Synopsis
Pain after thoracotomy is very severe, probably the most severe pain experienced after surgery. It is also unique as this pain state has multiple implications, including respiratory failure due to splinting; inability to clear secretions by effective coughing, with resulting pneumonia; and facilitation of the often incapacitating chronic pain: the post-thoracotomy pain syndrome. Thoracic epidural analgesia has greatly improved the pain experience and its consequences and has been considered the ‘gold standard’ for pain management after thoracotomy. This view has recently been challenged by the use of paravertebral nerve blocks. Nevertheless, severe ipsilateral shoulder pain and the prevention of the post-thoracotomy pain syndrome remain the most important challenges for post-thoracotomy pain management.
Introduction
A thoracotomy requires a very painful incision, involving multiple muscle layers, rib resection, and continuous motion as the patient breathes. Treatment of acute post-thoracotomy pain is particularly important not only to keep the patient comfortable but also to minimize pulmonary complications. It enables patients to ambulate and to breathe normally (without splinting) and deeply (to allow cough). The effects of chronic post-thoracotomy pain are generally less detrimental to respiration but can be incapacitating, making daily activities impossible.
Many methods of pain management, each with attendant problems, have been tried with varied success, for example: intercostal nerve block (1;2), intrapleural analgesia (3;4), cryoanalgesia (5;6), lumbar epidural (7;8), thoracic epidural (9;10), paravertebral block (11–13), IV narcotics (8;14;15), intrathecal (16–18) or epidural (2;15;19;20) narcotics, NSAIDS (1;21;22), and transcutaneous nerve stimulation (23–25).
Acute post-thoracotomy pain
Severe acute pain after thoracotomy due to retraction, resection, or fracture of ribs, dislocation of costovertebral joints, injury of intercostal nerves, and further irritation of the pleura by chest tubes is a normal response to all these insults (26). Acute pain after video-assisted thoracoscopic surgery is considered less severe.
Sub-optimal management of pain after thoracotomy (or after video-assisted thoracoscopic surgery in patients with severely limited respiratory reserve) has major respiratory consequences. Inspiration is limited by pain, which leads to reflex contraction of expiratory muscles, and consecutively to diaphragmatic dysfunction (decreased functional residual capacity or FRC and atelectasis, shunting, hypoxemia). In addition, most patients are extubated early to decrease the risk of pulmonary barotrauma (particularly “blowout” of the bronchial suture line) and to prevent respiratory sequelae like pulmonary infection. Deep breathing requires stretching the incision. As this may be extremely painful, patients without adequate analgesia try to prevent stretching of the skin incision by contracting their expiratory muscles, i.e., splinting, thus limiting the stretch on the incision during inspiration. This failure to inspire deeply before a forceful exhalation results in an ineffective cough, which in turn promotes retention of secretions, leading to airway closure and atelectasis, reinforcing the importance of adequate analgesia following thoracotomy to avoid the need for reintubation due to inadequate pulmonary toilet.
Diaphragmatic contraction is also impaired; however, thoracic epidural anesthesia has been shown to increase diaphragmatic shortening after thoracotomy in the awake lamb (27;28).
Chronic post-thoracotomy pain
Post-thoracotomy pain syndrome or PTPS (chronic post-thoracotomy pain or post-thoracotomy neuralgia) is defined by the International Association for the Study of Pain (IASP) as ‘pain that recurs or persists along a thoracotomy incision at least two months following the surgical procedure’. In general, it is burning and stabbing pain with dysesthesia and thus shares many features of neuropathic pain (29). PTPS is increasingly acknowledged by anesthesiologists and surgeons alike (30).
Prevalence of post-thoracotomy pain
Chronic post-thoracotomy pain was commonly noted by surgeons during the Second World War in men who had had a thoracotomy for chest trauma; it was called chronic intercostal pain. Unfortunately, not much has changed since then, as the majority of patients do not seek help for their pain, but mention it only when specifically asked. Furthermore, despite a commonly held belief that post-thoracotomy pain is transient, there is no evidence that the pain experience decreases significantly over time. For example, incidence of long-term post-thoracotomy pain has been reported to be 80% at 3 months, 75% at 6 months, and 61% at one year after surgery; incidence of severe pain is 3–5%, and pain that interferes with normal life is reported by about 50% of patients (31). In one study, 66% of the patients with PTPS received treatment for pain. (32) In another study, over 70% of the cases with PTPS received three or more of the treatment modalities and regimens that have been reported to be of value. More than 50% needed to be referred to three different types of specialists. Nevertheless, no patient claimed to have become free of symptoms as a result of treatment, and a significant proportion implied that therapy was either more disabling than PTPS or made it worse. (33) For many patients, even the gentlest stimulation provokes intense pain, making participation in routine daily activities impossible. The rate of long-term persistent pain (3–18 months) has been found to be the same after both thoracotomy and thoracoscopic procedures (34;35). However, other authors concluded that the use of video-assisted thoracic surgery for pulmonary resection may decrease the incidence of chronic pain and disability when compared with thoracotomy (36;37).
Although there is a wide variation in reported incidence (probably attributable to differences in the definition of pain), clearly post-thoracotomy pain can be seen as the commonest complication of thoracotomy (38).
Mechanism of post-thoracotomy pain
Mechanisms for chronic pain after thoracotomy are several, and no consensus exists regarding causality.
Intercostal nerve damage
Surgery routinely crushes the intercostal nerve, particularly as the nerve is quite exposed on the caudal side of the rib. It is also not uncommon for the nerve to be totally severed or included in a suture when closing the chest. Among the many possibilities for nerve injury are mechanical damage during rib resection and compression with a retractor. Furthermore, incidental rib fractures may damage the intercostal nerve immediately or entrap an intercostal nerve during healing, leading to neuropathic pain symptoms. The sensation of pain in response to a normally non-painful stimulus (allodynia) or an exaggerated response to a slightly painful stimulus (hyperalgesia), especially when accompanied by numbness, is considered diagnostic for nerve injury. These symptoms occur frequently along the distribution area/innervation area of the intercostal nerves and are the most frequent feature of post-thoracotomy pain (39). Neurophysiological assessment of the intercostal nerve during thoracotomy has demonstrated total conduction block, implying nerve injury during rib retraction (40;41). In another study the authors performed recordings on 24 patients 1 month after thoracotomy and found that patients with a higher degree of intercostal nerve impairment had greater postthoracotomy pain (42).
Tumor recurrence
Many studies have shown that increasing pain may also be an early sign of tumor recurrence (43;44).
Type of incision
Many surgical techniques have been correlated with the amount of postoperative pain. Even muscle-sparing incisions appear to have no major advantage over posterolateral incisions (45). Overall, variation in surgical techniques has not been shown to reduce subsequent pain (46).
Others
Studies suggest that personality traits are strong modulatory factors in the overall post-thoracotomy pain experience. Preoperative anxiety appears to play a major role (47). The costochondral and costovertebral junctions may be disarticulated due to extensive rib retraction, and ipsilateral shoulder disability is also common as a result of division of serratus anterior muscles and latissimus dorsi. Injuries to the muscles responsible for moving the shoulder as well as insufficiently treated pain lead to inadequate rehabilitation and may produce frozen shoulder.
Current treatment options and their associated problems
Epidural analgesia
Technique
Median or paramedian approach
Most practitioners prefer either the median or paramedian approach. There seems to be little difference in terms of patient safety. However, the paramedian approach makes it much easier to locate the epidural space, when overlapping spinous processes might prevent the operator from reaching the epidural space in the median plane.
Asleep vs. awake technique
However, the difference between an awake technique and an asleep technique should have a major impact on potential spinal cord cannulation. Surprisingly, a survey of the practice of thoracic epidural analgesia in the United Kingdom in the not-too-distant past revealed that thoracic epidural cannulation is most often (60%) performed following induction of general anesthesia (48). We strongly discourage insertion of thoracic epidural catheters in anesthetized/paralyzed patients in all but the rarest circumstances (for example, a thoracoscopic surgery that is converted to a thoracotomy in a patient with severely compromised respiratory reserve and extubation is most likely to fail) and then only by very experienced operators. Similarly, others also state that ‘techniques above the termination of the cord …should be avoided (in anesthetized patients)” (Horlocker TT. ASA newsletter April 2001, Vol 65). In addition, when epidural anatomy was examined by cryomicrotome section in humans, it was found that compared with the lumbar level, at the thoracic level the ligamentum flavum is more frequently discontinuous (49), further pointing out the high risk of inserting a catheter into the intrathecal space and even spinal cord in anesthetized patients (50).
Outcome
Many researchers have addressed outcomes after thoracic epidural anesthesia. Since the publication of an extensive review of techniques for pain control after thoracic surgery more than 10 years ago (51), no major new approaches have been proposed since then. Some strongly suggest improved outcomes with utilization of epidural catheters, while others found no improvement. In general, it is difficult to compare these studies. Some authors looked at the effects of different analgesia techniques on long-term post-thoracotomy pain (52), while others investigated the effect of epidural analgesia in the reduction of postoperative myocardial infarction via a meta-analysis(53). Similarly, in a cumulative meta-analysis of randomized, controlled trials, the comparative effects of postoperative analgesic therapies on pulmonary outcome were evaluated; it was concluded that ‘…epidural pain control can significantly decrease the incidence of pulmonary morbidity’ (54). However, some randomized studies looked at epidural vs. iv (or i.m.) on demand and, not surprisingly, found a superior outcome with the epidural group. Overall, it appears important not only whether the patient received epidural analgesia, but also how the epidural was managed: e.g., untreated hypotension with epidural analgesia is clearly detrimental for cardiovascular outcomes.
The shoulder pain reported by thoracotomy patients is mostly referred pain and is not covered by the epidural analgesia. Most surgeons would agree that shoulder pain is a major postoperative pain problem that deserves special attention.
Shoulder pain
Over 75% of thoracotomy patients report constant severe ache in the ipsilateral shoulder post-surgery (55). This pain is relatively resistant to intravenous opioids and is only partially relieved by NSAIDs. Postulated mechanisms include transection of a major bronchus, ligamentous strain from malposition or surgical mobilization of the scapula, pleural irritation due to the thoracostomy tube, or referred pain from irritation of the pericardium or mediastinal and diaphragmatic pleural surfaces.
Several methods have been investigated with varying results. Intrapleural bupivacaine did not provide effective pain relief (56). Superficial cervical plexus or interscalene brachial plexus blocks effectively reduced localized shoulder pain in some patients (57;58), whereas suprascapular nerve block was not helpful (59). Phrenic nerve block via intraoperative infiltration of the periphrenic fat pad with lidocaine reduced the incidence of shoulder pain from 85% to 33% and lowered overall pain scores (60). Ropivacaine 0.2% reduced the incidence and delayed the onset of shoulder pain for the first 24 hours postoperatively with no adverse effect on respiratory function (61).
It appears that the main origin of shoulder pain may be referred pain via the phrenic nerve (blocked by periphrenic infiltration and interscalene brachial plexus block) with contribution from positioning and surgery (coracoid impingement syndrome and coraco-clavicular ligament strain), which is partially relieved by the use of NSAIDS (58;62) and acetoaminophen (63). However, some patients who received phrenic nerve infiltration still reported pain. This could be due to anatomical variations in the emergence of the sensory fibers from the phrenic nerve, reaching the fibrous pericardium and parietal layers of the pleura.
The most effective management strategy would be multimodal, consisting of acetoaminophen (preemptive and regularly), NSAIDS if not contra-indicated, and infiltration of the phrenic nerve with a long-acting local anesthetic.
Intercostal nerve block
Intercostal nerve blockade is used routinely at some centers. The simplest method is a single injection of local anesthetics in multiple intercostal nerves before closure of a thoracotomy incision (five interspaces are usually blocked: two above, two below, and one at the site of the incision). However, single-shot intercostal nerve blocks with local anesthetic generally do not provide effective long-term analgesia and frequently have to be repeated. A longer-lasting method involves continuous infusion of local anesthetics for several days through an indwelling catheter placed in a subpleural/extrapleural pocket, allowing the local anesthetic to diffuse to the nerves. Most surgeons use this approach for thoracoscopic surgeries, although others argue that an intercostal catheter is equivalent to epidural analgesia combined with patient-controlled analgesia (64;65). Another approach is intraoperative cryoneurolysis of the intercostal nerves prior to closure of the thoracotomy incision. Similar to single-shot intercostal blocks, direct application of the probe onto the intercostal nerve at the site of the incision as well as the nerves two levels above and below leads to axon degeneration. Because endoneurium and perineural connective tissue are preserved, restoration of nerve structure occurs 1–3 months after freezing.
Although this has been shown to be effective in decreasing postoperative pain as well as the amount of oral and parenteral analgesics required post-procedure, long-term outcomes have been less positive, due to the high incidence of development of neuropathic pain, dysesthesia, and intercostal muscle paralysis (5;66–68).
As can be seen in fig. 1, an intercostal nerve block by a needle inserted perpendicular to the skin in the posterior axillary line does not cover pain in the more posterior parts of the back. In addition, if a chest tube is not in place, the risk of pneumothorax from the block needs to be considered.
Fig 1.
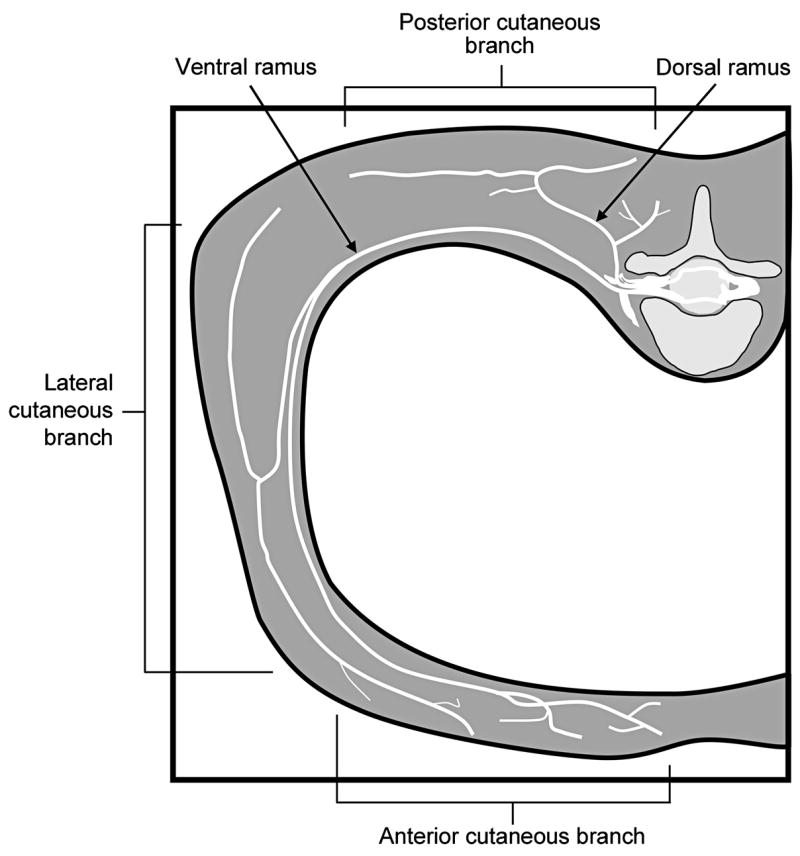
Drawing depicting a typical intercostal nerve and its branches
Paravertebral nerve block
Some authors consider paravertebral block nearly equivalent to epidural analgesia, without the detrimental effects of bilateral sympathetic blockade (69;70). It is somewhat surprising that this technique is not more widespread, though possibly because it has only recently become reintroduced into clinical practice. Fewer practitioners are therefore familiar with it, it is more difficult to thread the catheter compared to epidural, and a loss of resistance is not so much appreciable as with an epidural approach.
Another problem with paravertebral blocks with either the percutaneous approach or the open technique is the relatively high failure rate of ~10%. This might be due to the interference by the endothoracic fascia. Once the tip of the needle or of the catheter is ventral to this fascia, the diffusion of the local anesthetic back to the nerve(s) is severely hindered (71)
With the advent of video-assisted pulmonary resection, more patients undergoing thoracic procedures became ambulatory surgical candidates. Central neuraxial techniques, even those incorporating very low concentrations of neuraxial local anesthetics and opiates, do not appear to be useful in the ambulatory setting. Though paravertebral nerve blocks are a well-established and safe technique, most published results are from patients admitted to the hospital, and application of these principles in the ambulatory setting may not be feasible.
Preemptive analgesia and thoracotomy
Some of the mechanisms for the development of allodynia and hyperalgesia are well-known. The concept of sensitization has led to an increased effort to control acute pain by a more or less total afferent blockade, with the goal of reducing the development of post-thoracotomy pain.
Preemptive analgesia is intended to prevent the establishment of central sensitization caused by incisional and inflammatory injuries. Evidence from basic research has indicated that analgesic drugs are more effective if administered before, rather than after, a noxious stimulus (72;73). The benefit of pre-emptive analgesia has been supported by some clinical studies using local anesthetics (74;75), opioids, and non-steroidal anti-inflammatory drugs (76;77). However, the clinical usefulness of pre-emptive analgesia has remained controversial (78–80), probably due in part to the wide variation in study conditions such as surgery, drugs, doses, routes of administration, and treatment duration as well as pain assessment methods used in different studies (81–83).
Previous studies comparing the effects of pre-operative and post-operative epidural block in abdominal surgery have failed to demonstrate any benefit of pre-emptive analgesia (78;79). This lack of benefit was partly attributed to the less discrete, visceral nature of pain after abdominal surgery. Thoracotomy produces high-intensity noxious stimuli sufficient to cause central sensitization (84;85), and the area of post-thoracotomy pain is more discrete and largely restricted to the site of surgery. Hence, any benefit of preemptive epidural analgesia should, theoretically, be more apparent in thoracic surgery than in abdominal surgery.
Though results from clinical studies so far have not shown a major impact of pre-emptive epidural analgesia on postoperative pain after thoracic surgery (86;87), the concept holds promise, especially in preventing the development of chronic post-thoracotomy pain (52;88).
It has also been suggested that while preemptive analgesia is beneficial in some surgical procedures, it is ineffective in others (14). One explanation offered is that the surgical area is innervated by multiple segmental and cranial nerves (89).
It has also been shown that the degree of acute pain after thoracic surgery predicts long-term post-thoracotomy pain, and hence aggressive management of early postoperative pain may reduce the likelihood of long-term post-thoracotomy pain (84). A good analgesic regime not only reduces pulmonary complications in the immediate perioperative period (90), but also helps in early mobilization (91;92). The most common technique for pain relief is a thoracic epidural, with the catheter in the mid-thoracic region with a continuous infusion of local anesthetic and narcotics (93). Some recent studies (52;85;88;94) have shown beneficial effects (both immediate and late) when preemptive analgesia (nerve blockade either by epidural or intercostal nerve block) was begun before the surgical incision. However, other researchers have found marginal or no benefits even when a multimodal approach was used (86;87;95;96).
In summary, it appears that the most logical explanation for the failure of preemptive analgesia has two components: first, afferent impulse blockade should be complete, which is impossible given the variety of incoming stimuli, and should also last for at least several days postoperatively (97;98). Secondly, a complete “humoral blockade” would be necessary, as it has been shown that circulating pro-inflammatory cytokines lead to central COX-2 induction, e.g., Interleukin-1β-mediated induction of COX-2 in the CNS contributes to inflammatory pain hypersensitivity (99).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Reference List
- 1.Carretta A, Zannini P, Chiesa G, Altese R, Melloni G, Grossi A. Efficacy of ketorolac tromethamine and extrapleural intercostal nerve block on post-thoracotomy pain. A prospective, randomized study. Int Surg. 1996 July;81(3):224–8. [PubMed] [Google Scholar]
- 2.Dauphin A, Lubanska-Hubert E, Young JE, Miller JD, Bennett WF, Fuller HD. Comparative study of continuous extrapleural intercostal nerve block and lumbar epidural morphine in post-thoracotomy pain. Can J Surg. 1997 December;40(6):431–6. [PMC free article] [PubMed] [Google Scholar]
- 3.Broome IJ, Sherry KM, Reilly CS. A combined chest drain and intrapleural catheter for post-thoracotomy pain relief. Anaesthesia. 1993 August;48(8):724–6. doi: 10.1111/j.1365-2044.1993.tb07190.x. [DOI] [PubMed] [Google Scholar]
- 4.Inderbitzi R, Flueckiger K, Ris HB. Pain relief and respiratory mechanics during continuous intrapleural bupivacaine administration after thoracotomy. Thorac Cardiovasc Surg. 1992 April;40(2):87–9. doi: 10.1055/s-2007-1020119. [DOI] [PubMed] [Google Scholar]
- 5.Joucken K, Michel L, Schoevaerdts JC, Mayne A, Randour P. Cryoanalgesia for post-thoracotomy pain relief. Acta Anaesthesiol Belg. 1987;38(2):179–83. [PubMed] [Google Scholar]
- 6.Gough JD, Williams AB, Vaughan RS, Khalil JF, Butchart EG. The control of post-thoracotomy pain. A comparative evaluation of thoracic epidural fentanyl infusions and cryo-analgesia. Anaesthesia. 1988 September;43(9):780–3. doi: 10.1111/j.1365-2044.1988.tb05757.x. [DOI] [PubMed] [Google Scholar]
- 7.Hurford WE, Dutton RP, Alfille PH, Clement D, Wilson RS. Comparison of thoracic and lumbar epidural infusions of bupivacaine and fentanyl for post-thoracotomy analgesia. J Cardiothorac Vasc Anesth. 1993 October;7(5):521–5. doi: 10.1016/1053-0770(93)90306-6. [DOI] [PubMed] [Google Scholar]
- 8.Baxter AD, Laganiere S, Samson B, Stewart J, Hull K, Goernert L. A comparison of lumbar epidural and intravenous fentanyl infusions for post-thoracotomy analgesia. Can J Anaesth. 1994 March;41(3):184–91. doi: 10.1007/BF03009829. [DOI] [PubMed] [Google Scholar]
- 9.Burgess FW, Anderson DM, Colonna D, Cavanaugh DG. Thoracic epidural analgesia with bupivacaine and fentanyl for postoperative thoracotomy pain. J Cardiothorac Vasc Anesth. 1994 August;8(4):420–4. doi: 10.1016/1053-0770(94)90281-x. [DOI] [PubMed] [Google Scholar]
- 10.Hasenbos M, van EJ, Gielen M, Crul JF. Post-operative analgesia by high thoracic epidural versus intramuscular nicomorphine after thoracotomy. Part III. The effects of per- and post-operative analgesia on morbidity. Acta Anaesthesiol Scand. 1987 October;31(7):608–15. doi: 10.1111/j.1399-6576.1987.tb02630.x. [DOI] [PubMed] [Google Scholar]
- 11.Bimston DN, McGee JP, Liptay MJ, Fry WA. Continuous paravertebral extrapleural infusion for post-thoracotomy pain management. Surgery. 1999 October;126(4):650–6. [PubMed] [Google Scholar]
- 12.Marret E, Bazelly B, Taylor G, Lembert N, Deleuze A, Mazoit JX, Bonnet FJ. Paravertebral block with ropivacaine 0.5% versus systemic analgesia for pain relief after thoracotomy. Ann Thorac Surg. 2005 June;79(6):2109–13. doi: 10.1016/j.athoracsur.2004.07.030. [DOI] [PubMed] [Google Scholar]
- 13.Vogt A, Stieger DS, Theurillat C, Curatolo M. Single-injection thoracic paravertebral block for postoperative pain treatment after thoracoscopic surgery. Br J Anaesth. 2005 December;95(6):816–21. doi: 10.1093/bja/aei250. [DOI] [PubMed] [Google Scholar]
- 14.Della RG, Coccia C, Pompei L, Costa MG, Pierconti F, Di MP, Tommaselli E, Pietropaoli P. Post-thoracotomy analgesia: epidural vs intravenous morphine continuous infusion. Minerva Anestesiol. 2002 September;68(9):681–93. [PubMed] [Google Scholar]
- 15.Grant RP, Dolman JF, Harper JA, White SA, Parsons DG, Evans KG, Merrick CP. Patient-controlled lumbar epidural fentanyl compared with patient-controlled intravenous fentanyl for post-thoracotomy pain. Can J Anaesth. 1992 March;39(3):214–9. doi: 10.1007/BF03008779. [DOI] [PubMed] [Google Scholar]
- 16.Cohen E, Neustein SM. Intrathecal morphine during thoracotomy, Part I: Effect on intraoperative enflurane requirements. J Cardiothorac Vasc Anesth. 1993 April;7(2):154–6. doi: 10.1016/1053-0770(93)90208-3. [DOI] [PubMed] [Google Scholar]
- 17.Liu M, Rock P, Grass JA, Heitmiller RF, Parker SJ, Sakima NT, Webb MD, Gorman RB, Beattie C. Double-blind randomized evaluation of intercostals nerve blocks as an adjuvant to subarachnoid administered morphine for post-thoracotomy analgesia. Reg Anesth. 1995 September;20(5):418–25. [PubMed] [Google Scholar]
- 18.Askar FZ, Kocabas S, Yucel S, Samancilar O, Cetin H, Uyar M. The efficacy of intrathecal morphine in post-thoracotomy pain management. J Int Med Res. 2007 May;35(3):314–22. doi: 10.1177/147323000703500305. [DOI] [PubMed] [Google Scholar]
- 19.Brodsky JB, Kretzschmar KM, Mark JB. Caudal epidural morphine for post-thoracotomy pain. Anesth Analg. 1988 April;67(4):409–10. [PubMed] [Google Scholar]
- 20.Slinger PD. Pro: every postthoracotomy patient deserves thoracic epidural analgesia. J Cardiothorac Vasc Anesth. 1999 June;13(3):350–4. doi: 10.1016/s1053-0770(99)90276-8. [DOI] [PubMed] [Google Scholar]
- 21.McCrory C, Diviney D, Moriarty J, Luke D, Fitzgerald D. Comparison between repeat bolus intrathecal morphine and an epidurally delivered bupivacaine and fentanyl combination in the management of post-thoracotomy pain with or without cyclooxygenase inhibition. J Cardiothorac Vasc Anesth. 2002 October;16(5):607–11. doi: 10.1053/jcan.2002.126957. [DOI] [PubMed] [Google Scholar]
- 22.Perttunen K, Kalso E, Heinonen J, Salo J. IV diclofenac in post-thoracotomy pain. Br J Anaesth. 1992 May;68(5):474–80. doi: 10.1093/bja/68.5.474. [DOI] [PubMed] [Google Scholar]
- 23.Miller-Jones CM, Phillips D, Pitchford EA, Smallpeice CJ. Transcutaneous nerve stimulation in post-thoracotomy pain relief. Anaesthesia. 1980 October;35(10):1018. doi: 10.1111/j.1365-2044.1980.tb05013.x. [DOI] [PubMed] [Google Scholar]
- 24.Solak O, Turna A, Pekcolaklar A, Metin M, Sayar A, Solak O, Gurses A. Transcutaneous electric nerve stimulation for the treatment of postthoracotomy pain: a randomized prospective study. Thorac Cardiovasc Surg. 2007 April;55(3):182–5. doi: 10.1055/s-2006-924631. [DOI] [PubMed] [Google Scholar]
- 25.Erdogan M, Erdogan A, Erbil N, Karakaya HK, Demircan A. Prospective, Randomized, Placebo-controlled Study of the Effect of TENS on postthoracotomy pain and pulmonary function. World J Surg. 2005 December;29(12):1563–70. doi: 10.1007/s00268-005-7934-6. [DOI] [PubMed] [Google Scholar]
- 26.Ochroch EA, Gottschalk A. Impact of acute pain and its management for thoracic surgical patients. Thorac Surg Clin. 2005 February;15(1):105–21. doi: 10.1016/j.thorsurg.2004.08.004. [DOI] [PubMed] [Google Scholar]
- 27.Polaner DM, Kimball WR, Fratacci MD, Wain JC, Zapol WM. Thoracic epidural anesthesia increases diaphragmatic shortening after thoracotomy in the awake lamb. Anesthesiology. 1993 October;79(4):808–16. doi: 10.1097/00000542-199310000-00024. [DOI] [PubMed] [Google Scholar]
- 28.Fratacci MD, Kimball WR, Wain JC, Kacmarek RM, Polaner DM, Zapol WM. Diaphragmatic shortening after thoracic surgery in humans. Effects of mechanical ventilation and thoracic epidural anesthesia. Anesthesiology. 1993 October;79(4):654–65. doi: 10.1097/00000542-199310000-00005. [DOI] [PubMed] [Google Scholar]
- 29.Koehler RP, Keenan RJ. Management of postthoracotomy pain: acute and chronic. Thorac Surg Clin. 2006 August;16(3):287–97. doi: 10.1016/j.thorsurg.2006.05.006. [DOI] [PubMed] [Google Scholar]
- 30.Gottschalk A, Cohen SP, Yang S, Ochroch EA. Preventing and treating pain after thoracic surgery. Anesthesiology. 2006 March;104(3):594–600. doi: 10.1097/00000542-200603000-00027. [DOI] [PubMed] [Google Scholar]
- 31.Perttunen K, Tasmuth T, Kalso E. Chronic pain after thoracic surgery: a follow-up study. Acta Anaesthesiol Scand. 1999 May;43(5):563–7. doi: 10.1034/j.1399-6576.1999.430513.x. [DOI] [PubMed] [Google Scholar]
- 32.Kalso E, Perttunen K, Kaasinen S. Pain after thoracic surgery. Acta Anaesthesiol Scand. 1992 January;36(1):96–100. doi: 10.1111/j.1399-6576.1992.tb03430.x. [DOI] [PubMed] [Google Scholar]
- 33.Conacher ID. Therapists and therapies for post-thoracotomy neuralgia. Pain. 1992 March;48(3):409–12. doi: 10.1016/0304-3959(92)90093-Q. [DOI] [PubMed] [Google Scholar]
- 34.Furrer M, Rechsteiner R, Eigenmann V, Signer C, Althaus U, Ris HB. Thoracotomy and thoracoscopy: postoperative pulmonary function, pain and chest wall complaints. Eur J Cardiothorac Surg. 1997 July;12(1):82–7. doi: 10.1016/s1010-7940(97)00105-x. [DOI] [PubMed] [Google Scholar]
- 35.Maguire MF, Ravenscroft A, Beggs D, Duffy JP. A questionnaire study investigating the prevalence of the neuropathic component of chronic pain after thoracic surgery. Eur J Cardiothorac Surg. 2006 May;29(5):800–5. doi: 10.1016/j.ejcts.2006.02.002. [DOI] [PubMed] [Google Scholar]
- 36.Forster R, Storck M, Schafer JR, Honig E, Lang G, Liewald F. Thoracoscopy versus thoracotomy: a prospective comparison of trauma and quality of life. Langenbecks Arch Surg. 2002 April;387(1):32–6. doi: 10.1007/s00423-002-0287-9. [DOI] [PubMed] [Google Scholar]
- 37.Landreneau RJ, Mack MJ, Hazelrigg SR, Naunheim K, Dowling RD, Ritter P, Magee MJ, Nunchuck S, Keenan RJ, Ferson PF. Prevalence of chronic pain after pulmonary resection by thoracotomy or video-assisted thoracic surgery. J Thorac Cardiovasc Surg. 1994 April;107(4):1079–85. doi: 10.1097/00132586-199412000-00051. [DOI] [PubMed] [Google Scholar]
- 38.Karmakar MK, Ho AM. Postthoracotomy pain syndrome. Thorac Surg Clin. 2004 August;14(3):345–52. doi: 10.1016/S1547-4127(04)00022-2. [DOI] [PubMed] [Google Scholar]
- 39.Gotoda Y, Kambara N, Sakai T, Kishi Y, Kodama K, Koyama T. The morbidity, time course and predictive factors for persistent post-thoracotomy pain. Eur J Pain. 2001;5(1):89–96. doi: 10.1053/eujp.2001.0225. [DOI] [PubMed] [Google Scholar]
- 40.Rogers ML, Henderson L, Mahajan RP, Duffy JP. Preliminary findings in the neurophysiological assessment of intercostal nerve injury during thoracotomy. Eur J Cardiothorac Surg. 2002 February;21(2):298–301. doi: 10.1016/s1010-7940(01)01104-6. [DOI] [PubMed] [Google Scholar]
- 41.Maguire MF, Latter JA, Mahajan R, Beggs FD, Duffy JP. A study exploring the role of intercostal nerve damage in chronic pain after thoracic surgery. Eur J Cardiothorac Surg. 2006 June;29(6):873–9. doi: 10.1016/j.ejcts.2006.03.031. [DOI] [PubMed] [Google Scholar]
- 42.Benedetti F, Vighetti S, Ricco C, Amanzio M, Bergamasco L, Casadio C, Cianci R, Giobbe R, Oliaro A, Bergamasco B, Maggi G. Neurophysiologic assessment of nerve impairment in posterolateral and muscle-sparing thoracotomy. J Thorac Cardiovasc Surg. 1998 April;115(4):841–7. doi: 10.1016/S0022-5223(98)70365-4. [DOI] [PubMed] [Google Scholar]
- 43.Keller SM, Carp NZ, Levy MN, Rosen SM. Chronic post thoracotomy pain. J Cardiovasc Surg (Torino) 1994 December;35(6 Suppl 1):161–4. [PubMed] [Google Scholar]
- 44.Kanner R. Diagnosis and management of neuropathic pain in patients with cancer. Cancer Invest. 2001;19(3):324–33. doi: 10.1081/cnv-100102559. [DOI] [PubMed] [Google Scholar]
- 45.Ochroch EA, Gottschalk A, Augoustides JG, Aukburg SJ, Kaiser LR, Shrager JB. Pain and physical function are similar following axillary, muscle-sparing vs posterolateral thoracotomy. Chest. 2005 October;128(4):2664–70. doi: 10.1378/chest.128.4.2664. [DOI] [PubMed] [Google Scholar]
- 46.Khan IH, McManus KG, McCraith A, McGuigan JA. Muscle sparing thoracotomy: a biomechanical analysis confirms preservation of muscle strength but no improvement in wound discomfort. Eur J Cardiothorac Surg. 2000 December;18(6):656–61. doi: 10.1016/s1010-7940(00)00591-1. [DOI] [PubMed] [Google Scholar]
- 47.Bachiocco V, Morselli-Labate AM, Rusticali AG, Bragaglia R, Mastrorilli M, Carli G. Intensity, latency and duration of post-thoracotomy pain: relationship to personality traits. Funct Neurol. 1990 October;5(4):321–32. [PubMed] [Google Scholar]
- 48.Romer HC, Russell GN. A survey of the practice of thoracic epidural analgesia in the United Kingdom. Anaesthesia. 1998 October;53(10):1016–22. doi: 10.1046/j.1365-2044.1998.00525.x. [DOI] [PubMed] [Google Scholar]
- 49.Hogan QH. Epidural anatomy examined by cryomicrotome section. Influence of age, vertebral level, and disease. Reg Anesth. 1996 September;21(5):395–406. [PubMed] [Google Scholar]
- 50.Benumof JL. Permanent loss of cervical spinal cord function associated with interscalene block performed under general anesthesia. Anesthesiology. 2000 December;93(6):1541–4. doi: 10.1097/00000542-200012000-00033. [DOI] [PubMed] [Google Scholar]
- 51.Kavanagh BP, Katz J, Sandler AN. Pain control after thoracic surgery. A review of current techniques. Anesthesiology. 1994 September;81(3):737–59. doi: 10.1097/00000542-199409000-00028. [DOI] [PubMed] [Google Scholar]
- 52.Senturk M, Ozcan PE, Talu GK, Kiyan E, Camci E, Ozyalcin S, Dilege S, Pembeci K. The effects of three different analgesia techniques on long-term postthoracotomy pain. Anesth Analg. 2002 January;94(1):11–5. doi: 10.1213/00000539-200201000-00003. table. [DOI] [PubMed] [Google Scholar]
- 53.Beattie WS, Badner NH, Choi P. Epidural analgesia reduces postoperative myocardial infarction: a meta-analysis. Anesth Analg. 2001 October;93(4):853–8. doi: 10.1097/00000539-200110000-00010. [DOI] [PubMed] [Google Scholar]
- 54.Ballantyne JC, Carr DB, deFerranti S, Suarez T, Lau J, Chalmers TC, Angelillo IF, Mosteller F. The comparative effects of postoperative analgesic therapies on pulmonary outcome: cumulative meta-analyses of randomized, controlled trials. Anesth Analg. 1998 March;86(3):598–612. doi: 10.1097/00000539-199803000-00032. [DOI] [PubMed] [Google Scholar]
- 55.Burgess FW. Epidural versus intravenous fentanyl following thoracotomy. Anesthesiology. 1993 September;79(3):621–3. doi: 10.1097/00000542-199309000-00036. [DOI] [PubMed] [Google Scholar]
- 56.Pennefather SH, Akrofi ME, Kendall JB, Russell GN, Scawn ND. Double-blind comparison of intrapleural saline and 0.25% bupivacaine for ipsilateral shoulder pain after thoracotomy in patients receiving thoracic epidural analgesia. Br J Anaesth. 2005 February;94(2):234–8. doi: 10.1093/bja/aei030. [DOI] [PubMed] [Google Scholar]
- 57.Ng KP, Chow YF. Brachial plexus block for ipsilateral shoulder pain after thoracotomy. Anaesth Intensive Care. 1997 February;25(1):74–6. doi: 10.1177/0310057X9702500114. [DOI] [PubMed] [Google Scholar]
- 58.Barak M, Iaroshevski D, Poppa E, Ben-Nun A, Katz Y. Low-volume interscalene brachial plexus block for post-thoracotomy shoulder pain. J Cardiothorac Vasc Anesth. 2007 August;21(4):554–7. doi: 10.1053/j.jvca.2006.08.013. [DOI] [PubMed] [Google Scholar]
- 59.Tan N, Agnew NM, Scawn ND, Pennefather SH, Chester M, Russell GN. Suprascapular nerve block for ipsilateral shoulder pain after thoracotomy with thoracic epidural analgesia: a double-blind comparison of 0.5% bupivacaine and 0.9% saline. Anesth Analg. 2002 January;94(1):199–202. doi: 10.1097/00000539-200201000-00038. table. [DOI] [PubMed] [Google Scholar]
- 60.Scawn ND, Pennefather SH, Soorae A, Wang JY, Russell GN. Ipsilateral shoulder pain after thoracotomy with epidural analgesia: the influence of phrenic nerve infiltration with lidocaine. Anesth Analg. 2001 August;93(2):260–4. doi: 10.1097/00000539-200108000-00004. 1st. [DOI] [PubMed] [Google Scholar]
- 61.Danelli G, Berti M, Casati A, Bobbio A, Ghisi D, Mele R, Rossini E, Fanelli G. Ipsilateral shoulder pain after thoracotomy surgery: a prospective, randomized, double-blind, placebo-controlled evaluation of the efficacy of infiltrating the phrenic nerve with 0.2%wt/vol ropivacaine. Eur J Anaesthesiol. 2007 July;24(7):596–601. doi: 10.1017/S0265021507000178. [DOI] [PubMed] [Google Scholar]
- 62.Barak M, Ziser A, Katz Y. Thoracic epidural local anesthetics are ineffective in alleviating post-thoracotomy ipsilateral shoulder pain. J Cardiothorac Vasc Anesth. 2004 August;18(4):458–60. doi: 10.1053/j.jvca.2004.05.025. [DOI] [PubMed] [Google Scholar]
- 63.Mac TB, Girard F, Chouinard P, Boudreault D, Lafontaine ER, Ruel M, Ferraro P. Acetaminophen decreases early post-thoracotomy ipsilateral shoulder pain in patients with thoracic epidural analgesia: a double-blind placebo-controlled study. J Cardiothorac Vasc Anesth. 2005 August;19(4):475–8. doi: 10.1053/j.jvca.2004.11.041. [DOI] [PubMed] [Google Scholar]
- 64.Luketich JD, Land SR, Sullivan EA, velo-Rivera M, Ward J, Buenaventura PO, Landreneau RJ, Hart LA, Fernando HC. Thoracic epidural versus intercostal nerve catheter plus patient-controlled analgesia: a randomized study. Ann Thorac Surg. 2005 June;79(6):1845–9. doi: 10.1016/j.athoracsur.2004.10.055. [DOI] [PubMed] [Google Scholar]
- 65.Detterbeck FC. Efficacy of methods of intercostal nerve blockade for pain relief after thoracotomy. Ann Thorac Surg. 2005 October;80(4):1550–9. doi: 10.1016/j.athoracsur.2004.11.051. [DOI] [PubMed] [Google Scholar]
- 66.Trescot AM. Cryoanalgesia in interventional pain management. Pain Physician. 2003 July;6(3):345–60. [PubMed] [Google Scholar]
- 67.Yang MK, Cho CH, Kim YC. The effects of cryoanalgesia combined with thoracic epidural analgesia in patients undergoing thoracotomy. Anaesthesia. 2004 November;59(11):1073–7. doi: 10.1111/j.1365-2044.2004.03896.x. [DOI] [PubMed] [Google Scholar]
- 68.Ju H, Feng Y, Yang BX, Wang J. Comparison of epidural analgesia and intercostal nerve cryoanalgesia for post-thoracotomy pain control. Eur J Pain. 2007 September 14; doi: 10.1016/j.ejpain.2007.07.011. [DOI] [PubMed] [Google Scholar]
- 69.Davies RG, Myles PS, Graham JM. A comparison of the analgesic efficacy and side-effects of paravertebral vs epidural blockade for thoracotomy--a systematic review and meta-analysis of randomized trials. Br J Anaesth. 2006 April;96(4):418–26. doi: 10.1093/bja/ael020. [DOI] [PubMed] [Google Scholar]
- 70.Casati A, Alessandrini P, Nuzzi M, Tosi M, Iotti E, Ampollini L, Bobbio A, Rossini E, Fanelli G. A prospective, randomized, blinded comparison between continuous thoracic paravertebral and epidural infusion of 0.2% ropivacaine after lung resection surgery. Eur J Anaesthesiol. 2006 December;23(12):999–1004. doi: 10.1017/S0265021506001104. [DOI] [PubMed] [Google Scholar]
- 71.Karmakar MK, Chung DC. Variability of a thoracic paravertebral block. Are we ignoring the endothoracic fascia? Reg Anesth Pain Med. 2000 May;25(3):325–7. doi: 10.1016/s1098-7339(00)90028-2. [DOI] [PubMed] [Google Scholar]
- 72.Yashpal K, Katz J, Coderre TJ. Effects of preemptive or postinjury intrathecal local anesthesia on persistent nociceptive responses in rats. Confounding influences of peripheral inflammation and the general anesthetic regimen. Anesthesiology. 1996 May;84(5):1119–28. doi: 10.1097/00000542-199605000-00014. [DOI] [PubMed] [Google Scholar]
- 73.Dickenson AH, Sullivan AF. Subcutaneous formalin-induced activity of dorsal horn neurones in the rat: differential response to an intrathecal opiate administered pre or post formalin. Pain. 1987 September;30(3):349–60. doi: 10.1016/0304-3959(87)90023-6. [DOI] [PubMed] [Google Scholar]
- 74.Herroeder S, Pecher S, Schonherr ME, Kaulitz G, Hahnenkamp K, Friess H, Bottiger BW, Bauer H, Dijkgraaf OG, Durieux ME, Hollmann MW. Systemic lidocaine shortens length of hospital stay after colorectal surgery: a double-blinded, randomized, placebo-controlled trial. Ann Surg. 2007 August;246(2):192–200. doi: 10.1097/SLA.0b013e31805dac11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Fridrich P, Colvin HP, Zizza A, Wasan AD, Lukanich J, Lirk P, Saria A, Zernig G, Hamp T, Gerner P. Phase 1A safety assessment of intravenous amitriptyline. J Pain. 2007 July;8(7):549–55. doi: 10.1016/j.jpain.2007.02.433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Woolf CJ, Chong MS. Preemptive analgesia--treating postoperative pain by preventing the establishment of central sensitization. Anesth Analg. 1993 August;77(2):362–79. doi: 10.1213/00000539-199377020-00026. [DOI] [PubMed] [Google Scholar]
- 77.Dahl JB, Kehlet H. The value of pre-emptive analgesia in the treatment of postoperative pain. Br J Anaesth. 1993 April;70(4):434–9. doi: 10.1093/bja/70.4.434. [DOI] [PubMed] [Google Scholar]
- 78.Dahl JB, Hansen BL, Hjortso NC, Erichsen CJ, Moiniche S, Kehlet H. Influence of timing on the effect of continuous extradural analgesia with bupivacaine and morphine after major abdominal surgery. Br J Anaesth. 1992 July;69(1):4–8. doi: 10.1093/bja/69.1.4. [DOI] [PubMed] [Google Scholar]
- 79.Pryle BJ, Vanner RG, Enriquez N, Reynolds F. Can pre-emptive lumbar epidural blockade reduce postoperative pain following lower abdominal surgery? Anaesthesia. 1993 February;48(2):120–3. doi: 10.1111/j.1365-2044.1993.tb06848.x. [DOI] [PubMed] [Google Scholar]
- 80.Kissin I. Preemptive analgesia. Why its effect is not always obvious. Anesthesiology. 1996 May;84(5):1015–9. doi: 10.1097/00000542-199605000-00001. [DOI] [PubMed] [Google Scholar]
- 81.Erdek MA, Staats PS. Chronic pain and thoracic surgery. Thorac Surg Clin. 2005 February;15(1):123–30. doi: 10.1016/j.thorsurg.2004.10.001. [DOI] [PubMed] [Google Scholar]
- 82.Senturk M. Acute and chronic pain after thoracotomies. Curr Opin Anaesthesiol. 2005 February;18(1):1–4. doi: 10.1097/00001503-200502000-00002. [DOI] [PubMed] [Google Scholar]
- 83.Bong CL, Samuel M, Ng JM, Ip-Yam C. Effects of preemptive epidural analgesia on post-thoracotomy pain. J Cardiothorac Vasc Anesth. 2005 December;19(6):786–93. doi: 10.1053/j.jvca.2005.08.012. [DOI] [PubMed] [Google Scholar]
- 84.Katz J, Jackson M, Kavanagh BP, Sandler AN. Acute pain after thoracic surgery predicts long-term post-thoracotomy pain. Clin J Pain. 1996 March;12(1):50–5. doi: 10.1097/00002508-199603000-00009. [DOI] [PubMed] [Google Scholar]
- 85.Katz J, Kavanagh BP, Sandler AN, Nierenberg H, Boylan JF, Friedlander M, Shaw BF. Preemptive analgesia. Clinical evidence of neuroplasticity contributing to postoperative pain. Anesthesiology. 1992 September;77(3):439–46. doi: 10.1097/00000542-199209000-00006. [DOI] [PubMed] [Google Scholar]
- 86.Doyle E, Bowler GM. Pre-emptive effect of multimodal analgesia in thoracic surgery. Br J Anaesth. 1998 February;80(2):147–51. doi: 10.1093/bja/80.2.147. [DOI] [PubMed] [Google Scholar]
- 87.Kavanagh BP, Katz J, Sandler AN, Nierenberg H, Roger S, Boylan JF, Laws AK. Multimodal analgesia before thoracic surgery does not reduce postoperative pain. Br J Anaesth. 1994 August;73(2):184–9. doi: 10.1093/bja/73.2.184. [DOI] [PubMed] [Google Scholar]
- 88.Obata H, Saito S, Fujita N, Fuse Y, Ishizaki K, Goto F. Epidural block with mepivacaine before surgery reduces long-term post-thoracotomy pain. Can J Anaesth. 1999 December;46(12):1127–32. doi: 10.1007/BF03015520. [DOI] [PubMed] [Google Scholar]
- 89.Aida S, Baba H, Yamakura T, Taga K, Fukuda S, Shimoji K. The effectiveness of preemptive analgesia varies according to the type of surgery: a randomized, double-blind study. Anesth Analg. 1999 September;89(3):711–6. doi: 10.1097/00000539-199909000-00034. [DOI] [PubMed] [Google Scholar]
- 90.Eng J, Sabanathan S. Continuous extrapleural intercostal nerve block and post-thoracotomy pulmonary complications. Scand J Thorac Cardiovasc Surg. 1992;26(3):219–23. doi: 10.3109/14017439209099081. [DOI] [PubMed] [Google Scholar]
- 91.Byrd RB, Burns JR. Cough dynamics in the post-thoracotomy state. Chest. 1975 June;67(6):654–7. doi: 10.1378/chest.67.6.654. [DOI] [PubMed] [Google Scholar]
- 92.Schultz AM, Werba A, Ulbing S, Gollmann G, Lehofer F. Peri-operative thoracic epidural analgesia for thoracotomy. Eur J Anaesthesiol. 1997 November;14(6):600–3. doi: 10.1046/j.1365-2346.1994.00183.x. [DOI] [PubMed] [Google Scholar]
- 93.Cook TM, Riley RH. Analgesia following thoracotomy: a survey of Australian practice. Anaesth Intensive Care. 1997 October;25(5):520–4. [PubMed] [Google Scholar]
- 94.Richardson J, Sabanathan S, Mearns AJ, Evans CS, Bembridge J, Fairbrass M. Efficacy of pre-emptive analgesia and continuous extrapleural intercostal nerve block on post-thoracotomy pain and pulmonary mechanics. J Cardiovasc Surg (Torino) 1994 June;35(3):219–28. [PubMed] [Google Scholar]
- 95.Neustein SM, Kreitzer JM, Krellenstein D, Reich DL, Rapaport E, Cohen E. Preemptive epidural analgesia for thoracic surgery. Mt Sinai J Med. 2002 January;69(1–2):101–4. [PubMed] [Google Scholar]
- 96.Ochroch EA, Gottschalk A, Augostides J, Carson KA, Kent L, Malayaman N, Kaiser LR, Aukburg SJ. Long-term pain and activity during recovery from major thoracotomy using thoracic epidural analgesia. Anesthesiology. 2002 November;97(5):1234–44. doi: 10.1097/00000542-200211000-00029. [DOI] [PubMed] [Google Scholar]
- 97.Kissin I. Preemptive analgesia. Anesthesiology. 2000 October;93(4):1138–43. doi: 10.1097/00000542-200010000-00040. [DOI] [PubMed] [Google Scholar]
- 98.Kissin I. Study design to demonstrate clinical value of preemptive analgesia: is the commonly used approach valid? Reg Anesth Pain Med. 2002 May;27(3):242–4. doi: 10.1053/rapm.2002.31936. [DOI] [PubMed] [Google Scholar]
- 99.Samad TA, Moore KA, Sapirstein A, Billet S, Allchorne A, Poole S, Bonventre JV, Woolf CJ. Interleukin-1beta-mediated induction of Cox-2 in the CNS contributes to inflammatory pain hypersensitivity. Nature. 2001 March 22;410(6827):471–5. doi: 10.1038/35068566. [DOI] [PubMed] [Google Scholar]


