Abstract
Fesselin is a natively unfolded protein that is abundant in avian smooth muscle. Like many natively unfolded proteins, fesselin has multiple binding partners including actin, myosin, calmodulin and α-actinin. Fesselin accelerates actin polymerization and bundles actin. These and other observations suggest that fesselin is a component of the cytoskeleton. We have now cloned fesselin and have determined the cDNA derived amino acid sequence. We verified parts of the sequence by Edman analysis and by mass spectroscopy. Our results confirmed fesselin is homologous to human synaptopodin 2 and belongs to the synaptopodin family of proteins.
Keywords: Fesselin, synaptopodin, myopodin, actin binding protein, actin polymerization, Ca++-calmodulin regulation
Fesselin is an actin binding protein [1] that is rich in avian smooth muscle. Fesselin binds to both F-actin and to G-actin. When added to G-actin, fesselin induces rapid polymerization by increasing the rate of nucleation [2]. The rate of actin polymerization is inhibited by Ca2+-calmodulin to a rate that is lower than that of actin alone [3]. When added to F-actin fesselin initiates the formation of actin aggregates that sediment at low speed [1]. Fesselin inhibits actin activation of myosin S1 ATPase activity [4]. That inhibition is not reversed by Ca2+-calmodulin. Fesselin is natively unfolded [5] with little secondary or tertiary structure when in pure form at neutral pH and physiological ionic strength. Like other natively unfolded proteins fesselin has several binding partners and gains secondary structure when bound to one of those partners (calmodulin). In addition to binding actin and calmodulin [3], fesselin also binds to myosin [4] and α-actinin [6]. These abilities of fesselin to polymerize actin, organize actin filaments and bind to other actin associated proteins suggest a role of fesselin in organizing cellular actin. This hypothesis is supported by evidence that fesselin is localized in smooth muscle dense bodies [7].
Because actin binding proteins tend to be highly conserved it is likely that proteins similar to fesselin are found in mammals [8]. In an earlier analysis of 7 fesselin peptides (about 8% of the total fesselin sequence) we observed that three fragments were unique and 4 others had 57–70% identity to synaptopodin [1]. Synaptopodin is the eponym of a family of actin binding proteins that is formed by 3 protein subgroups: synaptopodin, the synaptopodin 2 proteins, to which myopodin belongs, and the synaptopodin-2 like proteins. Both fesselin and synaptopodin are rich in proline and have high isoelectric points [1, 9]. Synaptopodin family members also bind to actin [9, 10] and α-actinin [11, 12]). Synaptopodin has been linked with the organization of the cytoskeleton [13].
Synaptopodin family members are important since they have been associated with defects of the spine apparatus and learning deficiencies in knock out mice [14]. These proteins are also linked to some cancers. Myopodin suppresses cancers of bladder and prostate [15, 16] and its absence has been used as an indication for cancer staging.
Establishing a link between fesselin and synaptopodin family members is important because the ease in purification of avian fesselin facilitates biochemical and biophysical analyses of this protein. As such, fesselin could be a useful paradigm for the synaptopodin family. We have now cloned fesselin. From the derived sequence and from direct sequencing of the protein we now report that avian fesselin is homologous to mammalian synaptopodin 2.
Materials and Methods
Protein-Sequencing
Sequencing was performed by Edman degradation [17]. Proteins were separated on SDS-gels and transferred on Sequi-Blot™ PVDF Membrane (BIO-RAD) in a tank blot apparatus [18]. To obtain specific peptide fragments, the protein was digested with trypsin directly on the PVDF membrane [19]. The peptides obtained were separated by high pressure liquid chromatography (HPLC), on a Hypersil C18BDS 3-m LC-Packings column (150.1.0 mm; BIA, Bensheim, Germany) and a 130 A HPLC separation system (Applied Biosystems, Weiterstadt, Germany) with 0.1% trifluoric acid (TFA) as solvent A and 80% acetonitrile in 0.085% TFA as solvent B. The HPLC-separated fragments were sequenced on Polybrene-treated filters, using a Procise 494 A protein sequencer from Applied Biosystems. MALDI-TOF fingerprint analysis was performed by Dr. Monica Linder (Giessen, Germany). Proteins were precipitated with 8 volumes acetone at −20°C and then washed 3 times with acetone (−20°C) prior to shipping. Peptides were identified using the program Mascot Search Results from MATRIX SCIENCE Inc., Boston, MA, and the program ProteinProspector v4.0.8, http://prosprector.ucsf.edu/.
RNA-isolation, RT-PCR, cloning, and DNA-sequencing of turkey fesselin
Total-RNA was isolated from turkey gizzard tissue using an RNeasy Kit (Qiagen, Hilden, Germany). The RT-reaction was performed using dT18-oligos and Superscript II or Superscript III (Invitrogen, Carlsbad, CA). For the PCR reactions oligo nucleotides were selected based on the nucleotide sequence for a predicted protein similar to synaptopodin 2 from gallus gallus (GenBank accession no. XM_426324). Oligo nucleotides used were as following: 5’ primer 5’ AGA GAG AAT TCT CCT TCT CTG CCC TCC TTT G 3’ (bp 1316–1336), 3’ primer 5’ AGA GAG GAT CCT CAC CTG GAA TCC ATG GAC ATT ATA TCA G 3’ (bp 4099-4073). The 5’ and 3’ ends of the mRNA were obtained using a 5’/3’ RACE Kit, 2nd generation (Roche Applied Science, Indianapolis, IN). The gene specific 3’ oligo nucleotides used are listed in Table 1. The 5’ oligo nucleotide used was provided in the kit. The 3’RACE was performed as recommended by the manufacturer using the gene specific oligo nucleotide listed in Table 1. The amplification products were cloned in a pCR BLUNT vector (Invitrogen, Carlsbad, CA) and sequenced by cycle sequencing with the ABI Prism big dye terminator cycle sequencing ready reaction kit. The products were resolved on an ABI PRISM 377 automated sequencer (Perkin Elmer/Applied Biosystems).
Table 1.
Gene specific oligo nucleotides that were used in 5’ and 3’ RACES
| oligo nucleotide | Sequence |
|---|---|
| 5’ RACE | |
| 1st primer | 5’ ACC TGG AAT CCA TGG ACA TTA TAT CAG 3’ |
| 2nd primer | 5’ TCA CCT TCT TCA CCC TCG TC 3’ |
| 3rd primer | 5’ AAA CAT GAG GGC CCC TTT AC 3’ |
| 4th primer | 5’ GTT GCC CTG TGC TTG AGA G 3’ |
| 3’ RACE | |
| 1st primer | 5’ GAA AGC AAC CGG GTG TTA CAG 3’ |
Protein preparations
Turkey gizzard fesselin was purified as described by Leinweber et al. [1].
Western blot
Snap frozen tissues were ground under liquid nitrogen to a fine powder and suspended in a buffer containing 8 M urea, 0.1 M Tris pH 6.8, 2% SDS, 0.035M dithiothreitol, and protease inhibitor cocktail (SIGMA P2714) equivalent to 0.1 mg USP pancreatin/ml. The suspended tissue was incubated for 1 hour on ice, and clarified by centrifugation. A Bradford protein assay was used to determine the protein concentration in the supernatants. 50 µg proteins per lane were electrophoresed on a 10% SDS gel, transferred on nitrocellulose membrane, and probed with anti fesselin antibodies.
Results and Discussion
In our first description of turkey gizzard fesselin [1] we sequenced 7 short peptides of that new protein. We noted that 4 of these peptides had identities ranging from 57 to 70% with the previously published synaptopodin sequence [9]. Those regions of homology represented approximately 4% of the total fesselin sequence. While our limited sequence information showed some similarity to the synaptopodin family the distribution of fesselin is different from that reported for any of the synaptopodin family member proteins. Fesselin is abundant in avian smooth muscle tissue. Synaptopodin is found in kidney and brain [9]. Synaptopodin mRNA, but not the gene product, has been found in pancreas [20]. The splicing product of synaptopodin 2, myopodin, is expressed in many tissues and is particularly rich in skeletal muscles with smaller amounts in smooth muscles [10, 16]. None of the synaptopodin family members is abundant in mammalian smooth muscle.
To explore the possibility that fesselin is a member of the synaptopodin family we have determined the sequence of fesselin both at the protein and cDNA levels. Knowing this relationship is important for determining the function of fesselin and synaptopodin. More information is available on the biochemistry of the more readily purified fesselin while much is known about the cell biology of synaptopodin family members.
Avian fesselin is isolated as two polypeptide chains (103 and 79 kDa) having similar properties [1]. We analyzed the sequence of purified 103 kDa natural fesselin form by both Edman and MALDI-TOF analyses to explore the relationship to the synaptopodin family. Results from Edman analysis of internal peptides are shown in Table 2. Of the 116 residues analyzed, 112 were identical to predicted avian synaptopodin 2 (96.5% identity); 3 residues were conservative substitutions. The regions of homology extended from amino acid residues 498 to 1092 of the gallus gallus sequence (GenBank accession no XM_426324). Figure 1 shows the alignment of the cDNA derived amino acid sequence from turkey fesselin (GenBank accession nos. EU086520, ABU55374) with predicted synaptopodin 2 from gallus gallus (GenBank accession no. XM_426324). Amino acid residues that were confirmed by Edman analysis are shown as bold letters in that figure. Note that we were unable to identify the N-terminal residue by Edman analysis.
Table 2.
Peptides of turkey fesselin determined by Edman degradation a
| Protease | Peptide |
|---|---|
| Trypsin | TGILQEAK (681–688)b; STSKPMFSFK (691–700); APSF-APASPQAAYP (789–803); GAQLFAK (868–874); FARRHSRMEK (872–892); YVVDSDTVQAN (882–892); SLSLPGKPLFI (1082–1092) |
| Glu C | ACNFMQS (743–749); TVQANMARASSP (888–899); SNVRAPPPVA (911–920) |
| Arginase C | RQRMDQITAEQEE (498–510); EAPKVSPNPALLSL (701–714) |
Figure 1.
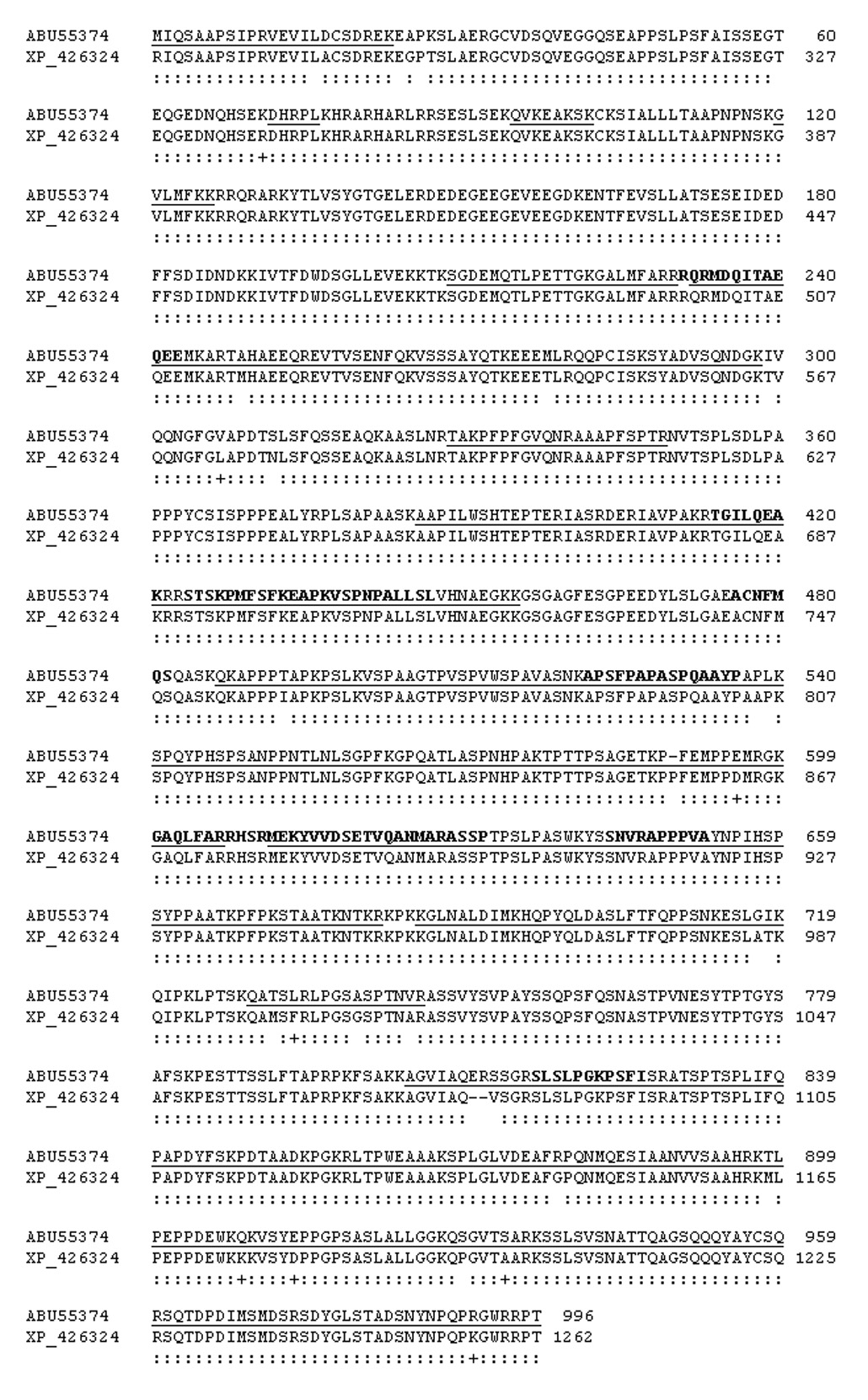
Alignment of the cDNA derived amino acid sequence of 103 kDa turkey fesselin (GenBank accession nos. EU086520, ABU55374) with that of predicted synaptopodin 2 from gallus gallus (GenBank accession no. XM_426324) starting with residue 268 in the chicken sequence. MALDI TOF fingerprint results are underlined. Identical residues are indicated by : and similarities are shown by +. Edman analyses are in bold letters.
We used MALDI-TOF MS analysis to compare 66 tryptic peptides of the 103 kDa fesselin polypeptide with the predicted synaptopodin 2 like protein from gallus gallus (GenBank accession no. XM_426324). The results of the MALDI-TOF analyses are underlined in Figure 1. The 66 peptides were distributed over the whole gallus gallus sequence allowing us to confirm 66% of the cDNA-derived sequence. The carboxyl terminus identified by mass spectroscopy from the 103 kDa polypeptide corresponded to the C-terminus of the predicted gallus gallus sequence. However, the first N-terminal residue identified by these peptides was equivalent to amino acid number 268 of the predicted gallus gallus sequence.
We confirmed the sequence of the 103 kDa fesselin by an analysis of the cDNA sequence. The region of known amino acid sequence was amplified by RT-PCR. Separation of the RT-PCR products performed on turkey gizzard RNA resulted in a band at about 2.8 kb (not shown) that, when sequenced, was found to be equivalent to nucleotides 941–3718 of predicted synaptopodin 2 from gallus gallus.
The N- and C- terminal regions of fesselin were defined using 5’ and 3’ RACE as described in Materials and Methods. We observed different N-termini with 3 independent 5’ RACE studies. Our earliest detected upstream nucleotides corresponded to amino acid residues 174 or 263 of the chicken sequence. This means that the likely start sites in mature 103 kDa fesselin are either methionine 194 or methionine 268. With MALDI-TOF analysis our first detected peptide corresponded to amino acid residues Met268-Arg289 indicating this is the most likely start point of mature fesselin. The molecular mass predicted from the start site at methionine 268 (107.702 kDa) is in agreement with our earlier estimate (103 kDa) [1].
The presumed synaptopodin 2 of the domestic chicken does not have a methionine residue at position 268. In fact, residue 268 comes in them middle of an exon of the chicken. Yet the apparent mobility of turkey and chicken fesselin’s on SDS gels are the same (see Figure 2). We must consider the possibility that the 103 kDa fesselin band is the product of proteolytic processing. In some preparations of fesselin we have detected larger forms of fesselin. Figure 2 shows extracts of both turkey and chicken gizzard muscle probed by anti-fesselin antibodies. The two higher forms have approximate apparent molecular weights of 140 and 160 kDa. We confirmed that these higher bands are in fact derived from fesselin by MALDI-TOF fingerprint analysis. The most N-terminal sequence identified to this point is residues 256 to 264. Further analyses of the function of these larger fesselin forms may lead to as yet unidentified binding partners. These larger fesselin forms may also provide clues to processing that gives rise to the major forms of fesselin that are isolated.
Figure 2.
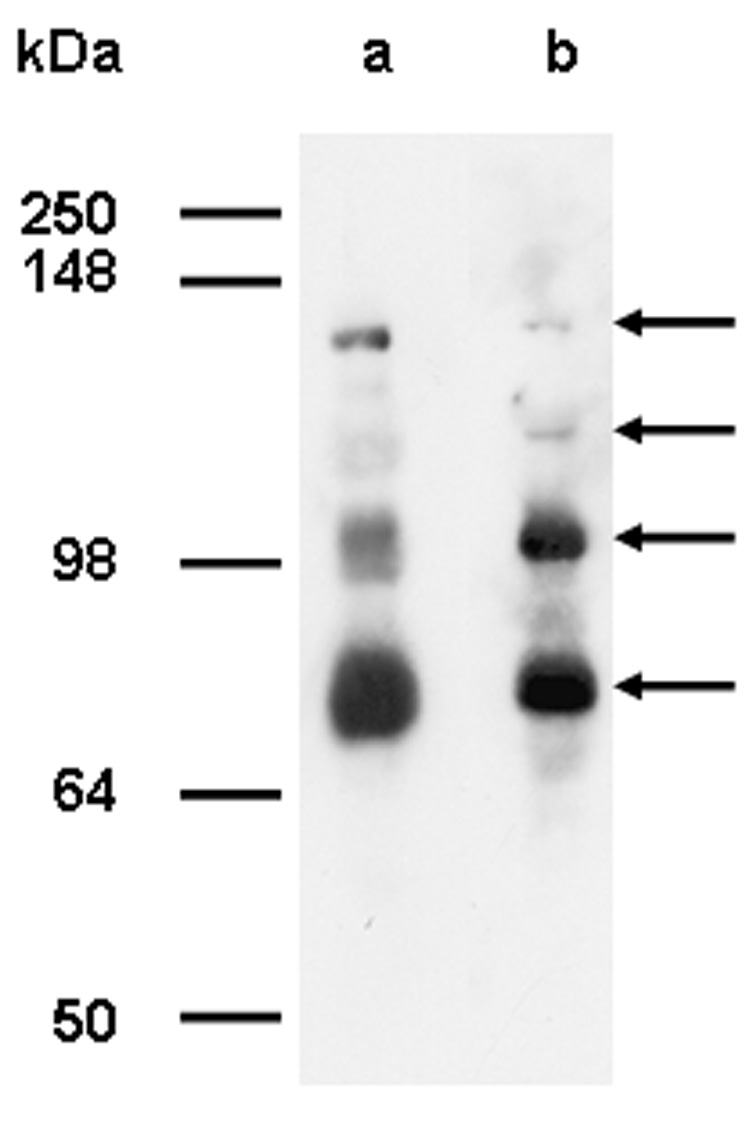
Western Blot of gizzard homogenate from turkey (lane a) and chicken (lane b) probed with polyclonal affinity purified anti-fesselin antibodies raised against gel excised fesselin purified from turkey.
The major fesselin forms having molecular masses of 79 and 103 kDa lack a PDZ domain that is present in the predicted synaptopodin 2 sequence from chicken (aa 7–85). PDZ domains are small globular protein-protein interacting modules consisting of six β-strands and two α-helices. The majority of the interactions consist of the binding to a carboxyl terminal pentapeptide of the target protein or the binding of C-terminus mimicking motifs. The structure of these highly specialized domains does not change significantly upon protein binding [21]. The larger fesselin precursors may therefore have interactions with proteins that are to this point unknown.
In summary, the 103 kDa fesselin band is an avian gene product of predicted synaptopodin 2 (GenBank accession no. XM_426324). We have shown this by comparing results obtained from analyses of the isolated protein (Edman and MALDI-TOF MS) and of the primary sequence derived from the cDNA of fesselin with the sequence of predicted avian synaptopodin 2 [XM_426324]. Further evidence of identity between fesselin and synaptopodin 2 is that the first 21 hits in a BLAST P search [22] performed with the cDNA derived amino acid sequence of turkey fesselin were synaptopodin 2 protein sequences with an E value of 0 indicating identity. Turkey fesselin has 60% identity and 72% similarity with human synaptopodin 2 (GenBank accession no. NP_597734). Fesselin is also 60% identical to human myopodin (GenBank accession no. CAB51856), an alternative splicing product of synaptopodin 2. In comparison, the similarity of fesselin to human synaptopodin (GenBank accession no. AAQ07402) is approximately 43%. This shows fesselin is homologous to mammalian synaptopodin 2. Furthermore, like synaptopodin 2, fesselin is rich in proline and contains multiple PXXP motifs. These PXXP motifs could function as potential peptide ligand motifs for SH3 domain containing proteins. Fesselin, like synaptopodin 2, contains one PPXY motif. These motifs function often as ligand peptides for proteins with WW domains as found in dystrophin [23].
Acknowledgements
We thank Dr. Rachel Roper for her helpful comments on the manuscript.
Abbreviations
- EDTA
ethylenediaminetetraacetic acid
- EGTA
ethylene glycol-bis(β-aminoethyl ether)- N,N,N’,N’-tetraacetic acid
- MOPS
3-(N-Morpholino)-propanesulfonic acid
Footnotes
Parts of this work were presented 50th Annual Biophysical Society Meeting, Salt Lake City, UT, February 2006 and at the European Muscle Conference in Heidelberg, Germany, September 2006.
Supported by grant AR035216 from the National Institutes of Health to J.M.C.
The turkey fesselin sequence reported in this paper has been deposited in GenBank under the name Turkey Synaptopodin 2 (GenBank accession nos. EU086520, ABU55374).
Myopodin was first described as a unique gene product (Weins et al. 2001) and was later shown to be an alternative splicing product of the synaptopodin 2 gene.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Leinweber BD, Fredricksen RS, Hoffman DR, Chalovich JM. Fesselin: a novel synaptopodin-like actin binding protein from muscle tissue. J. Muscle Res. Cell Motil. 1999;20:539–545. doi: 10.1023/a:1005597306671. [DOI] [PubMed] [Google Scholar]
- 2.Beall B, Chalovich JM. Fesselin, a synaptopodin-like protein, stimulates actin nucleation and polymerization. Biochemistry. 2001;40:14252–14259. doi: 10.1021/bi011806u. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Schroeter M, Chalovich JM. Ca++-calmodulin regulates fesselin-induced actin polymerization. Biochemistry. 2004;43:13875–13882. doi: 10.1021/bi0487490. [DOI] [PubMed] [Google Scholar]
- 4.Schroeter MM, Chalovich JM. Fesselin binds to actin and myosin and inhibits actin-activated ATPase activity. J. Muscle Res. Cell Motil. 2005;26:183–189. doi: 10.1007/s10974-005-9009-6. [DOI] [PubMed] [Google Scholar]
- 5.Khaymina SS, Kenney JM, Schroeter MM, Chalovich JM. Fesselin is a natively unfolded protein. J. Prot. Res. 2007;6:3648–3654. doi: 10.1021/pr070237v. [DOI] [PubMed] [Google Scholar]
- 6.Pham M, Chalovich JM. Smooth muscle alpha-actinin binds tightly to fesselin and attenuates its activity toward actin polymerization. J. Muscle Res. Cell Motil. 2006;27:45–51. doi: 10.1007/s10974-005-9053-2. [DOI] [PubMed] [Google Scholar]
- 7.Renegar RH, Chalovich JM, Leinweber BD, Zary JT, Schroeter MM. Localization of the actin-binding protein fesselin in chicken smooth muscle. doi: 10.1007/s00418-008-0508-6. submitted. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Pollard TD. Introduction to actin and actin-binding proteins. In: Kreis T, Vale R, editors. Guidebook to the Cytoskeletal and Motor Proteins. second ed. New York: Oxford University Press; 1999. pp. 3–11. [Google Scholar]
- 9.Mundel P, Heid HW, Mundel TM, Krüger M, Reiser J, Kriz W. Synaptopodin: an actin-associated protein in telencephalic dendrites and renal podocytes. J. Cell Biol. 1997;139:193–204. doi: 10.1083/jcb.139.1.193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Weins A, Schwarz K, Faul C, Barisoni L, Linke WA, Mundel P. Differentiation- and stress-dependent nuclear cytoplasmatic redistribution of myopodin, a novel actin-bundling protein. J. Cell Biol. 2001;155:393–404. doi: 10.1083/jcb.200012039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Asanuma K, Kim K, Oh J, Giardino L, Chabanis S, Faul C, Reiser J, Mundel P. Synaptopodin regulates the actin-bundling activity of alpha-actinin in an isoform-specific manner. J. Clin. Invest. 2005;115:1188–1198. doi: 10.1172/JCI23371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Faul C, Dhume A, Schecter SD, Mundel P. Protein kinase A, Ca2+/calmodulin-dependent kinase II, and calcineurin regulate the intracellular trafficking of myopodin between the Z-disc and the nucleus of cardiac myocytes. Mol. Cell. Biol. 2007;27:8215–8227. doi: 10.1128/MCB.00950-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mundel P, Reiser J, Zuniga Mejia Borga A, Pavenstadt H, Davidson GR, Kriz W, Zeller R. Rearrangements of the cytoskeleton and cell contacts induce process formation during differentiation of conditionally immortalized mouse podocyte cell lines. Exp. Cell Res. 1997;236:248–258. doi: 10.1006/excr.1997.3739. [DOI] [PubMed] [Google Scholar]
- 14.Deller T, Korte M, Chabanis S, Drakew A, Schwegler H, Stefani GG, Zuniga A, Schwarz K, Bonhoeffer T, Zeller R, Frotscher M, Mundel P. Synaptopodin-deficient mice lack a spine apparatus and show deficits in synaptic plasticity. Proc. Natl. Acad. Sci. USA. 2003;100:10494–10499. doi: 10.1073/pnas.1832384100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sanchez-Carbayo M, Schwarz K, Charytonowicz E, Cordon-Cardo C, Mundel P. Tumor suppressor role for myopodin in bladder cancer: loss of nuclear expression of myopodin is cell-cycle dependent and predicts clinical outcome. Oncogene. 2003;22:5298–5305. doi: 10.1038/sj.onc.1206616. [DOI] [PubMed] [Google Scholar]
- 16.Lin F, Yu YP, Woods J, Cieply K, Gooding B, Finkelstein P, Dhir R, Krill D, Becich MJ, Michalopoulos G, Finelkstein S, Luo JH. Myopodin, a synaptopodin homologue, is frequently deleted in invasive prostate cancers. Am. J. Pathol. 2001;159:1603–1612. doi: 10.1016/S0002-9440(10)63006-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Edman PA. Method for determination of the amino acid sequence in peptides. Acta Chem. Scan. 1950;4:283–293. [Google Scholar]
- 18.Towbin H, Staehelin T, Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc. Natl. Acad. Sci. U S A. 1979;76:4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Fernandez J, Andrews L, Mische SM. An improved procedure for enzymatic digestion of polyvinylidene difluoride-bound proteins for internal sequence analysis. Anal. Biochem. 1994;218:112–117. doi: 10.1006/abio.1994.1148. [DOI] [PubMed] [Google Scholar]
- 20.Rinta-Valkama J, Palmen T, Lassila M, Holthofer H. Podocyte-associated proteins FAT, alpha-actinin-4 and filtrin are expressed in langerhans islets of the pancreas. Mol. Cell. Biochem. 2007;294:117–125. doi: 10.1007/s11010-006-9251-2. [DOI] [PubMed] [Google Scholar]
- 21.Jelén F, Oleksy A, Smietana K, Otlewski J. PDZ domains - common players in the cell signalling. Acta Biochim. Pol. 2003;50:985–1017. [PubMed] [Google Scholar]
- 22.Altschul SF, Gish W, Miller W, Myers EW, Lipman DJ. Basic logical alignment search tool. J. Mol. Biol. 1990;215:403–410. doi: 10.1016/S0022-2836(05)80360-2. [DOI] [PubMed] [Google Scholar]
- 23.Kay BK, Williamson MP, Sudol M. The importance of being proline: the interaction of proline-rich motifs in signaling proteins with their cognate domains. FASEB J. 2000;14:231–241. [PubMed] [Google Scholar]


