Abstract
Rationale: Efficient removal of apoptotic cells is essential for the resolution of acute pulmonary inflammation. Alveolar macrophages ingest apoptotic cells less avidly than other professional phagocytes at rest but overcome this defect during acute inflammation. Surfactant protein (SP)-A and SP-D are potent modulators of macrophage function and may suppress clearance of apoptotic cells through activation of the transmembrane receptor signal inhibitory regulatory protein α (SIRPα).
Objectives: To investigate whether binding of SP-A and SP-D to SIRPα on alveolar macrophages suppresses apoptotic cell clearance.
Methods: Phagocytosis of apoptotic cells was assessed using macrophages pretreated with SP-A, SP-D, or the collectin-like molecule C1q. Binding of SP-A and SP-D to SIRPα was confirmed in vitro using blocking antibodies and fibroblasts transfected with active and mutant SIRPα. The effects of downstream molecules SHP-1 and RhoA on phagocytosis were studied using SHP-1–deficient mice, sodium stibogluconate, and a Rho kinase inhibitor. Lipopolysaccharide was given to chimeric mice to study the effects of SP-A and SP-D binding on inflammatory macrophages.
Measurements and Main Results: Preincubation of macrophages with SP-A or SP-D suppressed apoptotic cell clearance. Surfactant suppression of macrophage phagocytosis was reversed by blocking SIRPα and inhibiting downstream molecules SHP-1 and RhoA. Macrophages from inflamed lungs ingested apoptotic cells more efficiently than resting alveolar macrophages. Recruited mononuclear phagocytes with low levels of SP-A and SP-D mediated this effect.
Conclusions: SP-A and SP-D tonically inhibit alveolar macrophage phagocytosis by binding SIRPα. During acute pulmonary inflammation, defects in apoptotic cell clearance are overcome by recruited mononuclear phagocytes.
Keywords: macrophage, phagocytosis, cell surface molecules, inflammation, lung
AT A GLANCE COMMENTARY
Scientific Knowledge on the Subject
Apoptotic cell clearance is necessary for resolution of inflammation yet several studies show that resting alveolar macrophages ingest apoptotic cells poorly. Mechanisms to explain improved macrophage phagocytosis during inflammation are incomplete.
What This Study Adds to the Field
In the absence of inflammation, surfactant proteins A and D suppress alveolar macrophage phagocytosis. During acute inflammation, defects in apoptotic cell clearance are overcome by recruited mononuclear phagocytes.
Apoptotic cell clearance is essential for maintenance of lung structure and function. Timely removal of dead or dying cells prevents the release of their potentially toxic intracellular contents and promotes the production of antiinflammatory mediators, antiproteases, and growth factors from the phagocyte (1–3). Effective apoptotic cell removal is essential for the resolution of acute lung injury and hastens tissue repair (4–6). Conversely, impaired clearance of apoptotic cells renders the lung susceptible to chronic inflammation and contributes to the pathogenesis of pulmonary diseases, including cystic fibrosis, bronchiectasis, chronic obstructive pulmonary disease (COPD), and asthma (7–9).
The alveolar macrophage (AM) is considered to be the primary phagocyte in the lung. However, compared with other professional phagocytes, unstimulated AMs clear apoptotic cells poorly both in vivo and in vitro (10–12). During acute inflammation, this defect is overcome, enabling AMs to have a phagocytic capacity similar to that of other professional phagocytes (10). The intriguing hypothesis therefore arises that the normal environment of the lung suppresses phagocytosis, whereas the environment of an inflamed lung promotes apoptotic cell clearance.
This paradigm may reflect the unique environment in which the AM exists. The alveolus constitutes an aerobic environment in which oxygen partial pressures reach 100 mm Hg. In contrast, other macrophages (e.g., peritoneal macrophages [PMs]) may exist in anaerobic environments in which oxygen partial pressures measure 5 mm Hg. The alveolar macrophage is also bathed in fluid that contains high levels of surfactant proteins. Surfactant protein (SP)-A and SP-D in particular play an important role in immune modulation (13–15).
SP-A and SP-D are members of the collectin family of molecules that function as components of the innate immune system by recognizing pathogen-associated molecular patterns (PAMPs) on microorganisms. Collectins are comprised of N-terminal collagen-like domains linked to C-terminal lectin or carbohydrate recognition domains. Collectin monomers are highly oligomerized, first into trimers and then further to form complex sertiform or cruciate structures. SP-A and SP-D contribute to the innate immunologic response by facilitating removal of pathogens and enhancing the proinflammatory response to infection (14, 16). Conversely, other reports suggest that SP-A and SP-D inhibit proinflammatory mediator production by macrophages (13, 15). We have recently attempted to clarify this paradox by demonstrating that the orientation by which SP-A and SP-D bind to effector cells dictates their pro- or antiinflammatory potential (17). For example, ligation of the N-terminal collagen domains with the calreticulin/CD91 receptor complex is proinflammatory, whereas binding of the C-terminal heads to signal inhibitory regulatory protein α (SIRPα) prevents inflammation. We hypothesize that phagocytosis may be similarly regulated.
SP-A and SP-D have been shown to opsonize apoptotic cells in vitro and to facilitate their phagocytosis by resting AMs (18–20). This effect has been confirmed in vivo and is mediated through CD91 and calreticulin on the phagocyte surface (20). An opposing effect may be seen when SIRPα is activated by SP-A or SP-D. Ligation of this receptor blocks Fcγ receptor and complement-mediated cellular phagocytosis (21, 22). Thus, in the naive lung, binding of SP-A and SP-D to SIRPα on the AM may tonically inhibit apoptotic cell engulfment, resulting in the inefficient uptake observed for these cells. During inflammation, this inhibitory effect may be lifted to facilitate apoptotic cell removal. Herein, we describe the effects of SP-A and SP-D on AM uptake of apoptotic cells during resting and inflammatory conditions.
METHODS
Detailed methods are provided in the online supplement.
Human and Animal Experimentation
This study was approved by and performed in accordance with the ethical standards of the National Jewish Medical and Research Center Institutional Review Board and the Institutional Animal Care and Use Committees at the University of Colorado Health Sciences Center and National Jewish Medical and Research Center.
Animals
Institute for Cancer Research mice (Taconic, Germantown, NY) were used in all murine experiments unless otherwise indicated. SP-D knockout mice were a kind gift from Dr. James Fisher (23). Wild-type C57BL/6 mice, SHP-1−/− (Ptpn6mev/Ptpn6mev) mice, and green fluorescent protein (GFP)–expressing mice (C57BL/6-Tg [UBC-GFP] 30Scha/J) were obtained from Jackson Laboratory (Bar Harbor, ME). Additional details regarding these mouse strains and generation of GFP-expressing chimeric mice can be found in the online supplement.
Isolation and Purification of Human Collectins
SP-A was isolated from whole lung lavage fluid taken from patients with pulmonary alveolar proteinosis and stripped of LPS, as previously described (24). Human SP-D was isolated from transfected Chinese hamster ovary cells (17). Harvested medium was purified and stripped of LPS. Purified human C1q (10 μg/ml) was obtained from Quidel Corp. (San Diego, CA).
Isolation of Primary Cells
Human AMs were isolated by bronchoalveolar lavage (BAL) from normal volunteers (20). Human neutrophils were obtained from healthy donors and isolated via density centrifugation (25). Mouse AMs were isolated by lavage with phosphate-buffered saline (PBS) containing100 μM ethylenediaminetetraacetic acid. For experiments in which AMs were isolated from LPS-treated mice, purification was performed using density centrifugation (26). Specific details can be found in the online supplement.
Cell Culture and Transient Transfection
Murine J774A.1 macrophages (American Type Culture Collection, Manassas, VA) and Swiss 3T3 fibroblasts (a gift from Dr. A. J. Ridley, Ludwig Institute, London) were cultured in Dulbecco's modified Eagle medium supplemented with 10% fetal bovine serum plus penicillin-streptomycin-glutamine and incubated at 37°C in 10% CO2. Human Jurkat T cells were obtained from American Type Culture Collection (Manassas, VA) and were cultured in RPMI 1640 with 10% fetal bovine serum supplemented with penicillin-streptomycin-glutamine, and incubated at 37°C in 5% CO2. Transient cell transfection of Swiss 3T3 cells with SIRPα constructs was performed using Lipofectamine Plus reagent (Invitrogen, Carlsbad, CA) as detailed in the online supplement.
In Vitro Phagocytosis Assays
Human and murine AMs, murine PMs, and J774 A.1 macrophages were plated in 24-well plates at a concentration of 5 × 105 cells/well. After overnight culture, nonadherent cells were gently washed off and either fresh medium or medium containing apoptotic target cells were added. For collectin experiments, SP-A (10 μg/ml), SP-D (10 μg/ml), C1q (10 μg/ml), or CD47-Fc ligand (10 μg/ml) was added for 20 minutes. The cell culture supernatant was then removed, adherent macrophages were gently washed with medium, and apoptotic cells were added at a 10:1 ratio for 90 minutes. Samples were washed before Wright's Giemsa staining to remove uningested apoptotic cells. Phagocytosis was quantified using the phagocytic index previously described (27). For SHP inhibitor studies, sodium stibogluconate was added for 20 minutes at a concentration of 100 μg/ml. For Rho kinase inhibitor studies, Y27632 was added 20 minutes before the addition of the surfactant proteins at a concentration of 10 μM.
In Vivo Phagocytosis Assays
Mice were anesthetized with Avertin (Sigma-Aldrich, St. Louis, MO) after which 10 × 106 apoptotic thymocytes were instilled intratracheally in a volume of 50 μl PBS. Sixty minutes later, BAL was performed. Lavage cells were fixed and stained with modified Wright's Giemsa stain. Phagocytosis was determined by visual inspection and was expressed as a phagocytic index as previously described (27). For Rho kinase inhibitor studies, Y27632 was administered by gavage 4 hours before apoptotic cell instillation at a dose of 10 mg/kg.
Competitive Binding Experiments
To distinguish cultured neutrophils from macrophages with flow cytometry, J774 macrophages were labeled with PKH26-PCL fluorescent dye (Sigma-Aldrich) as per the manufacturer's instructions. Macrophages were washed in Dulbecco's modified Eagle medium with 10% fetal calf serum twice to remove excess dye. SP-D or SP-A was labeled with fluorescein isothiocyanate (Sigma-Aldrich) as per the manufacturer's instructions and then added directly to macrophages alone, viable neutrophils alone, apoptotic neutrophils alone, or to 1:1 mixtures containing neutrophils and macrophages for 30 minutes on ice. Samples were washed, fixed and analyzed by flow cytometry.
Statistics
Data are presented as mean ± SEM of at least three independent experiments. Statistical analysis was performed using two-tailed Student's t test of unpaired samples. For multiple comparisons, data were evaluated by analysis of variance with post hoc analysis by the two-tailed Dunnett test. Significant differences were defined as P < 0.05.
RESULTS
SP-A and SP-D Suppress AM Phagocytosis of Apoptotic Cells
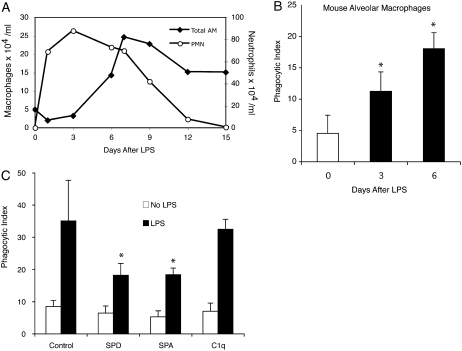
To confirm that resting AMs ingest apoptotic cells less efficiently than other phagocytes, murine alveolar and peritoneal macrophages were coincubated with apoptotic cells (Figure 1A). As shown, the phagocytic ability of AMs was significantly less than that of PMs.
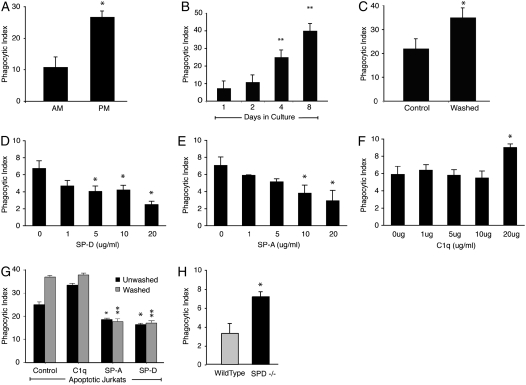
Figure 1.
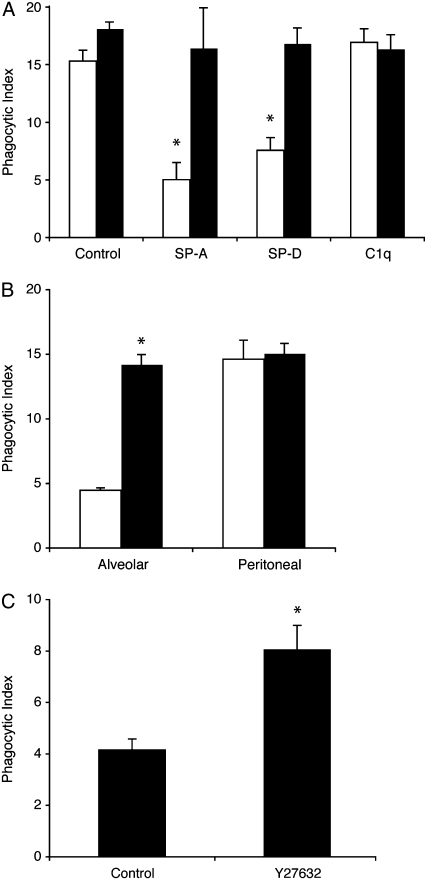
Lung collectins contribute to tonic suppression of alveolar macrophages (AMs). (A) Murine AMs and peritoneal macrophages (PMs) were isolated from Institute for Cancer Research mice and cocultured with apoptotic Jurkat T cells for 1 hour. *Significantly different from AMs (P < 0.05). (B) Murine AMs were cultured for the times indicated and then coincubated with apoptotic Jurkat T cells. **Significantly different from Day 1 phagocytic index (P < 0.05). (C) Human AMs were cultured directly (control) or after washing with phosphate-buffered saline (PBS). Washed and unwashed cells were incubated overnight. Phagocytosis was assessed after a 90-minute coculture with apoptotic human neutrophils. *Significantly different from unwashed AMs (P < 0.05). (D–F) Dose–response curves for the lung collectins were created by adding surfactant protein (SP)-D, SP-A, and C1q to cultured J774 macrophages at the indicated concentrations for 20 minutes. The cells were gently washed to remove unbound protein, and then fresh medium containing apoptotic Jurkat T cells was added. Phagocytosis was assessed 90 minutes later. *P < 0.05 versus control. (G) Human AMs were cultured directly after isolation or after washing with PBS. SP-A, SP-D, or C1q (10 μg/ml) was added for 20 minutes, media were removed, and fresh media containing viable or apoptotic Jurkat T cells were added. Phagocytosis was significantly reduced by SP-A and SP-D for washed (*P < 0.05) and unwashed cells (**P < 0.05) versus control. (H) Mouse AMs were isolated from wild-type or SP-D–deficient mice. The macrophages were plated overnight and then fed apoptotic Jurkat T cells for 90 minutes. *P < 0.05.
We hypothesized that the alveolar environment contributes to tonic AM suppression and that removal of AMs from the lungs would restore phagocytic function. To test this, we isolated murine AMs and cultured them for extended periods of time. The ability of cultured AMs to ingest apoptotic cells increased significantly after 4 days of culture, and this was further increased at 8 days (Figure 1B). As a second method of separating AMs from the effects of the alveolar environment, we isolated human AMs and cultured them directly or after vigorous washing. Washed AMs exhibited enhanced uptake of apoptotic Jurkat T cells (Figure 1C), suggesting removal of a soluble or easily dissociated factor.
The lung contains high levels of surfactant proteins. We therefore sought to determine if SP-A or SP-D could suppress the phagocytic function of collectin-naive macrophages. J774 mouse macrophages were incubated with SP-A, SP-D, or C1q at concentrations ranging from 1 to 20 μg/ml for 20 minutes. Unbound protein was gently washed away, and apoptotic cells were added (Figures 1D–1F). SP-D suppressed phagocytosis at concentrations of 5 μg/ml and greater. SP-A exhibited similar effects at concentrations of 10 and 20 μg/ml. The collectin-like protein C1q, whose globular head region does not bind to SIRPα (17), enhanced phagocytosis at a concentration of 20 μg/ml but had no effect at lower levels. Concentrations of 10 μg/ml were used for all subsequent experiments.
To determine if the lung collectins could also suppress AM phagocytosis, SP-A and SP-D were added to both washed and unwashed AMs before coincubation with apoptotic cells. For unwashed AMs, both SP-A and SP-D suppressed the phagocytic index to a level below control (Figure 1G). Washed AMs exhibited a higher phagocytic index than unwashed AMs at baseline but had equivalent suppression of phagocytosis once SP-A or SP-D was added. C1q exerted no effect.
SP-D–deficient mice have no detectable SP-D and reduced levels of SP-A (23). We therefore hypothesized that AMs from these mice would escape tonic suppression. To test this, AMs were obtained by BAL, cultured in vitro, and coincubated with apoptotic cells. As expected, AMs from SP-D–deficient mice had a significantly enhanced phagocytic index compared with AMs from wild-type mice (Figure 1H), again suggesting that lung collectins tonically inhibit AM phagocytic function.
SP-D–deficient mice develop chronic low-grade pulmonary inflammation, age-dependent emphysema, and pulmonary fibrosis (23). Increased numbers of apoptotic cells have also been reported in their airways (28). To address whether the enhanced phagocytic ingestions we observed in our ex vivo experiments resulted from in situ phagocytosis of endogenous airway cells, we performed BAL and analyzed the cells with light microscopy. AMs from SP-D–deficient mice were foamy in their appearance and greater in number than AMs lavaged from wild-type mice but contained no observable apoptotic bodies. Staining with annexin V and propidium iodide detected very few apoptotic cells in BAL from our SP-D–deficient mice and from wild-type control animals (data not shown).
SP-A and SP-D Serve Dual Roles in Regulation of Apoptotic Cell Phagocytosis
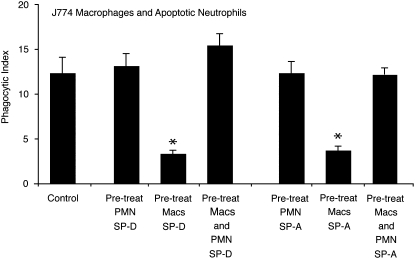
Several groups, including our own, have demonstrated that SP-A and SP-D can opsonize apoptotic cells and enhance their removal (18–20). This effect is mediated by a common CD91/calreticulin complex on the phagocyte surface. Because this result is in direct contrast to our data that show collectins suppress macrophage function, we sought to determine the dominant effect by adding collectins to either J774 macrophages or apoptotic neutrophils before performing phagocytosis assays (Figure 2). As with our previous experiments, pretreating macrophages with SP-A or SP-D significantly reduced phagocytosis. Pretreating apoptotic neutrophils with collectins did not enhance the phagocytic index above control levels but did serve to offset macrophage suppression. We suggest that this latter circumstance most closely approximates in vivo conditions in the alveolus in which collectin-opsonized apoptotic cells are the most common targets for collectin-suppressed AMs.
Figure 2.
Lung collectins serve dual regulatory roles in macrophage clearance of apoptotic cells. J774A.1 macrophages or apoptotic human neutrophils (PMN) were coincubated with SP-A or SP-D (10 μg/ml) for 20 minutes and then washed to remove excess, unbound collectins. Phagocytosis assays were performed after pairing: (1) collectin-naive macrophages + collectin-treated PMN, (2) collectin-treated macrophages + collectin-naive apoptotic PMN, or (3) collectin-treated macrophages + collectin-treated PMN. The control group is composed of collectin-naive macrophages and apoptotic neutrophils. Graphs represent the mean phagocytic index ± SEM of three independent experiments (*P < 0.05).
Inhibition of Apoptotic Cell Uptake by Surfactant Proteins Requires Active SIRPα
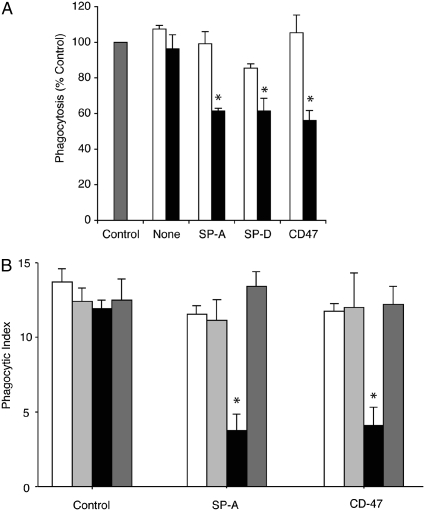
We have previously shown that surfactant proteins can bind to SIRPα (17). Others have shown that ligation of SIRPα by CD47 results in decreased clearance of C3 and IgG opsonized cells (21, 22). To confirm that the inhibitory effects of the lung collectins occur through SIRPα, J774 macrophages were treated with a blocking antibody to SIRPα before the addition of SP-A, SP-D, or CD47-Fc ligand (Figure 3A). As anticipated, SP-A and SP-D failed to reduce apoptotic cell ingestion in macrophages pretreated with the anti-SIRPα blocking antibody. In contrast, SP-A and SP-D significantly suppressed phagocytosis of control macrophages treated with a rat IgG isotype, suggesting that SP-A and SP-D mediate their suppressive effect through SIRPα.
Figure 3.
Signal inhibitory regulatory protein α (SIRPα) is required for collectin-mediated suppression of phagocytosis. (A) J774A.1 macrophages were incubated with a SIRPα blocking antibody or a rat IgG1 isotype control for 30 minutes before the addition of surfactant protein (SP)-A or SP-D (10 μg/ml). Excess media were removed and fresh media containing apoptotic Jurkat T cells were added for 90 minutes, then phagocytosis was assessed. Open bars, anti-SIRP antibody; solid bars, rat IgG isotype; shaded bar, control. *Significantly different from control (P < 0.05). (B) Swiss 3T3 fibroblasts were transiently transfected with an empty vector, an active SIRPα vector, or an inactive SIRPα construct. Forty-eight hours after transfection, culture media were changed and SP-A or CD47-Fc ligand (10 μg/ml) was added for 20 minutes. Cells were gently washed once and then apoptotic cells were added. Phagocytosis of apoptotic cells was only decreased in cells transfected with the active SIRPα construct and exposed to SP-A or CD47-Fc ligand (*P < 0.01). Results are expressed as the phagocytic index ± SEM for three separate experiments. Open bars, control; light shaded bars, empty vector; solid bars, SIRP; dark shaded bars, inactive SIRP.
To further verify that the pulmonary collectins inhibit apoptotic cell removal via SIRPα activation, we transfected Swiss 3T3 fibroblasts (a cell line that does not contain SIRPα de novo) with intact SIRPα or a mutant form in which all four tyrosines in the intracellular domain had been substituted with asparagine (29). SP-A and soluble CD47-Fc ligand, a known binding partner of SIRPα, significantly reduced apoptotic cell uptake in cells transfected with the intact SIRPα (Figure 3B). In contrast, SP-A and CD47-Fc ligand did not suppress phagocytosis in cells transfected with an empty vector or the altered, inactive SIRPα.
AM Suppression Is Mediated through SHPs
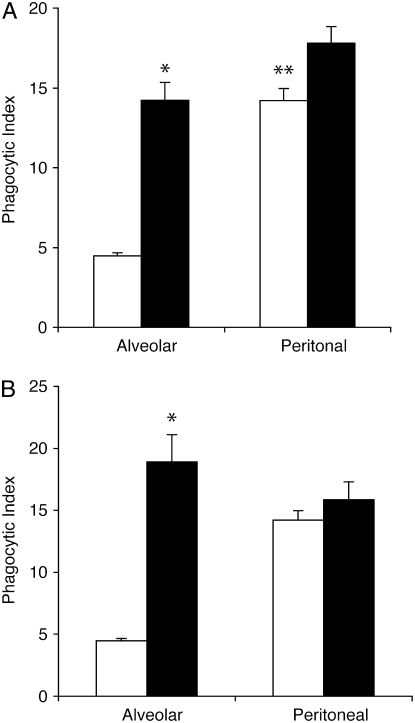
The cytoplasmic region of SIRPα contains two immunoreceptor tyrosine-based inhibitory motifs, which recruit and activate SHP-1 and SHP-2 (30). SHP-1 is the major phosphatase associated with SIRPα in macrophages (31), and its activation negatively regulates Fcγ receptor–mediated phagocytosis (32). We have previously demonstrated that SHP-1 is activated by SIRPα upon binding with SP-A or SP-D (17) and therefore sought to determine if SHP activation contributes to tonic suppression of AM phagocytosis. Murine AMs and PMs were cultured in the presence of sodium stibogluconate, a known inhibitor of SHPs (33), and then coincubated with apoptotic Jurkat T cells. Inhibition of SHPs restored the phagocytic ability of AMs to a level equal to that of resting PMs (Figure 4A). Interestingly, SHP inhibition had no effect on the phagocytic ability of PMs, which are not exposed in vivo to surfactant collectins.
Figure 4.
Src homology protein (SHP) inhibition restores phagocytic capacity of alveolar macrophages (AMs). (A) Murine alveolar and peritoneal macrophages were isolated from ICR mice and cultured overnight. Sodium stibogluconate was added to inhibit SHP activity. Media were removed and fresh media containing apoptotic Jurkat T cells were added. Compared with peritoneal macrophages (PMs), AMs exhibited significantly reduced phagocytosis at baseline (**P < 0.001). This defect was corrected by SHP inhibition (*P < 0.05 vs. resting control). Open bars, control; solid bars, SHP inhibitor. (B) AMs and PMs were isolated from 4-week-old motheaten mice or age-matched C57 BL/6 controls. After overnight culture, phagocytosis of apoptotic Jurkat T cells was assessed (*P < 0.05 for wild-type AMs compared with viable motheaten AMs). Open bars, wild-type; solid bars, motheaten.
To further verify that tonic inhibition of AM phagocytosis is mediated through SHP-1, AMs from viable motheaten mice (mev/mev) were used. These mice contain a point mutation in the SHP-1 gene and express catalytically defective SHP-1 (34). AMs from the motheaten mice had significantly enhanced phagocytic function compared with AMs from wild-type mice (Figure 4B). To verify that the SHP-1 defect did not result in a general phagocytic defect, we also isolated PMs and measured their phagocytic ability. No significant difference was observed in apoptotic cell clearance between wild-type PMs and PMs from motheaten mice. When considered in the context of previous publications (17, 29, 30, 35), our findings suggest that tonic suppression of AM phagocytic function results from collectin-mediated activation of SIRPα and its downstream effector, SHP-1.
Surfactant Protein Binding to SIRPα Inhibits Engulfment through Activation of RhoA
Rho-family GTPases are small GTP binding proteins essential for cytoskeletal reorganization and pseudopod formation. RhoA is a member of this family and is a negative regulator of phagocytosis through the activation of Rho kinase. Ligation of SIRPα with CD47 activates RhoA (36). In addition, SHP-1 phosphorylation impairs phagocytosis through inhibition of Rac, which has been shown to reciprocally activate RhoA (32). Because RhoA negatively regulates engulfment of apoptotic cells, we sought to determine if surfactant-mediated activation of RhoA is responsible for suppressed AM phagocytosis. As a first step, J774 macrophages were pretreated with a selective inhibitor of Rho kinase (Y27632) before addition of the surfactant proteins (Figure 5A). As with our previous experiments, SP-A and SP-D significantly reduced macrophage phagocytosis. However, this effect was fully abrogated by the Rho kinase inhibitor.
Figure 5.
Surfactant proteins inhibit efferocytosis through activation of RhoA. (A) J774A.1 macrophages were cultured in the presence of the Rho kinase inhibitor Y27632 (10 μM for 20 min) and then treated with 10 μg/ml surfactant protein (SP)-A, SP-D, or C1q. Phagocytosis of apoptotic Jurkat T cells was assessed after 90 minutes of coincubation; *P < 0.05 versus control. (B) Alveolar macrophages and peritoneal macrophages were isolated from ICR mice, cultured in the presence of Y27632, and then fed apoptotic Jurkat T cells. (A, B) Open bars, control; solid bars, Y27632, (C) Mice were treated with 10 mg/kg Y27632 or phosphate-buffered saline by gavage. Four hours later, apoptotic murine thymocytes were instilled directly into the lungs. Bronchoalveolar lavage was performed 60 minutes later and the phagocytic index was assessed on cytospin samples using light microscopy. n = 8 mice per group. *P < 0.05 versus control.
To investigate whether RhoA actively contributes to the tonic suppression exhibited by AMs, we performed phagocytosis assays on murine AMs and PMs in culture (Figure 5B). Phagocytosis was significantly enhanced in AMs treated with the inhibitor and reached levels equivalent to those of PMs. Importantly, inhibiting Rho kinase had no effect on PMs, a cell population that is not exposed to the alveolar environment. To further verify that RhoA contributes to AM suppression, we proceeded with in vivo experiments in which we instilled apoptotic thymocytes directly into the lungs of mice treated with the Rho kinase inhibitor. As with our in vitro studies, inhibiting Rho kinase significantly enhanced apoptotic cell clearance (Figure 5C). In total, these experiments suggest that the tonically inhibited phenotype of the AM results from SP-A and SP-D and is mediated through SHP-1 and activation of RhoA.
The Phagocytic Capacity of AMs Is Enhanced during Inflammation
Massive numbers of neutrophils migrate to the airspaces in community-acquired pneumonia and the acute respiratory distress syndrome (ARDS), yet the burden of apoptotic cells is surprisingly low (37–39). We therefore questioned if the tonic suppression displayed by resting AMs could be overcome during acute inflammation. To model bacterial pneumonia, LPS was delivered to mice via direct intratracheal instillation. Brisk neutrophil accumulation occurred in the lungs within 24 hours and peaked at 72 hours. Macrophage counts increased beginning at Day 3 and peaked at Day 7 (Figure 6A). We selected Post-LPS Days 3 and 6 as optimal time points to study apoptotic cell clearance because they represented peak inflammation and neutrophil decline. Because high numbers of neutrophils were present in BAL fluid at both times, AMs were purified by density centrifugation before coculture with apoptotic cells. In line with the findings of Newman and colleagues (10), phagocytosis was significantly enhanced in inflammatory macrophages compared with resting AMs (Figure 6B).
Figure 6.
Phagocytosis increases during inflammation. (A) Two hundred micrograms of LPS in 50 μl phosphate-buffered saline was administered to C57BL/6 mice by direct intratracheal instillation. Bronchoalveolar lavage (BAL) was performed at the times indicated. Absolute macrophage and neutrophil counts were obtained by multiplying BAL leukocyte counts by differential cell counts. Values represent the mean of three independent experiments with a minimum of four mice per group. (B) Macrophages from LPS-treated mice were purified from BAL by density centrifugation. After overnight culture, 5 × 106 apoptotic Jurkat T cells were added to each well. Phagocytosis was assayed 90 minutes later. (C) Macrophages were isolated from uninjured mice (no LPS) or 6 days after treatment with intratracheal LPS. Purified alveolar macrophages (AMs) were cultured overnight, and then treated with surfactant protein (SP)-A, SP-D, or C1q (10 μg/ml) for 20 minutes. Phagocytosis of apoptotic Jurkat T cells was assessed. (*P < 0.05 versus control.) PMN = neutrophils.
To determine if the phagocytic capacity of inflammatory AMs could be altered by SP-A or SP-D, we repeated the ex vivo phagocytosis assay using inflammatory AMs obtained on Post-LPS Day 6. Pretreating the macrophages with SP-A or SP-D (but not C1q) significantly reduced phagocytosis, although levels remained elevated above those of resting AMs (Figure 6C).
Recruited Macrophages Ingest Apoptotic Cells More Effectively than Resident AMs
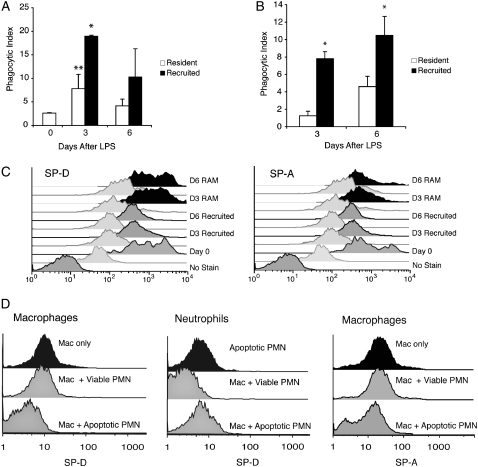
AM numbers increased fivefold in our model of LPS-induced inflammation (Figure 6A). We therefore asked whether the observed increase in phagocytosis during inflammation resulted from enhanced function of resident AMs (RAMs) or if mononuclear phagocytes recruited from the bloodstream were the prime contributors to apoptotic cell clearance. To answer this question, bone marrow transplantation was performed on γ-irradiated mice to create chimeras in which bone marrow–derived cells constitutively express GFP, but RAMs were GFP negative. A high percentage of macrophages in BAL expressed GFP after LPS (76.1 ± 13.4% on Day 3, 86.3 ± 4.3% on Day 6, vs. 14.3 ± 4.3% in untreated mice; P < 0.001), indicating that the majority of phagocytes in the airspaces were recruited mononuclear phagocytes rather then RAMs. When these two populations were separated via fluorescence-activated cell sorting and cultured ex vivo, recruited mononuclear phagocytes cleared apoptotic cells more effectively than resident macrophages (Figure 7A), suggesting that recruited mononuclear phagocytes play a major role in apoptotic cell clearance during inflammation. RAMs from LPS-injured lungs also exhibited enhanced phagocytic capacity, but to a lesser degree than recruited mononuclear phagocytes.
Figure 7.
Phagocytosis of apoptotic cells by resident and recruited macrophages during inflammation. LPS was administered to green fluorescent protein (GFP)–expressing chimeric mice. Resident alveolar macrophages (RAMs) and recruited mononuclear phagocytes were differentiated using flow cytometry based on GFP autofluorescence. (A) Resident and recruited AMs were fluorescently sorted, cultured overnight, and then coincubated with apoptotic Jurkat T cells. Phagocytosis was assessed 60 minutes later; *P < 0.05 for recruited versus resident macrophages; **P < 0.05 for inflammatory resident macrophages versus baseline. (B) Cytospin samples of freshly sorted resident and recruited macrophages were stained with Wright's Giemsa stain and analyzed by light microscopy for phagocytosis of endogenous apoptotic cells. The phagocytic index for this experiment represents macrophages that ingested apoptotic neutrophils in vivo; *P < 0.05 for recruited versus resident macrophages. (C) Flow cytometry was performed on lavage specimens from LPS-treated mice and controls (Day 0) using antibodies to surfactant protein (SP)-A and SP-D. RAMs (black) and recruited mononuclear phagocytes (gray) were identified by forward scatter, side scatter, and FL1 autofluorescence (GFP). Isotype controls are shown below each sample. Day 0 samples only contain RAMs. (D) Fluorescein isothiocyanate–labeled SP-D was added to J774 macrophages alone, apoptotic neutrophils alone, viable neutrophils alone, or to 1:1 mixtures containing neutrophils and macrophages for 30 minutes on ice. Samples were washed, fixed, and analyzed by flow cytometry. J774 macrophages were prelabeled with the fluorescent dye PKH26-PCL to permit discrimination between macrophages and neutrophils with flow cytometry. Experiments were repeated with SP-A. Histograms represent findings from three independent experiments. PMN = human neutrophils.
To further verify that recruited mononuclear phagocytes contribute to clearance of apoptotic neutrophils and aid in resolving inflammation, we again treated GFP-expressing chimeras with LPS. Bronchoalveolar specimens were taken directly to a fluorescence-activated cell sorter where GFP-negative (resident) and GFP-positive (recruited) macrophage populations were separated. Cytospins of sorted samples were then examined by light microscopy for the presence of endogenously ingested apoptotic cells. In concordance with our ex vivo phagocytosis assays, recruited mononuclear phagocytes contained significantly more apoptotic bodies then RAMs (Figure 7B), suggesting that they were the phagocytes responsible for the majority of apoptotic cell clearance.
Our previous experiments demonstrated that SP-A and SP-D suppress macrophage phagocytic function when bound to SIRPα. Therefore, we sought to determine if differences in binding of SP-A or SP-D to resident versus recruited macrophages would explain alterations in phagocytic capacity during inflammation. BAL was performed on GFP-expressing chimeric mice 3 and 6 days after LPS administration. Antibodies to SP-A and SP-D were added and cells were analyzed using flow cytometry. RAMs exhibited higher levels of SP-D and SP-A than recruited mononuclear phagocytes at rest (Day 0) and during inflammation (Figure 7C). In contrast, SIRPα levels were similar on both cell populations (data not shown).
Because large numbers of neutrophils turn over in the airspaces during inflammation and the lung collectins are capable of binding apoptotic cells (18, 20), we considered the possibility that free collectins preferentially bind apoptotic cells rather than incoming mononuclear phagocytes. To test this theory, we added fluorescently labeled SP-D to collectin-naive macrophages and apoptotic neutrophils (Figure 7D). In isolation, each cell type effectively bound SP-D. However, when SP-D was added to a mixed population of macrophages and apoptotic neutrophils, the apoptotic cells were preferentially opsonized. Experiments with SP-A yielded similar results, with SP-A preferentially binding to apoptotic neutrophils over macrophages. In the inflammatory milieu of the LPS-injured lung, this effect could promote apoptotic cell clearance by dual mechanisms that include diversion of free collectins from incoming mononuclear phagocytes and direct opsonization of apoptotic cells. The former would allow recruited mononuclear phagocytes to escape SIRPα-mediated suppression of phagocytic function, whereas the latter would facilitate ingestion of apoptotic neutrophils by both RAMs and recruited mononuclear phagocytes.
DISCUSSION
The human respiratory tract is constantly exposed to inhaled particles, environmental antigens, and microorganisms. These substances have the potential to induce massive inflammation and lung injury, yet under most circumstances their removal is virtually silent. In the proximal airways, clearance occurs via the mucociliary apparatus and cough reflex. More distally, pathogens and particles are engulfed by AMs and cleared in situ. To prevent collateral damage to the delicate gas exchange structures of the alveolus, the AM is held in a quiescent state in which inflammatory cytokine production and antigen presentation are tonically suppressed (40–42). Our previous work suggests that interaction of the C-terminal heads of SP-A and SP-D with SIRPα may in part drive this phenotype (17).
AMs ingest apoptotic cells less avidly than other tissue macrophages (10, 11). In the studies detailed herein, we show that removing the AM from the lung environment improves its ability to ingest apoptotic cells. Adding SP-A or SP-D back to the AM restores the suppressed phenotype. In a similar vein, the addition of SP-A or SP-D to collectin-naive macrophages also suppresses phagocytic function. On initial inspection, it appears that these findings contradict with those of Schagat and colleagues, Reidy and coworkers, and our own group who previously reported that SP-A and SP-D enhanced apoptotic cell clearance (18–20). An important distinction between the former studies and the current one is the order in which pulmonary collectins were added to culture conditions. In the current study, macrophages were coincubated with SP-A or SP-D and then washed to remove excess and unbound surfactant proteins before apoptotic cells were added. This resulted in suppression of AM function but did not allow for opsonization of the apoptotic targets. In comparison, prior studies added pulmonary collectins and apoptotic cells to cultured AMs simultaneously. This permitted opsonization of apoptotic targets and facilitated their ingestion. Importantly, when Vandivier and colleagues added target cells to macrophages that had been incubated on SP-A– or SP-D–coated plates, phagocytosis was significantly reduced (20), supporting our current findings. We suggest that in the resting, noninflamed lung, the lung collectins have dual effects. On one hand, they suppress AM phagocytic function through tonic interaction with SIRPα. On the other, they enhance apoptotic cell removal by opsonizing apoptotic cells and facilitating their removal through CD91. The net effect permits low-level phagocytosis of apoptotic cells by resting AMs during times of health, yet maintains their quiescent phenotype.
Studies with SP-D–deficient mice support this paradigm. Targeted inactivation of the SP-D gene results in AM activation, chronic low-grade pulmonary inflammation, age-dependent emphysema, and pulmonary fibrosis (23). Not surprisingly, we found that AMs from these animals had a greater ability to ingest apoptotic cells in vitro than AMs from wild-type control animals. We hypothesize that this activated macrophage phenotype results from loss of tonic SIRPα activation by the collectins. In vivo, AMs from SP-D–deficient mice remain activated but removal of apoptotic cells from the lungs is impaired, a finding that we have previously attributed to incomplete opsonization of apoptotic cells by the collectins (20). The end result is chronic AM activation and impaired apoptotic cell removal.
The use of purified SP-A and SP-D is a potential limitation of our study. In the lungs, SP-A and SP-D associate closely with other surfactant constituents, including phospholipids and carbohydrates. It is possible that these molecules may affect AM function, either through direct interaction with the cell or by altering collectin activity. Because phospholipids comprise 90% of the surfactant layer, future studies of these molecules are essential.
In the absence of inflammation, there may be little need for the AM to clear apoptotic cells. AMs and epithelial cells comprise the main cell types in contact with the alveolar lumen. The turnover rate of the AM is extremely low, with an estimated lifespan of over 6 months (43). Therefore, very few AMs undergo apoptosis at any given time. Little is known about the lifespan of type I and type II alveolar epithelial cells, but their turnover rate may be similarly low. In the rare instances in which apoptotic cells are present in the distal airspaces, epithelial cells may perform the lion's share of phagocytosis. Recently, we have reported that mammary epithelial cells are predominantly responsible for clearance of apoptotic cells during mammary involution (44), whereas others have noted robust clearance of apoptotic eosinophils by airway epithelial cells (45, 46).
During acute inflammation, removal of dying cells is a necessity. In ARDS and in community-acquired pneumonia, neutrophil concentrations in the airspaces can exceed 7×106/ml (39). Surprisingly, less than 1.5% of the observed cells are apoptotic and even fewer are necrotic (37, 39), suggesting robust apoptotic cell clearance. Our data support the findings of Newman and colleagues and Reidy and coworkers (10, 19) and indicate that inflammatory macrophages have enhanced phagocytic capacity. We have extended their findings to show that mononuclear phagocytes recruited from the bloodstream and bone marrow are significant contributors to this phenomenon. Previous studies have shown that monocyte-derived macrophages can develop the capacity to ingest apoptotic cells in vitro (47). Endotoxin accelerates the rate at which this occurs and can enhance phagocytic potential (48). Cytokines found in the lungs during inflammation, such as granulocyte-macrophage colony–stimulating factor (GM-CSF) and IL-1β can have similar effects (49). In our system, clearance of apoptotic neutrophils is maximal 3 to 6 days after LPS. At these time points, over 80% of macrophages in the airspaces are of bone marrow origin. Because their phagocytic capacity is two to five times greater than that of RAMs, they account for over 90% of the phagocytic ingestions.
Our experiments did not explore the clearance of apoptotic cells at the initiation of inflammation. In our model, recruited mononuclear phagocytes are absent from the airspaces for the first 48 hours after LPS administration, yet apoptotic neutrophils are rare. Inflammatory cytokines such as GM-CSF, IL-1β, and IL-8 are present in acute lung injury and may provide one explanation because they protect neutrophils from apoptosis. A second possibility is that RAMs are stimulated in early lung injury. Our ex vivo experiments support this notion and show enhanced phagocytic function of RAMs 3 days after LPS.
Enhanced phagocytosis by recruited mononuclear phagocytes may not hold true for all inflammatory stimuli. A recent study by Jennings and colleagues used sheep red blood cells to induce lymphocyte-predominant inflammation in the lungs (12). Under these circumstances, the phagocytic ability of recruited mononuclear phagocytes was poor and remained inferior to that of RAMs. Although differences in the initiating inflammatory stimulus and the degree of tissue injury between the two models may explain this effect, so may differences in apoptotic target cells, because macrophages may have unique mechanisms for recognizing apoptotic neutrophils (50). We cannot rule out the possibility that the radiation used to create our GFP-expressing chimeric mice affected RAM function, although we believe this is unlikely. The lungs of our mice were protected during radiation by lead shields and no evidence of radiation lung injury was detectable by BAL or histology (data not shown). Furthermore, RAMs from transplanted and wild-type mice exhibited similar levels of apoptotic cell clearance in vitro.
The roles of SP-A and SP-D in apoptotic cell clearance during inflammation remain undefined. In our system, cell surface levels of SP-A and SP-D remained constant on RAMs during inflammation, whereas levels on recruited mononuclear phagocytes were reduced. SIRPα levels were similar on both cell types, and we believe that the molecule is active because incubating inflammatory macrophages with SP-A and SP-D reduces their phagocytic capacity.
In an attempt to correlate cell surface levels of SP-A and SP-D with levels of free collectins in the airspaces, we performed Western blotting on cell-free supernatants from lavage fluid of LPS-treated mice (data not shown). Interestingly, SP-A and SP-D levels were increased 3 days after LPS, but decreased on Day 6. These levels may not provide an accurate reflection of total collectin content in the alveolus because SP-A is highly lipid bound and only a small portion is present in the aqueous phase. SP-D is more accessible in BAL, but total levels are 10-fold lower than SP-A. Moreover, neutrophil-derived proteases that are present during inflammation can alter collectin structure and function. Therefore, we do not know if the SP-A and SP-D measured from the BAL is capable of binding to macrophage receptors.
As inflammation progresses, the production of SP-A and SP-D may be reduced due to alveolar epithelial cell dysfunction. In addition, high numbers of cells rapidly undergoing apoptosis (and subsequent phagocytic cell clearance) may bind free collectins and serve as a sink for their removal. Indeed, SP-A levels are reduced in the lavage fluid of patients suffering from ARDS, acute lung injury after trauma, sepsis, and pneumonia (51, 52). SP-D has been less well studied in lung injury but may be reduced in some patients with ARDS (51). Decreased binding of SP-A or SP-D to SIRPα (as a result of competition from apoptotic cells or from reduced surfactant protein levels) provides one mechanism by which recruited mononuclear phagocytes escape collectin-mediated suppression. In addition, SIRPα receptor complexes on recruited mononuclear phagocytes and RAMs may have differential SP-A and SP-D binding affinity.
Our findings explain the longstanding dichotomous observations that AMs ingest apoptotic cells poorly but that few apoptotic cells are present in the lungs during inflammation. Herein we have demonstrated that, in the absence of inflammation, SP-A and SP-D suppress AM phagocytic function by binding to SIRPα and altering the activity of its downstream effectors SHP-1 and RhoA. During LPS-induced inflammation, recruited mononuclear phagocytes escape collectin-mediated inhibition and are major contributors to apoptotic cell clearance.
Supplementary Material
Supported by National Institutes of Health grants R01HL68864 and GM61031.
This article has an online supplement, which is accessible from this issue's table of contents at www.atsjournals.org
Originally Published in Press as DOI: 10.1164/rccm.200711-1661OC on April 17, 2008
Conflict of Interest Statement: W.J.J. does not have a financial relationship with a commercial entity that has an interest in the subject of this manuscript. K.A.M. does not have a financial relationship with a commercial entity that has an interest in the subject of this manuscript. M.G.D. has been reimbursed for enrolling patients in research trials for GlaxoSmithKline (GSK). D.J.L. does not have a financial relationship with a commercial entity that has an interest in the subject of this manuscript. K.M. does not have a financial relationship with a commercial entity that has an interest in the subject of this manuscript. Y.Q.X. does not have a financial relationship with a commercial entity that has an interest in the subject of this manuscript. K.M.O. has been reimbursed for enrolling patients in research trials for GSK. R.W.V. does not have a financial relationship with a commercial entity that has an interest in the subject of this manuscript. P.M.H. has been reimbursed by AstraZeneca for attending a conference. S.J.G. does not have a financial relationship with a commercial entity that has an interest in the subject of this manuscript.
References
- 1.Fadok VA, Bratton DL, Konowal A, Freed PW, Westcott JY, Henson PM. Macrophages that have ingested apoptotic cells in vitro inhibit proinflammatory cytokine production through autocrine/paracrine mechanisms involving TGF-beta, PGE2, and PAF. J Clin Invest 1998;101:890–898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Morimoto K, Amano H, Sonoda F, Baba M, Senba M, Yoshimine H, Yamamoto H, Ii T, Oishi K, Nagatake T. Alveolar macrophages that phagocytose apoptotic neutrophils produce hepatocyte growth factor during bacterial pneumonia in mice. Am J Respir Cell Mol Biol 2001;24:608–615. [DOI] [PubMed] [Google Scholar]
- 3.Golpon HA, Fadok VA, Taraseviciene-Stewart L, Scerbavicius R, Sauer C, Welte T, Henson PM, Voelkel NF. Life after corpse engulfment: phagocytosis of apoptotic cells leads to VEGF secretion and cell growth. FASEB J 2004;18:1716–1718. [DOI] [PubMed] [Google Scholar]
- 4.Cox G, Crossley J, Xing Z. Macrophage engulfment of apoptotic neutrophils contributes to the resolution of acute pulmonary inflammation in vivo. Am J Respir Cell Mol Biol 1995;12:232–237. [DOI] [PubMed] [Google Scholar]
- 5.Haslett C. Granulocyte apoptosis and its role in the resolution and control of lung inflammation. Am J Respir Crit Care Med 1999;160:S5–S11. [DOI] [PubMed] [Google Scholar]
- 6.Huynh ML, Fadok VA, Henson PM. Phosphatidylserine-dependent ingestion of apoptotic cells promotes TGF-beta1 secretion and the resolution of inflammation. J Clin Invest 2002;109:41–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hodge S, Hodge G, Scicchitano R, Reynolds PN, Holmes M. Alveolar macrophages from subjects with chronic obstructive pulmonary disease are deficient in their ability to phagocytose apoptotic airway epithelial cells. Immunol Cell Biol 2003;81:289–296. [DOI] [PubMed] [Google Scholar]
- 8.Huynh ML, Malcolm KC, Kotaru C, Tilstra JA, Westcott JY, Fadok VA, Wenzel SE. Defective apoptotic cell phagocytosis attenuates prostaglandin E2 and 15-hydroxyeicosatetraenoic acid in severe asthma alveolar macrophages. Am J Respir Crit Care Med 2005;172:972–979. [DOI] [PubMed] [Google Scholar]
- 9.Vandivier RW, Fadok VA, Ogden CA, Hoffmann PR, Brain JD, Accurso FJ, Fisher JH, Greene KE, Henson PM. Impaired clearance of apoptotic cells from cystic fibrosis airways. Chest 2002;121:89S. [DOI] [PubMed] [Google Scholar]
- 10.Newman SL, Henson JE, Henson PM. Phagocytosis of senescent neutrophils by human monocyte-derived macrophages and rabbit inflammatory macrophages. J Exp Med 1982;156:430–442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hu B, Sonstein J, Christensen PJ, Punturieri A, Curtis JL. Deficient in vitro and in vivo phagocytosis of apoptotic T cells by resident murine alveolar macrophages. J Immunol 2000;165:2124–2133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jennings JH, Linderman DJ, Hu B, Sonstein J, Curtis JL. Monocytes recruited to the lungs of mice during immune inflammation ingest apoptotic cells poorly. Am J Respir Cell Mol Biol 2005;32:108–117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bridges JP, Davis HW, Damodarasamy M, Kuroki Y, Howles G, Hui DY, McCormack FX. Pulmonary surfactant proteins A and D are potent endogenous inhibitors of lipid peroxidation and oxidative cellular injury. J Biol Chem 2000;275:38848–38855. [DOI] [PubMed] [Google Scholar]
- 14.LeVine AM, Whitsett JA. Pulmonary collectins and innate host defense of the lung. Microbes Infect 2001;3:161–166. [DOI] [PubMed] [Google Scholar]
- 15.Borron P, McIntosh JC, Korfhagen TR, Whitsett JA, Taylor J, Wright JR. Surfactant-associated protein A inhibits LPS-induced cytokine and nitric oxide production in vivo. Am J Physiol 2000;278:L840–L847. [DOI] [PubMed] [Google Scholar]
- 16.Kremlev SG, Phelps DS. Surfactant protein A stimulation of inflammatory cytokine and immunoglobulin production. Am J Physiol 1994;267:L712–L719. [DOI] [PubMed] [Google Scholar]
- 17.Gardai SJ, Xiao YQ, Dickinson M, Nick JA, Voelker DR, Greene KE, Henson PM. By binding SIRPalpha or calreticulin/cd91, lung collectins act as dual function surveillance molecules to suppress or enhance inflammation. Cell 2003;115:13–23. [DOI] [PubMed] [Google Scholar]
- 18.Schagat TL, Wofford JA, Wright JR. Surfactant protein A enhances alveolar macrophage phagocytosis of apoptotic neutrophils. J Immunol 2001;166:2727–2733. [DOI] [PubMed] [Google Scholar]
- 19.Reidy MF, Wright JR. Surfactant protein A enhances apoptotic cell uptake and TGF-beta1 release by inflammatory alveolar macrophages. Am J Physiol 2003;285:L854–L861. [DOI] [PubMed] [Google Scholar]
- 20.Vandivier RW, Ogden CA, Fadok VA, Hoffmann PR, Brown KK, Botto M, Walport MJ, Fisher JH, Henson PM, Greene KE. Role of surfactant proteins A, D, and c1q in the clearance of apoptotic cells in vivo and in vitro: calreticulin and CD91 as a common collectin receptor complex. J Immunol 2002;169:3978–3986. [DOI] [PubMed] [Google Scholar]
- 21.Okazawa H, Motegi S, Ohyama N, Ohnishi H, Tomizawa T, Kaneko Y, Oldenborg PA, Ishikawa O, Matozaki T. Negative regulation of phagocytosis in macrophages by the CD47-SHPS-1 system. J Immunol 2005;174:2004–2011. [DOI] [PubMed] [Google Scholar]
- 22.Oldenborg PA, Gresham HD, Lindberg FP. CD47-signal regulatory protein alpha (SIRPalpha) regulates fcgamma and complement receptor-mediated phagocytosis. J Exp Med 2001;193:855–862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Korfhagen TR, Sheftelyevich V, Burhans MS, Bruno MD, Ross GF, Wert SE, Stahlman MT, Jobe AH, Ikegami M, Whitsett JA, et al. Surfactant protein-D regulates surfactant phospholipid homeostasis in vivo. J Biol Chem 1998;273:28438–28443. [DOI] [PubMed] [Google Scholar]
- 24.Allen MJ, Voelker DR, Mason RJ. Interactions of surfactant proteins A and D with saccharomyces cerevisiae and aspergillus fumigatus. Infect Immun 2001;69:2037–2044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Whitlock BB, Gardai S, Fadok V, Bratton D, Henson PM. Differential roles for alpha(m)beta(2) integrin clustering or activation in the control of apoptosis via regulation of AKT and ERK survival mechanisms. J Cell Biol 2000;151:1305–1320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Suratt BT, Petty JM, Young SK, Malcolm KC, Lieber JG, Nick JA, Gonzalo JA, Henson PM, Worthen GS. Role of the CXCR4/SDF-1 chemokine axis in circulating neutrophil homeostasis. Blood 2004;104:565–571. [DOI] [PubMed] [Google Scholar]
- 27.Vandivier RW, Fadok VA, Hoffmann PR, Bratton DL, Penvari C, Brown KK, Brain JD, Accurso FJ, Henson PM. Elastase-mediated phosphatidylserine receptor cleavage impairs apoptotic cell clearance in cystic fibrosis and bronchiectasis. J Clin Invest 2002;109:661–670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Clark H, Palaniyar N, Strong P, Edmondson J, Hawgood S, Reid KB. Surfactant protein D reduces alveolar macrophage apoptosis in vivo. J Immunol 2002;169:2892–2899. [DOI] [PubMed] [Google Scholar]
- 29.Neznanov N, Neznanova L, Kondratov RV, O'Rourke DM, Ullrich A, Gudkov AV. The ability of protein tyrosine phosphatase SHP-1 to suppress NFkappaB can be inhibited by dominant negative mutant of SIRPalpha. DNA Cell Biol 2004;23:175–182. [DOI] [PubMed] [Google Scholar]
- 30.Kharitonenkov A, Chen Z, Sures I, Wang H, Schilling J, Ullrich A. A family of proteins that inhibit signalling through tyrosine kinase receptors. Nature 1997;386:181–186. [DOI] [PubMed] [Google Scholar]
- 31.Veillette A, Thibaudeau E, Latour S. High expression of inhibitory receptor SHPS-1 and its association with protein-tyrosine phosphatase SHP-1 in macrophages. J Biol Chem 1998;273:22719–22728. [DOI] [PubMed] [Google Scholar]
- 32.Kant AM, De P, Peng X, Yi T, Rawlings DJ, Kim JS, Durden DL. SHP-1 regulates fcgamma receptor-mediated phagocytosis and the activation of rac. Blood 2002;100:1852–1859. [PubMed] [Google Scholar]
- 33.Pathak MK, Yi T. Sodium stibogluconate is a potent inhibitor of protein tyrosine phosphatases and augments cytokine responses in hemopoietic cell lines. J Immunol 2001;167:3391–3397. [DOI] [PubMed] [Google Scholar]
- 34.Tsui HW, Siminovitch KA, de Souza L, Tsui FW. Motheaten and viable motheaten mice have mutations in the haematopoietic cell phosphatase gene. Nat Genet 1993;4:124–129. [DOI] [PubMed] [Google Scholar]
- 35.Fujioka Y, Matozaki T, Noguchi T, Iwamatsu A, Yamao T, Takahashi N, Tsuda M, Takada T, Kasuga M. A novel membrane glycoprotein, SHPS-1, that binds the SH2-domain-containing protein tyrosine phosphatase SHP-2 in response to mitogens and cell adhesion. Mol Cell Biol 1996;16:6887–6899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Motegi S, Okazawa H, Ohnishi H, Sato R, Kaneko Y, Kobayashi H, Tomizawa K, Ito T, Honma N, Buhring HJ, et al. Role of the CD47-SHPS-1 system in regulation of cell migration. EMBO J 2003;22:2634–2644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Droemann D, Aries SP, Hansen F, Moellers M, Braun J, Katus HA, Dalhoff K. Decreased apoptosis and increased activation of alveolar neutrophils in bacterial pneumonia. Chest 2000;117:1679–1684. [DOI] [PubMed] [Google Scholar]
- 38.Droemann D, Hansen F, Aries SP, Braun J, Zabel P, Dalhoff K, Schaaf B. Neutrophil apoptosis, activation and anti-inflammatory cytokine response in granulocyte colony-stimulating factor-treated patients with community-acquired pneumonia. Respiration 2006;73:340–346. [DOI] [PubMed] [Google Scholar]
- 39.Matute-Bello G, Liles WC, Radella F II, Steinberg KP, Ruzinski JT, Jonas M, Chi EY, Hudson LD, Martin TR. Neutrophil apoptosis in the acute respiratory distress syndrome. Am J Respir Crit Care Med 1997;156:1969–1977. [DOI] [PubMed] [Google Scholar]
- 40.Bilyk N, Holt PG. Inhibition of the immunosuppressive activity of resident pulmonary alveolar macrophages by granulocyte/macrophage colony-stimulating factor. J Exp Med 1993;177:1773–1777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Bilyk N, Holt PG. Cytokine modulation of the immunosuppressive phenotype of pulmonary alveolar macrophage populations. Immunology 1995;86:231–237. [PMC free article] [PubMed] [Google Scholar]
- 42.Holt PG, Oliver J, Bilyk N, McMenamin C, McMenamin PG, Kraal G, Thepen T. Downregulation of the antigen presenting cell function(s) of pulmonary dendritic cells in vivo by resident alveolar macrophages. J Exp Med 1993;177:397–407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Maus UA, Janzen S, Wall G, Srivastava M, Blackwell TS, Christman JW, Seeger W, Welte T, Lohmeyer J. Resident alveolar macrophages are replaced by recruited monocytes in response to endotoxin-induced lung inflammation. Am J Respir Cell Mol Biol 2006;35:227–235. [DOI] [PubMed] [Google Scholar]
- 44.Monks J, Rosner D, Geske FJ, Lehman L, Hanson L, Neville MC, Fadok VA. Epithelial cells as phagocytes: apoptotic epithelial cells are engulfed by mammary alveolar epithelial cells and repress inflammatory mediator release. Cell Death Differ 2005;12:107–114. [DOI] [PubMed] [Google Scholar]
- 45.Sexton DW, Al-Rabia M, Blaylock MG, Walsh GM. Phagocytosis of apoptotic eosinophils but not neutrophils by bronchial epithelial cells. Clin Exp Allergy 2004;34:1514–1524. [DOI] [PubMed] [Google Scholar]
- 46.Sexton DW, Blaylock MG, Walsh GM. Human alveolar epithelial cells engulf apoptotic eosinophils by means of integrin- and phosphatidylserine receptor-dependent mechanisms: a process upregulated by dexamethasone. J Allergy Clin Immunol 2001;108:962–969. [DOI] [PubMed] [Google Scholar]
- 47.Stern M, Savill J, Haslett C. Human monocyte-derived macrophage phagocytosis of senescent eosinophils undergoing apoptosis: mediation by alpha v beta 3/CD36/thrombospondin recognition mechanism and lack of phlogistic response. Am J Pathol 1996;149:911–921. [PMC free article] [PubMed] [Google Scholar]
- 48.Kramer BW, Joshi SN, Moss TJ, Newnham JP, Sindelar R, Jobe AH, Kallapur SG. Endotoxin-induced maturation of monocytes in preterm fetal sheep lung. Am J Physiol 2007;293:L345–L353. [DOI] [PubMed] [Google Scholar]
- 49.Ren Y, Savill J. Proinflammatory cytokines potentiate thrombospondin-mediated phagocytosis of neutrophils undergoing apoptosis. J Immunol 1995;154:2366–2374. [PubMed] [Google Scholar]
- 50.Moffatt OD, Devitt A, Bell ED, Simmons DL, Gregory CD. Macrophage recognition of ICAM-3 on apoptotic leukocytes. J Immunol 1999;162:6800–6810. [PubMed] [Google Scholar]
- 51.Greene KE, Wright JR, Steinberg KP, Ruzinski JT, Caldwell E, Wong WB, Hull W, Whitsett JA, Akino T, Kuroki Y, et al. Serial changes in surfactant-associated proteins in lung and serum before and after onset of ARDS. Am J Respir Crit Care Med 1999;160:1843–1850. [DOI] [PubMed] [Google Scholar]
- 52.Pison U, Obertacke U, Seeger W, Hawgood S. Surfactant protein A (SP-A) is decreased in acute parenchymal lung injury associated with polytrauma. Eur J Clin Invest 1992;22:712–718. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.