Abstract
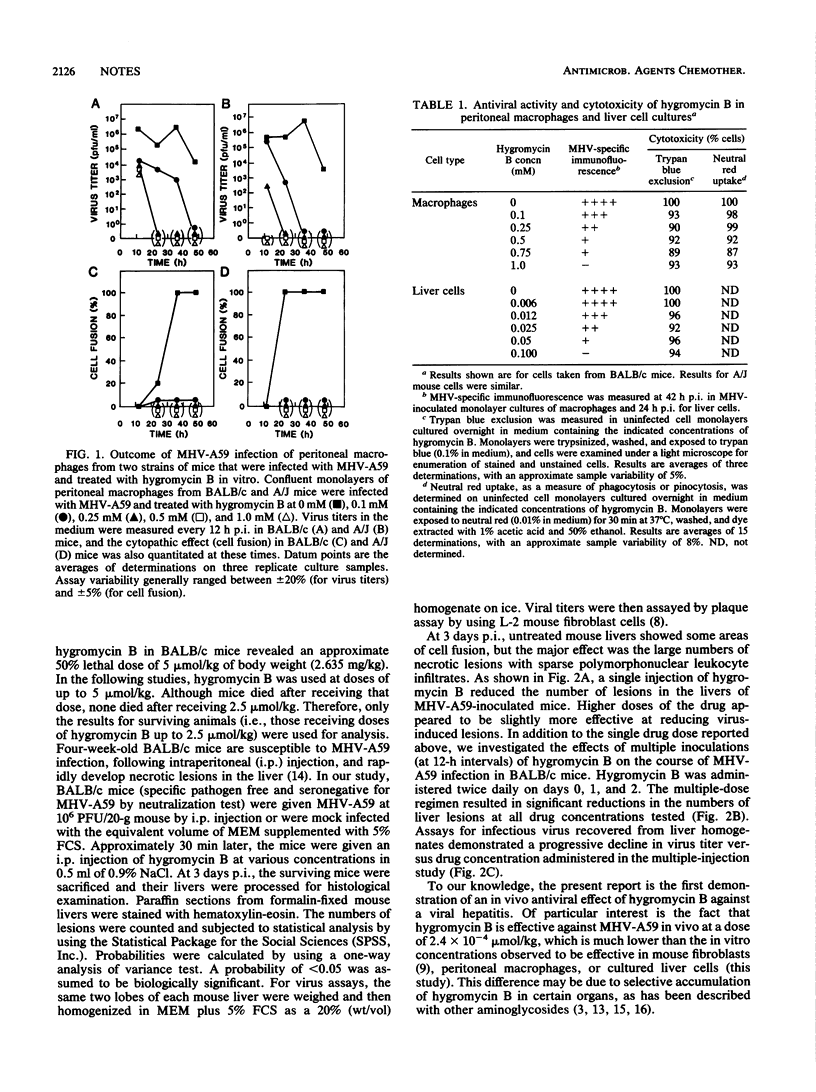
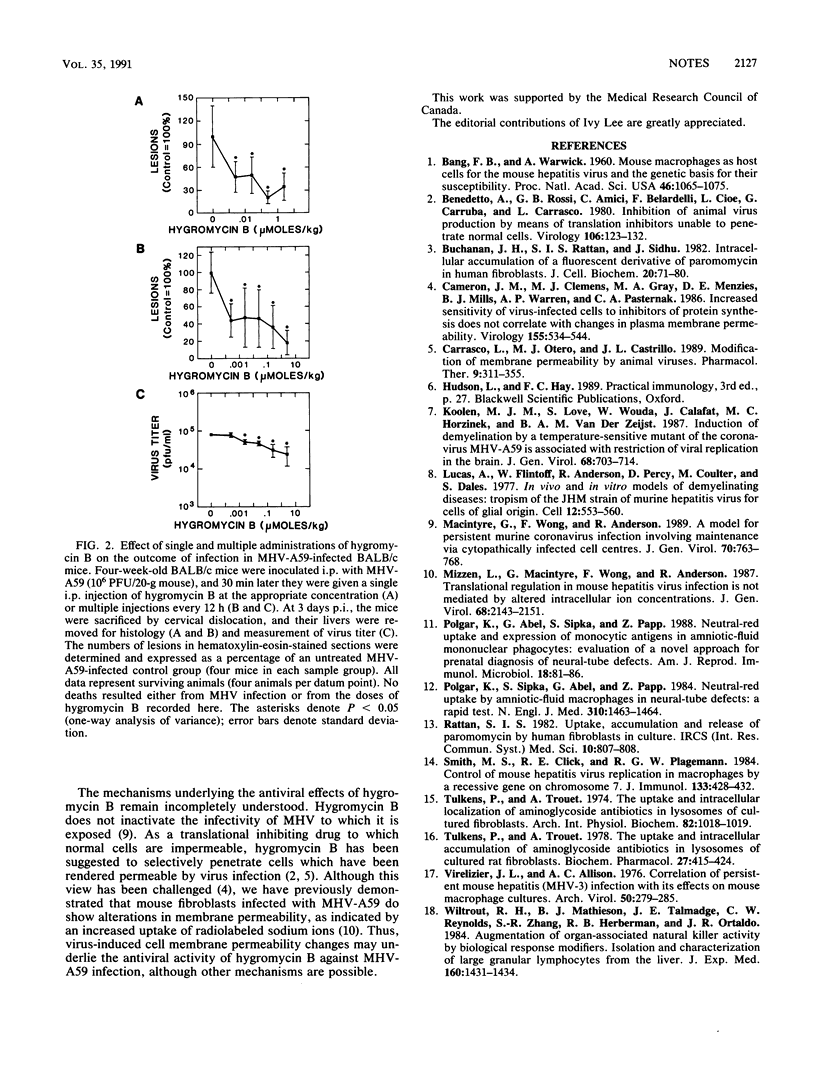
Hepatitis caused by mouse hepatitis virus (MHV-A59), a murine coronavirus, is accompanied by direct infection and replication of virus within the liver. We demonstrate here that the aminoglycoside hygromycin B is able to eliminate MHV-A59 infection from mouse peritoneal macrophages and cultured liver cells in vitro and is also able to reduce levels of virus replication and necrotic liver foci in vivo.
Full text
PDF
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bang F. B., Warwick A. MOUSE MACROPHAGES AS HOST CELLS FOR THE MOUSE HEPATITIS VIRUS AND THE GENETIC BASIS OF THEIR SUSCEPTIBILITY. Proc Natl Acad Sci U S A. 1960 Aug;46(8):1065–1075. doi: 10.1073/pnas.46.8.1065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benedetto A., Rossi G. B., Amici C., Belardelli F., Cioè L., Carruba G., Carrasco L. Inhibition of animal virus production by means of translation inhibitors unable to penetrate normal cells. Virology. 1980 Oct 15;106(1):123–132. doi: 10.1016/0042-6822(80)90227-5. [DOI] [PubMed] [Google Scholar]
- Buchanan J. H., Rattan S. I., Stevens A., Holliday R. Intracellular accumulation of a fluorescent derivative of paromomycin in human fibroblasts. J Cell Biochem. 1982;20(1):71–80. doi: 10.1002/jcb.240200108. [DOI] [PubMed] [Google Scholar]
- Cameron J. M., Clemens M. J., Gray M. A., Menzies D. E., Mills B. J., Warren A. P., Pasternak C. A. Increased sensitivity of virus-infected cells to inhibitors of protein synthesis does not correlate with changes in plasma membrane permeability. Virology. 1986 Dec;155(2):534–544. doi: 10.1016/0042-6822(86)90214-x. [DOI] [PubMed] [Google Scholar]
- Carrasco L., Smith A. E. Molecular biology of animal virus infection. Pharmacol Ther. 1980;9(3):311–355. doi: 10.1016/0163-7258(80)90022-4. [DOI] [PubMed] [Google Scholar]
- Koolen M. J., Love S., Wouda W., Calafat J., Horzinek M. C., van der Zeijst B. A. Induction of demyelination by a temperature-sensitive mutant of the coronavirus MHV-A59 is associated with restriction of viral replication in the brain. J Gen Virol. 1987 Mar;68(Pt 3):703–714. doi: 10.1099/0022-1317-68-3-703. [DOI] [PubMed] [Google Scholar]
- Lucas A., Flintoff W., Anderson R., Percy D., Coulter M., Dales S. In vivo and in vitro models of demyelinating diseases: tropism of the JHM strain of murine hepatitis virus for cells of glial origin. Cell. 1977 Oct;12(2):553–560. doi: 10.1016/0092-8674(77)90131-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macintyre G., Wong F., Anderson R. A model for persistent murine coronavirus infection involving maintenance via cytopathically infected cell centres. J Gen Virol. 1989 Mar;70(Pt 3):763–768. doi: 10.1099/0022-1317-70-3-763. [DOI] [PubMed] [Google Scholar]
- Mizzen L., Macintyre G., Wong F., Anderson R. Translational regulation in mouse hepatitis virus infection is not mediated by altered intracellular ion concentrations. J Gen Virol. 1987 Aug;68(Pt 8):2143–2151. doi: 10.1099/0022-1317-68-8-2143. [DOI] [PubMed] [Google Scholar]
- Polgár K., Abel G., Sipka S., Papp Z. Neutral-red uptake and expression of monocytic antigens in amniotic-fluid mononuclear phagocytes: evaluation of a novel approach for prenatal diagnosis of neural-tube defects. Am J Reprod Immunol Microbiol. 1988 Nov;18(3):81–86. doi: 10.1111/j.1600-0897.1988.tb00240.x. [DOI] [PubMed] [Google Scholar]
- Polgár K., Sipka S., Abel G., Papp Z. Neutral-red uptake by amniotic-fluid macrophages in neural-tube defects: a rapid test. N Engl J Med. 1984 May 31;310(22):1463–1464. doi: 10.1056/nejm198405313102217. [DOI] [PubMed] [Google Scholar]
- Smith M. S., Click R. E., Plagemann P. G. Control of mouse hepatitis virus replication in macrophages by a recessive gene on chromosome 7. J Immunol. 1984 Jul;133(1):428–432. [PubMed] [Google Scholar]
- Tulkens P., Trouet A. The uptake and intracellular accumulation of aminoglycoside antibiotics in lysosomes of cultured rat fibroblasts. Biochem Pharmacol. 1978 Feb 15;27(4):415–424. doi: 10.1016/0006-2952(78)90370-2. [DOI] [PubMed] [Google Scholar]
- Tulkens P., Trouet A. Uptake and intracellular localization of kanamycin and gentamycin in the lysosomes of cultured fibroblasts. Arch Int Physiol Biochim. 1974 Dec;82(5):1018–1019. [PubMed] [Google Scholar]
- Virelizier J. L., Allison A. C. Correlation of persistent mouse hepatitis virus (MHV-3) infection with its effect on mouse macrophage cultures. Arch Virol. 1976;50(4):279–285. doi: 10.1007/BF01317953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiltrout R. H., Mathieson B. J., Talmadge J. E., Reynolds C. W., Zhang S. R., Herberman R. B., Ortaldo J. R. Augmentation of organ-associated natural killer activity by biological response modifiers. Isolation and characterization of large granular lymphocytes from the liver. J Exp Med. 1984 Nov 1;160(5):1431–1449. doi: 10.1084/jem.160.5.1431. [DOI] [PMC free article] [PubMed] [Google Scholar]


