treatment commenced 16–24 hours after cell plating (day 0), and cultures were retreated when the media were changed every other day. Cells were trypsinized and counted on days 2, 4, and 6. Data from a representative experiment (mean ± duplicate counts) are shown. Inhibition of EGFR activity with 100 nM tyrphostin AG 1478 gave results comparable to mAb 225 (data not shown). (b) Effect of β1-integrin mAb AIIB2 or EGFR mAb 225 alone or in combination on T4–2 cell proliferation in monolayer, as assessed by BrdUrd labeling. Results shown are from day 2 of treatment and are expressed as the average of three experiments (mean ± SEM). An 80% reduction in T4–2 cell proliferation required the presence of both EGFR and β1-integrin inhibitors. (c) Immunoblot analysis of total and activated EGFR, β1-integrin, and E-cadherin expressed in T4–2 cells treated in monolayer with vehicle (T4–2), 100 μg/ml β1-integrin AIIB2 ascites mAb (T4 + AIIB2), 100 nM tyrphostin (T4 + tyrphostin), or AIIB2 ascites mAb as well as tyrphostin (T4 + tyr +AIIB2) and grown for 4 days. (d) Effects of EGFR overexpression on β1-integrin level and acinar formation in S1 nonmalignant cells. EGFR-infected S1 cells overexpressed total and activated EGFR in both the presence (3.1-fold increase over control) and absence of a BM (2.6-fold increase). Overexpression of EGFR in the S1 cells did not affect β1-integrin expression when the cells were grown as monolayers (1.1-fold increase). In contrast, when the EGFR-overexpressing cells were grown in the presence of a 3D BM, there was a 2.8-fold increase in β1-integrin expression (roughly equivalent to the fold increase in EGFR) compared with the noninfected S1 cells.
If the results described above for revertants were an indication of normal tissue behavior, they would predict that overexpression of either EGFR or β1-integrin in phenotypically normal S1 cells should lead to disruption of growth control and disorganization of tissue structure. They also would predict that there should be a concomitant increase in the levels of β1-integrin (when EGFR was overexpressed) or EGFR (when β1-integrin was overexpressed). Overexpression of β1-integrin is potentially complicated (32, 33); we thus overexpressed EGFR in S1 cells as proof of principle. S1 cells were infected with an EGFR retrovirus (pLWERNL vector), and G418-selected colonies exhibited 2- to 3-fold increase in EGFR expression, as shown by immunoblot analysis (Fig. 3d). To show that the increased total expression was accompanied with increased surface expression, FACS analysis was performed. The EGFR-infected S1 cells had 2.5-fold increase in cell surface EGFR expression (data not shown). When grown within the malleable BM, the EGFR-overexpressing cells formed large, disorganized colonies (Fig. 3d), which, in the presence of mAb 225, could be reverted to the organized, noninfected S1-like acini (not shown). These results establish a relationship between EGFR overexpression and the loss of mammary tissue phenotype. Immunoblot analysis of protein lysates prepared from the S1-EGFR infectants showed that, although EGFR levels (both activated and total) were always higher than the levels in the uninfected S1 cells (3.1-fold increase in 3D BM vs. 2.6-fold in monolayer), an up-regulation of β1-integrin was found only when the EGFR-overexpressing cells were grown within a 3D BM (2.8-fold increase in 3D BM vs. 1.1-fold in monolayer). Thus, EGFR and β1-integrin coupling and cross-talk exist also in functionally normal cells but require spatial orientation and the presence of a malleable BM. How the interaction with a BM is translated into changes in cellular structure and the cytoskeleton in these cells remains speculative and needs to be determined (34, 35).
Association of Attenuated Transient MAPK Activation with MEC Tissue Morphogenesis in 3D Cultures.
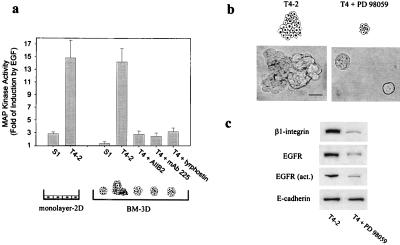
MAPK has been shown to serve as a common downstream effector of both β1-integrin and EGFR signaling (9, 36, 37). We reasoned that this pathway may serve as a molecular switch, sensing changes in the cell adhesion and growth factor levels in the cellular microenvironment to influence growth and differentiation decisions in the breast. Sustained MAPK activity has been shown to be associated with cell differentiation whereas transient stimulation, as induced by EGFR ligation, promotes proliferation in PC12 cells (9, 10). We investigated whether the BM-directed cross-modulation of β1-integrins and EGFRs and breast tissue morphogenesis were associated with an alteration in transient and/or sustained MAPK activation. Sustained (basal) MAPK activity was similar in S1 acini, the T4–2 colonies, and the mAb AIIB2, mAb 225, or tyrphostin AG 1478-reverted T4–2 structures in a 3D BM. When EGF was added to T4–2 cells, there was a dramatic increase in transient MAPK activity whether or not the cells were on monolayer or in a 3D BM. In contrast, EGF activation of MAPK was very low in the S1 acini in a 3D BM, and there was a marked reduction in the reverted T4–2 structures (Fig. 4a). However, when the S1 cells were cultured as monolayers, in the absence of an exogenous BM, EGF stimulation led to a measurable response in MAPK [compare S1 cells on monolayer to S1 cells on BM (Fig. 4a)]. This indicates that the EGF-inducible MAPK pathway is “on” in phenotypically normal cells in monolayer cultures whereas it is essentially switched “off” in 3D BM-organized tissue structures. These findings raised the possibility that inhibition of MAPK kinase, an upstream regulator of MAPK activation, may be sufficient to induce growth-arrest and tissue morphogenesis in the T4–2 cells in 3D. To test this, T4–2 cells were treated in the presence or absence of a 3D BM with a selective inhibitor of MAPK kinase (PD 98059; ref. 38). Inhibition of MAPK kinase activity in either monolayer or 3D BM cultures of T4–2 cells attenuated transient MAPK activation by EGF but did not influence basal activity (data not shown). Attenuation of MAPK activation induced only a 50% growth reduction in the cells on 2D monolayers (not shown) but led to complete growth-arrest and phenotypic reversion of the tumor cells when they were grown within 3D BMs (Fig. 4b). Moreover, the reversion in a 3D BM was associated with down-regulation of β1-integrin and EGFR (Fig. 4c). These results show that 3D BM-induced tissue morphogenesis of MECs is associated with an attenuation of EGF-induced transient stimulation of MAPK activity. Moreover, they show that repression of transient MAPK activation once more leads to different end points in monolayer as opposed to 3D BM-induced cultures.
Figure 4.
Attenuation of transient MAPK activation by EGF is associated with MEC tissue morphogenesis in a 3D BM. (a) Transient stimulation of MAPK activity by EGF in S1 and T4–2 cells grown as monolayers and in 3D with or without treatments of mAb AIIB2 and mAb 225. EGF induced a 3-fold transient increase in MAPK activity in S1 cells grown as monolayers and a 14-fold increase in T4–2 cells grown either as monolayers or within 3D BMs. In contrast, EGF was unable to induce an increase in MAPK activity in the organized S1 structures in 3D. Phenotypic reversion of the T4–2 cells by treatment with mAb AIIB2 or mAb 225 in the presence of a BM attenuated the EGF-induced transient stimulation of MAPK activity. (b) Phenotypic reversion of T4–2 cells cultured for 10 days in BMs in the absence (left) or presence (right) of 20 μM MAPK kinase inhibitor, PD 98059. (Bar = 30 μm.) (c) Immunoblot analysis of β1-integrin, total and activated EGFR, and E-cadherin protein expression in T4–2 cell colonies grown in the absence or presence of PD 98059 (T4 + PD 98059).
To ask whether other metabolic inhibitors also could cause reversion, we treated T4–2 cells with chelerythrine chloride (39) or GF 109203X (40), inhibitors of PKC. Although these treatments greatly inhibited the PKC activity of T4–2 cells (not shown), they neither influenced growth within 3D BM cultures nor induced phenotypic reversion or down-regulation of EGFR or β1-integrins (not shown). Thus, PKC signaling pathway is not involved in cross-modulation between β1-integrin and EGFR and phenotypic reversion of T4–2 cells.
In conclusion, we have found that regulation of EGFR (as well as β1-integrin) is linked to tissue morphogenesis. This was shown by restoration of the normal breast phenotype when EGFR was down-modulated in the tumor cells and the loss of tissue structure, when it was overexpressed in nonmalignant cells. Furthermore, we found qualitative differences in how EGFR and β1-integrin pathways are coupled via MAPK signaling when cells were organized spatially in a reconstituted BM matrix, as opposed to adhesion as monolayers on tissue culture plastic in 2D. The data presented here provide evidence for the stated hypothesis that, in nonmalignant breast epithelial tissue in 3D BMs (and presumably in vivo), growth and adhesion pathways are coupled dynamically and reciprocally. They further provide a mechanistic framework for the dramatic phenotypic reversion and growth-suppression observed in the tumor cells by β1-integrin inhibitory antibody treatment (14). These data may explain the recent observation of elevated MAPK in breast tumors (41) and the association between EGFR overexpression and the invasive behavior of poorly differentiated breast tumors in vivo (17). Finally, the data indicate that, as long as the essential components of the EGFR and β1-integrin pathways are not irreversibly altered or deleted, both “inside out” and “outside in” signaling can correct aberrant behavior and restore normal function to tumor cells in a 3D BM. As such, these results may prove useful in devising alternative therapeutic strategies for early stages of breast cancer progression.
Acknowledgments
We thank Z. Werb, J. Brugge, C. Roskelley, and K. Schmeichel for critical reading of the manuscript, X. Yue and M. Lund for technical assistance, E. Vladusic and F. Guerra-Vladusic for help with the RNA protection assay, H. Su Huang and S. Wiley for providing the pLWERNL vector, C. Damsky for providing the antibody AIIB2, R. Schwarz and H.-M. Chen for helpful comments, and B. Johansen for administrative support. This work was supported by grants from the U.S. Department of Energy Office of Biological and Environmental Research (Contract DE-AC03–76SF00098) and the National Institutes of Health (Grant CA-64786) to M.J.B., the University of California Breast Cancer Research Program (IFB-0400) to V.M.W., and the Danish Cancer Society (95-100-44), the Thaysen Foundation, the Novo Foundation, and the Danish Medical Research Council (9503681) to O.W.P. S.D. is a Terry Fox Cancer Scientist of the National Cancer Institute of Canada and is supported by the National Cancer Institute of Canada.
ABBREVIATIONS
- BM
basement membrane
- ECM
extracellular matrix
- EGF
epidermal growth factor
- EGFR
epidermal growth factor receptor
- FAK
focal adhesion kinase
- ILK
integrin-linked kinase
- MAPK
mitogen-activated protein kinase
- MEC
mammary epithelial cell
- 3D
three-dimensional
- PKC
protein kinase C
- FACS
fluorescence-activated cell sorter
References
- 1.Johnson G L, Vaillancourt R R. Curr Opin Cell Biol. 1994;6:230–238. doi: 10.1016/0955-0674(94)90141-4. [DOI] [PubMed] [Google Scholar]
- 2.Gumbiner B M. Cell. 1996;84:345–357. doi: 10.1016/s0092-8674(00)81279-9. [DOI] [PubMed] [Google Scholar]
- 3.Roskelley C D, Srebrow A, Bissell M J. Curr Opin Cell Biol. 1995;7:736–747. doi: 10.1016/0955-0674(95)80117-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Clark E A, Brugge J S. Science. 1995;268:233–239. doi: 10.1126/science.7716514. [DOI] [PubMed] [Google Scholar]
- 5.Sastry S K, Horwitz A F. Dev Biol. 1996;180:455–467. doi: 10.1006/dbio.1996.0319. [DOI] [PubMed] [Google Scholar]
- 6.Giancotti F G. Curr Opin Cell Biol. 1997;9:691–700. doi: 10.1016/s0955-0674(97)80123-8. [DOI] [PubMed] [Google Scholar]
- 7.Katz B Z, Yamada K M. Biochimie (Paris) 1997;79:467–476. doi: 10.1016/s0300-9084(97)82738-1. [DOI] [PubMed] [Google Scholar]
- 8.Schwartz M A. J Cell Biol. 1997;139:575–578. doi: 10.1083/jcb.139.3.575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Marshall C J. Cell. 1995;80:179–185. doi: 10.1016/0092-8674(95)90401-8. [DOI] [PubMed] [Google Scholar]
- 10.York R D, Yao H, Dillon T, Ellig C L, Eckert S P, McCleskey E W, Stork P J. Nature (London) 1998;392:622–626. doi: 10.1038/33451. [DOI] [PubMed] [Google Scholar]
- 11.Briand P, Petersen O W, van Deurs B. In Vitro Cell Dev Biol. 1987;23:181–188. doi: 10.1007/BF02623578. [DOI] [PubMed] [Google Scholar]
- 12.Briand P, Nielsen K V, Madsen M W, Petersen O W. Cancer Res. 1996;56:2039–2044. [PubMed] [Google Scholar]
- 13.Petersen O W, Ronnov-Jessen L, Howlett A R, Bissell M J. Proc Natl Acad Sci USA. 1992;89:9064–9068. doi: 10.1073/pnas.89.19.9064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Weaver V M, Petersen O W, Wang F, Larabell C A, Briand P, Damsky C, Bissell M J. J Cell Biol. 1997;137:231–245. doi: 10.1083/jcb.137.1.231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Okamoto S, Oka T. Proc Natl Acad Sci USA. 1984;81:6059–6063. doi: 10.1073/pnas.81.19.6059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fowler K J, Walker F, Alexander W, Hibbs M L, Nice E C, Bohmer R M, Mann G B, Thumwood C, Maglitto R, Danks J A. Proc Natl Acad Sci USA. 1995;92:1465–1469. doi: 10.1073/pnas.92.5.1465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Fox S B, Harris A L. J Mammary Gland Biol Neoplasia. 1997;2:131–141. doi: 10.1023/a:1026399613946. [DOI] [PubMed] [Google Scholar]
- 18.Takada Y, Puzon W. J Biol Chem. 1993;268:17597–17601. [PubMed] [Google Scholar]
- 19.Hannigan G E, Leung-Hagesteijn C, Fitz-Gibbon L, Coppolino M G, Radeva G, Filmus J, Bell J C, Dedhar S. Nature (London) 1996;379:91–96. doi: 10.1038/379091a0. [DOI] [PubMed] [Google Scholar]
- 20.Radeva G, Petrocelli T, Behrend E, Leung-Hagesteijn C, Filmus J, Slingerland J, Dedhar S. J Biol Chem. 1997;272:13937–13944. doi: 10.1074/jbc.272.21.13937. [DOI] [PubMed] [Google Scholar]
- 21.Huang H S, Nagane M, Klingbeil C K, Lin H, Nishikawa R, Ji X D, Huang C M, Gill G N, Wiley H S, Cavenee W K. J Biol Chem. 1997;272:2927–2935. doi: 10.1074/jbc.272.5.2927. [DOI] [PubMed] [Google Scholar]
- 22.Madsen M W, Lykkesfeldt A E, Laursen I, Nielsen K V, Briand P. Cancer Res. 1992;52:1210–1217. [PubMed] [Google Scholar]
- 23.Johnson G R, Kannan B, Shoyab M, Stromberg K. J Biol Chem. 1993;268:2924–2931. [PubMed] [Google Scholar]
- 24.Arteaga C L, Hurd S D, Dugger T C, Winnier A R, Robertson J B. Cancer Res. 1994;54:4703–4709. [PubMed] [Google Scholar]
- 25.Lupu R, Carillo M, Harris L, Hijazi M, Rosenberg K. Semin Cancer Biol. 1995;6:135–145. doi: 10.1006/scbi.1995.0016. [DOI] [PubMed] [Google Scholar]
- 26.Peng D, Fan Z, Lu Y, DeBlasio T, Scher H, Mendelsohn J. Cancer Res. 1996;56:3666–3669. [PubMed] [Google Scholar]
- 27.Damsky C H, Librach C, Lim K H, Fitzgerald M L, McMaster M T, Janatpour M, Zhou Y, Logan S K, Fisher S J. Development (Cambridge, UK) 1994;120:3657–3666. doi: 10.1242/dev.120.12.3657. [DOI] [PubMed] [Google Scholar]
- 28.Han Y, Caday C G, Nanda A, Cavenee W K, Huang H J. Cancer Res. 1996;56:3859–3861. [PubMed] [Google Scholar]
- 29.Richardson A, Parsons J T. BioEssays. 1995;17:229–236. doi: 10.1002/bies.950170309. [DOI] [PubMed] [Google Scholar]
- 30.Frisch S M, Vuori K, Ruoslahti E, Chan-Hui P Y. J Cell Biol. 1996;134:793–799. doi: 10.1083/jcb.134.3.793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Delcommenne M, Tan C, Gray V, Ruel L, Woodgett J, Dedhar S. Proc Natl Acad Sci USA. 1998;95:11211–11216. doi: 10.1073/pnas.95.19.11211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Heino J, Ignotz R A, Hemler M E, Crouse C, Massague J. J Biol Chem. 1989;264:380–388. [PubMed] [Google Scholar]
- 33.Reszka A A, Hayashi Y, Horwitz A F. J Cell Biol. 1992;117:1321–1330. doi: 10.1083/jcb.117.6.1321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Boudreau N, Myers C, Bissell M J. Trends Cell Biol. 1995;5:1–4. doi: 10.1016/s0962-8924(00)88924-2. [DOI] [PubMed] [Google Scholar]
- 35.Rana B, Mischoulon D, Xie Y, Bucher N L R, Farmer S R. Mol Cell Biol. 1994;14:5858–5869. doi: 10.1128/mcb.14.9.5858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Clark E A, Hynes R O. J Biol Chem. 1996;271:14814–14818. doi: 10.1074/jbc.271.25.14814. [DOI] [PubMed] [Google Scholar]
- 37.Chen Q, Kinch M S, Lin T H, Burridge K, Juliano R L. J Biol Chem. 1994;269:26602–26605. [PubMed] [Google Scholar]
- 38.Saltiel A R, Sawyer T K. Chem Biol. 1996;3:887–893. doi: 10.1016/s1074-5521(96)90177-5. [DOI] [PubMed] [Google Scholar]
- 39.Ventura C, Pintus G, Tadolini B. J Biol Chem. 1997;272:6693–6698. doi: 10.1074/jbc.272.10.6693. [DOI] [PubMed] [Google Scholar]
- 40.Kiss Z, Phillips H, Anderson W H. Biochim Biophys Acta. 1995;1265:93–95. doi: 10.1016/0167-4889(94)00242-7. [DOI] [PubMed] [Google Scholar]
- 41.Sivaraman V S, Wang H, Nuovo G J, Malbon C C. J Clin Invest. 1997;99:1478–1483. doi: 10.1172/JCI119309. [DOI] [PMC free article] [PubMed] [Google Scholar]