Abstract
Rationale: Preterm infants exposed to mechanical ventilation and oxygen are at risk for bronchopulmonary dysplasia (BPD), a multifactorial chronic lung disorder characterized by arrested alveolar development. Studies have described disruption of microvascular development in BPD, characterized by primitive angioarchitectural patterns reminiscent of the canalicular/saccular stages of lung development. The molecular regulation of this BPD-associated dysangiogenesis remains undetermined.
Objectives: Endoglin (CD105), a hypoxia-inducible transforming growth factor-β coreceptor, has been implicated as an important regulator of angiogenesis in various neoplastic and nonneoplastic conditions. The aim of this study was to investigate the expression of endoglin and other angiogenesis-related factors in ventilated preterm human lungs.
Methods: We have studied endoglin protein and mRNA expression in postmortem lungs of short-term and long-term ventilated preterm infants. Control subjects were age-matched infants who had lived for less than 1 hour.
Measurements and Main Results: Lungs of short-term ventilated preterm infants showed significant upregulation of endoglin mRNA and protein levels, immunolocalized to the microvasculature. Similar but more variable endoglin upregulation was noted in lungs of long-term ventilated infants with BPD. The mRNA levels of vascular endothelial growth factor, angiopoietin-1, and their respective receptors were significantly lower in ventilated lungs than in age-matched nonventilated control lungs.
Conclusions: BPD is associated with a shift from traditional angiogenic growth factors (vascular endothelial growth factor, angiopoietin-1) to alternative regulators such as endoglin, which may contribute to BPD-associated microvascular dysangiogenesis.
Keywords: chronic lung disease of prematurity, bronchopulmonary dysplasia, neonatal lung disease, angiogenesis
AT A GLANCE COMMENTARY
Scientific Knowledge on the Subject
Microvascular development is disrupted in premature infants with bronchopulmonary dysplasia (BPD), resulting in abnormalities of lung structure that occur during the late canalicular and early saccular stages of lung development. Mechanisms that impair angiogenesis in BPD are incompletely understood.
What This Study Adds to the Field
This study suggests that endoglin, an important regulator of angiogenesis in various neoplastic and nonneoplastic conditions, contributes to BPD-associated dysangiogenesis.
Preterm newborns who require mechanical ventilation and supplemental oxygen are at risk for bronchopulmonary dysplasia (BPD), a chronic lung disease of newborn infants associated with significant mortality and morbidity (1). BPD in the postsurfactant era is seen mainly in very low birthweight infants and affects 30% of infants born between 24 and 28 weeks of gestation, many of whom will require long-term respiratory support (2, 3).
The dominant pathological finding in postsurfactant BPD is an arrest in alveolar development, resulting in large and simplified airspaces that show varying degrees of interstitial fibrosis (2, 4–8). Studies have shown that, in addition to impaired alveolar development, there is also a disruption of pulmonary microvascular development in infants with BPD (8–10) or in BPD-like animal models such as chronically ventilated premature baboons (11, 12). In view of the intimate relation between alveolar and microvascular development during pulmonary morphogenesis (13–16), disruption of microvascular development in premature lungs has been implicated as a critical factor in the arrest of alveolar development that is characteristic of BPD (17, 18).
We have studied the growth kinetics of the pulmonary microvasculature in ventilated preterm infants (10). Using stereologic morphometric techniques and analysis of endothelial cell proliferation, we determined that the pulmonary microvasculature of ventilated preterm lungs undergoes significant expansion, rather than growth arrest, which is at least in part attributable to brisk endothelial cell proliferation during the early postnatal period (10).
Although the microvasculature in ventilated preterm lungs is quantitatively nearly normal, we (10) and others (8, 9) have described striking angioarchitectural abnormalities compared with age-matched nonventilated control lungs. Normal human lungs at term (36–40 wk of gestation) display thin alveolar septa with abundant secondary crest formation, characteristic of the early alveolar stage of lung development. Within the complex alveolar septa of normal lungs, the microvasculature forms a delicate network, characterized by extensive capillary sprouting. In contrast, we determined that the pulmonary microvasculature of long-term ventilated preterm infants at the same corrected postmenstrual age (36–40 wk) retains the vascular pattern of canalicular/saccular lungs, characterized by a persistent dual capillary pattern and primitive, nonbranching vessels.
The molecular regulation of this ventilation-induced dysangiogenesis remains undetermined. Previous studies of angiogenesis in BPD or BPD-like animal models have focused on the major factors traditionally linked with developmental angiogenesis, such as vascular endothelial growth factor (VEGF), angiopoietin-1 (Ang-1), and their respective receptors. In a fetal baboon model of BPD, ventilation was associated with significantly decreased levels of VEGF and its receptor Fms-like tyrosine kinase-1/VEGF receptor-1 (Flt-1) (12). Studies in human preterm infants have shown variable results, in part attributable to small sample sizes, high clinical variability, and possible detection of differing (VEGF) protein isoforms. Bhatt and coworkers (9) reported decreased levels of VEGF, Flt-1, and Ang-1 receptor TIE receptor tyrosine kinase-2 (TIE-2) mRNAs as well as decreased VEGF immunostaining in infants with BPD compared with infants without BPD. Similarly, Lassus and coworkers (19) described lower VEGF in tracheal aspirates of preterm infants with severe respiratory distress/BPD compared with control subjects. In contrast, studies by Ambalavanan and Novak (20), D'Angio and coworkers (21), and Currie and coworkers (22) found no correlation between levels of VEGF in tracheal aspirates of preterm infants and risk for development of BPD.
In this study, we focused on endoglin (CD105) as potential candidate regulator of ventilation-induced angiogenesis. Endoglin is an accessory receptor for transforming growth factor (TGF)-β that is emerging as a pivotal regulatory component of the gateway for TGF-β signaling in vascular endothelial cells (23, 24). Endoglin is expressed predominantly in proliferating endothelial cells in culture and in angiogenic blood vessels in vivo (25). High levels of endoglin expression have been described in vascular endothelial cells in tissues undergoing active angiogenesis, such as regenerating and inflamed tissues or tumors (26–30). In addition, endoglin is weakly expressed in selected nonendothelial cell types including syncytiotrophoblast of full-term placenta (31), stromal cells (32, 33), mesenchymal cells (fibroblasts and vascular smooth muscle cells) (34, 35), and some hematopoietic cells (36, 37). Several lines of evidence implicate endoglin as an important regulator of cardiovascular development, angiogenesis, and vascular remodeling. Endoglin null mice are embryonally lethal because of vascular and cardiac abnormalities (38–40). Moreover, the gene encoding endoglin is the target for a hereditary vascular disorder known as hereditary hemorrhagic telangiectasia type I (24, 41).
The aim of the present study was to investigate endoglin expression in ventilated preterm human lungs. We report that the levels of pulmonary endoglin, immunolocalized to the microvasculature, are markedly increased in short-term ventilated preterm lungs compared with nonventilated age-matched control subjects. In contrast, mRNA levels of the more traditional angiogenic factors VEGF, Ang-1, and their receptors were significantly decreased in ventilated lungs. We speculate that this shift in angiogenic regulators may contribute to ventilation-induced dysangiogenesis in infants at risk for BPD.
METHODS
Patients
Lung samples from ventilated and control infants were obtained from the Women and Infants Hospital (Providence, RI) perinatal autopsy files between 2001 and 2005. Informed consent was obtained in compliance with institutional guidelines. The study protocol was approved by the institutional review board. The patients' medical and autopsy records were reviewed. Infants with congenital, chromosomal, or cardiac anomalies or with other conditions potentially predisposing to pulmonary anomalies (42) were excluded. In addition, cases with documented lung hypoplasia, defined as a lung-to-body weight ratio below the 10th percentile for age (43) were excluded. Records were reviewed for postmenstrual age (PMA) at birth, postnatal age, and corrected postmenstrual age at death (gestational age at birth plus postnatal age). In all patients, the postmortem interval (i.e., time between death and autopsy) was recorded.
To study the early and late effects of ventilation on pulmonary expression of angiogenic factors, patients were divided in four groups: “short-term ventilated,” “long-term ventilated,” “early control,” and “late control.” The group of short-term ventilated infants was composed of very preterm infants (23–29 wk PMA at the time of death), who had lived for at least 4 days and had been ventilation dependent throughout life. The long-term ventilated group consisted of near-term or term infants (35–39 wk PMA at death) who had lived for prolonged periods of time (at least 6 wk), and had been ventilated for most of their life (at least 75% of their total life span). Pulmonary angiogenic expression of short- and long-term ventilated infants was compared with that of early and late control infants, respectively. Control patients consisted of age-matched nonmacerated fetuses whose intrauterine demise occurred immediately before delivery, or live-born infants who lived for less than 12 hours. Control subjects were age matched with the ventilated infants with respect to the time of death: early control infants or fetuses had died between 23 and 29 weeks PMA, late control subjects between 35 and 39 weeks PMA.
Lung Processing
Autopsies were performed at Women and Infants Hospital according to standard methods. After thorough in situ examination, the lungs were dissected and weighed. Biopsies taken from the right upper lobe were treated with RNAlater (Ambion, Inc., Austin, TX) for molecular analyses, as described previously (44). The remainder of the right lung was immersed in formalin. The left lung was inflation-fixed with formalin at a standardized pressure of 20 cm H2O.
Analysis of Angiogenic Factor Expression by RNase Protection Assay
The RNase protection assay (RPA) was used to detect and quantify angiogenesis-related mRNAs in lung tissue. Total cellular RNA was isolated from lung tissue according to the method of Chomczynski and Sacchi (45). Subsequently, RPA was performed with a RiboQuant RNase protection assay kit with the hAngio-1 human angiogenesis multiprobe template set (BD Biosciences, San Jose, CA) containing DNA templates for Flt-1, Flt-4, TIE, thrombin receptor, TIE-2, CD31 (platelet endothelial cell adhesion molecule [PECAM]-1), endoglin, Ang-1, VEGF, and VEGF-C and the housekeeping gene product, L32. Specific antisense cRNA probes were synthesized with [α-32P]UTP in an in vitro transcription reaction, and 10-μg samples of total RNA were hybridized for 16 hours at 56°C. After RNase and proteinase K treatment, the RNA:RNA duplexes were heat denatured and resolved on a 5% denaturing polyacrylamide gel. Dried gels were exposed to X-AR film (Kodak, Rochester, NY) at −70°C. The resulting bands were scanned and quantified with PhotoShop (Adobe Systems, San Jose, CA) and Image NIH software (National Institutes of Health, Bethesda, MD). Band intensities were normalized to that of L32 in the same reaction.
Western Blot Analysis of Endoglin Expression
Pulmonary endoglin protein levels were evaluated by Western blot analysis of whole lung lysates, as described in detail elsewhere (44, 46), using monoclonal mouse anti–human endoglin antibody (clone SN6h; DakoCytomation, Inc., Carpinteria, CA). Human umbilical vein endothelial cell (VEC Technologies, Inc., Rensselaer, NY) lysates were used as positive control.
Immunohistochemical Analysis
Sections of left lung were processed for avidin–biotin–immunoperoxidase staining, using anti-endoglin antibody and anti–PECAM-1 antibody (goat polyclonal anti–PECAM-1, M-20, sc-1506; Santa Cruz Biotechnology, Santa Cruz, CA). Binding was detected with 3,3′-diaminobenzidine tetrachloride (DAB). Sections were lightly counterstained with Mayer's hematoxylin, cleared, and mounted. Controls for specificity consisted of omission of the primary antibody, which abolished all immunoreactivity.
All lung sections were prepared and stained in a single session for immunohistochemical analysis. Semiquantitative analysis of the endoglin-immunostained sections was performed by a single observer blinded with respect to the nature of the study group. At least 25 randomly selected fields (magnification, ×200) were scored for intensity and distribution of staining (Table 1). The intensity and distribution scores were combined to obtain a cumulative immunoreactivity score for each case.
TABLE 1.
SYSTEM FOR SCORING IMMUNOREACTIVITY
| 0 | 1 | 2 | 3 | |
|---|---|---|---|---|
| Intensity | Negative | Weak | Moderate | Strong |
| Distribution (% of ×20 power fields) | <10 | 10–25 | 26–50 | >50 |
Data Analysis
Values are expressed as means ± SD. The significance of differences between ventilated and control groups was determined by unpaired Student's t test or Mann-Whitney U test, where applicable. The significance level was set at P < 0.05. StatView software (Abacus, Berkeley, CA) was used for all statistical work.
RESULTS
Patients
The age and sex distribution, relevant clinical data, and general autopsy findings of control and ventilated infants analyzed by RPA are summarized in Table 2. The mean postmortem interval (time interval between death and autopsy) ranged from 8 to 63 hours and was not significantly different between the various study groups.
TABLE 2.
CLINICAL DATA OF PATIENTS STUDIED BY RNase PROTECTION ASSAY
| Early (23–29 wk)
|
Late (32–39 wk)
|
|||
|---|---|---|---|---|
| Control | Ventilated | Control | Ventilated | |
| (n =10) | (n = 7) | (n = 5) | (n = 5) | |
| Age at birth*, wk | 25.7 ± 2.3 | 24.6 ± 2.0 | 34.5 ± 3.7 | 27.0 ± 4.2 |
| Postnatal age, d | <0.5 | 8.9 ± 4.7 | <0.5 | 58.3 ± 26.2 |
| Corrected age at death*, wk | 25.8 ± 2.4 | 26.1 ± 2.3 | 35.0 ± 3.8 | 36.8 ± 4.2 |
| Sex | 5 M/5 F | 5 M/2 F | 3 M/2 F | 3 M/2 F |
| Body weight at autopsy, g | 725 ± 184 | 782 ± 265 | 2,616 ± 753 | 3,053 ± 862 |
| Postmortem interval, h | 27.3 ± 13.2 | 35.8 ± 20.9 | 31.8 ± 13.3 | 21.8 ± 9.5 |
| (8–48) | (16–63) | (22–51) | (10–32) | |
| Clinical/autopsy diagnosis | PROM (3); chorioamnionitis ± sepsis (4); placental insufficiency (1); COD undetermined (2) | Early BPD with complications of prematurity (3); early BPD with sepsis (4) | Sepsis (2); COD undetermined (2); abruption (1) | BPD (1); BPD with sepsis/pneumonia (1); BPD with NEC (1); BPD with viral infection (2) |
Definition of abbreviations: BPD = bronchopulmonary dysplasia; COD = cause of death; F = female; M = male; NEC = necrotizing enterocolitis; PROM = premature rupture of membranes.
Values represent means ± SD of (n) patients.
Age and corrected age reflect postmenstrual age.
RNase Protection Assay of Pulmonary Angiogenesis–related Gene Expression
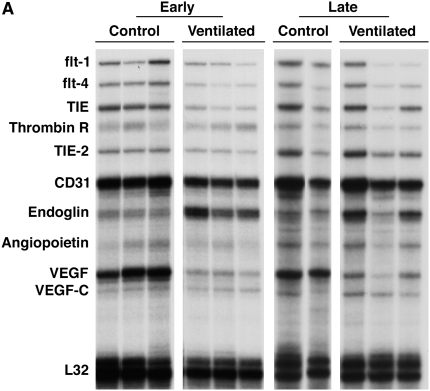
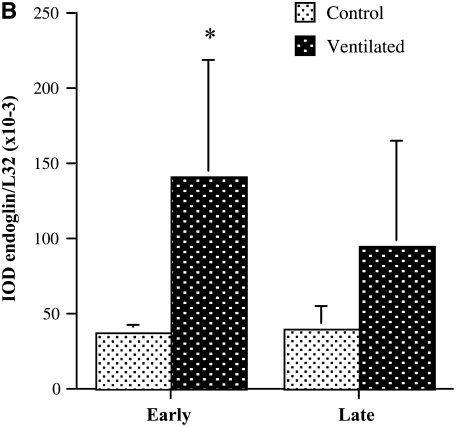
The mRNA levels of angiogenesis-related genes were assayed by multiprobe RPA. As shown in Figure 1, endoglin mRNA levels, normalized to the constitutively expressed housekeeping gene L32, were significantly more than threefold higher in short-term ventilated lungs (“early”) compared with control subjects (P < 0.05). In sharp contrast to the endoglin up-regulation, the levels of VEGF and its receptor, Flt-1, were markedly lower in short-term ventilated lungs compared with nonventilated control subjects (Table 3). Levels of Ang-1 were low in both control and short-term ventilated groups, although densitometric analysis of a longer exposed radiograph revealed significantly decreased levels in ventilated lungs compared with control subjects. Similarly, the mRNA level of the Ang-1 receptor, TIE-2, were lower in ventilated lungs (Table 3).
Figure 1.
RNase protection assay of angiogenesis-related gene expression. (A) Representative RNase protection assay (RPA) of angiogenesis-related gene expression in whole lung homogenates of control and ventilated preterm infants. (B) Densitometry of endoglin RPA analysis. Values represent means ± SD. *P < 0.05 versus control.
TABLE 3.
DENSITOMETRY OF VASCULAR ENDOTHELIAL GROWTH FACTOR AND ANGIOPOIETIN RNase PROTECTION ASSAY
| Early Control | Short-Term Ventilated | |
|---|---|---|
| (n = 10) | (n = 7) | |
| VEGF | 283.9 ± 95.4 | 22.3 ± 4.7* |
| Flt-1 | 22.2 ± 18.1 | 4.8 ± 4.2† |
| Ang-1 | 24.0 ± 15.8 | 10.7 ± 4.7† |
| TIE-2 | 15.3 ± 2.0 | 5.6 ± 2.9* |
Definition of abbreviations: Ang-1 = angiopoietin-1; Flt-1 = Fms-like tyrosine kinase-1/VEGF receptor-1; TIE-2 = TIE receptor tyrosine kinase-2 (angiopoietin-1 receptor); VEGF = vascular endothelial growth factor.
Values represent mean integrated optical density (IOD)/IOD L32 ± SD (×10−3) of (n) samples.
P < 0.01 versus control.
P < 0.05 versus control.
Endoglin mRNA levels in long-term ventilated lungs (“late”) were overall higher than in lungs of age-matched late control subjects (Figure 1). However, greater variability between individual cases precluded statistical significance with the sample sizes available for analysis. As in short-term ventilated lungs, the VEGF mRNA levels were consistently lower in long-term ventilated lungs with established BPD compared with late control subjects (Figure 1).
Western Blot Analysis of Pulmonary Endoglin Expression
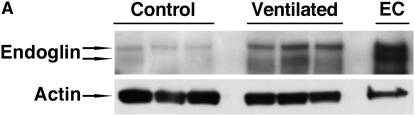
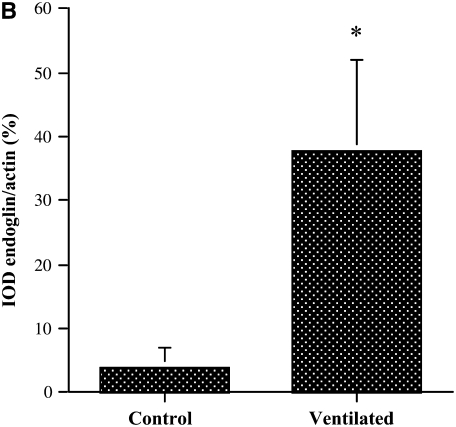
Endoglin protein expression in lungs of short-term ventilated and early control infants was assayed by Western blot analysis of whole lung homogenates, according to methods described in detail elsewhere (44). Samples (n = 5 in each group) were obtained from the patients described in Table 2, based on the availability of good-quality protein. Representative results are shown in Figure 2. Appropriately sized (∼170 and ∼160 kD) endoglin-immunoreactive protein bands were detected, consistent with the L- and S-endoglin isoforms, respectively. The pulmonary L-endoglin levels of ventilated infants were more almost 10-fold higher than those of age-matched control subjects (P < 0.01). A smaller increase was noted for the S-endoglin isoform. Results of densitometric quantitation of L-endoglin band intensities, normalized to actin, are shown in Figure 2B.
Figure 2.
Western blot analysis of pulmonary endoglin protein levels in early control subjects and short-term ventilated patients. (A) Representative Western blot analysis of endoglin protein expression in whole lung homogenates. Appropriately sized bands were detected for endoglin (approximately 170 and 160 kD). Actin (42 kD) served as internal loading control. Human umbilical vein endothelial cells (EC) served as positive control. (B) Densitometry of 170-kD L-endoglin Western blot analysis. Values represent means ± SD of five samples in each group. *P < 0.01 versus control. IOD = integrated optical density.
Endoglin and PECAM-1 Immunohistochemistry
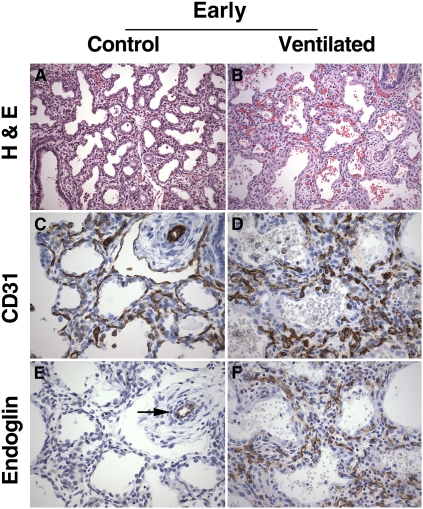
Morphologic and immunohistochemical analysis was performed in a slightly different patient group, as dictated by the availability of high-quality paraffin-embedded material. Table 4 shows the relevant clinical data of patients included for immunohistochemical analysis. Lungs of early control and short-term ventilated infants (23 to 29 wk of gestational age) showed histologic features characteristic of the early saccular stage of lung development: the peripheral lung parenchyma was composed of large-sized primitive acini, separated by relatively wide septa, showing focal septation of the acinar units by vascularized ridges (“secondary crests”) (Figure 3A). Compared with control lungs, the lungs of short-term ventilated infants showed varying degrees of septal widening and hypercellularity, often associated with focal pulmonary hemorrhage and interstitial emphysema (Figure 3B).
TABLE 4.
CLINICAL DATA OF PATIENTS STUDIED BY IMMUNOHISTOCHEMISTRY
| Early (23–29 wk)
|
Late (32–39 wk)
|
|||
|---|---|---|---|---|
| Control | Ventilated | Control | Ventilated | |
| (n = 6) | (n = 5) | (n = 4) | (n = 5) | |
| Age at birth*, wk | 25.3 ± 1.9 | 25.8 ± 2.4 | 37.0 ± 2.7 | 27.2 ± 2.7 |
| Postnatal age, d | <0.5 | 11.6 ± 5.4 | <0.5 | 54.0 ± 6.5 |
| Corrected age at death*, wk | 24.8 ± 2.0 | 27.2 ± 3.4 | 37.4 ± 2.9 | 35.8 ± 3.1 |
| Sex | 2 M/4 F | 2 M/3 F | 2 M/2 F | 3 M/2 F |
| Body weight at autopsy, g | 692 ± 180 | 853 ± 235 | 2,808 ± 484 | 3,492 ± 967 |
| Clinical/autopsy diagnosis | PROM/extreme prematurity (3); chorioamnionitis ± sepsis (1); COD undetermined (2) | Early BPD with complications of prematurity (3); early BPD with sepsis (2) | Sepsis (1); intrapartum death/placental insufficiency (1); maternal fatty liver of infancy (1); abruption (1) | BPD (1); BPD with sepsis/pneumonia (1); BPD with NEC (1); BPD with viral infection (2) |
For definition of abbreviations, see Table 2.
Values represent means ± SD of (n) patients.
Age and corrected age reflect postmenstrual age.
Figure 3.
Lung histology and platelet endothelial cell adhesion molecule (PECAM)-1/endoglin immunohistochemistry of early control subjects and short-term ventilated patients. (A) Early control lung showing large-sized, simple airspaces, relatively wide septa, and focal early secondary crest formation, characteristic of late canalicular/early saccular stage of lung development (infant born at 24.5 wk of gestation, lived for 30 min). (B) Short-term ventilated lung showing widening and increased cellularity of the septa, associated with focal hemorrhages within the air spaces (infant born at 23 wk, lived for 5 d, ventilated). (C) PECAM-1 (CD31) immunostaining of early control lung, highlighting the double subepithelial capillary network characteristic of the canalicular/saccular stage of lung development. (D) PECAM-1 (CD31) immunostaining of short-term ventilated lung, showing abundant, disarrayed capillary structures within the expanded septa. (E) Endoglin immunostaining of early control lung corresponding to (C), showing endoglin immunoreactivity in association with a peripheral arteriole (arrow). Endoglin staining is virtually absent in capillary structures within the septa. (F) Endoglin immunostaining of short-term ventilated lung corresponding to (D), showing abundant endoglin-positive capillary structures within the widened septa. (A and B) Hematoxylin–eosin staining (H&E); (C and D) PECAM-1 (CD31) immunohistochemistry, DAB (3,3′-diaminobenzidine tetrachloride) with hematoxylin counterstain; (E and F) endoglin (CD105) immunohistochemistry, DAB with hematoxylin counterstain. Original magnification: (A and B) ×200; (C–F) ×400.
PECAM-1 (CD31) immunostaining of early control lungs at 23–29 weeks (late canalicular/saccular stage) revealed a double capillary network within the septa that was frequently localized immediately beneath the alveolar epithelial lining (Figure 3C). Lungs of short-term ventilated infants of the same age displayed abundant microvascular structures within the septa, both subepithelially and centrally (Figure 3D). Although the size of the septal microvessels varied greatly, they were mostly of small caliber and were haphazardly arranged within the interstitium.
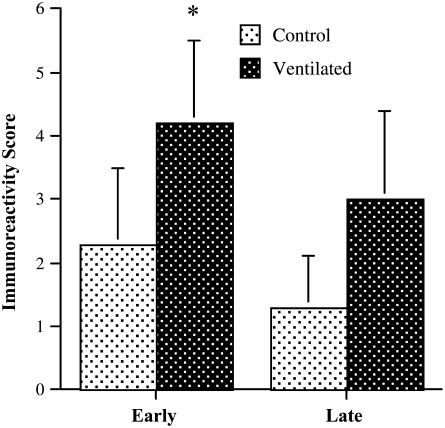
The cellular distribution of endoglin was determined by immunohistochemical analysis, using a monoclonal anti–human endoglin antibody (Figures 3E and 3F). Endoglin immunoreactivity was associated with microvascular structures within the peripheral lung parenchyma, as well as larger sized central vascular structures. Considerable variability in endoglin immunostaining was noted between patients of the same study group, which may, at least in part, be due to differences in protein degradation and length of fixation inherent in autopsy material. Overall, however, the cumulative immunoreactivity score, which takes into account both intensity and distribution of staining, was higher in the lungs of short-term ventilated patients compared with control patients (see Figure 5).
Figure 5.
Endoglin immunoreactivity score. Cumulative immunoreactivity score, obtained by adding distribution and intensity scores. Values represent means ± SD. *P < 0.05 (Mann-Whitney U test).
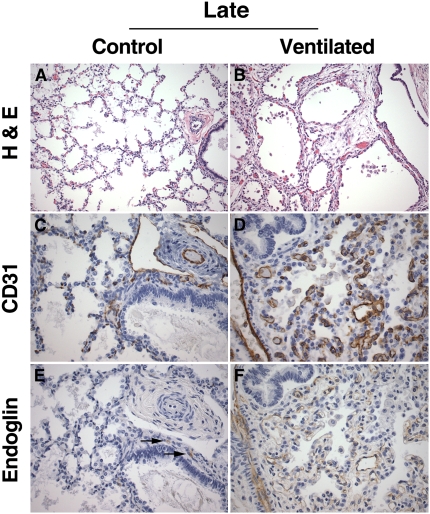
Lungs of late control patients showed thin septa with abundant secondary crests and focal evidence of early alveolarization, consistent with the late saccular/early alveolar stage of development (Figure 4A). The lungs of age-matched long-term ventilated patients showed large and simple airspaces, separated by wide and hypercellular septa, characteristic of fully established BPD (Figure 4B). PECAM-1 immunostaining of late control lungs revealed a delicate single capillary network within the thin septa, displaying abundant branching points into secondary crests (Figure 4C). The PECAM-immunoreactive microvascular network of long-term ventilated BPD lungs exhibited the double “tram-track” appearance typical of more primitive stages, associated with a striking paucity of sprouting (Figure 4D). Endoglin immunoreactivity in late control lungs was weak and limited to scattered centrally located microvascular structures (Figure 4E). Endoglin staining in long-term ventilated BPD lungs was highly variable. Overall, the immunoreactivity score tended to be higher in patients with BPD than in late control subjects (Figures 4E and 5). However, this difference did not reach significance, which may be due, at least in part, to the relatively small sample size available for analysis.
Figure 4.
Lung histology and platelet endothelial cell adhesion molecule (PECAM)-1/endoglin immunohistochemistry of late control subjects and long-term ventilated patients. (A) Late control lung showing late saccular/early alveolar architecture characterized by thin alveolar septa and abundant secondary crests (nonmacerated stillborn delivered at 36 weeks gestation, cause of death unknown). (B) Long-term ventilated lung showing simple, large-sized airspaces with wide and hypercellular septa (infant born at 24 weeks, lived for 56 days, ventilated). (C) PECAM-1 (CD31) immunostaining of late control lung, showing branching capillary structures within the alveolar septa and immunoreactive endothelium in central arterial and lymphatic structures. (D) PECAM-1 (CD31) immunostaining of long-term ventilated lung, showing abundant, primitive-appearing and predominantly nonbranching capillary structures within the expanded septa. (E) Endoglin immunostaining of late control lung corresponding to (C), showing weak endoglin immunoreactivity limited to rare central capillary structures (arrows). Endoglin staining is absent in the interstitial microvasculature. (F) Endoglin immunostaining of long-term ventilated lung corresponding to (D), showing diffuse endoglin immunoreactivity in association with capillary structures in the widened septa. (A and B) Hematoxylin–eosin staining (H&E); (C and D) PECAM-1 (CD31) immunohistochemistry, DAB (3,3′-diaminobenzidine tetrachloride) with hematoxylin counterstain; (E and F) endoglin (CD105) immunohistochemistry, DAB with hematoxylin counterstain. Original magnification: (A and B) ×200; (C–F) ×400.
DISCUSSION
We have previously demonstrated that the pulmonary microvasculature of ventilated preterm infants undergoes marked growth, nearly proportionate to the expansion of the distal air-exchanging lung parenchyma (10). Although quantitatively almost normal, the microvasculature of ventilated lungs displays a primitive, nonbranching architecture, suggestive of a failure of angiogenesis (10). Angiogenesis, here defined as the sprouting of new vessels from preexisting ones, is a complex and highly coordinated process that requires endothelial cell activation, proliferation and directional migration, extracellular matrix generation and remodeling, recruitment of mural cells (pericytes and smooth muscle cells), and specialization of the vessel wall for regulation of vessel function (47–49). Orchestration of these complex functions is regulated by various pro- and antiangiogenic growth factors, angiogenic chemokines/cytokines, matrix-degrading proteases, and cell–extracellular matrix interactions (47–50).
Previous studies of the molecular regulation of angiogenesis in BPD lungs have focused mainly on the major angiogenic growth factors traditionally associated with vascular morphogenesis, such as VEGF and Ang-1 (9, 12, 19–22). In view of the important role of the accessory TGF-β receptor endoglin (CD105) in a variety of neoplastic and nonneoplastic angiogenic conditions (23–30), we speculated that endoglin may be implicated in ventilation-induced angiogenesis in preterm lungs as well.
Endoglin expression was studied in postmortem lung samples from short- and long-term ventilated preterm infants. We determined that endoglin mRNA and protein levels were significantly higher in short-term ventilated lungs than in age-matched control lungs; endoglin was immunolocalized to the peripheral microvasculature. A similar tendency to increased endoglin mRNA and protein levels was noted in long-term ventilated lungs with fully established BPD. In this group, however, a considerably wider range of endoglin levels was noted, which may be related to the greater clinical variability associated with a more protracted stay in neonatal intensive care. In concordance with previous reports in humans and BPD-like animal models, we determined that pulmonary levels of VEGF and Ang-1 and of their respective receptors, Flt-1 and TIE-2, were significantly lower in ventilated infants (9, 12, 19).
The significant up-regulation of pulmonary endoglin in short-term ventilated lungs, especially in the context of down-regulated VEGF and Ang-1, suggests that endoglin may be an important angiogenic factor in ventilation-induced vascular remodeling. Similarly, endoglin up-regulation has been described in a rapidly increasing number of neoplastic and nonneoplastic conditions characterized by prominent angiogenesis (26–30). Endoglin is an accessory receptor for TGF-β (51), a pleiotropic multifunctional cytokine involved in cellular proliferation, differentiation, and migration (52, 53). Endoglin is believed to stimulate endothelial proliferation by interference with inhibitory TGF-β signaling, possible by modulating the balance between the TGF-β/activin receptor-like kinase-1 (ALK1) and TGF-β/ALK5 signaling pathways (23, 24, 54–56).
The functional involvement of endoglin in angiogenesis is based mainly on evidence derived from loss-of-function clinical or experimental conditions. Mutations in the endoglin gene have been implicated in the autosomal dominant vascular disorder termed hereditary hemorrhagic telangiectasia type I (41). Endoglin null mice (Eng−/−) are embryonally lethal, dying in utero around midgestation because of defects in cardiovascular development and angiogenesis, leading to dilated vessels prone to rupture and hemorrhages (38–40). Mice heterozygous at the endoglin locus (Eng+/−) mature to adulthood and can develop hereditary hemorrhagic telangiectasia (38).
The in vivo effects of endoglin overexpression on capillary growth patterns are, to our knowledge, unknown. Endoglin has been shown to regulate the expression of various components of the extracellular matrix including fibronectin, collagen, plasminogen activator inhibitor-1, and lumican (57–60), suggesting a crucial role of endoglin in cellular transmigration. In mouse fibroblasts in culture, endoglin overexpression has been shown to alter cellular morphology, migration, and intercellular cluster formation and to modulate extracellular matrix production (58). Although these studies suggest that endoglin may play a role in cellular migration and extracellular matrix remodeling essential for capillary sprouting, the functional contribution of endoglin upregulation to the primitive, nonbranching angioarchitecture of BPD lungs remains to be determined.
Endoglin is a disulfide-linked homodimeric membrane glycoprotein. Molecular cloning of the human endoglin cDNA has demonstrated the existence of two protein isoforms, arising by alternative splicing. L-endoglin, the predominant isoform, has a cytoplasmic domain of 47 residues, whereas the minor isoform, S-endoglin, contains a cytoplasmic tail of only 14 amino acids (61, 62). Both endoglin isoforms can bind ligand (62), but differ in their capacity to regulate TGF-β–dependent responses (63). Similar L- and S-endoglin variants have been identified in murine tissues (64). In mice, S-endoglin is believed to have antiangiogenic functions, in contrast to the proangiogenic influence of L-endoglin (64). In our study, Western blot analysis identified two endoglin-immunoreactive bands of approximately 170 and 160 kD, consistent with the L-endoglin and S-endoglin dimers, respectively. In accordance with previous reports, L-endoglin was the dominant form in human lung homogenates. Although both isoforms appeared to be upregulated in short-term ventilated lungs, the increase was more pronounced for the L-isoform. Whether this implies that the net effect of pulmonary endoglin upregulation in ventilated preterm lungs is proangiogenic remains undetermined.
In addition to its known role in cardiovascular development and remodeling (38–41), endoglin has other functions that may potentially contribute to the pathogenesis of BPD in ventilated preterm lungs. Endoglin has been shown to play a role in actin cytoskeletal organization (65). In endothelial cells, where endoglin is highly expressed, actin filaments play a crucial role in angiogenesis and vascular permeability. It is possible that upregulated pulmonary endoglin may modulate vascular permeability in ventilated preterm lungs, as interstitial edema is a morphologic hallmark of early BPD (10).
The regulation of endoglin overexpression in ventilated preterm lungs remains unclear. The levels of endoglin protein, mRNA, and promoter activity are known to be up-regulated by hypoxia (66) and by TGF-β1 (63), which cooperate to induce the expression of endoglin at the transcriptional level (66). Although hypoxia may play a role in BPD-associated endoglin upregulation, the importance of other factors implicated in BPD, such as oxygen toxicity, infection/inflammation, glucocorticoids, and mechanical stretch/strain2 remains to be determined. In this context, it needs to be emphasized that the terms “short-term ventilated” and “long-term ventilated,” used in this study to describe preterm infants at risk for BPD and older post-premature infants with established BPD, respectively, are mainly terms of convenience and not meant to imply that ventilation is deemed the sole or major cause of endoglin up-regulation or BPD.
Of note, this study was entirely based on postmortem human lung tissues, traditionally regarded as unsuitable for molecular analyses. For this study, no attempt was made to expedite the postmortem examination, which resulted in postmortem intervals (time between death and autopsy) often longer than 24 hours. Of interest, several samples obtained more than 24 hours after death still yielded high-quality protein and RNA, adequate for Western blot analysis and RPA.
In summary, we have documented upregulation of endoglin in the pulmonary microvasculature of ventilated preterm infants at risk for BPD. This study, performed with postmortem lung tissues, addresses the most severe (fatal) end of lung injury produced by ventilation and preterm birth. However, it is likely that similar events may occur in less severe nonlethal preterm lung injury. Although we focused on endoglin as a potential alternative angiogenic factor in BPD, it is conceivable that other factors such as angiogenic growth factors, proinflammatory chemokines, and extracellular matrix components may contribute to BPD-associated dysangiogenesis.
Acknowledgments
The authors thank Terry Pasquariello for assistance with immunohistochemical analyses. The authors are also grateful to Francois I. Luks, M.D., Ph.D., for insightful discussions and review of the manuscript.
Supported in part by National Institutes of Health P20-RR18728 (M.E.D.P.).
Originally Published in Press as DOI: 10.1164/rccm.200608-1240OC on April 17, 2008
Conflict of Interest Statement: None of the authors has a financial relationship with a commercial entity that has an interest in the subject of this manuscript.
References
- 1.Jobe AH, Ikegami M. Mechanisms initiating lung injury in the preterm. Early Hum Dev 1998;53:81–94. [DOI] [PubMed] [Google Scholar]
- 2.Jobe AH, Bancalari E. Bronchopulmonary dysplasia. Am J Respir Crit Care Med 2001;163:1723–1729. [DOI] [PubMed] [Google Scholar]
- 3.Lemons JA, Bauer CR, Oh W, Korones SB, Papile LA, Stoll BJ, Verter J, Temprosa M, Wright LL, Ehrenkranz RA, et al.; NICHD Neonatal Research Network. Very low birth weight outcomes of the National Institute of Child Health and Human Development Neonatal Research Network, January 1995 through December 1996. Pediatrics 2001;107:E1. [DOI] [PubMed] [Google Scholar]
- 4.Erickson AM, de la Monte SM, Moore GW, Hutchins GM. The progression of morphologic changes in bronchopulmonary dysplasia. Am J Pathol 1987;127:474–484. [PMC free article] [PubMed] [Google Scholar]
- 5.Bonikos DS, Bensch KG, Northway WH Jr, Edwards DK. Bronchopulmonary dysplasia: the pulmonary pathologic sequel of necrotizing bronchiolitis and pulmonary fibrosis. Hum Pathol 1976;7:643–666. [DOI] [PubMed] [Google Scholar]
- 6.Husain AN, Siddiqui NH, Stocker JT. Pathology of arrested acinar development in postsurfactant bronchopulmonary dysplasia. Hum Pathol 1998;29:710–717. [DOI] [PubMed] [Google Scholar]
- 7.Jobe AJ. The new BPD: an arrest of lung development. Pediatr Res 1999;46:641–643. [DOI] [PubMed] [Google Scholar]
- 8.Coalson JJ. Pathology of chronic lung disease in early infancy. In: Bland RD, Coalson JJ, editors. Chronic lung disease in early infancy. New York: Marcel Dekker; 2000. pp. 85–124.
- 9.Bhatt AJ, Pryhuber GS, Huyck H, Watkins RH, Metlay LA, Maniscalco WM. Disrupted pulmonary vasculature and decreased vascular endothelial growth factor, Flt-1, and TIE-2 in human infants dying with bronchopulmonary dysplasia. Am J Respir Crit Care Med 2001;164:1971–1980. [DOI] [PubMed] [Google Scholar]
- 10.De Paepe ME, Mao Q, Powell J, Rubin SE, DeKoninck P, Appel N, Dixon M, Gundogan F. Growth of pulmonary microvasculature in ventilated preterm infants. Am J Respir Crit Care Med 2006;173:204–211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Coalson JJ, Winter VT, Siler-Khodr T, Yoder BA. Neonatal chronic lung disease in extremely immature baboons. Am J Respir Crit Care Med 1999;160:1333–1346. [DOI] [PubMed] [Google Scholar]
- 12.Maniscalco WM, Watkins RH, Pryhuber GS, Bhatt A, Shea C, Huyck H. Angiogenic factors and alveolar vasculature: development and alterations by injury in very premature baboons. Am J Physiol Lung Cell Mol Physiol 2002;282:L811–L823. [DOI] [PubMed] [Google Scholar]
- 13.Jakkula M, Le Cras TD, Gebb S, Hirth KP, Tuder RM, Voelkel NF, Abman SH. Inhibition of angiogenesis decreases alveolarization in the developing rat lung. Am J Physiol Lung Cell Mol Physiol 2000;279:L600–L607. [DOI] [PubMed] [Google Scholar]
- 14.Le Cras TD, Markham NE, Tuder RM, Voelkel NF, Abman SH. Treatment of newborn rats with a VEGF receptor inhibitor causes pulmonary hypertension and abnormal lung structure. Am J Physiol Lung Cell Mol Physiol 2002;283:L555–L562. [DOI] [PubMed] [Google Scholar]
- 15.Gerber HP, Hillan KJ, Ryan AM, Kowalski J, Keller GA, Rangell L, Wright BD, Radtke F, Aguet M, Ferrara N. VEGF is required for growth and survival in neonatal mice. Development 1999;126:1149–1159. [DOI] [PubMed] [Google Scholar]
- 16.Galambos C, Ng YS, Ali A, Noguchi A, Lovejoy S, D'Amore PA, DeMello DE. Defective pulmonary development in the absence of heparin-binding vascular endothelial growth factor isoforms. Am J Respir Cell Mol Biol 2002;27:194–203. [DOI] [PubMed] [Google Scholar]
- 17.Abman SH. Bronchopulmonary dysplasia: a vascular hypothesis. Am J Respir Crit Care Med 2001;164:1755–1756. [DOI] [PubMed] [Google Scholar]
- 18.Thebaud B, Abman SH. Bronchopulmonary dysplasia: where have all the vessels gone? Roles of angiogenic growth factors in chronic lung disease. Am J Respir Crit Care Med 2007;175:978–985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lassus P, Turanlahti M, Heikkila P, Andersson LC, Nupponen I, Sarnesto A, Andersson S. Pulmonary vascular endothelial growth factor and Flt-1 in fetuses, in acute and chronic lung disease, and in persistent pulmonary hypertension of the newborn. Am J Respir Crit Care Med 2001;164:1981–1987. [DOI] [PubMed] [Google Scholar]
- 20.Ambalavanan N, Novak ZE. Peptide growth factors in tracheal aspirates of mechanically ventilated preterm neonates. Pediatr Res 2003;53:240–244. [DOI] [PubMed] [Google Scholar]
- 21.D'Angio CT, Maniscalco WM, Ryan RM, Avissar NE, Basavegowda K, Sinkin RA. Vascular endothelial growth factor in pulmonary lavage fluid from premature infants: effects of age and postnatal dexamethasone. Biol Neonate 1999;76:266–273. [DOI] [PubMed] [Google Scholar]
- 22.Currie AE, Vyas JR, MacDonald J, Field D, Kotecha S. Epidermal growth factor in the lungs of infants developing chronic lung disease. Eur Respir J 2001;18:796–800. [DOI] [PubMed] [Google Scholar]
- 23.Bernabeu C, Conley BA, Vary CP. Novel biochemical pathways of endoglin in vascular cell physiology. J Cell Biochem 2007;102:1375–1388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lebrin F, Mummery CL. Endoglin-mediated vascular remodeling: mechanisms underlying hereditary hemorrhagic telangiectasia. Trends Cardiovasc Med 2008;18:25–32. [DOI] [PubMed] [Google Scholar]
- 25.Gougos A, Letarte M. Identification of a human endothelial cell antigen with monoclonal antibody 44G4 produced against a pre-B leukemic cell line. J Immunol 1988;141:1925–1933. [PubMed] [Google Scholar]
- 26.Burrows FJ, Derbyshire EJ, Tazzari PL, Amlot P, Gazdar AF, King SW, Letarte M, Vitetta ES, Thorpe PE. Up-regulation of endoglin on vascular endothelial cells in human solid tumors: implications for diagnosis and therapy. Clin Cancer Res 1995;1:1623–1634. [PubMed] [Google Scholar]
- 27.Miller DW, Graulich W, Karges B, Stahl S, Ernst M, Ramaswamy A, Sedlacek HH, Muller R, Adamkiewicz J. Elevated expression of endoglin, a component of the TGF-β–receptor complex, correlates with proliferation of tumor endothelial cells. Int J Cancer 1999;81:568–572. [DOI] [PubMed] [Google Scholar]
- 28.Torsney E, Charlton R, Parums D, Collis M, Arthur HM. Inducible expression of human endoglin during inflammation and wound healing in vivo. Inflamm Res 2002;51:464–470. [DOI] [PubMed] [Google Scholar]
- 29.Fonsatti E, Del Vecchio L, Altomonte M, Sigalotti L, Nicotra MR, Coral S, Natali PG, Maio M. Endoglin: an accessory component of the TGF-β–binding receptor–complex with diagnostic, prognostic, and bioimmunotherapeutic potential in human malignancies. J Cell Physiol 2001;188:1–7. [DOI] [PubMed] [Google Scholar]
- 30.Fonsatti E, Altomonte M, Nicotra MR, Natali PG, Maio M. Endoglin (CD105): a powerful therapeutic target on tumor-associated angiogenetic blood vessels. Oncogene 2003;22:6557–6563. [DOI] [PubMed] [Google Scholar]
- 31.Gougos A, St Jacques S, Greaves A, O'Connell PJ, d'Apice AJ, Buhring HJ, Bernabeu C, van Mourik JA, Letarte M. Identification of distinct epitopes of endoglin, an RGD-containing glycoprotein of endothelial cells, leukemic cells, and syncytiotrophoblasts. Int Immunol 1992;4:83–92. [DOI] [PubMed] [Google Scholar]
- 32.St-Jacques S, Cymerman U, Pece N, Letarte M. Molecular characterization and in situ localization of murine endoglin reveal that it is a transforming growth factor-β binding protein of endothelial and stromal cells. Endocrinology 1994;134:2645–2657. [DOI] [PubMed] [Google Scholar]
- 33.Robledo MM, Hidalgo A, Lastres P, Arroyo AG, Bernabeu C, Sanchez-Madrid F, Teixido J. Characterization of TGF-β1–binding proteins in human bone marrow stromal cells. Br J Haematol 1996;93:507–514. [DOI] [PubMed] [Google Scholar]
- 34.Adam PJ, Clesham GJ, Weissberg PL. Expression of endoglin mRNA and protein in human vascular smooth muscle cells. Biochem Biophys Res Commun 1998;247:33–37. [DOI] [PubMed] [Google Scholar]
- 35.Chen K, Mehta JL, Li D, Joseph L, Joseph J. Transforming growth factor β receptor endoglin is expressed in cardiac fibroblasts and modulates profibrogenic actions of angiotensin II. Circ Res 2004;95:1167–1173. [DOI] [PubMed] [Google Scholar]
- 36.Lastres P, Bellon T, Cabanas C, Sanchez-Madrid F, Acevedo A, Gougos A, Letarte M, Bernabeu C. Regulated expression on human macrophages of endoglin, an Arg-Gly-Asp–containing surface antigen. Eur J Immunol 1992;22:393–397. [DOI] [PubMed] [Google Scholar]
- 37.Buhring HJ, Muller CA, Letarte M, Gougos A, Saalmuller A, van Agthoven AJ, Busch FW. Endoglin is expressed on a subpopulation of immature erythroid cells of normal human bone marrow. Leukemia 1991;5:841–847. [PubMed] [Google Scholar]
- 38.Bourdeau A, Dumont DJ, Letarte M. A murine model of hereditary hemorrhagic telangiectasia. J Clin Invest 1999;104:1343–1351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Li DY, Sorensen LK, Brooke BS, Urness LD, Davis EC, Taylor DG, Boak BB, Wendel DP. Defective angiogenesis in mice lacking endoglin. Science 1999;284:1534–1537. [DOI] [PubMed] [Google Scholar]
- 40.Arthur HM, Ure J, Smith AJ, Renforth G, Wilson DI, Torsney E, Charlton R, Parums DV, Jowett T, Marchuk DA, et al. Endoglin, an ancillary TGFβ receptor, is required for extraembryonic angiogenesis and plays a key role in heart development. Dev Biol 2000;217:42–53. [DOI] [PubMed] [Google Scholar]
- 41.McAllister KA, Grogg KM, Johnson DW, Gallione CJ, Baldwin MA, Jackson CE, Helmbold EA, Markel DS, McKinnon WC, Murrell J, et al. Endoglin, a TGF-β binding protein of endothelial cells, is the gene for hereditary haemorrhagic telangiectasia type 1. Nat Genet 1994;8:345–351. [DOI] [PubMed] [Google Scholar]
- 42.De Paepe ME. Lung growth and development. In: Churg AM, Myers JL, Tazelaar HD, Wright JL, editors. Thurlbeck's pathology of the lung. New York: Thieme Medical Publishers; 2005.
- 43.De Paepe ME, Friedman RM, Gundogan F, Pinar H. Postmortem lung weight/body weight standards for term and preterm infants. Pediatr Pulmonol 2005;40:445–448. [DOI] [PubMed] [Google Scholar]
- 44.De Paepe ME, Mao Q, Huang C, Zhu D, Jackson CL, Hansen K. Postmortem RNA and protein stability in perinatal human lungs. Diagn Mol Pathol 2002;11:170–176. [DOI] [PubMed] [Google Scholar]
- 45.Chomczynski P, Sacchi N. Single-step method of RNA isolation by acid guanidinium thiocyanate–phenol–chloroform extraction. Anal Biochem 1987;162:156–159. [DOI] [PubMed] [Google Scholar]
- 46.De Paepe ME, Mao Q, Embree-Ku M, Rubin LP, Luks FI. Fas/FasL-mediated apoptosis in perinatal murine lungs. Am J Physiol Lung Cell Mol Physiol 2004;287:L730–L742. [DOI] [PubMed] [Google Scholar]
- 47.Jain RK. Molecular regulation of vessel maturation. Nat Med 2003;9:685–693. [DOI] [PubMed] [Google Scholar]
- 48.Risau W. Mechanisms of angiogenesis. Nature 1997;386:671–674. [DOI] [PubMed] [Google Scholar]
- 49.Carmeliet P. Mechanisms of angiogenesis and arteriogenesis. Nat Med 2000;6:389–395. [DOI] [PubMed] [Google Scholar]
- 50.Adams RH, Alitalo K. Molecular regulation of angiogenesis and lymphangiogenesis. Nat Rev Mol Cell Biol 2007;8:464–478. [DOI] [PubMed] [Google Scholar]
- 51.Cheifetz S, Bellon T, Cales C, Vera S, Bernabeu C, Massague J, Letarte M. Endoglin is a component of the transforming growth factor-β receptor system in human endothelial cells. J Biol Chem 1992;267:19027–19030. [PubMed] [Google Scholar]
- 52.Govinden R, Bhoola KD. Genealogy, expression, and cellular function of transforming growth factor-β. Pharmacol Ther 2003;98:257–265. [DOI] [PubMed] [Google Scholar]
- 53.Bobik A. Transforming growth factor-βs and vascular disorders. Arterioscler Thromb Vasc Biol 2006;26:1712–1720. [DOI] [PubMed] [Google Scholar]
- 54.Lebrin F, Goumans MJ, Jonker L, Carvalho RL, Valdimarsdottir G, Thorikay M, Mummery C, Arthur HM, ten Dijke P. Endoglin promotes endothelial cell proliferation and TGF-β/ALK1 signal transduction. EMBO J 2004;23:4018–4028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Li C, Hampson IN, Hampson L, Kumar P, Bernabeu C, Kumar S. CD105 antagonizes the inhibitory signaling of transforming growth factor β1 on human vascular endothelial cells. FASEB J 2000;14:55–64. [DOI] [PubMed] [Google Scholar]
- 56.Warrington K, Hillarby MC, Li C, Letarte M, Kumar S. Functional role of CD105 in TGF-β1 signalling in murine and human endothelial cells. Anticancer Res 2005;25:1851–1864. [PubMed] [Google Scholar]
- 57.Diez-Marques L, Ortega-Velazquez R, Langa C, Rodriguez-Barbero A, Lopez-Novoa JM, Lamas S, Bernabeu C. Expression of endoglin in human mesangial cells: modulation of extracellular matrix synthesis. Biochim Biophys Acta 2002;1587:36–44. [DOI] [PubMed] [Google Scholar]
- 58.Guerrero-Esteo M, Lastres P, Letamendia A, Perez-Alvarez MJ, Langa C, Lopez LA, Fabra A, Garcia-Pardo A, Vera S, Letarte M, et al. Endoglin overexpression modulates cellular morphology, migration, and adhesion of mouse fibroblasts. Eur J Cell Biol 1999;78:614–623. [DOI] [PubMed] [Google Scholar]
- 59.Botella LM, Sanz-Rodriguez F, Sanchez-Elsner T, Langa C, Ramirez JR, Vary C, Roughley PJ, Bernabeu C. Lumican is down-regulated in cells expressing endoglin: evidence for an inverse correlationship between endoglin and lumican expression. Matrix Biol 2004;22:561–572. [DOI] [PubMed] [Google Scholar]
- 60.Obreo J, Diez-Marques L, Lamas S, Duwell A, Eleno N, Bernabeu C, Pandiella A, Lopez-Novoa JM, Rodriguez-Barbero A. Endoglin expression regulates basal and TGF-β1–induced extracellular matrix synthesis in cultured L6E9 myoblasts. Cell Physiol Biochem 2004;14:301–310. [DOI] [PubMed] [Google Scholar]
- 61.Gougos A, Letarte M. Primary structure of endoglin, an RGD-containing glycoprotein of human endothelial cells. J Biol Chem 1990;265:8361–8364. [PubMed] [Google Scholar]
- 62.Bellon T, Corbi A, Lastres P, Cales C, Cebrian M, Vera S, Cheifetz S, Massague J, Letarte M, Bernabeu C. Identification and expression of two forms of the human transforming growth factor-β–binding protein endoglin with distinct cytoplasmic regions. Eur J Immunol 1993;23:2340–2345. [DOI] [PubMed] [Google Scholar]
- 63.Lastres P, Letamendia A, Zhang H, Rius C, Almendro N, Raab U, Lopez LA, Langa C, Fabra A, Letarte M, et al. Endoglin modulates cellular responses to TGF-β1. J Cell Biol 1996;133:1109–1121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Perez-Gomez E, Eleno N, Lopez-Novoa JM, Ramirez JR, Velasco B, Letarte M, Bernabeu C, Quintanilla M. Characterization of murine S-endoglin isoform and its effects on tumor development. Oncogene 2005;24:4450–4461. [DOI] [PubMed] [Google Scholar]
- 65.Sanz-Rodriguez F, Guerrero-Esteo M, Botella LM, Banville D, Vary CP, Bernabeu C. Endoglin regulates cytoskeletal organization through binding to ZRP-1, a member of the Lim family of proteins. J Biol Chem 2004;279:32858–32868. [DOI] [PubMed] [Google Scholar]
- 66.Sanchez-Elsner T, Botella LM, Velasco B, Langa C, Bernabeu C. Endoglin expression is regulated by transcriptional cooperation between the hypoxia and transforming growth factor-β pathways. J Biol Chem 2002;277:43799–43808. [DOI] [PubMed] [Google Scholar]