Abstract
Two sequences of major histocompatibility complex (MHC) regions in the domestic cat, 2.976 and 0.362 Mbps, which were separated by an ancient chromosome break (55–80 MYA) and followed by a chromosomal inversion were annotated in detail. Gene annotation of this MHC was completed and identified 183 possible coding regions, 147 human homologues, possible functional genes and 36 pseudo/unidentified genes) by GENSCAN and BLASTN, BLASTP RepeatMasker programs. The first region spans 2.976 Mbp sequence, which encodes six classical class II antigens (three DRA and three DRB antigens) lacking the functional DP, DQ regions, nine antigen processing molecules (DOA/DOB, DMA/DMB, TAPASIN, and LMP2/LMP7,TAP1/TAP2), 52 class III genes, nineteen class I genes/gene fragments (FLAI-A to FLAI-S). Three class I genes (FLAI-H, I-K, I-E) may encode functional classical class I antigens based on deduced amino acid sequence and promoter structure. The second region spans 0.362 Mbp sequence encoding no class I genes and 18 cross-species conserved genes, excluding class I, II and their functionally related/associated genes, namely framework genes, including three olfactory receptor genes. One previously identified feline endogenous retrovirus, a baboon retrovirus derived sequence (ECE1) and two new endogenous retrovirus sequences, similar to brown bat endogenous retrovirus (FERVmlu1, FERVmlu2) were found within a 140 Kbp interval in the middle of class I region. MHC SNPs were examined based on comparisons of this BAC sequence and MHC homozygous 1.9× WGS sequences and found that 11,654 SNPs in 2.84 Mbp (0.00411 SNP per bp), which is 2.4 times higher rate than average heterozygous region in the WGS (0.0017 SNP per bp genome), and slightly higher than the SNP rate observed in human MHC (0.00337 SNP per bp).
Introduction
The major histocompatibility complex (MHC) is one of the most extensively analyzed regions in the genome due to the fact that this region encodes the most important molecules in immune function, namely class I and class II antigens, and also other important molecules such as chemical sensing genes (olfactory receptor gene complex), its escort gene, and POU5F1 gene involved in iPS stem cells [1]–[4]. Recently, the human MHC, HLA haplotypes were sequenced in the HLA haplotype project [5]–[10]. Eight different HLA – homozygous haplotypes' DNA sequences were determined in order to shed a light on MHC–linked diseases and evolutionary history. These BAC-based sequencings are necessary to examine the details in the regions of the genome, where gene duplications, deletions and selections occurred many times, because the genome project, especially in the human genome, was carried out using a mixture of DNA sources [11]. The same will be true in genome projects in other outbred species. The domestic cat serves excellent animal models to study at least three RNA viruses in humans. Feline leukemia virus (FeLV) shares similarly to human leukemia viruses (HTLV I & II) [12]. Feline immunodeficiency virus is considered to cause similar symptoms to human AIDS in a natural host, the domestic cat [13]–[16]. Feline infectious peritonitis virus belongs to the same virus group (corona virus) as human SARS virus [17]. To study host-defense mechanisms, in this animal model, we previously analyzed and reported (i) approximately 750 kbp class II region in feline MHC (FLA) [18], (ii) the unique FLA structure with a single chromosomal split at the TRIM gene family region, and chromosome inversion [19], and (iii) comparison of three MHCs, HLA, DLA, and FLA using human sequence, canine MHC homozygous genomic sequence and feline 3.3 Mbp draft sequence based on BAC shotgun sequences [20]. In this manuscript, much detail of FLA gene contents, promoter structures of predicted functional class I and class II genes, proportional scale comparisons of four mammalian MHCs (domestic cat, human, mouse, dog) and one marsupial MHC (opossum) are presented. SNPs (single nucleotide polymorphisms) between the MHC homozygous sequence of the lightly covered (1.9×) domestic cat genome shotgun sequence and this BAC-based MHC sequence were also analyzed to compare the degree and mode of the MHC divergence. In addition, two haplotype BAC-based sequences in functional class II DR region in the domestic cat were analyzed.
Materials and Methods
BAC sequencing and assembly
BAC clones from RPCI86 domestic cat BAC library [21] were selected based on FLA BAC map previously described [21]. Shotgun libraries were made using the sonication method [18]. Sequencing reactions were made from both ends of the plasmid vector using BigDye v1.0 chemistry (ABI). Electrogram files (ab1 files) were ftp-transferred to an ABCC ncisgi high speed computer, analyzed by Phred base caller, assembled by Phrap assembler and finished sequence assembly by Consed13 autofinish programs [22]–[25]. The final assembly of these BAC sequence contigs were made using Crossmatch program (http://www.phrap.org/phredphrapconsed.html). The following BAC clones were analyzed for class III and proximal, central class I FLA regions in fcaB2qcen; 181p11, 116b21, 539f24, 162h14, 207i7, 20f19, 18a04, 141b1, 97q9, 410h15, 261j7, 469m20, 515g14, 167d5, 117c16, 27j10, 194g24, 253j16, 292m22, 455a7, 454a5, 148o13, 117e16, 329i22. The following BAC clones were analyzed for class I distal region in fcaB2pter with the order from a telomere of fcaB2 short arm, 46j10, 596j24, 269n17, 221p5. More than sequence quality value 20 was used for the final assembly. The first assembly from class III through central class I regions was connected with previously published [18] class II region sequence (758 Kbp) using Crossmatch program.
Gene annotation
Sequences were first masked by Repeatmasker program. Gene annotation was made using GENSCAN [26] coding prediction plus BLASTP and BLASTN programs [27], also using megablast for the entire sequences against the latest human Refseq database. Class I, MIC, BAT1, olfactory receptor, MOG, TRIM26, 15, 10 gene annotation was made using human transcripts or FLA class I mRNA sequence (FLAI-A24) [28] by bl2seq [29] and results were parsed using Perl scripts. Repeat sequences were analyzed using Repeatmasker and STR finder programs. These data was graphically presented using Advanced PIPmaker program [30].
Dotplot analysis
Blastz program [31] was used to generate raw blastz output with parameters: Y = 3400, H = 2200, W = 8, B = 2, K = 3000, C = 0, M = 83886080, P = 0 and this output and two sequences were submitted to Advanced PIPmaker website (http://pipmaker.bx.psu.edu/cgi-bin/pipmakeradvanced).
SNP analysis
The 1.9× feline WGS contigs [32] were aligned with BAC MHC sequence using CROSSMATCH program and SNP was found between sequences selected by reciprocal best matches (>90% sequence identity) and with more than Quality value = 15 [33].
DR haplotypes
BAC clones of 152N13–244j14–16i4 from B2qCen side were sequenced by the shotgun method described above and analyzed by the methods of GENSCAN, Spidey [34] and Genwise [35] for annotation. A sequence assembled from this DR haplotype 2 region was compared with a sequence from DR haplotype 1 region previously published [18].
Comparisons of MHC structures
Sequences of MHC from four species: human, mouse, dog, and opossum which span from KIFC1 gene through UBD plus three olfactory receptor genes were extracted from UCSC Genome Browser. Gene coordinates tables from UCSC site were parsed by Perl script and gene organizations were graphically plotted by R script (http://cran.R-project.org).
Transcription factor binding sites in promoter regions of predicted functional feline class I and class II DR genes
Sequences totaling 6 kb (5 kb upstream and 1 kb downstream) from a potential translation start site (ATG) of predicted functional feline class I genes (FLAI-E, I-H, I-K) and class II DR genes (FLA-DRA1, DRA2, DRA3, DRB1, DRB3, DRB4) in addition to human HLA-A, -B, -C class I genes and HLA-DRA, DRB1, DRB3 class II genes were analyzed for the presence of potential transcription factor binding sites using Match TM program with TRANSFAC 7.0 database (http://www.gene-regulation.com/pub/databases.html). In addition, S-Y-module sequences of human HLA class II and I genes were used to screen above 6 kb sequences with b12 seq [29] with parameters, MATCH = 1, MISMATCH = −1, GAP OPEN 5, GAP EXTENSION 2, X_DROP OFF 0, EXPECT 10.00, WORDSIZE 7.
Results
Sequence
2,975,516 bp and 381,545 bp sequences were assembled for two FLA regions on the pericentromeric and subtelomeric positions of feline chromosome FcaB2. The first sequence covers from KIFC1 gene in the extended class II region through the entire class II, class III and a part of class I regions from the point adjacent to BAT1 gene through HLA-B, -C class I corresponding region, TRIM39 plus HLA-92 (HLA-L) region to alpha satellite-rich region. The second sequence covers from telomeric repeats rich region through TRIM 26/15/10 genes to the third olfactory receptor like gene (GenBank accession Nos. EU153401, EU153402).
Annotation
The entire gene coordinates, and possible functions are listed in Table 1. Gene organization and GC level was depicted in Figure 1. Detailed graphic presentation for exon-intron structure, orientation, repeat sequence, CpG island and sequence identity level to human HLA-6 COX 4.72 Mbp sequence was organized in Figures 2. (Figure 2A-1 was shown in the main text. Please see Figure S1 supporting file).
Table 1. Predicted Genes, their Functions and Coordinates in FLA.
| Gene | Functional/physiological properties/other name/structure | Orientation | Start | End | Length |
| KIFC1 | kinesin family member C1 | − | 1243 | 13226 | 11983 |
| RPS28 | ribomal protein S28 | + | 25869 | 27731 | 1862 |
| X1 | unknown: cfa chr12.6 - 029.a N-SCAN gene prediction | + | 66551 | 66793 | 242 |
| DAXX | death-associated protein 6 | + | 74219 | 77417 | 3198 |
| ZBTB22 | zinc finger and BTB domain containing 22 | + | 79170 | 80865 | 1695 |
| TAPBP | TAP binding protein (tapasin) | + | 82211 | 92070 | 9859 |
| RGL2 | ral guanine nucleotide dissociation stimulator-like 2 | + | 96755 | 101963 | 5208 |
| BING4 | WD domain | + | 105084 | 112722 | 7638 |
| B3GALT4 | UDP-Gal:betaGlcNAc beta 1,3-galactosyltransferase, polypeptide 4 | − | 113431 | 114583 | 1152 |
| RPS28[KE3] | ribomal protein S28 | − | 115583 | 120594 | 5011 |
| ARE1 | Yeast sac2 homolog, suppressor of actin mutant 2, Sacm2l, coiled coil structure | + | 121032 | 136177 | 15145 |
| RING1 | ring finger protein 1 | − | 141255 | 167593 | 26338 |
| KE6 | Steroid dehydrogenase-like protein (estradiol 17 beta-dehydrogenase 8) | − | 170695 | 172885 | 2190 |
| KE4 | Transmembrane protein with histidine-rich charge clusters | − | 173342 | 175211 | 1869 |
| RXRB | retinoid X receptor, beta | + | 177083 | 181628 | 4545 |
| COL11A2 | collagen, type XI, alpha 2 | + | 184143 | 212170 | 28027 |
| X2 | unknown: cfa chr12: 5,766,607–5,773,167 | − | 216881 | 223065 | 6184 |
| DPBp | Class II antigen beta chain, pseudogene | − | 231611 | 242652 | 11041 |
| DPAp | Class II antigen alpha chain, pseudogene | + | 245150 | 268064 | 22914 |
| DNA | DOA, heterodimerize with DOB in pre-B cells, peptide loading for class II antigen at low PH | + | 278617 | 289676 | 11059 |
| BRD2 | bromodomain containing 2 | − | 303996 | 327744 | 23748 |
| DMA | Nonclassical class II antigen, alpha chain, peptide loading for class II antigen | + | 329474 | 332504 | 3030 |
| DMB | Nonclassical class II antigen, beta chain, heterodimer with DMA,peptide loading for class II antigen | + | 342380 | 351767 | 9387 |
| LMP2 | Proteosome subunit to cleave peptides for class I antigen | − | 416025 | 419082 | 3057 |
| TAP1 | transporter 1, ATP-binding cassette, sub-family B (MDR/TAP) | + | 421610 | 428032 | 6422 |
| LMP7 | Proteosome subunit to cleave peptides for class I antigen | + | 431491 | 433586 | 2095 |
| TAP2 | transporter 2, ATP-binding cassette, sub-family B (MDR/TAP) | + | 435278 | 446029 | 10751 |
| DOB | Nonclassical class II antigen, beta chain, heterodimer with DNA, H2-IAB2 in mouse | + | 456252 | 465656 | 9404 |
| DRB4 | Class II antigen, beta chain | − | 509012 | 514820 | 5808 |
| GAPDH | Glycerol aldehyde phosphodehydrase, pseudogene | − | 563173 | 575620 | 12447 |
| DRB1 | Class II antigen, beta chain | + | 596698 | 607755 | 11057 |
| DRA1 | Class II antigen, alpha chain | − | 621375 | 624059 | 2684 |
| RPS28p | ribomal protein S28 gene fragment | + | 631565 | 633427 | 1862 |
| DRB2p | Class II antigen, beta chain pseudogene with intron 1 and exon 2 | + | 652000 | 659950 | 7950 |
| DRB3 | Class II antigen, beta chain | + | 664799 | 679467 | 14668 |
| DRA2 | Class II antigen, alpha chain | − | 688714 | 691459 | 2745 |
| DRA3 | Class II antigen, alpha chain | − | 722112 | 725240 | 3128 |
| BTL2 | butyrophilin-like 2 (MHC class II associated) | + | 735556 | 754731 | 19175 |
| BTL2 | butyrophilin-like 2 (MHC class II associated) | − | 765965 | 777458 | 11493 |
| RPS28 | ribomal protein S28 | + | 782864 | 783037 | 173 |
| HNRPA2B1 | heterogeneous nuclear ribonucleoprotein A2/B1 | − | 829773 | 830860 | 1087 |
| RPS28 | ribomal protein S28 | + | 984596 | 984769 | 173 |
| NOTCH4 | Notch homolog 4 (Drosophila) | + | 1206316 | 1229484 | 23168 |
| GPSM3 | G-protein signalling modulator 3 (AGS3-like, C. elegans) | − | 1230443 | 1231562 | 1119 |
| PBX2 | pre-B-cell leukemia transcription factor 2 | + | 1234538 | 1236556 | 2018 |
| AGER | advanced glycosylation end product-specific receptor | + | 1238751 | 1241285 | 2534 |
| AGPAT1 | 1-acylglycerol-3-phosphate O-acyltransferase 1 (lysophosphatidic acid acyltransferase, alpha) | + | 1250819 | 1252976 | 2157 |
| EGFL8 | EGF-like-domain, multiple 8 | − | 1254290 | 1255788 | 1498 |
| PPT2 | palmitoyl-protein thioesterase 2 | − | 1258652 | 1263918 | 5266 |
| PRRT1 | proline-rich transmembrane protein 1 | + | 1267796 | 1269170 | 1374 |
| FKBPL | FK506 binding protein like | + | 1284306 | 1285355 | 1049 |
| CREBL1 | cAMP responsive element binding protein-like 1 | + | 1285915 | 1300603 | 14688 |
| TNXB | tenascin XB | + | 1310433 | 1357909 | 47476 |
| CYP21A2 | cytochrome P450, family 21, subfamily A, polypeptide 2 | − | 1358128 | 1360631 | 2503 |
| C4A | complement component 4A (Rodgers blood group) | − | 1363648 | 1378177 | 14529 |
| STK19 | serine/threonine kinase 19 | − | 1379597 | 1384827 | 5230 |
| DOM3Z | dom-3 homolog Z (C. elegans) | + | 1385598 | 1387305 | 1707 |
| SKIV2L | superkiller viralicidic activity 2-like (S. cerevisiae) | − | 1387465 | 1394012 | 6547 |
| CFB | complement factor B | − | 1394591 | 1402014 | 7423 |
| C2 | complement component 2 | − | 1402258 | 1415710 | 13452 |
| ZBTB12 | zinc finger and BTB domain containing 12 | + | 1448172 | 1449551 | 1379 |
| EHMT2 | euchromatic histone-lysine N-methyltransferase 2 | + | 1454322 | 1466627 | 12305 |
| SLC44A4 | solute carrier family 44, member 4 | + | 1467409 | 1479530 | 12121 |
| NEU4 | Neuraminidase 4 | + | 1480105 | 1483273 | 3168 |
| RPS28 | ribomal protein S28 | + | 1488926 | 1489106 | 180 |
| HSPA1A | heat shock 70 kDa protein 1A | − | 1513315 | 1515240 | 1925 |
| HSPA1A | heat shock 70 kDa protein 1A | − | 1524090 | 1526015 | 1925 |
| HSPA1A | heat shock 70 kDa protein 1A | + | 1528138 | 1529946 | 1808 |
| LSM2 | LSM2 homolog, U6 small nuclear RNA associated (S. cerevisiae) | + | 1532633 | 1537716 | 5083 |
| VARS | valyl-tRNA synthetase | + | 1539067 | 1553500 | 14433 |
| C6orf27 | chromosome 6 open reading frame 27 | + | 1555291 | 1563506 | 8215 |
| C6orf26 | chromosome 6 open reading frame 26 | − | 1564291 | 1565750 | 1459 |
| MSH6 | mutS homolog 6 (E. coli) | − | 1566395 | 1590460 | 24065 |
| CLIC1 | chloride intracellular channel 1 | + | 1594157 | 1598924 | 4767 |
| DDAH2 | dimethylarginine dimethylaminohydrolase 2 | + | 1600599 | 1602528 | 1929 |
| C6orf25 | chromosome 6 open reading frame 25 | − | 1604591 | 1606252 | 1661 |
| LY6G6C | lymphocyte antigen 6 complex, locus G6C | + | 1607799 | 1610137 | 2338 |
| LY6G6D | lymphocyte antigen 6 complex, locus G6D | − | 1611498 | 1613549 | 2051 |
| LY6G6E | lymphocyte antigen 6 complex, locus G6E | + | 1615087 | 1616230 | 1143 |
| C6orf21 | chromosome 6 open reading frame 21 | − | 1618100 | 1620941 | 2841 |
| BAT5 | HLA-B associated transcript 5 | + | 1623128 | 1636811 | 13683 |
| LY6G5C | lymphocyte antigen 6 complex, locus G5C | + | 1642789 | 1645897 | 3108 |
| LY6G5B | lymphocyte antigen 6 complex, locus G5B | − | 1649307 | 1650335 | 1028 |
| CSNK2B | casein kinase 2, beta polypeptide | − | 1651325 | 1654509 | 3184 |
| BAT4 | HLA-B associated transcript 4 | + | 1656917 | 1658511 | 1594 |
| C6orf47 | chromosome 6 open reading frame 47 | + | 1660571 | 1661426 | 855 |
| LTB | lymphotoxin beta (TNF superfamily, member 3) | + | 1663590 | 1665305 | 1715 |
| TNF | tumor necrosis factor (TNF superfamily, member 2) | − | 1668300 | 1670066 | 1766 |
| LTA | lymphotoxin alpha (TNF superfamily, member 1) | − | 1672461 | 1673412 | 951 |
| NFKBIL1 | nuclear factor of kappa light polypeptide gene enhancer in B-cells inhibitor-like 1 | − | 1685899 | 1694249 | 8350 |
| ATP6V1G2 | ATPase, H+ transporting, lysosomal 13 kDa, V1 subunit G2 | + | 1695942 | 1697113 | 1171 |
| BAT1 | HLA-B associated transcript 1 | + | 1699863 | 1710709 | 10846 |
| MCCD1 | mitochondrial coiled-coil domain 1 | − | 1711244 | 1714362 | 3118 |
| X3 | unknown: cfa chr12: 4,026,686–4,028,708 | − | 1716465 | 1722099 | 5634 |
| LOC345645 | similar to peptidase (prosome, macropain) 26S subunit, ATPase 1 | − | 1722382 | 1723468 | 1086 |
| FLAI-A | non classical class I molecule | + | 1727339 | 1733904 | 6565 |
| RPS28 | ribomal protein S28 | + | 1741091 | 1741264 | 173 |
| FLAI-Bp | Classical class I antigen gene fragment | + | 1754279 | 1755539 | 1260 |
| FLAI-C | non classical class I molecule | − | 1778815 | 1781240 | 2425 |
| RPS28 | ribomal protein S28 | + | 1791524 | 1791697 | 173 |
| MIC1 | MHC class I releated gene 1 | − | 1802050 | 1803673 | 1623 |
| FLAI-Dp | Classical class I antigen gene fragment | − | 1812028 | 1826561 | 14533 |
| X4 | unknown | − | 1816059 | 1819441 | 3382 |
| X5 | unknown: cfa chr12: 3,304,294–3,451,219 | + | 1822307 | 1852375 | 30068 |
| BAT1p | BAT1 fragement | − | 1854809 | 1855450 | 641 |
| FLAI-E | Classical class I antigen ( with long cytoplasmic tail) | + | 1859534 | 1862925 | 3391 |
| X6 | unknown | − | 1865368 | 1878940 | 13572 |
| MIC2p | MHC class I releated gene 2 fragment | + | 1880418 | 1880645 | 227 |
| RPS28 | ribomal protein S28 | + | 1892390 | 1892515 | 125 |
| FLAI-F | non classical class I molecule | + | 1918438 | 1921808 | 3370 |
| FLAI-Gp | Classical class I antigen gene fragment | − | 1938101 | 1940148 | 2047 |
| MIC3 | MHC class I releated gene 3 | − | 1962637 | 1964257 | 1620 |
| FLAI-H | Classical class I antigen | − | 1973577 | 1976991 | 3414 |
| BAT1p | BAT1 fragement | + | 1979546 | 1980192 | 646 |
| FLAI-Ip | Classical class I antigen gene fragment | + | 2003006 | 2003225 | 219 |
| FLAI-J | non classical class I molecule | − | 2011852 | 2015260 | 3408 |
| BAT1p | BAT1 fragement | + | 2017763 | 2018402 | 639 |
| BAT1p | BAT1 fragement | − | 2059127 | 2059750 | 623 |
| FLAI-K | Classical class I antigen | − | 2083736 | 2087122 | 3386 |
| BAT1p | BAT1 fragement | + | 2089903 | 2090242 | 339 |
| FERVmlu2 | endogenous retrovirus similar to brown bat (Motis Lucifugus) endogenous retrovirus 2 | − | 2103256 | 2105890 | 2634 |
| MIC4p | MHC class I releated gene 4 fragment | − | 2117309 | 2117536 | 227 |
| X7 | unknown: cfa chr10: 6,669,201–6,794,639 | − | 2117453 | 2120737 | 3284 |
| FLAI-L | non classical class I molecule | − | 2145008 | 2148428 | 3420 |
| BAT1p | BAT1 fragement | + | 2150915 | 2151554 | 639 |
| FLAI-M | non classical class I molecule | − | 2183520 | 2187094 | 3574 |
| BAT1p | BAT1 fragement | − | 2205884 | 2206254 | 370 |
| RD114(ECE1) | baboon retrovius related endogenous retrovirus | + | 2212532 | 2215463 | 2931 |
| FERVmlu1 | endogenous retrovirus similar to brown bat (Motis Lucifugus) endogenous retrovirus 1 | + | 2219742 | 2244701 | 24959 |
| FLAI-Np | Classical class I antigen gene fragment | + | 2221196 | 2221264 | 68 |
| BAT1p | BAT1 fragement | − | 2258148 | 2258782 | 634 |
| FLAI-O | non classical class I molecule | + | 2260882 | 2264315 | 3433 |
| BAT1p | BAT1 fragement | + | 2293369 | 2294534 | 1165 |
| FLAI-Pp | Classical class I antigen gene fragment | − | 2301870 | 2302131 | 261 |
| FLAI-Q | non classical class I molecule | + | 2329070 | 2332537 | 3467 |
| POU5F1 | POU domain, class 5, transcription factor 1, OCT3 | + | 2354289 | 2362844 | 8555 |
| SC1 | TCF19, transcription factor 19 | − | 2364737 | 2367161 | 2424 |
| CCHCR1 | coiled-coil alpha-helical rod protein 1 | + | 2370568 | 2382864 | 12296 |
| CDSN | corneodesmosin | + | 2401577 | 2410158 | 8581 |
| X8 | unknown: chr12: 3,698,941–3,701,819 | + | 2446230 | 2449015 | 2785 |
| VARSL | valyl-tRNA synthetase like | − | 2449807 | 2461727 | 11920 |
| GTF2H4 | general transcription factor IIH, polypeptide 4, 52 kDa | − | 2461975 | 2465896 | 3921 |
| DDR1 | discoidin domain receptor family, member 1 | + | 2466245 | 2488040 | 21795 |
| TAXREB107 | TAX response lement-binding protein | + | 2527942 | 2547435 | 19493 |
| IER3 | immediate early response 3 | + | 2607659 | 2607996 | 337 |
| FLOT1 | flotillin 1 | + | 2610071 | 2622235 | 12164 |
| TUBB | tubulin, beta | − | 2625114 | 2628405 | 3291 |
| MDC1 | mediator of DNA damage checkpoint 1 | + | 2630309 | 2644393 | 14084 |
| NRM | nurim (nuclear envelope membrane protein) | + | 2648569 | 2653735 | 5166 |
| KIAA1949 | KIAA1949 | + | 2653850 | 2660634 | 6784 |
| DHX16 | DEAH (Asp-Glu-Ala-His) box polypeptide 16 | + | 2663783 | 2678921 | 15138 |
| C6orf136 | chromosome 6 open reading frame 136 | − | 2679243 | 2683432 | 4189 |
| CG3967-PC | Drosophila melanogaster protein Cg3967-pc homolog | − | 2683765 | 2697339 | 13574 |
| MRPS18B | mitochondrial ribosomal protein S18B | − | 2698088 | 2703714 | 5626 |
| PPP1R10 | protein phosphatase 1, regulatory subunit 10 | + | 2711308 | 2722165 | 10857 |
| ABCF1 | ATP-binding cassette, sub-family F (GCN20), member 1 | − | 2724683 | 2735486 | 10803 |
| PRR3 | proline rich 3 | − | 2743092 | 2747113 | 4021 |
| GNL1 | guanine nucleotide binding protein-like 1 | + | 2748233 | 2755021 | 6788 |
| EEF1A1 | eukaryotic translation elongation factor 1 alpha 1 | − | 2779084 | 2786223 | 7139 |
| RPS28 | ribomal protein S28 | + | 2800184 | 2807333 | 7149 |
| PABPC4 | poly(A) binding protein, cytoplasmic 4 (inducible form) | + | 2818343 | 2818789 | 446 |
| PABPC4 | poly(A) binding protein, cytoplasmic 4 (inducible form) | + | 2820533 | 2821355 | 822 |
| X9 | unknown: PLEC1, PLECTIN1 | − | 2825427 | 2840447 | 15020 |
| X10 | unknown: SLC12AL Intron, sodium potassium chloride cotransporter2 | + | 2842351 | 2843559 | 1208 |
| RPS28 | ribomal protein S28 | + | 2850617 | 2852479 | 1862 |
| RNASE | Ribonuclease | + | 2867225 | 2867494 | 269 |
| TRIM39 | tripartite motif-containing 39 | − | 2870168 | 2879926 | 9758 |
| FLAI-Rp | Classical class I antigen gene fragment | + | 2905161 | 2905383 | 222 |
| FLAI-S | non classical class I molecule | − | 2910524 | 2913237 | 2713 |
| PNPLA6 | Patatin-like phospholipase domain containing 6 | − | 2956238 | 2961356 | 5118 |
| PeriCentromic Region and chromosomal break and inversion | |||||
| Subtelomeric Region | |||||
| X11 | unknown: cfa chr6: 27,705,326–27,717,672 | − | 7700 | 29900 | 22200 |
| TRIM26 | tripartite motif-containing 26 protein | + | 102400 | 111040 | 8640 |
| TRIM15 | tripartite motif-containing 26 protein | − | 125740 | 132100 | 6360 |
| TRIM10 | tripartite motif-containing 26 protein | + | 134820 | 140560 | 5740 |
| X12 | unknown: cfa chr35: 29,351,535–29,362,511 | − | 142140 | 158060 | 15920 |
| PPP1R11 | protein phosphatase 1, regulatory (inhibitor) subunit 11 | − | 189360 | 193120 | 3760 |
| MOG | myelin oligodendrocyte glycoprotein | − | 219300 | 228580 | 9280 |
| GABBR1 | gamma-aminobutyric acid (GABA) B receptor 1 | + | 248040 | 268920 | 20880 |
| OLFR1 | Olfactory receptor | − | 282860 | 289000 | 6140 |
| UBD | ubiquitin | + | 299600 | 301440 | 1840 |
| X13 | unknown | − | 303120 | 303740 | 620 |
| OLFR2 | Olfactory receptor | − | 316640 | 317400 | 760 |
| OLFR3 | Olfactory receptor | + | 330520 | 352180 | 21660 |
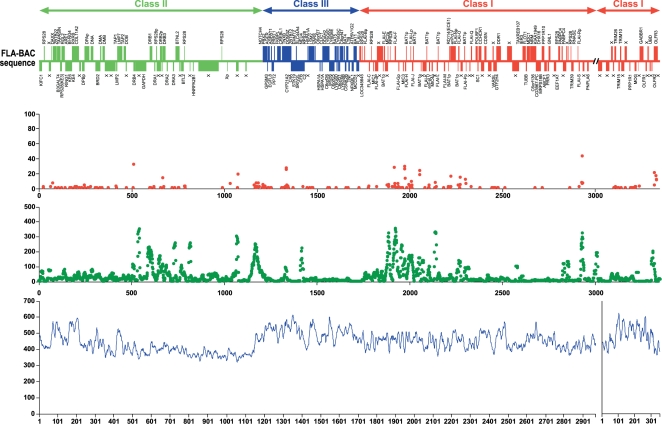
Figure 1. Gene Organization, SNP level, GC contents in FLA.
(A) Gene organization of FLA. Genes with forward orientation, which towards to telomere in human HLA, but towards to centromere in FLA, and away from telomere in distal class I region in FLA were placed above the solid line. A position of the ancient chromosome break and an inversion was indicated by double slashed lines and genes with opposite orientation were placed below the solid line. (B) Coding (CDS) SNPs. CDS SNPs were counted based on exon structure of each gene. Pseudogenes CDS SNPs were omitted. No. of SNPs per 10 kbp were plotted. (C) Single nucleotide polymorphism (SNP). SNP was counted in 10 Kbp window and shift 1 Kb. (D) GC content. GC content was counted in 10 Kbp window and shift 1 Kb and number was plotted.
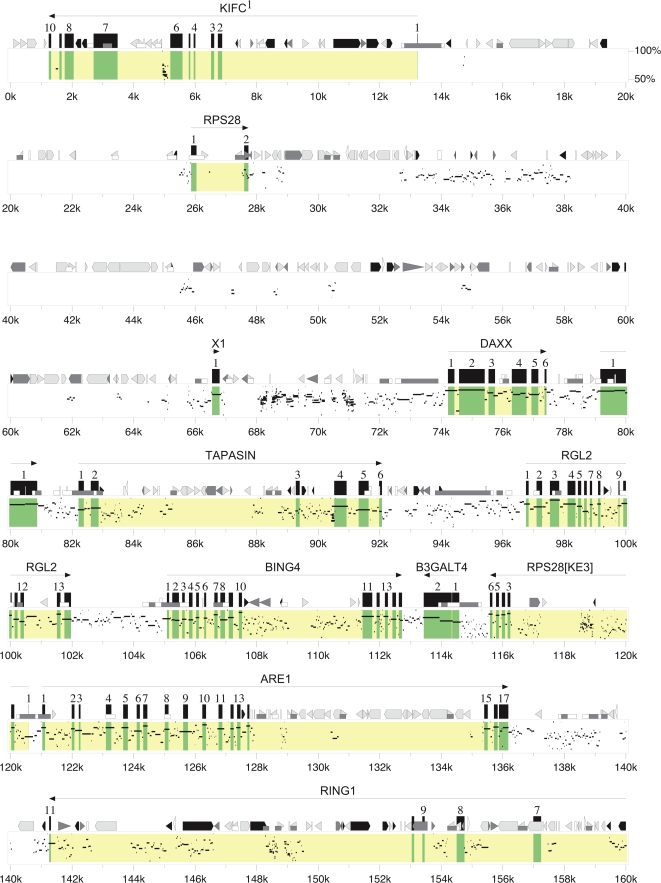
Figure 2. Percent Identity Plots between FLA and HLA.
(A) Percent identity Plot of FLA extend class II, classical class II, class III, proximal and central class I regions. Genes and exons were highlighted with yellow and green colors, respectively. Gene, exon, UTR, simple repeats, MIR, other SINE, LINE1, LINE2, LTR, other repeats, CpG/GpC ratios were indicated. FLA sequence was compared with human HLA 6COX sequence. (B) Percent Identity Plot of FLA distal class I region. Same methods and criterions were used as in Figure 2(A). Only Figure 2A-1 was included in this text. The rest of Figure 2A and 2B can be found in Supporting Information File, Figure 2AB_All.tar.bzip2.
Extended and classical class II region
Extended class II region spans 230 Kbp from KIFC1 gene to the point adjacent to DPB pseudogene. Fourteen human gene homologues and 2 unknown coding regions were found. Classical class II region spans 884 Kbp. Twenty-five human gene homologues were found in the region defined from DPB pseudogene to the point adjacent region of NOTCH4 gene. Annotation and sequence of a part of this region, (KIFC1, previously assigned as HSET to BTNL2), were described elsewhere [18]. Briefly, a pair of DPA, B pseudogenes, 3 pairs of DRA, B genes were identified with one DRB pseudogene. A set of genes which are involved in antigen processing, including a pair of DOA, DOB, DMA, and B genes, two antigen transporter genes, TAP1, 2, and protease genes, LMP2, 7 were found. In addition, two butylophillin genes, BTNL2, BTL2, and BRD2 (previously assigned as RING3) genes were found.
Class III region
FLA class III region spans 520 Kbp which encodes fifty-one human gene homologues and two unknown coding regions.
Class I region
FLA class I region were classified as three subregions based on chromosomal localization and gene contents. The first class I region, proximal class I region spans 600 Kbp from the first class I gene (FLAI-A) to the last class I gene (FLAI-Q) in this HLA-B, -C corresponding region adjacent to the class III region. This region encodes seventeen class I genes/gene fragments based on sequence alignments with full length feline class I cDNA sequence. Eight BAT1 gene fragments are located in the vicinity of class I genes. Three RPS28 gene fragments, four class I-related (MIC) genes or gene fragments and four unknown coding regions were also identified.
The second class I subregion, a central class I region, spans 600 kb region from POU5F1 (previously assigned as OCT3) gene to the alpha satellite repeat-rich pericentromeric region. There are 32 human gene homologues including two class I gene/gene fragments in HLA-92 (HLA-L) region, three unknown coding regions. The third class I subregion, distal class I region, spans 360 Kbp from 47 telomere repeats of (TTAGGG) through the third olfactory receptor like gene. This region encodes ten human gene homologues and three unknown coding regions. Three TRIM genes, TRIM26, TRIM15, TRIM10 were identified, however, TRIM40, 31 gene homologues were not recognized. PPPR11 and MOG genes are located in 26 Kbp interval, while in human HLA, these two genes are located with 340 kb interval due to the existence of eleven class I genes/gene fragments as HLA-A region.
GC contents
GC contents nearly reached at 60% level in the extended class II, class III, and the distal class I regions. The lowest GC content of nearly 40% was found in the classical class II region and sharply increased at the border of class II and class III regions. The proximal/central class I regions kept GC content at 50% level (Figure 1).
Repeats
Interspersed repeats
Interspersed repeats occupied about thirty-four percentages of MHC region, which is approximately the same level as found in the cat genome (32%), but significantly fewer than human HLA region (48%) or human genome (46%). Table 2 summarized the repeat components in each FLA (sub) class. Though SINE repeat contents are relatively equal in each region ranging from 8 to 14%, the LINE repeat contents are significantly different. The highest LINE contents were found in classical class II and proximal class I regions, (more than 60% of total sequences), where major functional MHC gene amplifications have occurred. The lowest LINE contents were observed in the gene-rich extended class II and class III regions, at approximately 20% level. An intermediate level of LINE contents was found in central and distal class I regions at approximately 40% level.
Table 2. Interspersed Elements in FLA subregions.
| extended class II | classical class II | class III | proximal class I | central class I | distal class I | FLA | cat genome | HLA | |
| SINES: | 14.86 | 10.19 | 11.16 | 8.04 | 10.93 | 8.82 | 8.53 | 11.2 | 17.59 |
| MIRs | 2.29 | 1.69 | 2.46 | 0.44 | 2.07 | 1.56 | 1.05 | 3.10 | 16.06 |
| LINES: | 12.57 | 34.82 | 10.84 | 32.07 | 23.41 | 21.02 | 21.31 | 14.26 | 16.59 |
| LINE 1 | 8.61 | 32.88 | 6.86 | 29.13 | 19.76 | 19.39 | 18.63 | 10.79 | 13.35 |
| LINE 2 | 3.87 | 1.79 | 3.58 | 2.88 | 3.18 | 1.44 | 2.54 | 2.82 | 3.09 |
| L3/CR1 | 0.07 | 0.14 | 0.40 | 0.05 | 0.36 | 0.13 | 0.14 | 0.36 | 0.16 |
| LTR elements: | 2.84 | 4.49 | 1.39 | 5.17 | 4.51 | 4.93 | 2.69 | 4.44 | 10.55 |
| MaLRs | 1.90 | 0.79 | 0.82 | 1.51 | 1.57 | 0.91 | 1.04 | 2.14 | 2.61 |
| ERVL | 0.34 | 0.88 | 0.27 | 1.79 | 0.57 | 1.59 | 0.81 | 1.21 | 2.11 |
| ERV classI | 0.60 | 2.74 | 0.29 | 1.87 | 2.32 | 2.43 | 0.81 | 1.05 | 4.25 |
| DNA elements: | 5.23 | 1.56 | 1.63 | 2.56 | 1.72 | 1.43 | 1.62 | 2.19 | 2.64 |
| MER1_type | 2.30 | 1.16 | 0.97 | 1.73 | 1.07 | 0.81 | 1.31 | 1.26 | 1.52 |
| MER2_type | 1.46 | 0.17 | 0.26 | 0.83 | 0.33 | 0.23 | 0.14 | 0.39 | 0.88 |
| Total of Interspersed | 35.48 | 51.07 | 25.07 | 47.84 | 40.57 | 36.19 | 34.14 | 32.1 | 48.14 |
Percentage of sequence (%) was shown in each subregion.
Simple Tandem Repeats (STRs)
Frequency of STRs was calculated in each FLA subregion and was compared with results obtained from human HLA 6COX haplotype sequence. These results were summarized in Table 3. A total of 541 STRs (di-, tri-, tetra-, penta-) with more than 12 and 5 perfect repeats for di- and others, e.g. (CA)12 and (GGA)5, respectively were found in FLA. The frequency of STRs (1 every 6.17 kb) was 50% higher than that in human HLA (1 every 9.93 kb) due to at least 3 times higher frequency of dinucleotide repeats. This trend was more obviously observed in the classical class II region. Approximately 4 times more occurrence of dinucleotide STRs was found in this FLA subregion.
Table 3. Simple Tandem Repeats (STRs) in FLA.
| FLA | ||||||||||||||
| Extended class II | Classical class II | Class III | Proximal class I | Central class I | Distal class I | Total | ||||||||
| DI | 25a | 9.26b | 118 | 8.26 | 30 | 17.36 | 47 | 13.34 | 52 | 11.95 | 26 | 13.91 | 298 | 11.20 |
| TRI | 11 | 21.05 | 19 | 51.30 | 13 | 40.07 | 10 | 62.70 | 13 | 47.79 | 10 | 36.15 | 76 | 43.91 |
| TETRA | 9 | 25.73 | 37 | 26.34 | 13 | 40.07 | 39 | 16.08 | 23 | 27.01 | 17 | 21.27 | 138 | 24.18 |
| PENTA | 2 | 115.79 | 7 | 139.24 | 8 | 65.11 | 7 | 89.58 | 4 | 155.31 | 1 | 361.55 | 29 | 115.07 |
| TOTAL | 47 | 4.93 | 181 | 5.39 | 64 | 8.14 | 103 | 6.09 | 92 | 6.75 | 54 | 6.70 | 541 | 6.17 |
| size (bp) | 231580 | 974699 | 520919 | 627039 | 621255 | 361545 | 3337061 | |||||||
No. of more than 12 perfect repeats or 5 repeats were counted for dinucleotide (DI) and other STRs (TRI, TETRA, PENTA), respectively.
Average interval (kbp) of occurrence of STR was shown.
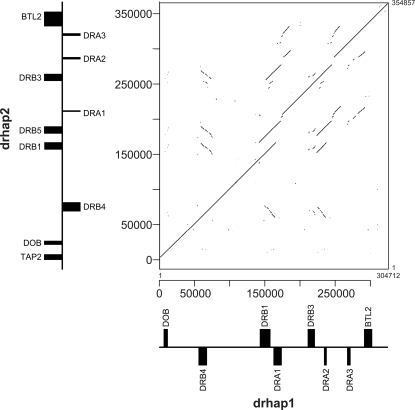
Dotplot analyses of HLA, DLA, FLA
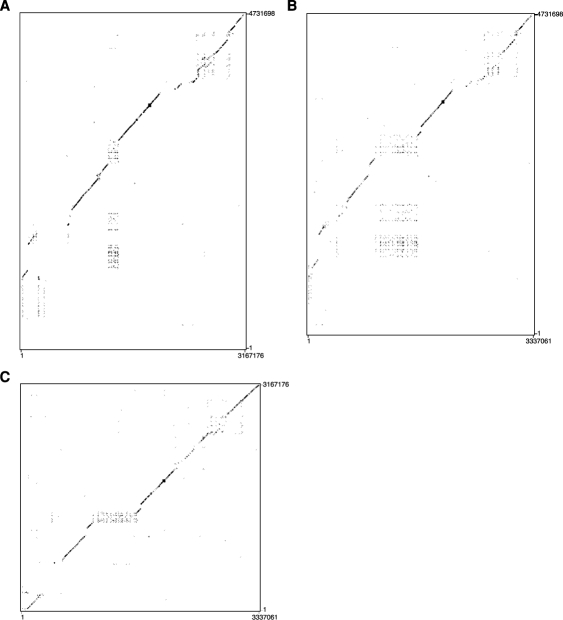
MHC sequences spanning from UBD plus three olfactory receptor genes to KIFC1 in HLA, DLA, FLA were compared in pairwise fashion. These analyses, DLA vs. HLA (Figure 3A), FLA vs. HLA (Figure 3B) and FLA vs. DLA (Figure 3C) revealed mosaic structures of highly conserved regions (solid lines), gene duplication (square with dots), deletions (spaces between solid lines in one axis but not in other) and divergent regions (broken lines). Figure 3A and 3B showed similar patterns between DLA vs. HLA and FLA vs. HLA, indicating conserved class II, III, and central class I regions plus class I gene amplifications, though the level of class I gene amplification was lower in DLA due to the fact that only 3 class I genes exit in HLA-B, -C corresponding region. The observation that DLA and FLA lack HLA-A class I region was also evident in this analysis. Figure 3C also showed that FLA and DLA were highly conserved in gene contents and organization except that the level of class I gene amplification was higher in FLA and sequences around pericentromere and subtelomere had highly divergent sequence due to the numerous and different types of gene translocations from other genome sites, resulting in a large broken solid line in these regions.
Figure 3. Dotplot analyses.
Two FLA sequences were connected based on HLA organization and oriented as follows; Telomeric side of FcaB2q→B2qcen→B2pter→B2p. The centromeric side of two DLA sequences, one on cfa12qcen and the other on cfa35qter were also connected based on HLA organization as follows; telomeric side of cfa12q→cfa12qcen→cfa35qter→cfa35q. (A) Dotplot analysis between DLA (KIFC1 to the third olfactory receptor genes from MOG) and HLA 6COX sequences (X axis vs. Y axis). (B) Dotplot analysis between FLA (KIFC1 to the third olfactory receptor genes from MOG) and HLA 6 COX sequences (X axis vs. Y axis). (C) Dot plot analysis between FLA (X axis) and DLA (Y axis).
Endogenous retrovirus sequences
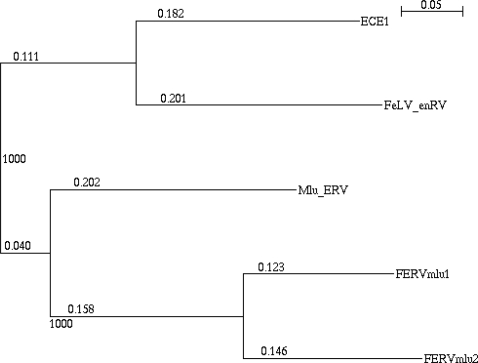
One of the baboon-derived endogenous retroviruses, ECE1 (RD114) which had 99% sequence identity (1631/1633) with GenBank RD114 (ECE1) AF155060 and two new types of endogenous retroviruses FERVmlu1 and 2, which showed high sequence similarity with recently submitted sequences by an NISC Comparative Sequencing Initiative project of brown bat (Myotis lucifugus) BAC clone (95% sequence identity with 83% coverage, and >85% sequence identity with 83% coverage, respectively) were also recognized within 1401kb region (Figure 4). Detailed open reading frame (ORF) analyses showed FERVmlu1 and 2 have 140 and 19 ORFs which sizes range from 102–900 and 102–516, respectively. The largest ORF of FERVmlu1 encodes 324 amino acid residues which have 70% similarity to a part of recombinant mouse-MuLV/RaLV Pol region, half of retroviral aspartyl protease, DNA binding region and a half of putative active site, however, other ORFs have no significant homology to gag, pol, env regions.
Figure 4. Neighbor-Joining Tree with 1,000 bootstrap for domestic cat endogeneous retrovirus sequences.
ECE1 represent RD114 endogeneous retrovirus transmitted from baboon, enFeLV represent a full length FeIV endogeneous retrovirus. enRVMlu represent brown bat retrovirus sequence and FERVmlu1, FERVmlu2 represent new endogeneous genes found in the proximal class I region of FLA in this study.
The FERVmlu2 have two Pol-like ORFs. The ORF1 is similar to reverse transcriptase like sequence, in which encodes a DNA binding domain and a putative active site. ORF2 has similarity to an integrase core domain. The third ORF showed a gag – p30 superfamily motif. Nine LTR like sequences were recognized in FERVmlu1 by Repeatmasker program, indicating sequence divergences ranging from 13 to 32% in canine, baboon, chimpanzee endogenous retroviral LTRs.
Single nucleotide polymorphism (SNP)
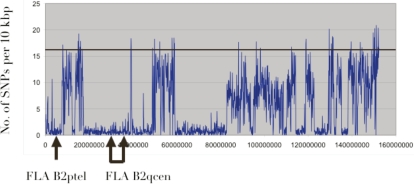
The SNP count plot (Figure 5) in the MHC region from 1.9× cat whole genome shotgun sequence indicated that this region is homozygous. Therefore, MHC BAC sequences were aligned with MHC homozygous 1.9× whole genome shotgun sequence contigs to examine SNP levels in FLA [32]. A total of 2,835,361 bps were aligned with sequence quality value, more than 15 by reciprocal best matches (>90% homology) using the algorithm of Smith-Waterman. This covers more than 85% of the entire FLA region. Distributions of these SNPs and coding SNPs were plotted in Figure 1 and the summary was presented in Table 4. A total of 11,654 SNPs were identified by this method. FLA SNP rate was slightly higher than the rate of two HLA haplotypes (0.00411 vs. 0.00337), and more than 2 times higher than genome wide regions of SNP rate (0.0017) found in regions of heterozygous cat WGS result. Ten to 20 times higher SNP rate than average FLA SNP rate was found in class II DR region, class II/III border region, proximal class I region and pericentromeric and subtelomeric regions. Clustered high coding SNP rates were observed in the proximal class I region.
Figure 5. Single Nucleotide Polymorphism (SNP) plot on cat chromosome B2 coordinates.
Number of SNPs were counted based on whole genome shotgun sequences and the number of SNPs per 10 Kbp were plotted. A solid line represents average SNP rate (per 10 Kbp) in heterozygous regions of a female Abyssinian cat genome. Areas of FLA were indicated as brackets.
Table 4. Characterization of 19 Class I Genes in FLA.
| Methods Applied | |||||||
| Gene | Dotplota with full length cDNA | Coding Prediction | Sequenced homology with cDNA | 31 conservede Amino acid residues in α1/α2 domains | Assignmentf | BAT1p g association | |
| Ib | IIc | ||||||
| FLA I-A | + | − | − | nonclassical | − | ||
| FLA I-B | − | gene fragment | − | ||||
| FLA I-C | + | − | − | nonclassical | − | ||
| FLA I-D | − | gene fragment | − | ||||
| FLA I-E | + | − | ++ | ++ (All) | classical | + | |
| FLA I-F | + | − | ++ | − (−5) | nonclassical | − | |
| FLA I-G | − | gene fragment | − | ||||
| FLA I-H | + | + | + | + | + (−1) | classical | + |
| FLA I-I | − | gene fragment | − | ||||
| FLA I-J | + | + | + | − (−3) | nonclassical | + | |
| FLA I-K | + | + | ++ | + | ++ (All) | classical | + |
| FLA I-L | + | − | + | − (−5) | nonclassical | + | |
| FLA I-M | + | − | + | − (−6) | nonclassical | + | |
| FLA I-N | − | gene fragment | − | ||||
| FLA I-O | + | + | − | − (−5) | nonclassical | + | |
| FLA I-P | − | gene fragment | + | ||||
| FLA I-Q | + | − | − | nonclassical | − | ||
| FLA I-R | − | gene fragment | − | ||||
| FLA I-S | + | − | − | nonclassical | − | ||
PIPmaker dotplot ( ) was used. + and − represent full-length and partial length, respectively compared with full length FLAIA24 cDNA.
GENSCAN was used to predict coding region for only full-length class I genes. + and − symbols represent right and wrong prediction of exon and intron boundaries in each gene.
Spidey was used to examine sequence alignment of genomic cDNA class I sequences and splicing donor/acceptor sites. ++, +, and − symbols represent typical class I exon/intron structures reported in human class I genes with all correct splicing donor/acceptor sites, with one or two missing splicing donor/acceptor sites, and atypical exon/intron structures, respectively.
Class I cDNA sequences from MHC homozygous feline fibroblast cells were compared with all class I genomic sequences by Megablast Search ( ). + symbol represents >99% sequence identity.
Thirty-one highly conserved amino acid residues found in α1 and α2 domains of human and cat class I antigens were examined. ++, +, − numbers represent all 31 conserved residues, one substitution and more than one substitutions, respectively.
Assignment of classical/nonclassical/gene fragment class I genes based on this study.
Symbols + and − represent presence and absence of BAT1gene fragment in vicinity of class I gene.
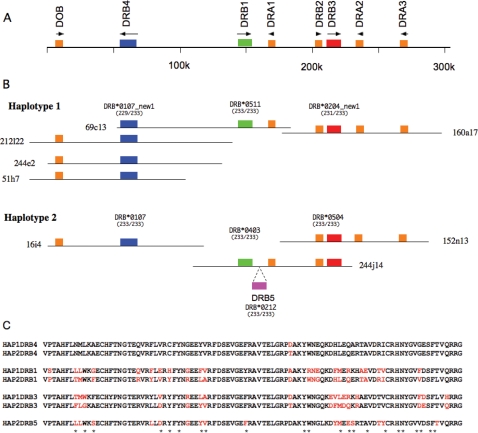
Two DR haplotypes in a single BAC library
We previously constructed a composite nucleotide sequence of the domestic cat MHC class II region spanning 758 kb from HSET to BTLII that included the DR region [18]. As shown in Figure 6A the DR region spans approximately 250 Kb and consists of 3 DRA and 4 DRB genes, both gene families are encoded by 5 exons with the exception of DRB2 which lacks the full complement of exons and thus represents a pseudogene. We determined the sequence of the second haplotype of a domestic cat DR region using BAC clones (152n13–244j14–16i4) from a single individual. The DR haplotype 2 contained three complete DRA genes (DRA1, DRA2 and DRA3), five DRB genes (four complete and one partial) similar to DRB4, DRB1, DRB3 and DRB2 plus new DRB gene, namely DRB5 as well as a BTLII gene all with the same order and orientation as observed in the DR haplotype 1 (except DRB5 adjacent to DRB1 with same orientation) (Figure 6B). To determine if DRB1 and DRB4 also displayed allelic variation, we aligned the genomic sequences of DRB1, 3 and 4 in the region of exon 2 and flanking introns 1 and 2. To assign these exon 2 sequences to specific DRB alleles we compared them to 71 different domestic cat DRB exon 2 alleles of 238 bp in length present in the NCBI nucleotide sequence database (nr/nt). The results summarized in Figure 6B show that DR haplotype 2 contains DRB3 exon 2 identical to DRB*0504 whereas DR haplotype 1 contained a DRB3 exon 2 that differed by 2 bp from DRB*0204, and thus represents a new domestic cat DRB allele (DRB*0204_new1) but differ from nucleotides of 70 nts between DR hap1 and DR hap2. Similarly, DRB1 exon2 sequences in haplotypes 1 and 2 contain the alleles which were identical to DRB*0511 and DRB*0403, respectively, but differ in 31 nucleotides of 233 nts. In addition, haplotype 1 was also positive for DRB4 which showed 229/233 nucleotide sequence identities with DRB*0107_new1. In contrast, the haplotype 2 DRB4 sequence was identical (233/233) to DRB*0107. The DR haplotype 2 contains additional DRB genes designated DRB5 that was not observed in haplotype 1 that displays identical exon 2 sequences with DRB*0212. In summary, this data show that a single domestic cat (Fca273) contains three or four DRB genes in the order DRB4-DRB1-(DRB5)-DRB3, that the three loci are heterozygous, and resolve into 2 distinct haplotypes consisting of DRB*0107_new1-DRB*0511-DRB*0204_new1 (haplotype 1) and DRB*0107-DRB*0403-DRB*0212-DRB*0504 (haplotype 2). Dotplot analysis confirmed this conclusion, indicating duplicated DRB genes adjacent to DRB1 gene (Figure 7). Deduced amino acid sequences of above alleles were compared in each DRB loci (Figure 6C). Of these loci, DRB1 alleles were the most polymorphic containing 23 different residues. DRB3 was the second most polymorphic loci, maintaining 17 different residues. In contrast, DRB4 had only one amino acid substitution.
Figure 6. Haplotype analysis of the domestic cat MHC class II DR region.
(A) Gene organization of the domestic cat MHC class II DR region based on the nucleotide sequence of a composite haplotype as previously reported in Yuhki et al. [14]. The location of eight DR genes is shown with the transcriptional orientation indicated by arrows. (B) Analysis of the haplotype structure of Fca273 (used to make the BAC library) based on mapping of gene content of individual BAC clones by hybridization and sequence-based typing of exon 2 of BAC clones. DRB alleles were identified based on comparison to 71 domestic cat DRB exon 2 sequences spanning 233 bp (after removal of primer sequences) present in the NCBI nucleotide database. (C) Deduced amino acid sequences from two haplotypes were aligned in each loci and different residues in each loci were depicted in red. Antigen recognition sites were shown as asterisks below the sequence alignment.
Figure 7. Dotplot analysis of two DR haplotype sequences from a single BAC library.
The additional DRB loci found in haplotype 2, named as DRB5 had 16 different residues compared with DRB1 loci in haplotype 2. On antigen recognition site (ARS) defined by X-ray crystallography [36], 22 sites forms ARS. Of these sites, 15 sites were found highly polymorphic in FLA.
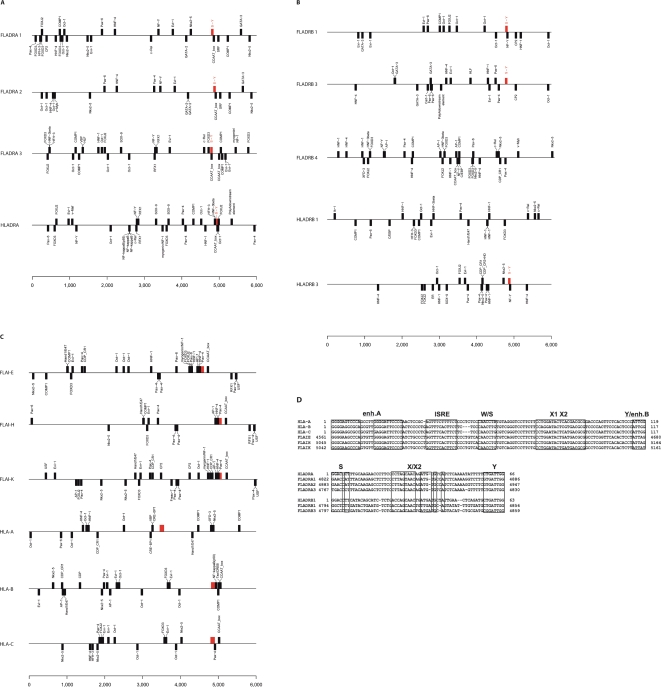
Transcription factor (TF) binding sites in predicted classical genes
We have analyzed transcription factor binding sites a total of 6 kbp (5 kb upstream and 1 kb downstream of ATG putative translation start site) of predicted feline classical class II genes (DRA1, 2, 3, DRB1, 3, 4: Figure 8A, Figure 8B) and classical class I gene candidate genes (I-E, I-H, I-K: Figure 8C), plus human classical class II and I genes (DRA, DRB1, DRB3, HLA-A, -B, -C). Figure 8A depicts the result of DRA genes. All three feline DRA genes have CCAAT-box. The DRA1 and DRA2 genes have striking similarity with TF binding sites up to about 4 kb upstream of ATG site and at least NF-Y binding site, indicating recent gene duplication. In contrast, the DRA3 gene has distinct TF binding sites from the other two genes and is relatively similar to those of the human DRA gene, (e.g., NFY-RFX1-RFX1 sites, Oct-1 sites, sox-9 sites). It may be suggested that the expression pattern is different in these two groups of DRA genes.
Figure 8. Transcriptional factor binding site prediction.
A total of 6 kb sequence spanning 1 kb downstream and 5 kb upstream from translation start site (ATG) were analyzed for (A) DRA genes, (B) DRB genes, and (C) Classical class I genes. The S-X/X2-Y module and enh.A-ISRE-W/S-X1-X2-Y.enh.B were depicted as a red box. HLA-DRB1, FLA- DRB4 modules were located at 52 Kb, 7 Kb upstream from ATG site, respectively. Forward and reverse orientation of TF binding sites were depicted above and below lines respectively: (D) enh.A-ISRE-W/S-X1-X2-Y/enh.B module sequences found in FLA-E, -H, -K and HLA-A, -B, -C genes and S-X1-X2-T module sequences found in FLA-DRA1, -DRA2, -DRA3, -DRB1, -DRB3 and HLA-DRA, -DRB1, were aligned and each promoter/enhancer cis-motifs were boxed. Coordinates of FLA were based on 6 Kb sequence described above.
Figure 8B depicts the difference between TF binding sites in feline DRB1, -3, -4 genes, and human DRB1 and -3. All of these genes lack the CCAAT-box site. The NF-Y site was found in FLA-DRB1, DRB4, and HLA-DRB3. However, no apparent similar TF binding site patterns were found. In class I genes, all predicted feline classical class I genes have a CCAAT-box site plus a unit of AP1-HNF4-Pax-4 sites adjacent to the CCAAT-box (Figure 8C). FLA I-H, I-K had relatively similar TF binding sites (e.g., Pax-4-Pax4, Evi-1, FOXD3-COMP1-Hand1/E47, Nkx2-5). Human HLA-B, -C had relatively similar TB binding sites (e.g., CCAAT-box, Oct1, Evi-1-FOXD3, Evi-1) however, the HLA-A gene had no CCAAT-box and was quite different in TF site pattern from HLA-B, -C genes. No apparent similar TF binding patterns were found in the FLA and HLA classical class I genes.
MHC class I and class II gene promoter structures were well documented and intensely analyzed by many molecular biological methods [37], [38]. MHC class II genes are regulated by a complex system containing two gene-specific transcription factors, regulatory factor X complex (RFX) and CIITA, and maintain an approximately 67 bp sequence, a strictly conserved regulatory module (S-X1-X2-Y) immediately upstream of the promoters [37]. In contrast, MHC class I genes are regulated by NFκB2, NFκB1, interferon-γ, RFX, and CIITA, and form an approximately 120 bp conserved regulatory module sequence, enh.A-ISRE-W/S-X1-X2/site α-Y/enh.B [38].
Similar conserved regulatory modules were identified in most of FLA class I and II genes analyzed here and summarized in Figure 8D.
Discussion
We report here annotation and SNP analysis of cat MHC (FLA). This study revealed one hundred forty-seven human gene homologues with mostly conserved gene order in five subregions, extended class II, class III, proximal class I, central class I, and distal class I regions. Extensive rearrangement events were obvious in classical class II and class I regions by dotplot analyses of three mammalian MHC, human HLA, canine DLA, and feline FLA (Figure 3). Especially, deletion of HLA-A and -E regions in both DLA and FLA, and expansion of the regions in FLA corresponding to HLA-B, -C were clearly observed (Figure 3A, B). A dotplot between DLA and FLA (Figure 3C) suggests that these two MHC systems are more syntenic than those to HLA. However, the manner of class I rearrangement was unique in each DLA and FLA. Each DLA and FLA also had unique sequences near the heterochromatin regions (near telomere and centromere) in canine chromosome cfa35ter/cfa12cen and feline chromosome B2pter/qcen regions. Among mammalian and MHC class I regions reported so far, only mammals which belong to the group Euarchontoglires (Primates and Rodentia) have class I E and A subgroups, plus the evidence of the recombinant origin of the class I E gene between class I A and B/C [39] suggests that the formation of these two class I subgroups (A, E) occurred after the split of two major mammalian groups, Euarchontoglires and Laurasiatheria [Carnivora (dog and cat), Perissodactyla (horse), Certartiodactyla (pig and cattle)].
Class II genes in FLA
Unlike all other mammalian MHCs which have a single DRA gene, FLA maintains three possible functional DRA genes due to two possible duplication events and one inversion [18]. The deduced amino acid sequences coding a mature DRA peptide are identical in these three DRA genes. However, significant levels of difference in amino acid sequences in the signal peptide region, which may suggest distinct roles in this region. In addition, distinct TF binding sites in DRA1/2 and DRA3 may suggest distinct expression patterns. All three DRB genes, common in two haplotypes examined had significant levels of polymorphism in exon 2 sequence which encodes peptides forming antigen binding and T cell receptor recognition sites. The well documented S-X1-X2-Y promoter module sequences were found in all DRA and DRB genes immediate upstream of CCAAT-box site , except that DRB4, which maintains this module sequence 7 kb upstream from ATG site and 5.5 kb upstream of CCAAT-box. FLA is also unique among mammalian MHC due to the fact that the entire DQ region is deleted. Since canine MHC (DLA) maintains a pair of A and B genes in its DQ region, this deletion event may occur after the split of canids and felids (55MYA).
Class I genes in FLA
Class I gene amplicon of a combination of class I and BAT1 gene fragments are found here in FLA-specific manner, though the human HLA-A region has two BAT1 gene fragments, suggesting that relatively new origins of multiple class I genes than classical class II families (DP, DQ, DR), which were estimated more than 80 MYA [40]. Gene structure of 19 FLA class I genes was characterized and summarized in Table 5. Eleven class I genes maintained full-length exons by dotplot, when compared with FLA class I cDNA sequence and their coding sequences were predicted by GENSCAN. Of those, six class I genes had intact splicing donor/acceptor sites. Three genes (FLA I-E, I-H, I-K) had 31–32 highly conserved amino acid residues in α1 and α2 domains which were reported in deduced amino acid sequences of FLA class I transcripts from fibroblast cell lines [41]. Analysis of FLA class I transcripts of a fibroblast cell line from MHC homozygous Abyssinian cat used for cat genome project indicated that these transcripts are derived from FLA I-H and I-K. In addition, all three of these class I genes maintain the conserved enh.A-ISRE-W/S-X1/X2-Y/enh.B promoter motif immediately upstream of CCAAT-box. Together, we tentatively assigned FLA I-E, I-H, I-K as classical class I genes and nine other genes as nonclassical class I genes.
Table 5. Single Nucleotide Polymorphism (SNP)s.
| FLA | HLA | |
| Size (Mbp) compared | 2.84 Mbp | 4.75 Mbp |
| No. of SNPs | 11,654 | 16,013 |
| SNP rate (per bp) | 0.00411 | 0.00337 |
| No. of CDS SNPs | 732 | 341 |
| Class I & II genes S/N | 48/145 | 48/68 |
This promoter analysis also revealed potentially distinct gene regulation of other FLA class I genes. For example, FLA I-S and I-O genes had an intact conserved promoter motif. However, I-S class I gene did not have intact coding region nor expression in fibroblast (Table 5). Also I-O class I gene did not maintain 5 highly conserved deduced amino acid residues in its peptide binding groove. Other class I genes, FLA I-A, I-Q lacked NFκB1, 2 and IFN-γ binding sites, and FLA I-J, I-L lacked Y/enh.B site.
Single Nucleotide Polymorphism (SNP)s
Overall, the SNP rate found in FLA (BAC sequence versus MHC homozygous 1.9× WGS contigs) was at least twice as much higher than the SNP rate in average heterozygous region in the WGS cat genome, (0.00411 versus 0.0017) and slightly higher but nearly equivalent to the SNP rate found in two human HLA haplotypes (6COX and 6QBL) (Table 4). A total number of coding SNP (CDS SNP) is higher than human HLA (732 versus 341). A total of 193 CDS SNPs were found in class II and class I genes. Of these, both class II DRB4 and DRB1 genes had a higher number of nonsynonymous CDS SNPs than synonymous ones, and two class I genes (FLA-I, -F, -H) had similar tendencies (Table 6). These data suggest that those genes are under positive selection.
Table 6. Nonsynonymous and Synonymous Coding SNPs in FLA class I and II genes.
| Class | FLA class I/II genes | No.of Synonymous and Nonsynonymous SNPs (S/N) |
| II | DRB4 | 8/25 |
| II | DRB1 | 1/2 |
| II | DRB3 | 5/13 |
| II | DRA1 | 2/0 |
| II | DRA2 | 0/0 |
| II | DRA3 | 0/0 |
| I | I-A | 1/0 |
| I | I-C | 2/1 |
| I | I-E | 0/0 |
| I | I-F | 4/40 |
| I | I-H | 15/50 |
| I | I-J | 6/7 |
| I | I-K | 0/1 |
| I | I-L | 1/1 |
| I | I-M | 0/1 |
| I | I-O | 3/4 |
New Endogenous Retrovirus Sequences
Phylogenetic analysis of three FLA endogenous retrovirus sequences using the neighbor-joining method (Figure 4) suggested that in addition to previously described baboon-derived RD114 retrovirus (or ECE1) [42]–[44] the other two sequences showed equidistance to FeLV derived [45], [46] and RD114 endogenous sequences, but more similar to the sequence recently submitted to GenBank as comparative genome initiative research derived from brown bat (Myotis Lucifugus) BAC sequence. Because all sequences described above maintained retrovirus POL region, newly identified feline retrovirus sequences was assigned as FERVmlu1 (previously FERV1) and FERVmlu2.
MHC Class I Related Genes
Of four MHC class I-related genes (MIC) which encodes c-lectin type NK receptor ligands in HLA, none of them had full length exon sequences when compared with human MICA transcripts (data not shown). Interestingly, neither cat nor dog genomes maintain multigene families of KIR and Ly49 found in primates and rodents genomes, respectively. These evidences may suggest distinct control systems for NK cells in cats and dogs.
Comparison of genomic structures in cat, dog, human, mouse, and opossum MHC genes
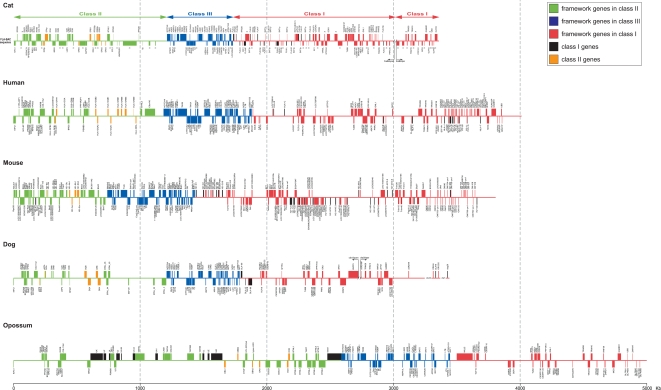
A proportionally scaled MHC genomic structure was presented for four mammalian genomes (cat, human, mouse, and dog) and one marsupial genome (opossum) (Figure 9). The MHC region spanning from KIFC (except mouse H2 which has a translocation in this region, so that H2 here compared from Rps 28) to UBD plus 3 olfactory receptor genes was compared in these MHCs. The result depicts striking similarity in gene contents and order of framework genes from marsupial through mammalian evolution. Three MHC (opossum, human, and mouse) have one contiguous gene content, suggesting depiction of an ancestral form of MHC, while two MHC (cat and dog) have a same split form of MHC at TRIM31 and TRIM26 in the class I region as compared with human HLA. In dog MHC, these two pieces were located on two chromosomes (cfa12qcen, cfa35qter), while in cat MHC, these were located on a single chromosome by an inversion (FcaB2 qcen, FcaB2pter) as previously described [19]. Further, two class I genes in dog MHC were located with two additional chromosomes (cfa7, cfa18). The size variation of MHC from about 3.3 Mbp (cat and dog excluding percentromeric, subtelomeric regions) to about 5 Mbp in opossum was also seen in this analysis. The difference in size observed here is mainly due to the magnitude of class I gene amplification and size of class II/III border regions. Cat MHC consists of 650 Kbp class I gene region, spanning from BAT1 to POU5F1 maintaining 17 class I genes/gene fragments, while human and dog MHC have only 2–3 class I genes in this region. Mouse H2 has 7 class I genes and there are no class I genes in opossum MHC in this region. Accordingly, class I gene amplification seemed to have occurred in a species-specific fashion. Additional evidences that e.g. opossum MHC, class I genes were amplified in the class II region, human HLA have at least 11 class I genes in the HLA-A region between the ZNRD1 and MOG genes and in mouse H2, at least 15 class I genes were found between Abcf1 and Trim26 genes, all support adaptive evolution of this importance immune system. Interestingly, the sizes of class II/III border regions vary in each MHC. Cat and dog MHC have approximately 400 Kbp in these regions. In cat MHC, this region was occupied with LINE repeats however, in dog and opossum there are multiple BTNL genes. These evidences reaffirmed the dynamic nature of evolution and maintenance of genome organizations in MHC.
Figure 9. Comparisons of MHC genomic structures in cat, human, mouse, dog, and opossum.
Framework genes in class II, III, I regions were shown as green, blue, red boxes, respectively. Forward and reverse orientations of each gene were shown above and below line, respectively. Classical class II antigen coding genes/gene fragments were shown in orange and classical and non-classical class I genes were shown in black.
Supporting Information
(3.98 MB BZ2)
Acknowledgments
The authors thank Lisa Maslan, and Beena Neelam for technical and computer support. Authors also thank all Werner-Kirsten high school student interns in Laboratory of Genomic Diversity from, Frederick County, Maryland, especially: Margaret Clotter, USC graduate school, Hue Banh, National Library of Medicine, Sabrina Selway, McGill University, Columbia University graduate School, Candis Jones, Lehigh University, Emily Howe, Davidson College, Nicholas Pinkin, UMBC, and Katherine Kelley, Arcadia University. The authors thank Joan Pontius for her suggestions to script Perl programs used to analyze data. The authors thank Joan Boxell for typing this manuscript.
Footnotes
Competing Interests: The authors have declared that no competing interests exist.
Funding: This project has been funded in whole or in part with federal funds from the National Cancer Institute, National Institutes of health, under contract N01-CO-12400. The content of this publication does not necessarily reflect the views or policies of the Department of Health and Human Services, nor does mention of trade names, commercial products, or organizations imply endorsement by the U.S. Government. This research was supported in part by the Intramural Research Program of the National Human Genome Research Institute, National Institutes of Health.
References
- 1.Klein J. Natural history of the major histocompatibility complex. New York: John Wiley and Sons; 1986. [Google Scholar]
- 2.Younger RM, Amadou C, Bethel G, Ehlers A, Fischer Lindahl K, et al. Characterization of Clustered MHC-Linked Olfactory Receptor Genes in Human and Mouse. Genome Res. 2001;11(4):519–530. doi: 10.1101/gr.160301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Loconto J, Papes F, Chang E, Stowers L, Jones EP, et al. Functional expression of murine V2R pheromone receptors involves selective association with the M10 and M1 families of MHC class Ib molecules. Cell. 2003;112(5):6-8–612. doi: 10.1016/s0092-8674(03)00153-3. [DOI] [PubMed] [Google Scholar]
- 4.Okita K, Ichisaka T, Yamanaka S. Generation of germline-competent induced pluripotent stem cells. Nature. 2007;448(7151):313–317. doi: 10.1038/nature05934. [DOI] [PubMed] [Google Scholar]
- 5.The MHC Sequencing Consortium. Complete sequence and gene map of a human major histocompatibility complex. Nature. 1999;401:921–923. doi: 10.1038/44853. [DOI] [PubMed] [Google Scholar]
- 6.Horton R, Gibson R, Coggill P, Miretti M, Allcock RJ, et al. Variation analysis and gene annotation of eight MHC haplotypes: The MHC Haplotype Project. Immunogenet. 2008;60:1–18. doi: 10.1007/s00251-007-0262-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Traherne JA, Horton R, Roberts AN, Miretti MM, Hurles, et al. Genetic analysis of completely sequenced disease-associated MHC haplotypes identifies shuffling of segments in recent human history. PLoS Genet. 2006;2:e9. doi: 10.1371/journal.pgen.0020009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Horton R, Wilming LRV, Lovering RC, Bruford EA, Khodiyar VK, et al. Gene map of the extended human MHC. Nat Rev Genet. 2004;5:889–899. doi: 10.1038/nrg1489. [DOI] [PubMed] [Google Scholar]
- 9.Stewart CA, Horton R, Allcock RJ, Ashurst JL, Atrazhev AM, et al. Complete MHC haplotype sequencing for common disease gene mapping. Gen Res. 2004;14:1176–1187. doi: 10.1101/gr.2188104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Allcock RJ, Atrazhev AM, Beck S, de Jong PJ, Elliott JF, et al. The MHC haplotype project: a resource for HLA-linked association studies. Tissue Antigens. 2002;59:520–521. doi: 10.1034/j.1399-0039.2002.590609.x. [DOI] [PubMed] [Google Scholar]
- 11.International Human Genome Sequencing Consortium. Initial sequencing and analysis of the human genome. Nature. 2001;409:860–921. doi: 10.1038/35057062. [DOI] [PubMed] [Google Scholar]
- 12.Hardy WD, Essex M, McClelland A. Feline Leukemia Virus. New York: Elsevier Publishers; 1980. [Google Scholar]
- 13.Brown EW, Yuhki N, Packer C, O'Brien SJ. A lion lentivirus related to feline immunodeficiency virus: epidemiologic and phylogenetic aspects. J Virol. 1994;68(9):5953–5968. doi: 10.1128/jvi.68.9.5953-5968.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Carpenter MA, Brown EW, Culver M, Johnson WE, Pecon-Slattery J, et al. Genetic and phylogenetic divergence of feline immunodeficiency virus in the puma (Puma concolor). J Virol. 1996;70(10):6682–6693. doi: 10.1128/jvi.70.10.6682-6693.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Troyer JL, Pecon-Slattery J, Roelke ME, Black L, Packer C, et al. Patterns of feline immunodeficiency virus multiple infection and genome divergence in a free-ranging population of African lions. J Virol. 2004;78(7):3777–3791. doi: 10.1128/JVI.78.7.3777-3791.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Troyer JL, Pecon-Slattery J, Roelke ME, Johnson W, VandeWoude S, et al. Seroprevalence and genomic divergence of circulating strains of feline immunodeficiency virus among Felidae and Hyaenidae species. J Virol. 2005;79(13):8282–8294. doi: 10.1128/JVI.79.13.8282-8294.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Pearks Wilkerson AJ, Teeling EC, Troyer JL, Bar-Gal GK, Roelke M, et al. Coronavirus outbreak in cheetahs: lessons for SARS. Curr Biol. 2004;14(6):R227–228. doi: 10.1016/j.cub.2004.02.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Yuhki N, Beck T, Stephens RM, Nishigaki Y, Newmann Y, et al. Comparative genome organization of human, murine and feline major histocompatibility complex class II region. Genome Res. 2003;13:1169–1179. doi: 10.1101/gr.976103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Beck T, Menninger J, Murphy WJ, Nash WG, O'Brien SJ, et al. The feline major histocompatibility complex is rearranged by inversion with a breakpoint in the distal class I region. Immunogenet. 2005;56:702–709. doi: 10.1007/s00251-004-0742-6. [DOI] [PubMed] [Google Scholar]
- 20.Yuhki N, Beck T, Stephens R, Neelam B, O'Brien SJ. Comparative genomic structure of human, dog, and cat MHC: HLA DLA, and FLA. J Hered. 2007;98(5):390–399. doi: 10.1093/jhered/esm056. [DOI] [PubMed] [Google Scholar]
- 21.Beck TW, Menninger J, Voight G, Newmann K, Nishigaki Y, et al. Comparative feline genomics: A BAC/PAC contig map of the major histocompatibility complex class II region. Genomics. 2001;71:282–295. doi: 10.1006/geno.2000.6416. [DOI] [PubMed] [Google Scholar]
- 22.Ewing B, Green P. Base calling of automated sequencer traces using phred II error probabilities. Gen Res. 1998a;8:186–194. [PubMed] [Google Scholar]
- 23.Ewing B, Hillier L, Wendi M, Green P. Basecalling of automated sequencer traces using phred I accuracy assessment. Gen Res. 1998;8:175–185. doi: 10.1101/gr.8.3.175. [DOI] [PubMed] [Google Scholar]
- 24.Gordon D, Desmarais C, Green P. Automated finishing with Autofinish. Gen Res. 2001;11:614–625. doi: 10.1101/gr.171401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gordon D, Abajian C, Green P. Consed: a graphical tool for sequence finishing. Genome Res. 1998;8:195–202. doi: 10.1101/gr.8.3.195. [DOI] [PubMed] [Google Scholar]
- 26.Burge C, Karlin S. Prediction of complete gene structures in human genomic DNA. J Mol Biol. 1997;268:78–94. doi: 10.1006/jmbi.1997.0951. [DOI] [PubMed] [Google Scholar]
- 27.Altschul SF, Gish W, Miller W, Myers EW, Lipman DJ. Basic local alignment search tool. J Mol Biol. 1990;268:78–94. doi: 10.1016/S0022-2836(05)80360-2. [DOI] [PubMed] [Google Scholar]
- 28.Yuhki N, Heidecker GF, O'Brien SJ. Characterization of major histocompatibility complex cDNA clones in the domestic cat: Diversity and evolution of MHC class I genes. J Immunol. 1989;142:3676–3682. [PubMed] [Google Scholar]
- 29.Tatusova TA, Madden TL. Blast 2 sequences - a new tool for comparing protein and nucleotide sequences. FEMS Microbiol Lett. 1999;174:247–250. doi: 10.1111/j.1574-6968.1999.tb13575.x. [DOI] [PubMed] [Google Scholar]
- 30.Schwartz S, Zhang Z, Frazer KA, Smit A, Riemer C, et al. PipMaker – A web server for aligning two genomic DNA sequences. Gen Res. 2000;10(4):577–586. doi: 10.1101/gr.10.4.577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Schwartz S, Kent WJ, Smit A, Zhang Z, Baertsch R, et al. Human-mouse alignments with BLASTZ. Gen Res. 2003;13:103–107. doi: 10.1101/gr.809403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Pontius J, Mullikin JC, Smith D, Lindblad-Toh K, Gnerre S, et al. The domestic cat genome sequence: Annotation and comparative inferences. Genome Res: Submitted; 2007. [Google Scholar]
- 33.Mullikin JC, Hunt SE, Cole CG, Mortimore BJ, Rice CM, et al. An SNP map of human chromosome 22. Nature. 2000;407(6803):516–520. doi: 10.1038/35035089. [DOI] [PubMed] [Google Scholar]
- 34.Wheelan SJ, Church DM, Ostell JM. Spidey: a tool for mRNA-to-genomic alignments. Gen Res. 2001;11:1952–1957. doi: 10.1101/gr.195301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Birney E, Clamp M, Durbin R. GeneWise and Genomewise. Gen Res. 2004;14:988–995. doi: 10.1101/gr.1865504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Yuhki N, O'Brien SJ. Nature and origin of polymorphism in feline MHC class II DRA and DRB genes. J Immunol. 1997;158:2822–2833. [PubMed] [Google Scholar]
- 37.Krawczyk M, Peyraud N, Rybtsova N, Masternak K, Bucher P, et al. Long distance control of MHC class II expression by multiple distal enhancers regulated by regulatory factor X complex and CIITA. J Immunol. 2004;173:6200–6210. doi: 10.4049/jimmunol.173.10.6200. [DOI] [PubMed] [Google Scholar]
- 38.van den Elsen PJ, obin SJP, van Eggermond MCAJ, Peijnenburg A. Regulation of MHC class I and class II gene transcription: differences and similarities. Immunogenet. 1998;48:208–221. doi: 10.1007/s002510050425. [DOI] [PubMed] [Google Scholar]
- 39.Koller BH, Geraghty DE, Shimizu Y, DeMars R, Orr HT. HLA-E, A novel HLA class I gene expressed in resting T lymphocytes. J Immunol. 1988;141(3):897–904. [PubMed] [Google Scholar]
- 40.Takahashi K, Rooney AP, Nei M. Origins and divergence times of mammalian class II MHC gene clusters. J Hered. 2000;91(3):198–204. doi: 10.1093/jhered/91.3.198. [DOI] [PubMed] [Google Scholar]
- 41.Yuhki N, O'Brien SJ. DNA recombination and natural selection pressure sustain genetic sequence diversity of the feline MHC class I genes. J Exp Med. 1990;172(2):621–630. doi: 10.1084/jem.172.2.621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Roca AL, Pecon-Slattery J, O'Brien SJ. Intact endogenous feline leukemia viruses of recent origin. J Virol. 2004;78(8):4370–4375. doi: 10.1128/JVI.78.8.4370-4375.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Roca AL, Nash WG, Menninger JC, Murphy WJ, O'Brien SJ. Insertional polymorphisms of endogenous feline leukemia viruses. J Virol. 2005;79(7):3979–3986. doi: 10.1128/JVI.79.7.3979-3986.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Klement V, McAllister RM. Syncytial cytopathic effect in KB cells of a C-type RNA virus isolated from human rhabdomyosarcoma. Virology. 1972;50:305–308. doi: 10.1016/0042-6822(72)90379-0. [DOI] [PubMed] [Google Scholar]
- 45.Nelson-Rees WA, Klement V, Peterson WD, Jr, Weaver JF. Comparative study of two RD 114 virus-indicator cell lines, KC and KB. J Natl. Cancer Inst. 1973;50:1129–1135. doi: 10.1093/jnci/50.5.1129. [DOI] [PubMed] [Google Scholar]
- 46.Niman HL, Stephenson JR, Gardner MB, Roy-Burman P. RD-114 and feline leukaemia virus genome expression in natural lymphomas of domestic cats. Nature (London) 1977;266:357–360. doi: 10.1038/266357a0. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(3.98 MB BZ2)