Abstract
We have investigated the activity and function of mitogen-activated protein kinase (MAPK) during neural specification in Xenopus. Ectodermal MAPK activity increased between late blastula and midgastrula stages. At midgastrula, MAPK activity in both newly induced neural ectoderm and ectoderm overexpressing the anterior neural inducer noggin was 5-fold higher than in uninduced ectoderm. Overexpression of MAPK phosphatase-1 (MKP-1) in ectoderm inhibited MAPK activity and prevented neurectoderm-specific gene expression when the ectoderm was recombined with dorsal mesoderm or treated with fibroblast growth factor (FGF). Neurectoderm-specific gene expression was observed, however, in ectoderm overexpressing both noggin and MKP-1. To evaluate the role of MAPK in posterior regionalization, ectodermal isolates were treated with increasing concentrations of FGF and assayed for MAPK activity and neurectoderm-specific gene expression. Although induction of posterior neural ectoderm by FGF was accompanied by an elevation of MAPK activity, relative MAPK activity associated with posterior neural fate was no higher than that of ectoderm specified to adopt an anterior neural fate. Thus, increasingly posterior neural fates are not correlated with quantitative increases in MAPK activity. Because MAPK has been shown to down-regulate Smad1, MAPK may disrupt bone morphogenetic protein 4 (BMP-4) signaling during neural specification. Our results suggest that MAPK plays an essential role in the establishment of neural fate in vivo.
The initiation of neural development in vertebrates is governed by a combination of positive and negative signals that regulate commitment to neural fate and the establishment of anteroposterior neural pattern. Signals that induce and pattern the neural ectoderm are produced by the dorsal mesoderm (1). In amphibian embryos, several lines of evidence indicate that neural fate is repressed by bone morphogenetic protein 4 (BMP-4) and anterior neural specification occurs via a reduction in signaling through the BMP-4 receptor (2, 3). Disruption of BMP-4 signaling via prolonged dissociation (4–7) or overexpression of a dominant-negative activin receptor (8, 9) or a dominant-negative BMP-4 (10) leads to neural specification in isolated ectoderm. BMP-4 represses neural specification and induces epidermis in dissociated animal cap cells (11). Finally, the anterior neural inducers noggin (12) and chordin (13) act as BMP-4 antagonists, bringing about anterior neural specification via sequestration of BMP-4 (14, 15). Increasing evidence that disruption of BMP-4 signaling is insufficient to initiate neural development in other vertebrate embryos (16–18), however, suggests that additional signals regulating neural fate have yet to be identified.
Activation of the fibroblast growth factor (FGF) receptor-signaling pathway can also initiate neural development and the establishment of posterior neural identity. Treatment of dissociated gastrula ectoderm with FGF leads to neuronal differentiation (19) and the establishment of posterior neural fate (20). Moreover, FGF elicits posterior regionalization in presumptive anterior neural ectoderm (21) or ectoderm overexpressing noggin (22). Disruption of FGF signaling via overexpression of a dominant-negative FGF receptor (XFD) has produced inconsistent results, e.g., although XFD overexpression in ectoderm can inhibit neural induction by noggin or endogenous signals (23), uniform expression in germ-line transgenic embryos disrupts posterior regionalization (24), although neural specification is not affected (25). One explanation is that signaling pathways activated by exogenous FGF mediate the establishment of anteroposterior neural pattern; however, the endogenous signals activating these pathways are unknown (25).
Because FGF signaling is mediated largely by the mitogen-activated protein kinase (MAPK) pathway (26, 27), these studies suggest that MAPK may play a critical role in the specification of neural fate and anteroposterior pattern. In this paper, we show that overexpression of a MAPK antagonist prevents neural specification in response to endogenous signals. Moreover, quantitative increases in MAPK activity are not required for the establishment of increasingly posterior neural fates.
MATERIALS AND METHODS
Embryos, Microsurgery, and Microinjection.
Preparation of embryos, tissue isolations, and explant recombinations were performed as described (28). Stages refer to those of Nieuwkoop and Faber (29). Capped RNA was prepared by in vitro transcription by using a mMessage mMachine kit (Ambion, Austin, TX) and linearized DNA as a template. Embryos were microinjected in each cell at the 2-cell stage via pressure injection of approximately 10 nl per blastomere. Isolates of animal cap ectoderm were held in very low Ca2+, Mg2+ Ringer medium (VLCMR, ref. 22) before and during treatment with FGF or PD098059 (Calbiochem). PD098059 was administered in 0.1% dimethyl sulfoxide + 0.05% BSA; control isolates were treated with dimethyl sulfoxide + BSA alone. Cycloheximide was administered as described (30).
MAPK Assays.
Either 15 animal caps or 4 neural plates (approximately 100 μg of total protein) were lysed in 20 μl of kinase buffer (KB: 20 mM Hepes, pH 7.5/40 mM MgCl2/1 mM EGTA/1 mM dithiothreitol/80 mM glycerol 2-phosphate/2 mM phenylmethylsulfonyl fluoride (PMSF)/3 μg/ml leupeptin/50 mM NaF/1 mM sodium orthovanadate/1 μM microcystin/20 μg/ml aprotinin) and centrifuged at 15,300 × g for 20 min. A 5-μl aliquot of supernatant was diluted to 20 μl with KB containing 0.1 μg/ml substrate, 50 μM ATP, 0.225 μCi/ml [γ-32P]ATP). A 2.8-kDa peptide containing the consensus MAPK phosphorylation site from Xnf7 (31, 32) was used as a substrate. Reactions were incubated at 24°C for 12 min and then stopped with the addition of 20 μl of 2× SDS sample buffer. Proteins were separated on a 4.5% Tris:10% N-[tris(hydroxymethyl)]glycine (Tricine):17% polyacrylamide gel (33). The lower half of the gel was subjected to autoradiography, and the upper half was blotted and probed with anti-MAPK (generously provided by J. Maller, University of Colorado Health Sciences Center, Denver, CO and J. Ferrell, Stanford University School of Medicine, Palo Alto, CA) and visualized by chemiluminescent detection. The intensities of the MAPK and phosphorylated substrate bands were quantified by using scanning densitometry. The ratio of phosphorylated substrate to the amount of MAPK present in a sample provides a measure of the relative specific activity of MAPK.
Reverse Tanscription–PCR (RT-PCR) Assays.
RNA isolation and RT-PCR assays were performed as described in ref. 34 using the primers listed therein. PCR primers for XANF 1/2 produce an amplified fragment of 451 bases and use the following sequences: U, ACT GAC CTA CAA GAG AGA AC; D, AGT GCA TCA TTG TTC CAC AG.
RESULTS
We developed an assay for MAPK activity that can be used to measure activity in as little as 25 μg of protein from a cell lysate. The assay measures phosphorylation of a peptide that includes only the consensus MAPK phosphorylation site from Xenopus nuclear factor 7 (Xnf7; ref. 32). The ratio of the phosphorylated substrate and the amount of MAPK present in a single reaction serves as a measure of relative specific activity. This assay is linear over at least a 16-fold range (data not shown). Phosphorylation of this peptide is primarily caused by MAPK with a minor contribution from cyclin B/cdc2 kinase. Immunodepletion of MAPK and cyclin B/cdc2 from Xenopus egg extracts removes all kinase activity directed against this site (32).
As a control, we examined the effects of the MAP or extracellular-signal-regulated kinase (MEK) antagonist PD098059 (35) on relative MAPK activity in FGF-treated ectoderm (Fig. 1A). PD098059 sequesters unphosphorylated MEK, preventing its activation, but has no effect on the activity of phosphorylated MEK. To confirm that phosphorylation of the Xnf7 substrate peptide is caused by MAPK activity, isolates of gastrula ectoderm were exposed to 50 μM PD098059 before treatment with recombinant Xenopus basic FGF. Pretreatment of ectoderm with PD098059 before addition of FGF reduces the amount of phosphorylated substrate by nearly 90%. This result shows that this assay can be used as a conservative indicator of relative MAPK activity. Addition of PD098059 to lysates of FGF-treated ectoderm did not reduce the level of MAPK activity (data not shown).
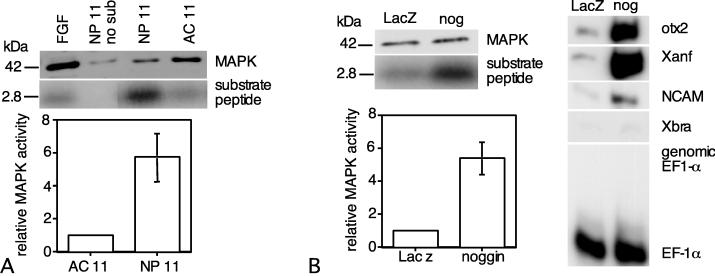
Figure 1.
Ectodermal MAPK activity before and during gastrulation. (A) Effects of PD098059 on FGF-induced MAPK activity. Animal caps were isolated at stage 8, aged in VLCMR until stage 11, and treated with 50 μM PD098059. Animal caps were pretreated with PD098059 in dimethyl sulfoxide or dimethyl sulfoxide alone for 30 min before addition of 15 ng/ml Xenopus bFGF + 0.5 mg/ml BSA. After 1 hr of bFGF treatment, the animal caps were lysed and assayed for phosphorylation of the Xnf7 substrate peptide. Pretreatment with PD098059 reduces the amount of substrate phosphorylated by lysates of FGF-treated ectoderm by approximately 90% (n = 4). (B) MAPK activity in ectoderm isolated from midblastula (stage 8) and early gastrula (stage 10) embryos and in neural ectoderm isolated at midgastrula (NP-11). Positive controls include thiophosphorylated MAPK (thio-phos MAPK) and midblastula animal cap ectoderm treated with 100 ng/ml bFGF (FGF). The lysate of FGF-treated ectoderm was also run in the absence of substrate (FGF-no sub). Autoradiograms in A and B show an immunoblot probed with anti-MAPK 42-kDa antibody (Upper) and an autoradiogram of the phosphorylated 2.8-kDa peptide (Lower). A ratio of the intensity of the phosphorylated substrate band to that of the MAPK protein provides a relative measure of MAPK specific activity. Relative specific activity for MAPK in ectoderm increases 6-fold between stage 8 and stage 10, with an additional increase during neural specification. (Mean ± SEM, n = 5).
We compared MAPK activity in ectoderm isolated at midblastula stage (stage 8), early gastrulation (stage 10), and midgastrulation (stage 11; see Fig. 1B). Animal cap ectoderm was isolated from stage 8 and stage 10 embryos, whereas presumptive neural ectoderm was isolated at stage 11. Ectodermal MAPK activity increases approximately 5-fold between late blastula and early gastrula stages. By midgastrula stage, MAPK activity in the neural ectoderm is approximately 7.5-fold higher than that observed in midblastula ectoderm. Equimolar mixing of the lysates from ectoderm at stages 8 and 11 produced levels of MAPK activity that were within 10% of the mean value for the individual lysates, indicating the absence of an inhibitor of MAPK activity that can act in vitro (data not shown).
Embryonic dissection leads to a spurious activation of MAPK, although this effect is significantly lower in the animal cap than in the marginal zone (36). Wound-induced activation can be avoided by dissecting tissues over ice. Because these experiments required dissection and prolonged culture before the MAPK assay, however, all dissections were performed at 23°C to preserve the viability of tissue isolates, and tissues were dissected carefully to avoid unnecessary wounding. The very low levels of MAPK activity observed in midblastula animal cap ectoderm suggest that any wound-induced MAPK activity has minimal effect on these assays.
To examine MAPK activity during neural specification, samples of newly induced neural ectoderm were isolated at stage 11 and assayed for MAPK activity together with animal cap ectoderm isolated at stage 8 and cultured until stage 11. MAPK activity is elevated >5-fold in newly specified neural ectoderm relative to uninduced ectoderm (Fig. 2A). Mixing of neural plate and aged animal cap lysates yielded a value for MAPK activity that was within 5% of the mean values of the two lysates (data not shown). These results indicate that whereas MAPK activity increases before gastrulation, endogenous neurectoderm-inducing signals are required to sustain elevated MAPK activity.
Figure 2.
MAPK activity increases during neural specification. (A) MAPK assays were performed on animal cap ectoderm isolated at stage 8 and cultured until midgastrulation (AC 11), and midgastrula neural-plate ectoderm (NP 11). MAPK activity is >5-fold higher in newly induced neural ectoderm than in uninduced ectoderm at the same stage. (B) Embryos were microinjected with 100 pg per embryo noggin or LacZ mRNA at the 2-cell stage. Animal caps were isolated at the midblastula stage and cultured until midgastrulation, when they were assayed for MAPK activity. MAPK activity is ≈5-fold higher in midgastrula ectoderm overexpressing noggin (nog) than in control ectoderm (LacZ) at this stage. The induction of anterior neural-gene expression in ectoderm overexpressing noggin was confirmed by RT-PCR. The assay for Xbra provides a way to detect mesoderm, whereas EF-1α serves as an indicator of RNA recovery and the presence of genomic DNA.
Similar experiments examined the effects of overexpression of the anterior neural inducer noggin on MAPK activity (Fig. 2B). Animal cap ectoderm was isolated at stage 8 from embryos injected with 100 pg of either noggin or LacZ mRNA. The ectodermal isolates were cultured until sibling control embryos reached stage 11 and then were assayed for MAPK activity. Additional isolates were cultured until control embryos reached stage 15–16 and then were assayed by using RT-PCR to verify the induction of neurectoderm-specific gene expression by noggin. MAPK activity in midgastrula ectoderm overexpressing noggin is 5-fold higher than that of ectoderm expressing LacZ. Because noggin is a BMP-4 antagonist (14), these results suggest that signaling through the BMP-4 receptor leads to an inhibition of MAPK activity.
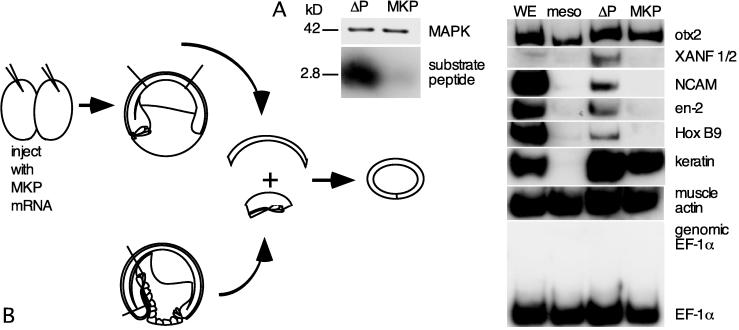
To investigate the role of MAPK in the response to neural induction, we overexpressed MKP-1 (37, 38) in ectoderm, recombined it with dorsal mesoderm, and examined neurectoderm-specific gene expression in the resulting recombinates (Fig. 3B). MAPKs are the principal substrate of MKP-1, although MKPs also act on the MAPK-related Jun N-terminal kinases (39). Embryos were injected with 4 ng of mRNA encoding either MKP-1 or a phosphatase-dead MKP-1 (ΔP-MKP-1). At late stage 9, the ectoderm was removed and recombined with involuted dorsal mesoderm from uninjected embryos at late stage 10.5 (28). Recombinates were cultured until sibling controls reached late neurulation (stage 18) and collected for RT-PCR. Additional embryos overexpressing MKP-1 or ΔP-MKP-1 were cultured until stage 10; animal cap ectoderm was then isolated and assayed for MAPK activity. MAPK activity was negligible in early gastrula ectoderm overexpressing MKP-1 (Fig. 3A). Ectoderm with exogenous MKP-1 mRNA did not express several neural genes, including NCAM, XANF 1/2, en-2, and Hox B9, in response to inductive signals produced by the dorsal mesoderm. The otx2 detected in the MKP recombinates reflects mesodermal otx2 expression. Epidermal keratin is strongly expressed in the MKP recombinates, attesting to the lack of nonspecific effects of MKP overexpression. All genes tested were expressed normally in recombinates prepared from ectoderm overexpressing ΔP-MKP-1. These findings indicate that neural specification in response to endogenous inductive signals requires MAPK or Jun N-terminal kinase.
Figure 3.
MAPK activity is required for neural induction by endogenous signals. (A) Ectoderm from embryos microinjected with 4 ng per embryo ΔMKP-1 or ΔP-MKP-1 mRNA was isolated at stage 10 and assayed for MAPK activity. Overexpression of MKP leads to a loss of MAPK activity. (B) Ectoderm from embryos overexpressing MKP-1 was isolated at stage 10 and recombined with involuted dorsal mesoderm from midgastrula embryos; recombinates were cultured until controls reached midneurulation (stage 15) and subjected to RT-PCR assays (n = 3).
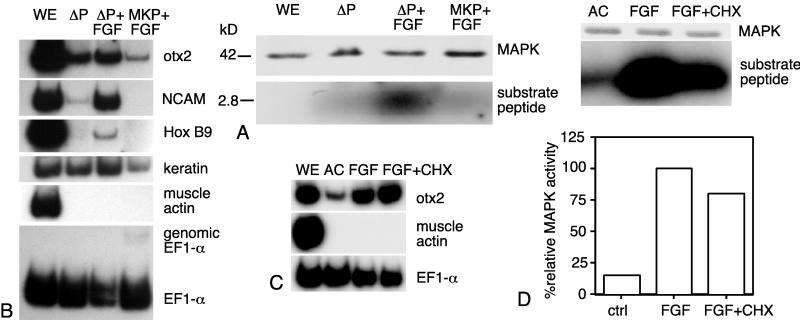
Additional experiments confirmed a role for MAPK in FGF-mediated neural induction. Animal cap ectoderm was isolated from midblastula embryos that had been microinjected with 4 ng of MKP-1 or ΔP-MKP-1 mRNA. Animal cap isolates were held in VLCMR until stage 10.5 (early midgastrula), when they were treated with 150 ng/ml Xenopus FGF. MAPK assays were performed after 1 hr (Fig. 4A); the remaining isolates were collected for RT-PCR when controls reached the mid-late neurula stage (Fig. 4B). MAPK activity in mixed lysates of FGF-treated ectoderm expressing MKP-1 or ΔP-MKP-1 was 14% higher than the mean MAPK activity of the individual lysates (data not shown). FGF induced expression of otx2, NCAM, and Hox B9 in ectoderm expressing ΔP-MKP-1. In FGF-treated ectoderm overexpressing MKP-1, however, otx2 expression was clearly reduced, and neither NCAM nor Hox B9 was detectable.
Figure 4.
MAPK activity is required for neural induction by bFGF. (A and B) Ectoderm from embryos microinjected with 4 ng of MKP-1 (WE) or ΔP-MKP-1 mRNA was isolated at stage 8 and treated with 150 ng/ml FGF at stage 10.5. At this stage, treatment with FGF does not elicit muscle actin expression. MAPK assays were performed after 1 hr (A), and gene expression was assayed by RT-PCR at stage 24 (B). (C and D) Ectoderm also was treated at stage 11 with 150 ng/ml FGF ± 5 μg/ml cycloheximide (CHX). Assays for gene expression (C) and MAPK activity (D) were performed after 1 hr. Ac juntreated animal cap ectoderm.
To determine whether the initial steps in FGF-mediated neural induction require protein synthesis, animal caps were isolated from midblastula embryos, treated for 1 hr at stage 11 with 150 ng/ml FGF ± 5 μg/ml cycloheximide, and collected for MAPK assays (Fig. 4C) and RT-PCR (Fig. 4D). MAPK activity in ectoderm treated with FGF plus cycloheximide was 80% that of ectoderm treated with FGF alone. The dramatic up-regulation of otx2 in response to FGF was largely unaffected by cycloheximide, indicating that protein synthesis is not required for the initiation of FGF-mediated neural induction.
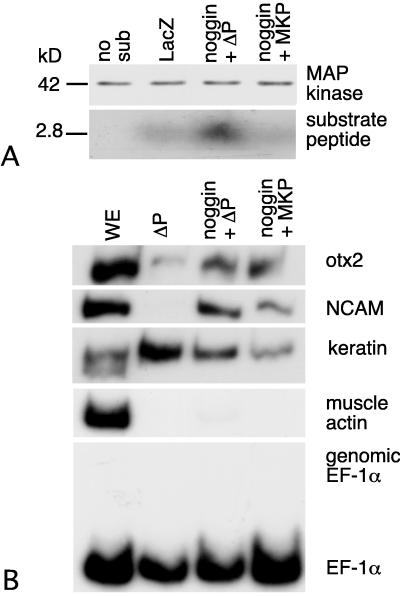
We examined the effects of MKP overexpression on neural specification in ectoderm overexpressing noggin. Animal caps from embryos coinjected with noggin and either MKP-1 or ΔP-MKP-1 mRNA were assayed for MAPK activity at stage 11 and for neurectoderm-specific gene expression at stage 13. MKP overexpresssion leads to a significant reduction in the MAPK activity of ectoderm expressing noggin (Fig. 5A). MAPK activity in combined lysates of ectoderm expressing noggin and either MKP-1 or ΔP-MKP-1 was within 5% of the mean MAPK activity of the individual lysates (data not shown). Overexpression of noggin and MKP-1 did not block the expression of anterior neural genes or the repression of epidermal keratin (Fig. 5B).
Figure 5.
MKP-1 does not prevent neurectoderm-specific gene expression in noggin-overexpressing ectoderm. Animal caps were isolated at stage 8 from embryos coinjected with 100 pg of noggin plus 4 ng of MKP-1 or ΔP-MKP mRNA. Half of them were assayed for MAPK activity when controls reached stage 11, and half of them were used for RT-PCR assays when controls reached stage 13. (A) Overexpression of MKP-1 decreases MAPK activity in expressing noggin-injected animal cap ectoderm. (B) Expression of NCAM and otx2 is not affected by MKP-1 (n = 6).
To determine whether MAPK activity quantitatively regulates anteroposterior pattern, animal caps were treated with 0.15–150 ng/ml Xenopus FGF at the early-midgastrula stage. MAPK activity was assessed 1 hr later (stage 11.5; Fig. 6A), and neurectoderm-specific gene expression was evaluated when controls reached stage 24 (Fig. 6B). Ectodermal isolates treated with 15 or 150 ng/ml FGF exhibited maximal levels of MAPK activity, a 4-fold increase over untreated controls. Whereas NCAM and otx2 were expressed in all FGF-treated isolates, Hox B9 was easily detected only in isolates treated with 15 or 150 ng/ml FGF. Although the threshold FGF concentration for activation of Hox B9 lies between 1.5 and 15 ng/ml, there was no significant difference in the MAPK activity at these concentrations. Moreover, the maximal MAPK activation was lower than that observed in midgastrula neural ectoderm or noggin-expressing ectoderm. This result suggests that the quantitative regulation of posterior neural fate in response to FGF does not occur via quantitative changes in the level of MAPK activity.
Figure 6.
MAPK and the induction of anteroposterior positional identity by bFGF. Animal caps were isolated at stage 8, held in VLCMR until intact control embryos reached stage 11, and treated with increasing concentrations of FGF. (A) Each sample was assayed for MAPK activity when control embryos reached stage 11. The maximal activation of MAPK observed over a range of 0.15 to 150 ng/ml was ≈4-fold. (B) The remainder were collected for RT-PCR assays when control embryos reached stage 24 (n = 6).
DISCUSSION
Our results indicate that a MAPK family member participates in neural specification during gastrulation. First, MAPK activity is elevated 5-fold in midgastrula neural ectoderm or noggin-overexpressing ectoderm in comparison with isolated uninduced ectoderm. Second, an increase in MAPK activity accompanies the activation of neurectoderm-specific gene expression in FGF-treated ectoderm. Third, overexpression of MKP-1 prevents the initiation of neurectoderm-specific gene expression in response to endogenous inductive signals or FGF. Although overexpression of MKP-1 had previously been shown to cause a wide range of abnormalities including defects in eye development (38), our findings specifically implicate MAPK or the related Jun N-terminal kinase in the response to neural induction.
In contrast, MKP-1 does not prevent anterior neural gene expression in ectoderm overexpressing noggin. This latter result has two implications. First, an increase in MAPK activity is not required to initiate anterior neural gene expression, although it may act to stabilize this transcriptional activation. Neural genes are not expressed in ectoderm expressing noggin or XFD after culture to postneurula stages (23). Second, MAPK may not be required for the initiation of anterior neural development under conditions in which the BMP-4 signaling pathway is already down-regulated, because mRNA-mediated overexpression of noggin should result in a precocious accumulation of noggin protein, inhibiting BMP-4. This parallels an earlier finding that disruption of FGF signaling affects the response to noggin more severely when noggin expression is delayed until after the midblastula transition (23). However, a possible function for MAPK in neural specification emerges from recent work showing that MAPK phosphorylates the BMP-4 transducer Smad1 (40), blocking its translocation to the nucleus. Thus, activation of MAPK in gastrula ectoderm may lead to the phosphorylation of Smad1 and the down-regulation of BMP-4 signals.
Previous studies have suggested that the establishment of posterior neural pattern occurs via quantitative increases in an unidentified regulator (41). Although retinoids (42, 43) and wnt 3a (44) have been implicated in the posterior regionalization of induced neural tissue, only FGF has been shown to act as both a direct neural inducer (19) and a posterior regionalizing factor (20–22). MAPK is a major transducer of signaling through the FGF receptor (26, 27), suggesting the hypothesis that FGF could elicit posterior regionalization via incremental increases in MAPK activity. Our results are inconsistent with this hypothesis for two reasons. First, the threshold FGF concentration that induces posterior neural gene expression does not elicit a greater increase in MAPK activity than that produced by subthreshold concentrations of FGF. Second, the increase in MAPK activity observed in response to overexpression of the anterior neural inducer noggin is greater than that occurring in response to FGF. Thus, although MAPK may participate in the establishment of posterior neural fate, it does not do so via incremental increases in activity that lead to increasingly posterior identities. Posterior regional identity may emerge via modulations in the duration of MAPK activation rather than the level of activity. Alternatively, downstream effectors other than MAPK may be responsible for the quantitative regulation of posterior neural fate in response to FGF.
The MAPK pathway is essential for the establishment of mesoderm in Xenopus. MAPK activity is strongly up-regulated in response to endogenous mesoderm-inducing signals (45). Expression of a constitutively active MEK leads to expression of the mesoderm-specific gene xbra (38, 46, 47). Overexpression of dominant-negative Ras, raf, or MEK blocks mesoderm induction by growth factors (48–50). Finally, overexpression of MKP-1 prevents expression of xbra in response to growth factors and disrupts posterior axial development (38, 46, 47). Although MAPK may regulate transcriptional activation of mesoderm-specific genes, it may also act to modulate BMP-4 signaling within the marginal zone.
Finally, our result that noggin overexpression leads to an elevation of MAPK activity suggests that MAPK is inhibited by signaling through the BMP-4 receptor. Transforming growth factor-β-family signaling pathways have been shown to regulate the MAPK signaling cascade in both positive and negative ways (51–53).
Because MAPK disrupts BMP-4 signaling by inhibiting the nuclear translocation of Smad1, our results lead to the speculation that the BMP-4 and MAPK pathways are mutually antagonistic in gastrula ectoderm. This antagonism could serve as a precondition for positive feedback during neural specification: an initial disruption in BMP-4 signaling caused by sequestration of BMP-4 by extracellular BMP-4 antagonists would lead to a rise in MAPK activity, which in turn further blocks BMP-4 signaling via its effects on Smad1. This antagonistic relationship between the signaling pathways could underlie many instances in which FGFs and BMPs have opposite effects in embryonic tissues, such as the positioning of tooth primordia (54) or the control of cell proliferation in the progress zone of the developing limb bud (55).
Acknowledgments
The authors thank M. Whitman and R. Harland for clones, J. Maller and J. Ferrell for MAPK antibodies, J. Kuang for thiophosphorylated MAPK, N. Buffinger for purified recombinant Xenopus bFGF, J. Gerhart for critical comments on the manuscript, and L. Etkin for suggestions. H.M.E. was supported by a National Institutes of Health training grant to the Department of Molecular Genetics, M. D. Anderson Cancer Center, University of Texas. This work was supported by the National Science Foundation and the Environmental Institute of Houston.
ABBREVIATIONS
- MAPK
mitogen-activated protein kinase
- VLCMR
very low Ca2+, Mg2+ Ringer
- MKP-1
MAP kinase phosphatase-1
- ΔP-MKP-1
phosphatase-inactive MKP-1
- FGF
fibroblast growth factor
- BMP-4
bone morphogenetic protein 4
- XFD
dominant-negative FGF receptor
- MEK
MAP or extracellular-signal-regulated kinase
References
- 1.Harland R, Gerhart J. Annu Rev Cell Biol. 1997;11:611–667. doi: 10.1146/annurev.cellbio.13.1.611. [DOI] [PubMed] [Google Scholar]
- 2.Wilson P A, Hemmati-Brivanlou A. Neuron. 1997;18:699–710. doi: 10.1016/s0896-6273(00)80311-6. [DOI] [PubMed] [Google Scholar]
- 3.Sasai Y, DeRobertis E M. Dev Biol. 1997;182:5–20. doi: 10.1006/dbio.1996.8445. [DOI] [PubMed] [Google Scholar]
- 4.Sato S, M, Sargent T D. Dev Biol. 1989;134:263–266. doi: 10.1016/0012-1606(89)90096-1. [DOI] [PubMed] [Google Scholar]
- 5.Godsave S F, Slack J M W. Dev Biol. 1989;134:486–490. doi: 10.1016/0012-1606(89)90122-x. [DOI] [PubMed] [Google Scholar]
- 6.Grunz H, Tacke L. Cell Differ Dev. 1989;28:211–218. doi: 10.1016/0922-3371(89)90006-3. [DOI] [PubMed] [Google Scholar]
- 7.Godsave S F, Slack J M W. Development (Cambridge, UK) 1991;111:523–530. doi: 10.1242/dev.111.2.523. [DOI] [PubMed] [Google Scholar]
- 8.Hemmati-Brivanlou A, Melton D A. Nature (London) 1992;359:609–614. doi: 10.1038/359609a0. [DOI] [PubMed] [Google Scholar]
- 9.Hemmati-Brivanlou A, Melton D A. Cell. 1994;77:273–282. doi: 10.1016/0092-8674(94)90319-0. [DOI] [PubMed] [Google Scholar]
- 10.Hawley S H B, Wunnenberg-Stapleton K, Hashimoto C, Laurent M N, Watabe T, Blumberg B W, Cho K W Y. Genes Dev. 1995;9:2923–2935. doi: 10.1101/gad.9.23.2923. [DOI] [PubMed] [Google Scholar]
- 11.Wilson P A, Hemmati-Brivanlou A. Nature (London) 1995;376:331–333. doi: 10.1038/376331a0. [DOI] [PubMed] [Google Scholar]
- 12.Lamb T M, Knecht A K, Smith W C, Stachel S E, Economides A N, Stahl N, Yancopolous G D, Harland R M. Science. 1993;262:713–718. doi: 10.1126/science.8235591. [DOI] [PubMed] [Google Scholar]
- 13.Sasai Y, Lu B, Steinbesser H, De Robertis E M. Nature (London) 1995;376:333–336. doi: 10.1038/376333a0. [DOI] [PubMed] [Google Scholar]
- 14.Zimmerman L B, DeJesús-Escobar J M, Harland R M. Cell. 1996;86:599–606. doi: 10.1016/s0092-8674(00)80133-6. [DOI] [PubMed] [Google Scholar]
- 15.Piccolo S, Sasai Y, Lu B, DeRobertis E M. Cell. 1996;86:589–598. doi: 10.1016/s0092-8674(00)80132-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Connolly D J, Patel K, Cooke J. Int J Dev Biol. 1997;41:389–396. [PubMed] [Google Scholar]
- 17.Schulte-Merker S, Lee K J, McMahon A P, Hammerschmidt M. Nature (London) 1997;387:862–863. doi: 10.1038/43092. [DOI] [PubMed] [Google Scholar]
- 18.Streit A, Lee K J, Woo I, Roberts C, Jessell T M, Stern C D. Development (Cambridge, UK) 1998;125:507–519. doi: 10.1242/dev.125.3.507. [DOI] [PubMed] [Google Scholar]
- 19.Kengaku M, Okamoto H. Development (Cambridge, UK) 1993;119:1067–1078. doi: 10.1242/dev.119.4.1067. [DOI] [PubMed] [Google Scholar]
- 20.Kengaku M, Okamoto H. Development (Cambridge, UK) 1995;121:3121–3130. doi: 10.1242/dev.121.9.3121. [DOI] [PubMed] [Google Scholar]
- 21.Cox W G, Hemmati-Brivanlou A. Development (Cambridge, UK) 1995;121:4349–4347. doi: 10.1242/dev.121.12.4349. [DOI] [PubMed] [Google Scholar]
- 22.Lamb T M, Harland R M. Development (Cambridge, UK) 1995;121:3627–3636. doi: 10.1242/dev.121.11.3627. [DOI] [PubMed] [Google Scholar]
- 23.Launay C, Fromentoux V, Shi D-L, Boucaut J-C. Development (Cambridge, UK) 1996;122:869–880. doi: 10.1242/dev.122.3.869. [DOI] [PubMed] [Google Scholar]
- 24.Pownall M E, Isaacs H V, Slack J M W. Curr Biol. 1998;8:673–676. doi: 10.1016/s0960-9822(98)70257-x. [DOI] [PubMed] [Google Scholar]
- 25.Kroll K, Amaya E. Development (Cambridge, UK) 1996;122:3173–3183. doi: 10.1242/dev.122.10.3173. [DOI] [PubMed] [Google Scholar]
- 26.Blenis J. Proc Natl Acad Sci USA. 1993;90:5889–5892. doi: 10.1073/pnas.90.13.5889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Seger R, Krebs E G. FASEB J. 1995;9:726–735. [PubMed] [Google Scholar]
- 28.Sater A K, Steinhardt R A, Keller R. Dev Dyn. 1993;197:268–280. doi: 10.1002/aja.1001970405. [DOI] [PubMed] [Google Scholar]
- 29.Nieuwkoop P D, Faber J. Normal table of Xenopus laevis (Daudin) Amsterdam: North–Holland; 1967. [Google Scholar]
- 30.Cascio S, Gurdon J B. Development (Cambridge, UK) 1987;100:297–305. doi: 10.1242/dev.100.2.297. [DOI] [PubMed] [Google Scholar]
- 31.Reddy B A, Kloc M, Etkin L. Dev Biol. 1991;148:107–116. doi: 10.1016/0012-1606(91)90321-s. [DOI] [PubMed] [Google Scholar]
- 32.El-Hodiri H M, Che S, Nelman-Gonzalez M, Kuang J, Etkin L D. J Biol Chem. 1997;272:20463–20470. doi: 10.1074/jbc.272.33.20463. [DOI] [PubMed] [Google Scholar]
- 33.Schagger H, von Jagow G. Anal Biochem. 1987;166:368–379. doi: 10.1016/0003-2697(87)90587-2. [DOI] [PubMed] [Google Scholar]
- 34.Uzman J A, Patil S, Uzgare A R, Sater A K. Dev Biol. 1998;193:10–20. doi: 10.1006/dbio.1997.8782. [DOI] [PubMed] [Google Scholar]
- 35.Alessi D R, Cuenda A, Cohen P, Dudley D T, Saltie A R. J Biol Chem. 1995;270:27489–27494. doi: 10.1074/jbc.270.46.27489. [DOI] [PubMed] [Google Scholar]
- 36.LaBonne C, Whitman M. Dev Biol. 1997;183:9–20. doi: 10.1006/dbio.1996.8497. [DOI] [PubMed] [Google Scholar]
- 37.Sun H, Charles C, Lau L, Tonks N K. Cell. 1993;75:487–493. doi: 10.1016/0092-8674(93)90383-2. [DOI] [PubMed] [Google Scholar]
- 38.LaBonne C, Burke B, Whitman M. Development (Cambridge, UK) 1995;121:1475–1486. doi: 10.1242/dev.121.5.1475. [DOI] [PubMed] [Google Scholar]
- 39.Hirsch D D, Stork P J. J Biol Chem. 1997;272:4568–4575. doi: 10.1074/jbc.272.7.4568. [DOI] [PubMed] [Google Scholar]
- 40.Kretzschmar M, Doody J, Massagué J. Nature (London) 1997;389:618–622. doi: 10.1038/39348. [DOI] [PubMed] [Google Scholar]
- 41.Doniach T, Musci T J. Mech Dev. 1995;53:403–413. doi: 10.1016/0925-4773(95)00457-2. [DOI] [PubMed] [Google Scholar]
- 42.Ruiz i Altaba A, Jessell T M. Genes Dev. 1991;5:175–187. doi: 10.1101/gad.5.2.175. [DOI] [PubMed] [Google Scholar]
- 43.Papalopulu N, Clarke J D W, Bradley L, Wilkinson D, Krumlauf R, Holder N. Development (Cambridge, UK) 1991;113:1145–1158. doi: 10.1242/dev.113.4.1145. [DOI] [PubMed] [Google Scholar]
- 44.McGrew L L, Lai C J, Moon R T. Dev Biol. 1995;172:337–342. doi: 10.1006/dbio.1995.0027. [DOI] [PubMed] [Google Scholar]
- 45.Hartley R S, Lewellyn A L, Maller J L. Dev Biol. 1994;163:521–524. doi: 10.1006/dbio.1994.1168. [DOI] [PubMed] [Google Scholar]
- 46.Umbhauer M, Marshall C J, Mason C S, Old R W, Smith J C. Nature (London) 1995;376:58–62. doi: 10.1038/376058a0. [DOI] [PubMed] [Google Scholar]
- 47.Gotoh Y, Masuyama N, Suzuki A, Ueno N, Nishida E. EMBO J. 1995;14:2491–2498. doi: 10.1002/j.1460-2075.1995.tb07246.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.LaBonne C, Whitman M. Development (Cambridge, UK) 1994;120:463–472. doi: 10.1242/dev.120.2.463. [DOI] [PubMed] [Google Scholar]
- 49.Cornell R A, Kimelman D. Development (Cambridge, UK) 1994;120:453–462. doi: 10.1242/dev.120.2.453. [DOI] [PubMed] [Google Scholar]
- 50.MacNicol A M, Muslin A J, Williams L T. Cell. 1993;73:571–584. doi: 10.1016/0092-8674(93)90143-e. [DOI] [PubMed] [Google Scholar]
- 51.Yamaguchi K, Shirakabe K, Shibuya H, Irie K, Oshi I, Ueno N, Taniguchi T, Nishida E, Matsumoto K. Science. 1995;270:2008–2011. doi: 10.1126/science.270.5244.2008. [DOI] [PubMed] [Google Scholar]
- 52.Pazdrak K, Justement L, Alam R. J Immunol. 1995;155:4454–4458. [PubMed] [Google Scholar]
- 53.King A G, Ozanne B W, Smythe C, Ashworthe A. Oncogene. 1995;11:2553–2563. [PubMed] [Google Scholar]
- 54.Neubuser A, Peters H, Balling R, Martin G R. Cell. 1997;90:247–255. doi: 10.1016/s0092-8674(00)80333-5. [DOI] [PubMed] [Google Scholar]
- 55.Niswander L, Martin G R. Nature (London) 1993;361:68–71. doi: 10.1038/361068a0. [DOI] [PubMed] [Google Scholar]