Abstract
Plants use sophisticated photosensing mechanisms to maximize their utilization of the available sunlight and to control developmental processes. The plant blue-light receptors of the phot family mediate plant phototropism and contain two light, oxygen, and voltage (LOV) sensitive domains as photoactive elements. Here, we report combined quantum mechanical/molecular mechanical simulations of the photocycle of a complete Phot-LOV1 domain from C. reinhardtii. We have investigated the electronic properties and structural changes that follow blue-light absorption. This permitted us to characterize the pathway for flavin-cysteinyl adduct formation, which was found to proceed via a neutral radical state generated by hydrogen atom transfer from the reactive cysteine residue, Cys57, to the chromophore flavin mononucleotide. Interestingly, we find that adduct formation does not cause any larger scale conformational changes in Phot-LOV1 which suggests that dynamical effects mediate signal transmission following the initial photoexcitation event.
Keywords: phototropin, photoreceptor, FMN, LOV1, QM/MM, molecular mechanics
1 Introduction
The conversion of sunlight into energy storage by the photosynthetic apparatus of plants is ubiquitous and its importance for all of life cannot be overemphasized. However, in addition to the ability to efficiently store light energy, organisms have to be able to respond to light cues from their environment, e.g., to adjust to changes in light conditions and to avoid damaging overexposure to light. Photosynthetic organisms have met these requirements by evolving sophisticated photosensing mechanisms. The responsible photoreceptors are the red-light sensitive phytochromes [7] and the blue-light receptors of the cryptochrome and phototropin family [6].
The plant phototropin family comprises phot1 and phot2 [3, 4, 5], that mediate light induced responses such as phototropism [9, 8], stomatal opening and closing [30], chloroplast relocation [25, 26], and gametogenesis [21]. Both phot1 and phot2 have two non-identical light, oxygen, and voltage (LOV) sensing domains, LOV1 and LOV2, as photoactive elements on the N-terminal side and a serine-threonine kinase moiety on the C-terminal side. The LOV domains are members of the well characterized PER-ARNT-SIM [42] family of sensor proteins that bind non-covalently the chromophore flavin mononucleotide (FMN).
Spectroscopically, there are significant differences in the photochemical properties of LOV1 and LOV2, e.g., in regard to their quantum efficiencies and photoproduct decay times. Insight into the function of LOV domains has been gained through the determination of X-ray crystallographic structures of phy3-LOV2 from A. capillus-veneris [11, 12] and of Phot-LOV1 from the algae C. reinhardtii [16].
For the Phot-LOV1 domains of C. reinhardtii, experimental studies have provided a detailed characterization of its photocycle: Upon excitation of the chromophore FMN into an excited singlet state, fast intersystem crossing (ISC) on the time scale of a few ns leads to an excited triplet state absorbing in the red at 715 nm [31]. This triplet state then decays over the course of ~4 μs into a blue-shifted species absorbing at 390 nm that is thought to represent a flavin-cysteinyl adduct between the C4a atom of FMN and the sulfur of a nearby cysteine residue, Cys57. This adduct is long lived and decays on a time scale of several seconds to minutes back to the original dark state.
The authors of [16] solved the structure of both the dark and the light state of Phot-LOV1 in C. reinhardtii, the latter evolving from the former upon light irradiation. The atomic resolution structures provided clear evidence for a flavin-cysteinyl adduct in the illuminated state, thereby, confirming spectroscopic data. Furthermore, these structures allowed the interpretation of the roles played by specific protein residues located close to the chromophore, particularly in terms of their possible influence on the photoreaction itself. Important for our purposes is the fact that the relatively high resolution at which both structures were determined establishes an ideal starting point for computer simulations.
Several computational studies of the electronic properties of flavins, including lumiflavin, have been reported in the literature. The authors of [16] conducted quantum chemical studies determining the change in electron distribution during the photocycle. Neiss et al. have performed ab initio calculations of the excited state properties of flavin related molecules [37] and also conducted a model study of the first steps of LOV’s photocycle [35]. Recently, the authors of [36] reported a molecular dynamics (MD) study of the LOV2 domain from A. capillus-veneris that compared the dynamical behavior of the dark and light states of the system.
However, despite the availability of a significant amount of spectroscopic and structural data as well as, recently, computational results, we still lack a detailed understanding of the photocycle. Of particular interest is the pathway for flavin-cysteinyl adduct formation, which is currently not very well characterized on an electronic level. In fact, several reaction mechanisms have been put forward: Swartz et al. proposed an ionic mechanism involving a thiolate anion and a cationic FMN that was assumed to be protonated by one of the amino acids in the binding pocket [41]. This suggestion was later refuted by infrared spectroscopy [1]. The authors of [13] and [16] proposed a concerted mechanism in which proton transfer from the cysteine to FMN-N5 is accompanied by nucleophilic attack of the thiolate anion onto FMN-C4a. Kennis et al. [29] suggested that the initial proton transfer from the cysteine to FMN-N5 is a separate event which is then followed by nucleophilic attack of the sulphur on FMN-C4a. Finally, the authors of [32, 39] proposed a radical-pair mechanism for adduct formation either via electron transfer or movement of a hydrogen atom from the cysteine to the FMN moiety and subsequent bond formation after ISC has taken place. On a more macroscopic scale, it is at present not known how flavin-cysteinyl adduct formation in LOV is coupled to the signaling action of the whole phot protein.
Here, we report combined quantum mechanical/molecular mechanical (QM/MM) simulations of the photocycle of the Phot-LOV1 domain of C. reinhardtii that allowed us to resolve the structural changes and electronic processes accompanying flavin-cysteinyl adduct formation. We have investigated the LOV1 ground and flavin-cysteinyl adduct state as well as several intermediates in the triplet excited state occurring during the photocycle. Our simulations provide electronic level insight into the reaction pathways and we find the adduct-formation reaction to be of the radical-pair type.
2 Methods
System Preparation
The simulations reported here are based on the dark state crystal structure of Phot-LOV1 in C. reinhardtii by Fedorov et al. [16] solved at 1.9 Å resolution (pdb code 1N9L). All water molecules as well as a sulphate ion present in the original structure were removed and missing hydrogen atoms were added to the FMN-protein complex. The mono-phosphate group of FMN was chosen to be de-protonated and, therefore, has a net charge of −2 e. The complex was then solvated in a 32.6 Å sphere of 6024 TIP3P water molecules yielding a final system size of 19804 atoms and is shown in Figure 1.
Figure 1.
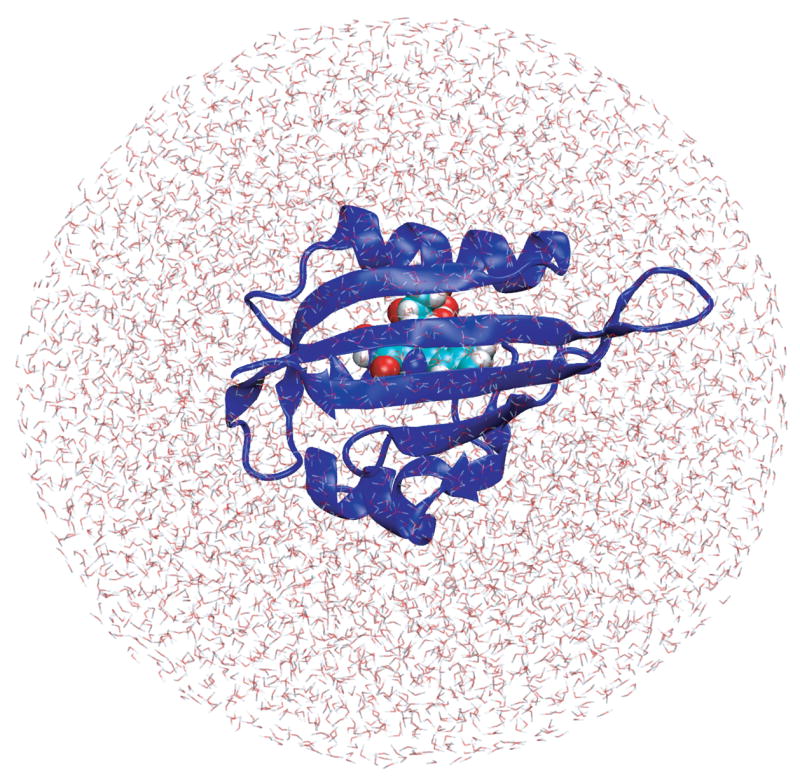
LOV1 domain simulation system. Shown is the Phot-LOV1 protein in cartoon representation with the FMN chromophore depicted as vdW spheres located inside the binding pocket. Also shown is the solvation sphere consisting of 6024 TIP3P water molecules.
The system was then minimized and equilibrated for 2.4 ns in the NVT ensemble with the molecular dynamics program NAMD2 [27] and the AMBER94 [10] forcefield. The parameters employed for the FMN part of the system were the ones developed by Schneider et al. [40]. During minimization and equilibration, spherical boundary conditions as implemented in NAMD2 where used to preserve the overall system shape and prevent the evaporation of water molecules. In order to obtain a properly minimized structure, the system was then subjected to further simulated cooling from 298.15K to a final temperature of 20K in steps of 10K and an equilibration time of 40ps per cycle followed by 500000 steps of conjugate gradient minimization using NAMD2. In all subsequent QM/MM simulations, the spherical boundary conditions where replaced by fixing all water molecules in a shell extending from a radius of 28 Å to the exterior of the water sphere at 32.6 Å. The system was then subjected to further minimization using a steepest descent algorithm until a final gradient smaller than 10−5 Bohr/Hartree was reached.
QM/MM simulations
The classically optimized structure described above provided the starting configuration for a series of QM/MM simulations. The quantum mechanically treated core segment is shown in Figure 2 and encompasses the lumiflavin part of FMN up to the C1′ atom of the ribityl chain and the sidechain of Cys57 up to Cβ.
Figure 2.
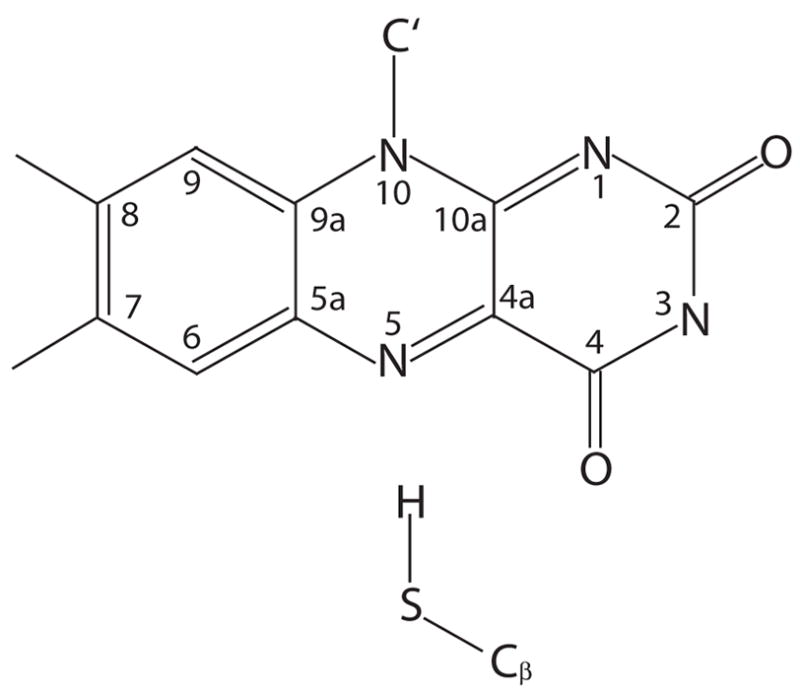
QM core region. Shown is a schematic view of the quantum mechanically treated core region, consisting of the lumiflavin part of FMN and the sidechain of Cys57. The figure also provides the atom numbering used in the remainder of the paper.
The frontier orbitals at the boundary between the quantum and classical regions were treated using the link atom approach and the free valences were capped with hydrogen atoms. Our QM/MM interface uses a RESP charge algorithm for the electrostatic coupling between the quantum and classical regions and is described in detail in [19, 20]. All geometry optimizations and single point calculations of the singlet and excited triplet states were conducted using a Hartree-Fock (HF) description at the RHF/6–31G(2d,2p) and ROHF/6–31G(2d,2p) [14, 15, 17, 22] level of theory, respectively. The use of restricted open-shell HF (ROHF) wavefunctions for the triplet excited states was necessary since the unrestricted HF wavefunctions exhibited a significant degree of spin contamination (>25%). In order to further corroborate our HF results, all calculations reported here with the exception of the transition state determination were repeated using density functional theory (DFT) at the B3LYP/6–31G(d) and U-B3LYP/6–31G(d) level of theory and were found to be in excellent qualitative agreement with our HF data. However, since the triplet excited state belongs to the same irreducible representation as the singlet ground state, the DFT approach is, formally, not rigorously defined and we therefore report the HF results only.
All geometry optimized conformations were confirmed to be true minima by calculating the Hessian matrix and analyzing its eigenvectors.
The transition state between T1 and Tradical was determined via constrained optimizations along the approximate reaction coordinate for hydrogen transfer given by the Cys57-H – FMN-N5 distance vector. The resulting conformation was validated to be a true transition state by the presence of a single imaginary frequency in the Hessian matrix and intrinsic reaction coordinate calculations connecting the transition state to T1 and Tradical, respectively. We did not, however, attempt to determine the transition state separating Sadduct and the singlet ground state, S0.
3 Results
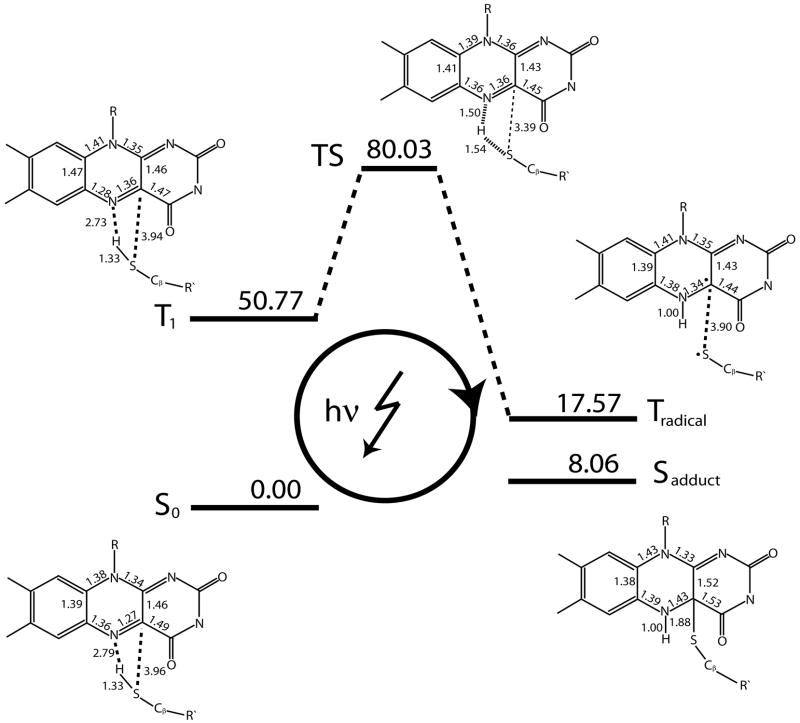
Here, we report our findings for the reaction pathway of flavin-cysteinyl adduct formation in Phot-LOV1 upon photoexcitation obtained via QM/MM simulations. We present the computational results that characterize the important states along this pathway in their proper reaction order.
FMN Binding Pocket Conformation
The structural organization of the binding pocket is shown in Figure 3, which depicts the FMN chromophore and residues lining the pocket. One can clearly discern the presence of polar residues adjacent to the pyrimidine side of FMN, whereas the di-methyl benzol ring is surrounded by non-polar residues. During the initial classical equilibration phase several water molecules migrated into the FMN binding pocket. The water molecule approaching the closest to the chromophore is hydrogen-bonded to Cys32 and the ribityl chain of FMN (c.f. Figure 3) and, hence, too far away from the reactive thiol group to contribute to adduct formation. Interestingly, the equilibration causes the rotation of the sidechain of Gln120 with respect to its position in the crystal structure such that eventually the amide oxygen is pointing toward FMN as opposed to the amide nitrogen in the original structure.
Figure 3.
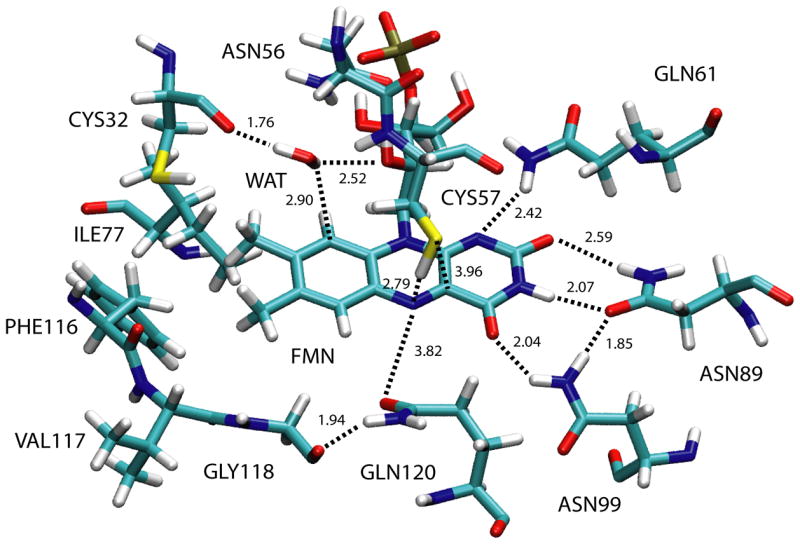
FMN binding pocket. Shown are the FMN chromophore and residues of the LOV-protein lining the binding pocket together with key interatomic distances.
Singlet Ground State, S0
Figure 3 depicts the conformation of FMN and several surrounding protein residues in the singlet ground state, S0, i.e. before photon absorption has taken place. FMN’s lumiflavin moiety is planar forming three hydrogen bonds with protein sidechains at its pyrimidine side, namely Asn89-Oδ1 – FMN-H3 (2.07 Å), Asn89-H δ21 – FMN-O2 (2.59 Å), and Asn99-H δ21 – FMN-O4 (2.04 Å). The sulfur of Cys57 is located almost directly above FMN-N5 with respect to the lumiflavin plane at a distance to FMN-C4a of 3.96 Å. The thiol hydrogen is pointing toward FMN-N5 and located 2.79 Å from the nitrogen atom. The intra-FMN bond lengths depicted in Figure 4 show that the N5-C4a bond has a double bond character compared to the single N5-C5a bond, with bond orders of 1.67 and 1.13 for the two bonds, respectively (data not shown).
Figure 4.
LOV1 photocycle. Shown is a schematic representation of the computed structures and energies of S0, T1, the transition state (TS), Tradical, and Sadduct determined at the RHF/6–31G(2d,2p) and ROHF/6–31G(2d,2p) level of theory.
Triplet Reactant State, T1
The triplet state T1 is reached via intersystem crossing (ISC) and possible further vibrational relaxation from an excited singlet state Sn generated by photon absorption. As shown in Figure 4, at the ROHF/6–31G(2d,2p) level of theory T1 is energetically 50.77 kcal/mol above S0.
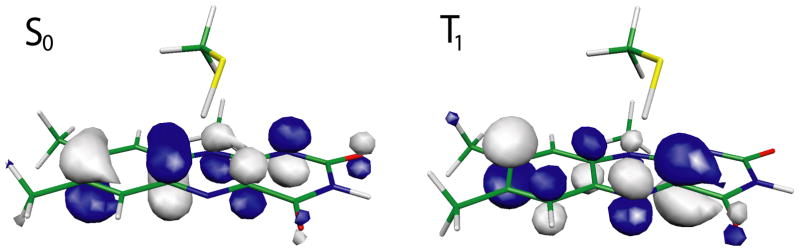
To characterize the electronic character of this state further, Figure 5 depicts the highest occupied molecular orbitals (HOMOs) of S0 and T1. An examination of the respective molecular orbitals reveals that T1 is of π–π* type.
Figure 5.
Highest occupied molecular orbitals. Depicted are the HOMOs of the quantum mechanically treated core region in S0 and T1 determined at the RHF/6–31G(2d,2p) and ROHF/6–31G(2d,2p) level of theory, respectively. The HOMO of S0 is a bonding π orbital, the one of T1 an anti-bonding π* orbital.
Table 1 lists the change in Mulliken charges with respect to S0, revealing a shift of negative charge toward N5 that originates mostly from C5a and from C4a. This shift is accompanied by lengthening of the N5-C4a bond (c.f. Figure 4) from 1.27 Å to 1.36 Å due to a reduction in its double bond character (bond order 1.15). At the same time, the N5-C5a bond contracts from 1.36 Å to 1.28 Å caused by an increase in its double bond character (bond order 1.60).
Table 1.
Mulliken charges on important atoms. Given are the changes in Mulliken charges with respect to S0 of key atoms in FMN and Cys57 calculated at the RHF/6–31G(2d,2p) and ROHF/6–31G(2d,2p) level of theory for S0, T1, the transition state (TS), Tradical, and Sadduct
| atom name | S0 | T1 | TS | Tradical | Sadduct |
|---|---|---|---|---|---|
| FMN-C4 | 0.00 | 0.00 | 0.01 | −0.01 | 0.03 |
| FMN-O4 | 0.00 | 0.00 | −0.01 | −0.07 | −0.03 |
| FMN-C4a | 0.00 | 0.07 | 0.08 | 0.05 | −0.13 |
| FMN-N5 | 0.00 | −0.14 | −0.28 | −0.13 | −0.20 |
| FMN-C5a | 0.00 | 0.06 | 0.08 | 0.09 | 0.10 |
| FMN-C9a | 0.00 | −0.03 | 0.03 | −0.05 | −0.06 |
| FMN-C10a | 0.00 | 0.01 | 0.04 | 0.01 | 0.02 |
| Cys57-Cβ | 0.00 | 0.00 | 0.02 | −0.01 | −0.05 |
| Cys57-S | 0.00 | −0.01 | −0.20 | 0.04 | 0.17 |
| Cys57-HS | 0.00 | 0.01 | 0.17 | – | – |
| FMN-H5 | – | – | – | 0.22 | 0.19 |
The increase in negative charge on the N5 nitrogen is accompanied by a slight movement of the thiol group toward FMN (by ~0.02 Å for Cys57-S) and leads to a slight increase in the charge of the thiol hydrogen itself. Apart from these charge rearrangement and structural changes involving the thiol group and the chromophore atoms C4a, N5, and C5a, our simulations reveal only minor differences between S0 and T1.
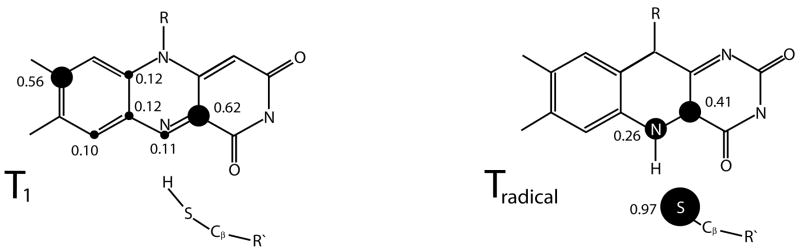
In the left panel of Figure 6, the most significant values for the atomic spin populations Δρ = ρ↑–ρ↓ in the triplet reactant state T1 are shown. Clearly, one observes a spin density which is distributed over most of the lumiflavin ring with no significant contribution by the thiol group, the largest share being provided by atoms C4a and C8.
Figure 6.
Mulliken spin populations. Shown are the atomic Mulliken spin populations Δρ = ρ↑–ρ↓for T1 and Tradical. Only the contributions with |Δρs| ≥ 0.1 at the ROHF/6–31G(2d,2p) level of theory are given.
Triplet Radical State, Tradical
Interestingly, our QM/MM simulations reveal the existence of a triplet state, Tradical, in which the thiol group hydrogen has transfered from Cys57-S to FMN-N5 and is now located 1.00 Å away from N5 in the lumiflavin plane. This is shown in Figure 4.
Tradical evolved from T1 via hydrogen transfer along a reaction coordinate given approximately by the Cys57-S - FMN-N5 distance vector. The conformation of the corresponding transition state structure is shown in Figure 4 and exhibits an energy of 81.03 kcal/mol; it is, therefore, ~30 kcal/mol above T1. Table 1 lists the Mulliken charges on important atoms in the transition state and reveals a significant charge polarization between Cys57-HS (+0.17 e) and Cys57-S (−0.20 e) and FMN-N5 (−0.28 e).
Energetically, Tradical was found 33 kcal/mol below the triplet reactant state but still 17 kcal/mol above the singlet ground state, S0 (c.f. Figure 4).
The observed movement of negative charge toward the N5 nitrogen in T1 facilitates this hydrogen transfer process. An analysis of the total Mulliken charge on FMN and Cys57 given in Table 2 clearly shows that Tradical evolves from T1 through transfer of a hydrogen atom as opposed to proton transfer, and, therefore, constitutes a neutral radical species.
Table 2.
Total Mulliken charges on FMN and Cys57. Given are the total Mulliken charges on FMN and Cys57 calculated at the RHF/6–31G(2d,2p) and ROHF/6–31G(2d,2p) level of theory for S0, T1, the transition state (TS), Tradical, and Sadduct.
| fragment | S0 | T1 | TS | Tradical | Sadduct |
|---|---|---|---|---|---|
| FMNa | 0.01 | 0.01 | 0.08 | 0.01 | −0.12 |
| Cys57b | −0.01 | −0.01 | −0.08 | −0.01 | 0.12 |
FMNH for Tradical and Sadduct
Cys57 without hydrogen for Tradical and Sadduct
In this context, it is important to note that the triplet radical character of the electronic wavefunction in Tradical represents an intrinsic property of the FMNH - Cys57-S system in the conformation studied by us. In other words, once hydrogen transfer has taken place, the triplet radical state corresponds to the electronic ground state with triplet spin symmetry of the system. Even though it might, in principle, be possible that a different structural arrangement of FMNH and Cys57-S leads to a wavefunction with, e.g., ionic character, we were not able to identify such a state. Based on our analysis, we do not find it likely that a state other than the neutral triplet radical observed in this study exists in the conformation of Phot-LOV1 studied by us.
Bond formation between FMN-N5 and the transfered thiol hydrogen atom pushes negative charge mostly toward FMN-O4 leading to an increased negative charge on this atom. Finally, an examination of the spin populations given in Figure 6 reveals a localization of the unpaired spin on FMN-C4a and N5 as well as, interestingly, the sulfur atom of Cys57.
To investigate the electronic properties of the singlet state coupled to Tradical via ISC, we examined the triplet and singlet state potential energy surfaces in the vicinity of Tradical by constrained optimizations along the approximate reaction coordinate for the transition from Tradical to Sadduct given by the Cys57-S - FMN-C4a distance. This is shown in Figure 7. Even though a vertical transition at the Tradical geometry yields a high energy conformation of ~82 kcal/mol, our calculations predict a much more moderate upper limit for the energy required to cross from the triplet to the singlet state potential energy surface of approximately 36 kcal/mol.
Figure 7.
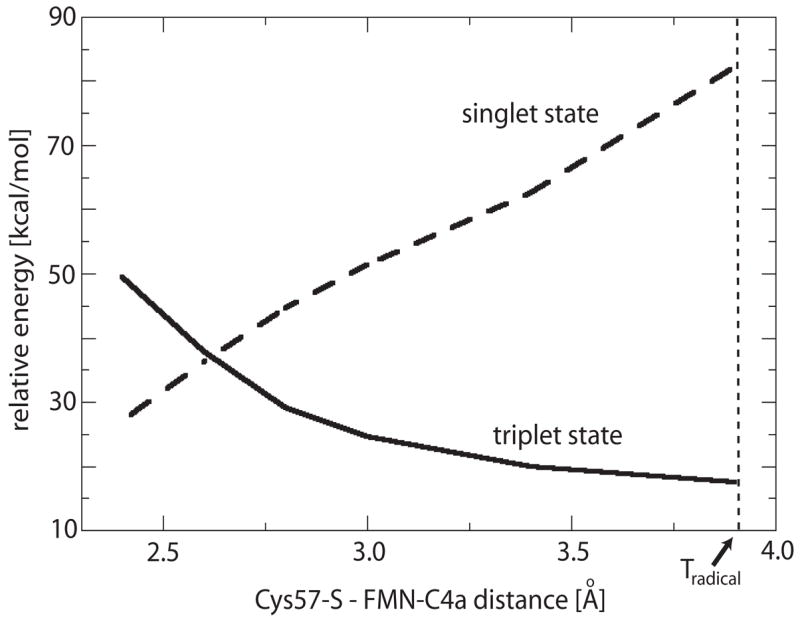
Energetics of triplet-singlet intersystem crossing. Shown are the approximate potential energy profiles measured with respect to S0 of the triplet and singlet states in the neighborhood of Tradical. The latter is located at a Cys57-S – FMN-C4a distance of 3.90 Å.
Singlet Flavin-Cysteinyl Adduct State, Sadduct
Figure 4 shows the conformation of the flavin-cysteinyl adduct state that evolves from the triplet excited state upon ISC to the singlet configuration. Sadduct was obtained from the conformation of Tradical via geometry optimization after changing the multiplicity of the wavefunction, thereby, establishing a direct connection on the potential energy surface between those two states (c.f. Figure 7).
The previously planar lumiflavin part in the state Sadductis now tetrahedrally distorted due to the sp3 hybridization of C4a caused by bond formation between the carbon atom and Cys57-S. The newly formed carbon-sulfur bond has a length of 1.88 Å. However, due partly to the tetrahedral distortion of FMN, Cys57 approaches FMN only slighty, e.g., by ~0.54 Å for the distance between Cys57-Cβ - FMN-N10. Hence, the adduct formation does not lead to any significant structural changes beyond the distortion of FMN itself.
Energetically, the flavin-cysteinyl adduct state lies approximately 10 kcal/mol below Tradical and 8 kcal/mol above S0.
Excitation Induced Protein Structural Changes
With the exception of FMN and the reactive Cys57 residue, the protein undergoes only very minor structural changes while traversing the four states of the photocycle described here, despite the fact that the protein is able to move freely inside the solvent sphere. This is clearly reflected in the root mean square deviations between the conformations of T1, Tradical, and Sadduct with respect to S0, which are 0.00 (0.01), 0.03 (0.08), and 0.02 (0.08), respectively. Here, the numbers in brackets denote the root mean square deviation for the complete protein including FMN, whereas the first number excludes FMN and the atoms of Cys57.
The protein conformational changes between S0 and T1 are completely confined to the lumiflavin ring and the thiol group of Cys57 described above. In Tradical we observe a motion of the sidechain of Gln120 toward N5 on the lumiflavin ring (c.f. Figure 3). The distance between Gln120-O3 and FMN-N5 decreases by 0.41 Å caused by the transfer of the thiol hydrogen to the lumiflavin ring and the resulting positive charge on the hydrogen atom (c.f. Table 1). Apart from this movement, there are no other significant changes in Tradical. Finally, in the flavin-cysteinyl state, Sadduct, the major conformational change is again confined to the FMN ring, and, due to bond formation, to residue Cys57. However, the secondary structural elements connected to Cys57 are only slightly affected. The sidechain of Gln120 moves back toward its position in the reactant state and assumes a final position ~0.21 Å from its initial state.
4 Discussion
Our computational findings provide new electronic level insight into the photocycle and into flavin-cysteinyl adduct formation in Phot-LOV1. The results are in good overall agreement with published experimental and theoretical data and their implications for the mode of operation of this remarkable photosensor will be discussed below.
Energetics of the Phot-LOV1 Photocycle
From an energetic point of view, our results suggest the following order of events upon photon absorption in the singlet ground state, S0. ISC from an excited singlet state relaxes the system into an excited triplet reactant state, T1, that subsequently converts into a neutral triplet radical state, Tradical. A second ISC event then leads to triplet-singlet conversion followed by bond formation between the sulfur atom of Cys57 and FMN-C4a to yield a flavin-cysteinyl adduct state. The latter conformation eventually converts back to the singlet ground state.
At the ROHF/6–31G(2d,2p) level of theory, the energy of the excited triplet state is 50.77 kcal/mol above the singlet ground state. For lumiflavin in the gas phase, Neiss et al. [35, 37] report a singlet-triplet splitting of 2.07 eV (47.7 kcal/mol) at the B3LYP/6–31G* level of theory. Hence, our results indicate that the presence of the protein environment does not give rise to a significant change in the singlet-triplet excitation energy compared to the gas phase. A value of ~50 kcal/mol is also in good agreement with a recent energy content analysis of this state by Losi et al. [33]. One can, therefore, conclude that the main role of the protein environment is the provision of a favorable arrangement of protein residues, particularly that of the reactive thiol group of Cys57, to facilitate bond formation rather than to change the overall reaction energetics. Using electron paramagnetic resonance (EPR), Schleicher et al. [39] arrived at a similar conclusion based on their observed small change in the zero-field splitting parameter |D|.
The triplet reactant state, T1, then converts exothermically into a triplet radical species, Tradical, that will be discussed in more detail below. Energetically, the radical state is approximately 33 kcal/mol below the initial triplet state and, therefore, 17 kcal/mol above the singlet ground state. We were able to identify the reaction pathway linking T1 and Tradical, even though the transition state barrier height of ~30 kcal/mol is overestimated by our HF method.
ISC leads subsequently to triplet-singlet conversion and flavin-cysteinyl adduct formation. This step also proceeds exothermically, since our calculations predict an energy of 8 kcal/mol for the flavin-cysteinyl adduct state, which corresponds to ~16% of the initial triplet excitation energy. Such a low energy conformation of Sadduct is in agreement with a quantum chemical study of adduct formation for several FMN model compounds [35]. The latter study reports, e.g., an energy of 2.51 kcal/mol for the adduct state between 8-methyl lumazine and the molecule HSCH3. A low energy conformation for the flavin-cysteinyl adduct state is, however, in stark contrast to findings by Losi et al. [34, 33] for YtvA-LOV and Phot-LOV1 of C. reinhardtii. These authors used laser-induced optoacustic spectroscopy to probe the energy content of various intermediates of the LOV photocycle. They find that in both systems the flavin-cysteinyl adduct assumes a high energy, strained conformation with an energy of 43 kcal/mol for Phot-LOV1. The analysis of protein structural changes observed during our simulations (c.f. Results) clearly shows that LOV does not assume any strained conformations since all structural changes are fully confined to the immediate vicinity of FMN. Furthermore, in light of the observed small structural differences between the X-ray crystal structures of the dark and light state of LOV1 from C. reinhardtii [16] it is unlikely that such a high energy strained conformation could arise. The cause of this discrepancy between experiment and computational studies is unclear and deserves further investigation.
To complete the photocycle, a transformation of the flavin-cysteinyl adduct state to the initial singlet ground state needs to take place. According to our results, this process is exothermic by ~ 8 kcal/mol and will, therefore, proceed spontaneously with a rate constant determined by the height of the transition state barrier.
Triplet-Singlet Intersystem Crossing
A thorough analysis of the wavefunction for T1 and Tradical provides a clear picture of how triplet-singlet intersystem crossing in Phot-LOV1 is effected. The atoms that harbor a significant fraction of the unpaired spin are shown in Figure 6. A close look at the data shows that, initially, the unpaired spin is distributed over most of the lumiflavin plane. Furthermore, due to the absence of aromatic protein residues in the vicinity of the lumiflavin ring, the triplet state can be assumed to be confined to the chromophore itself, as suggested in [39] based on EPR studies. In this context, it would be interesting to conduct a study similar to the one reported here for LOV2, for which an X-ray crystal structure [11] revealed the presence of a phenylalanine residue in π-stacking distance just below the lumiflavin ring.
Upon formation of Tradical the unpaired spin localizes on the atoms primarily involved in adduct formation, i.e., Cys57-S, FMN-C4a, and FMN-N5. These are ideal conditions for sulfur generated ISC. Due to its strong spin-orbit coupling constant, the sulfur atom is able to initiate efficient triplet-singlet intersystem crossing of the nearby located unpaired spin [2], leading to a rapid decay of the triplet radical state. The resulting short lifetime of Tradical could explain why spectroscopically only a single triplet species, corresponding to T1, without spectral evolution indicating the presence of Tradical is observed [29]. Furthermore, the existence of such an efficient spin conversion channel in the triplet radical state suggests that ISC occurs before flavin-cysteinyl bond formation takes place. The ISC event then paves the way for bond formation between Cys57-S and FMN-C4a in the singlet state.
Adduct Formation Pathway
The mechanism for flavin-cysteinyl adduct formation has been hotly debated in the literature and several different pathways have been proposed. Initially, adduct formation was suggested to proceed via a thiolate anion and a cationic FMN [41], protonation/deprotonation of which was proposed to be due to protein residues located nearby. Such an ionic mechanism, however, has been refuted based on experimental data [1, 24] and was, therefore, not investigated here. A neutral and protonated thiol group and a neutral and unprotonated FMN were assumed as initial state in all our simulations.
Three additional mechanisms have been invoked to explain flavin-cysteinyl adduct formation in LOV domains. An ionic mechanism has been suggested [29, 13] that involves proton abstraction from the thiol group by FMN-N5 in the excited triplet state followed by nucleophilic attack of the thiolate on the C4a position of FMN.
Based on their crystal structure of phy3-LOV2 from A. capillus-veneris in the dark state, Crosson et al. [11] proposed a concerted mechanism for adduct formation in which FMN-N5 is protonated while the thiol sulfur carries out a nucleophilic attack onto the C4a position. Similarly, Fedorov et al. [16] suggest a concerted mechanism based on their crystal structures for Phot-LOV1 of C. reinhardtii and theoretical Mulliken charge distributions obtained via quantum chemical calculations.
Our QM/MM simulations of the photocycle in Phot-LOV1 identify a third mechanism as the relevant pathway for thiol-cysteinyl adduct formation in Phot-LOV1: a radical-pair mechanism as evidenced by the neutral radical state Tradical (c.f. Table 1) that evolves from T1 via hydrogen atom transfer from the thiol group of Cys57 to FMN-N5.
The energy of the transition state separating T1 and Tradical was found to be ~80 kcal/mol above S0, corresponding to a barrier height of ~30 kcal/mol with respect to T1. In light of the experimentally measured transition times from the triplet toward the adduct state, which are on the microsecond timescale, this barrier is clearly too high. This could be due to the chosen HF method, which might not be able to properly describe the electronic wavefunction at the transition state conformation. However, we also cannot rule out the presence of other lower energy transition state conformations connecting T1 and Tradical.
Analysis of the Mulliken charge distribution calculated for the transition state structure (c.f. Table 1) suggests that the hydrogen atom is transfered from the cysteine to FMN via a coupled proton-electron transfer, rather than that of a hydrogen radical. Such a hydrogen transfer process is also in accord with the π–π* character of T1 and gives rise to the observed radical pair state Tradical.
A radical-pair mechanism has also been suggested by optical and EPR spectroscopy of Kay at al. [28] and Schleicher et al. [39], and by quantum chemical simulations of flavin-cysteinyl adduct formation between isolumazine and HSCH3 of Neiss et al. [35]. In principle, such a radical mechanism can evolve either via electron transfer from the thiol group to FMN giving rise to a zwitterionic radical species or via transfer of the thiol hydrogen yielding a neutral radical. Based on their data, Schleicher et al. [39] favor a zwitterionic radical species whereas Neiss et al. [35] find that a neutral radical mechanism proceeding via hydrogen atom transfer is energetically most favorable. Our results agree with the latter view, but it is evident that this triplet radical state deserves further experimental and theoretical investigation.
Constrained optimizations along the approximate reaction coordinate for adduct formation given by the Cys57-S – FMN-C4a distance provided an upper limit for the barrier height of 36 kcal/mol for the crossing between the triplet and singlet potential energy surfaces. Hence, even though the true barrier may be significantly lower, dynamic e3ects can be expected to play a role in the ISC event between Tradical and Sadduct, e.g., fluctuation driven shortening of the Cys57-S - FMN-C4a distance.
Protein Structural Changes
One of the central questions of LOV1 function is the coupling of its photocycle to the kinase activity of the full protein, which eventually relays the excitation by light to the signaling activity of Phot-LOV1 in C. reinhardtii. A comparison of the structures for the dark and light state of LOV1 in C. reinhardtii [16] reveals that all significant conformational changes are confined to the region immediately surrounding FMN. On the other hand, genetic analysis and comparison of available crystal structures for different species led Crosson et al. [13] to suggest a series of interconnected residues extending from FMN to the outside of the protein to be responsible for signal transduction. Our simulations support the findings of Federov et al. [16] since they do not reveal any significant structural changes beyond the immediate vicinity of FMN and the reactive residue Cys57 (c.f. Results). It has to be kept in mind, however, that our QM/MM approach is not able to overcome barriers separating local minima on the potential energy surface, which might be necessary to allow for such larger structural changes. The signaling to the kinase domain of the protein could, e.g, be mediated by interactions with the surface of the LOV1 domain that are altered through slight rearrangements of the FMN moiety in the protein core, possibly involving secondary structural elements like the β-sheet of LOV domains or the Jα helix suggested by Harper et al. [18] for the phot1-LOV2 domain of A. sativa.
5 Conclusions
Here, we report the results of an ab initio QM/MM study of the photocycle in the Phot-LOV1 domain of C. reinhardtii. Our calculations provide structures, energies, and electronic properties of important states traversed during the photocycle of Phot-LOV1. Our findings shed light on the nature of the exited triplet state and flavin-cysteinyl adduct formation is found to proceed via a neutral triplet radical species. The accumulation of the unpaired spin in Tradical close to the thiol sulfur of Cys57 primes the system for efficient and fast triplet-singlet intersystem crossing and likely is the reason why such a radical state so far has eluded experimental investigation. Surprisingly, all conformational changes observed during the photocycle are confined to the chromophore itself and protein residues in its immediate vicinity. This can be taken as further evidence for the suggestion that photo-induced signal transmission is not due to conformational changes of the LOV1 domain itself, but rather caused by a change in its dynamical properties resulting from covalent bond formation between Cys57 and FMN, and a likely small, but significant shift of FMN in its binding pocket.
Acknowledgments
The molecular images in this paper were created with the molecular graphics program VMD [23] with exception of Figure 5, which was created using Molekel [38]. We would like to thank the Pittsburgh Supercomputer Center for continuous support and acknowledge the use of the Center’s GS1280 computational facilities made available by the NCRR Research Resource RR06009.
This work is supported by grants from the National Institutes of Health PHS-5-P41-RR05969 and the National Science Foundation MCB02-34938. The authors gladly acknowledge supercomputer time provided by Pittsburgh Supercomputer Center and the National Center for Supercomputing Applications via National Resources Allocation Committee grant MCA93S028.
Footnotes
This PDF receipt will only be used as the basis for generating PubMed Central (PMC) documents. PMC documents will be made available for review after conversion (approx. 2-3 weeks time). Any corrections that need to be made will be done at that time. No materials will be released to PMC without the approval of an author. Only the PMC documents will appear on PubMed Central -- this PDF Receipt will not appear on PubMed Central.
References
- 1.Ataka K, Hegemann P, Heberle J. Vibrational spectroscopy of an algal Phot-LOV1 domain probes the molecular changes associated with blue-light reception. Biophys J. 2003;84:466–74. doi: 10.1016/S0006-3495(03)74866-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bittl Robert, Kay Christopher WM, Weber Stefan, Hegemann Peter. Characterization of a flavin radical product in a C57M mutant of a LOV1 domain by electron paramagnetic resonance. Biochemistry. 2003;42:8506–12. doi: 10.1021/bi034123i. [DOI] [PubMed] [Google Scholar]
- 3.Briggs Winslow R, Christie John M. Phototropins 1 and 2: versatile plant blue-light receptors. Trends Plant Sci. 2002;7:204–10. doi: 10.1016/s1360-1385(02)02245-8. [DOI] [PubMed] [Google Scholar]
- 4.Briggs WR, Beck CF, Cashmore AR, Christie JM, Hughes J, Jarillo JA, Kagawa T, Kanegae H, Liscum E, Nagatani A, Okada K, Salomon M, Rüdiger W, Sakai T, Takano M, Wada M, Watson JC. The phototropin family of photoreceptors. Plant Cell. 2001;13:993–7. doi: 10.1105/tpc.13.5.993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Briggs WR, Christie JM, Salomon M. Phototropins: a new family of flavin-binding blue light receptors in plants. Antioxid Redox Signal. 2001;3:775–88. doi: 10.1089/15230860152664975. [DOI] [PubMed] [Google Scholar]
- 6.Briggs WR, Huala E. Blue-light photoreceptors in higher plants. Annu Rev Cell Dev Biol. 1999;15:33–62. doi: 10.1146/annurev.cellbio.15.1.33. [DOI] [PubMed] [Google Scholar]
- 7.Briggs WR, Olney MA. Photoreceptors in plant photomorphogenesis to date. Five phytochromes, two cryptochromes, one phototropin, and one superchrome. Plant Physiol. 2001;125:85–8. doi: 10.1104/pp.125.1.85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Christie JM, Briggs WR. Blue light sensing in higher plants. J Biol Chem. 2001;276:11457–60. doi: 10.1074/jbc.R100004200. [DOI] [PubMed] [Google Scholar]
- 9.Christie JM, Salomon M, Nozue K, Wada M, Briggs WR. LOV (light, oxygen, or voltage) domains of the blue-light photoreceptor phototropin (nph1): binding sites for the chromophore flavin mononucleotide. Proc Natl Acad Sci U S A. 1999;96:8779–83. doi: 10.1073/pnas.96.15.8779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cornell WD, Cieplak P, Bayly CI, Gould IR, Jr, Merz KM, Ferguson DM, Spellmeyer DC, Fox T, Caldwell JW, Kollman PA. A second generation force field for the simulation of proteins, nucleic acids, and organic molecules. J Am Chem Soc. 1995;117:5179–5197. [Google Scholar]
- 11.Crosson S, Moffat K. Structure of a flavin-binding plant photoreceptor domain: insights into light-mediated signal transduction. Proc Natl Acad Sci U S A. 2001;98:2995–3000. doi: 10.1073/pnas.051520298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Crosson Sean, Moffat Keith. Photoexcited structure of a plant photoreceptor domain reveals a light-driven molecular switch. Plant Cell. 2002;14:1067–75. doi: 10.1105/tpc.010475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Crosson Sean, Rajagopal Sudarshan, Moffat Keith. The LOV domain family: photoresponsive signaling modules coupled to diverse output domains. Biochemistry. 2003;42:2–10. doi: 10.1021/bi026978l. [DOI] [PubMed] [Google Scholar]
- 14.Ditchfield R, Hehre WJ, Pople JA. Self-consistent molecular-orbital methods. ix. An extended gaussian-type basis for molecular-orbital studies of organic molecules. J Chem Phys. 1971;54:724–728. [Google Scholar]
- 15.Ditchfield R, Hehre WJ, Pople JA. Self-consistent molecular orbital methods. XII. Further extensions of gaussian-type basis sets for use in molecular orbital studies of organic molecules. J Chem Phys. 1972;56:2257–2261. [Google Scholar]
- 16.Fedorov Roman, Schlichting Ilme, Hartmann Elisabeth, Domratcheva Tatjana, Fuhrmann Markus, Hegemann Peter. Crystal structures and molecular mechanism of a light-induced signaling switch: The Phot-LOV1 domain from Chlamydomonas reinhardtii. Biophys J. 2003;84:2474–82. doi: 10.1016/S0006-3495(03)75052-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Francl Michelle M, Pietro William J, Hehre Warren J, Binkley J Stephen, Gordon Mark S, DeFrees Douglas J, Pople John A. Self-consistent molecular orbital methods. XXIII. A polarization-type basis set for second-row elements. J Chem Phys. 1982;77:3654–3665. [Google Scholar]
- 18.Harper Shannon M, Neil Lori C, Day Iain J, Hore PJ, Gardner Kevin H. Conformational changes in a photosensory LOV domain monitored by time-resolved NMR spectroscopy. J Am Chem Soc. 2004;126:3390–1. doi: 10.1021/ja038224f. [DOI] [PubMed] [Google Scholar]
- 19.Hayashi S, Ohmine I. Proton transfer in bacteriorhodopsin: Structure, excitation and IR spectra, and potential energy surface analyses by an ab initio QM/MM method. J Phys Chem B. 2000;104:10678–10691. [Google Scholar]
- 20.Hayashi Shigehiko, Tajkhorshid Emad, Schulten Klaus. Structural changes during the formation of early intermediates in the bacteriorhodopsin photocycle. Biophys J. 2002;83:1281–1297. doi: 10.1016/S0006-3495(02)73900-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hegemann Peter, Fuhrmann Markus, Kateriya Suneel. Algal sensory photoreceptors. J Phycol. 2001;37:668–676. [Google Scholar]
- 22.Hertwig Roland H, Koch Wolfram. On the parametrization of the local correlation functional. what is Becke-3-LYP? Chem Phys Lett. 1997;268:345–351. [Google Scholar]
- 23.Humphrey William, Dalke Andrew, Schulten Klaus. VMD – Visual Molecular Dynamics. J Mol Graphics. 1996;14:33–38. doi: 10.1016/0263-7855(96)00018-5. [DOI] [PubMed] [Google Scholar]
- 24.Iwata Tatsuya, Tokutomi Satoru, Kandori Hideki. Photoreaction of the cysteine S-H group in the LOV2 domain of Adiantum phytochrome3. J Am Chem Soc. 2002;124:11840–1. doi: 10.1021/ja020834c. [DOI] [PubMed] [Google Scholar]
- 25.Jarillo JA, Gabrys H, Capel J, Alonso JM, Ecker JR, Cashmore AR. Phototropin-related NPL1 controls chloroplast relocation induced by blue light. Nature. 2001 Apr;410:952–4. doi: 10.1038/35073622. [DOI] [PubMed] [Google Scholar]
- 26.Kagawa T, Sakai T, Suetsugu N, Oikawa K, Ishiguro S, Kato T, Tabata S, Okada K, Wada M. Arabidopsis NPL1: a phototropin homolog controlling the chloroplast high-light avoidance response. Science. 2001;291:2138–41. doi: 10.1126/science.291.5511.2138. [DOI] [PubMed] [Google Scholar]
- 27.Kalé Laxmikant, Skeel Robert, Bhandarkar Milind, Brunner Robert, Gursoy Attila, Krawetz Neal, Phillips James, Shinozaki Aritomo, Varadarajan Krishnan, Schulten Klaus. NAMD2: Greater scalability for parallel molecular dynamics. J Comp Phys. 1999;151:283–312. [Google Scholar]
- 28.Kay Christopher WM, Schleicher Erik, Kuppig Andreas, Hofner Heidi, Rüdiger Wolfhart, Schleicher Michael, Fischer Markus, Bacher Adelbert, Weber Stefan, Richter Gerald. Blue light perception in plants. Detection and characterization of a light-induced neutral flavin radical in a C450A mutant of phototropin. J Biol Chem. 2003;278:10973–82. doi: 10.1074/jbc.M205509200. [DOI] [PubMed] [Google Scholar]
- 29.Kennis John TM, Crosson Sean, Gauden Magdalena, van Stokkum Ivo HM, Moffat Keith, van Grondelle Rienk. Primary reactions of the LOV2 domain of phototropin, a plant blue-light photoreceptor. Biochemistry. 2003;42:3385–92. doi: 10.1021/bi034022k. [DOI] [PubMed] [Google Scholar]
- 30.Kinoshita T, Doi M, Suetsugu N, Kagawa T, Wada M, Shimazaki K. Phot1 and phot2 mediate blue light regulation of stomatal opening. Nature. 2001;414:656–60. doi: 10.1038/414656a. [DOI] [PubMed] [Google Scholar]
- 31.Kottke Tilman, Heberle Joachim, Hehn Dominic, Dick Bernhard, Hegemann Peter. Phot-LOV1: photocycle of a blue-light receptor domain from the green alga Chlamydomonas reinhardtii. Biophys J. 2003;84:1192–201. doi: 10.1016/S0006-3495(03)74933-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kowalczyk Radoslaw M, Schleicher Erik, Bittl Robert, Weber Stefan. The photoinduced triplet of flavins and its protonation states. J Am Chem Soc. 2004;126:11393–9. doi: 10.1021/ja049554i. [DOI] [PubMed] [Google Scholar]
- 33.Losi Aba, Kottke Tilman, Hegemann Peter. Recording of blue light-induced energy and volume changes within the wild-type and mutated phot-LOV1 domain from Chlamydomonas reinhardtii. Biophys J. 2004;86:1051–60. doi: 10.1016/S0006-3495(04)74180-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Losi Aba, Quest Benjamin, Gärtner Wolfgang. Listening to the blue: the time-resolved thermodynamics of the bacterial blue-light receptor YtvA and its isolated LOV domain. Photochemical & Photobiological Sciences. 2003;2:759–766. doi: 10.1039/b301782f. [DOI] [PubMed] [Google Scholar]
- 35.Neiss Christian, Saalfrank Peter. Ab initio quantum chemical investigation of the first steps of the photocycle of phototropin: a model study. Photochem Photobiol. 2003;77:101–9. doi: 10.1562/0031-8655(2003)077<0101:aiqcio>2.0.co;2. [DOI] [PubMed] [Google Scholar]
- 36.Neiss Christian, Saalfrank Peter. Molecular dynamics simulation of the LOV2 domain from Adiantum capillus-veneris. J Chem Inf Comput Sci. 2004;44(5):1788–93. doi: 10.1021/ci049883u. [DOI] [PubMed] [Google Scholar]
- 37.Neiss Christian, Saalfrank Peter, Parac Maja, Grimme Stefan. Quantum chemical calculation of excited states of flavin-related molecules. J Phys Chem A. 2003;107:140–147. [Google Scholar]
- 38.Portmann Stefan, Lüthi Hans Peter. Molekel: An interactive molecular graphics tool. CHIMIA. 2000;54:766–770. [Google Scholar]
- 39.Schleicher Erik, Kowalczyk Radoslaw M, Kay Christopher WM, Hegemann Peter, Bacher Adelbert, Fischer Markus, Bittl Robert, Richter Gerald, Weber Stefan. On the reaction mechanism of adduct formation in LOV domains of the plant blue-light receptor phototropin. J Am Chem Soc. 2004;126:11067–76. doi: 10.1021/ja049553q. [DOI] [PubMed] [Google Scholar]
- 40.Schneider C, Sühnel J. A molecular dynamics simulation of the flavin mononucleotide-RNA aptamer complex. Biopolymers. 1999;50:287–302. doi: 10.1002/(SICI)1097-0282(199909)50:3<287::AID-BIP5>3.0.CO;2-G. [DOI] [PubMed] [Google Scholar]
- 41.Swartz TE, Corchnoy SB, Christie JM, Lewis JW, Szundi I, Briggs WR, Bogomolni RA. The photocycle of a flavin-binding domain of the blue light photoreceptor phototropin. J Biol Chem. 2001;276:36493–500. doi: 10.1074/jbc.M103114200. [DOI] [PubMed] [Google Scholar]
- 42.Vreede Jocelyne, van der Horst Michael A, Hellingwerf Klaas J, Crielaard Wim, van Aalten Daan MF. PAS domains. Common structure and common flexibility. J Biol Chem. 2003;278:18434–9. doi: 10.1074/jbc.M301701200. [DOI] [PubMed] [Google Scholar]