Abstract
The competitive ability of hybrids, compared with their parental taxa, can cover a wide fitness range from poor to superior. For example communities of the Daphnia galeata–hyalina–cucullata species complex often show hybrid dominance. We tested whether taxa composition of 43 European lakes inhabited by this species complex can be explained by habitat characteristics (e.g. size descriptors, trophy level) or geography. We found that D. galeata occurs more frequently south of the Alps, whereas D. hyalina and D. cucullata are found more in the north. Lakes with D. galeata dominance had higher temperatures whereas D. hyalina dominance could be attributed to low phosphorus loads. The dominance of F1-hybrids, however, was not explainable with current environmental variables. In a subset of 28 lakes, we studied the impact of eutrophication history on F1-hybrid success. Lakes with the highest trophic state in the past tended to be dominated by F1-hybrids. Our data demonstrate that human-mediated habitat disturbance (eutrophication) has facilitated hybrid success and altered the Daphnia taxon composition across lakes. At the same time, specific habitat conditions might provide a refuge from hybridization for native genotypes.
Keywords: hybrid superiority, environmental measures, coexistence, disturbance, eutrophication
1. Introduction
Natural hybridization, resulting in crosses between genetically distinct populations of the same or different species (Arnold 1997), is a common phenomenon for species inhabiting both aquatic and terrestrial habitats (reviewed by Dowling & Secor 1997). Fitness of hybridizing taxa is influenced by environment-independent endogenous and environment-specific exogenous selection (reviewed by Burke & Arnold 2001). It has been shown that fitness of hybrids, compared with their parental species, can cover wide fitness ranges from poor to superior (reviewed by Arnold & Hodges 1995).
Three niche-based models exist to explain the coexistence of hybrid and parental species. The tension zone model (Barton & Hewitt 1985) assumes a balance between dispersal into the hybrid zone and selection against hybrids. By contrast, the bounded hybrid superiority model (Moore 1977) supposes that in intermediate environments, hybrids are fitter than either parental species. Other zones are known in which hybrids have a mosaic-like distribution within heterogeneous parental habitats (mosaic model, e.g. Harrison 1986). As such, each habitat patch has a unique set of environmental conditions, increasing the probability that natural selection can pick out the most fit genotypes, even though they might be rarely produced (Barton 2001). If the fitness of hybrids depends on environmental conditions, as the last two models propose, anthropogenic disturbance or fragmentation of natural systems will influence the structure of hybrid zones. A non-niche-based explanation of long-term coexistence is the neutral theory (Hubbell 2001), which assumes that species diversity is caused by drift. If species do show niche structures, the core assumption of the neutral theory is violated.
Hybrids that can propagate both sexually and asexually (e.g. plants with ramet production, cyclical parthenogenetic animals) can be especially successful owing to limited need for sexual reproduction, which otherwise causes serious problems in many hybrid taxa (i.e. recombination and segregation that break apart F1-hybrid genotypes; e.g. Emms & Arnold 1997). Cyclical parthenogens with regularly observed interspecific hybridization can be found within the genus Daphnia (Crustacea, Cladocera). In many natural Daphnia communities, hybrids coexist with their parental species (Wolf & Mort 1986; Taylor & Hebert 1992) and F1-hybrids are often the most abundant taxon (Taylor & Hebert 1992; Spaak & Hoekstra 1995; Hobæk et al. 2004). Spaak & Hoekstra (1995) proposed the temporal hybrid superiority model to explain long-term taxa coexistence and hybrid success in Daphnia. This model assumes that certain environmental conditions favour hybrids over their parental species and therefore ‘relative taxon fitness’ fluctuates in time with changing environments. Daphnia galeata, Daphnia cucullata and Daphnia hyalina (i.e. D. galeata–hyalina–cucullata species complex) and their hybrids are commonly found in sympatry in permanent lakes all across Europe (Spaak 1996; Schwenk & Spaak 1997). Daphnia galeata has a broad ecological niche, D. hyalina is most abundant in deep, oligo- to mesotrophic lakes, and D. cucullata has its core distribution in deep, moderately meso- to eutrophic lakes with average temperature and food availability (Flößner & Kraus 1986). Coexistence of parental species and hybrids can be explained by taxon-specific fitness differences indicated by different niche breadths (Weider 1993). Daphnia are sensitive to factors altered by the trophic state of lakes such as predation pressure (Spaak & Hoekstra 1995; Kerfoot & Weider 2004), food quantity (DeMott et al. 2001) and quality (Hairston et al. 1999). Biogeographic patterns in European lakes showed that parental species and hybrids occur in a rather patchy distribution with large differences in taxon compositions (Schwenk & Spaak 1997), and to date no clear habitat preferences for parental and hybrid classes have been formulated.
Many lakes experienced extensive trophy changes during the last century, which are known to disturb entire lake ecosystems (Schindler 2006). Disturbance has been described to facilitate the success and spread of hybrids (reviewed by Allendorf et al. 2001). The purpose of this study was to investigate the differences in Daphnia taxon composition and hybrid success across lakes with various habitat conditions, especially with different trophic histories. Specifically, we addressed the following questions: (i) how much of the variation in the taxon composition can be attributed to environmental and spatial variables (EV; SV); (ii) do D. galeata, D. hyalina and their F1-hybrids differ in responses to EV and SV; (iii) can the current D. galeata-, D. hyalina- and F1 dominance be explained by current EV and SV or by the past eutrophication.
2. Material and methods
(a) Study sites and field sampling
Seventeen European lakes south and 26 lakes north of the Alps inhabited by the D. galeata–hyalina–cucullata species complex were screened for their Daphnia taxon composition (table 1). All the lakes are at elevations below 900 metres above sea level (m a.s.l.), except four lakes that are situated in an inner alpine Swiss valley above 1760 m a.s.l. Lakes were sampled in spring and autumn. Lakes south of the Alps were sampled twice (May 2004 and September 2004) and lakes north of the Alps were sampled two to four times (May–July 2003, August–October 2003 and August–October 2004). From three more intensively studied lakes (Brienzersee, Greifensee and Pfäffikersee) comparable sample dates were chosen. Samples of adult Daphnia with a body size of 1 mm or above (see Wolinska et al. 2007a) were collected using zooplankton nets with mesh sizes well below this threshold, so that all individuals of target size classes were collected (lakes south of the Alps: 126 μm mesh; lakes north of the Alps: 250 μm). The samples were taken at the area of maximum depth (DM; table 1) from the upper 50 m in deep lakes or, in shallower lakes, over the entire water column. Approximately 70–100 adult asexual females were genotyped per sample. The samples from spring and autumn were pooled because we were interested in the overall taxon composition. Taxon composition in hybridizing Daphnia populations may vary seasonally; however, annualized taxon composition remains rather stable over several years (Keller & Spaak 2004; De Meester et al. 2006).
Table 1.
List with lake names, corresponding to the numbers in figure 1 and values of EV and SV. (Data sources: n.d., no data available; references (a) Federal Office for the Environment (FOEN; P. Liechti), (b) http://www.ise.cnr.it/limno/limno.htm, (c) BUWAL (2002), (d) Liechti (1994), (e) Osservatorio dei Laghi Lombardi (2005), (f) Ohlendorf (1998), (g) Elber et al. (2001), (h) Ludovisi et al. (2004), (i) Garibaldi et al. (1997), (j) Keller et al. (2002), (k) Keller (2003), (l) Ribi et al. (2001), (m) Kiefer (1987), (n) W. Steiner Nordostschweizerische Kraftwerke (NOK 2004, personal communication), and (o) field sampling June/July 2003, upper 20 m.)
| corresponding no. (figure 1) | lake name | ‘historic dataset’ | total phosphorus load (PT, μg l−1) | maximal phosphorus load (PM, μg l−1) | years since PM (YE) | volume (VO; 106 m3) | maximum depth (DM; m) | surface area (SU; km2) | elevation (EL; m a.s.l.) | longitude (LO) | latitude (LA) | references |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| lakes north of the Alps | ||||||||||||
| 1 | Ägerisee | X | 10 | 23 | 29 | 353 | 83 | 7 | 724 | 8.618 | 47.127 | a, d, k |
| 2 | Baldeggersee | X | 52 | 517 | 28 | 173 | 66 | 5 | 463 | 8.258 | 47.207 | a, c, d |
| 3 | Bielersee | X | 13 | 140 | 33 | 1240 | 74 | 40 | 429 | 7.163 | 47.071 | a, d |
| 4 | Brienzesee | X | 3 | 25 | 22 | 5170 | 261 | 30 | 564 | 7.966 | 46.728 | a, c, d |
| 5 | Greifensee | X | 63 | 535 | 30 | 148 | 32 | 8 | 435 | 8.677 | 47.353 | a, d, j |
| 6 | Hallwillersee | X | 45 | 270 | 26 | 280 | 47 | 10 | 449 | 8.211 | 47.297 | a, d |
| 7 | Klöntalersee | 12 | n.d. | n.d. | 56 | 47 | 3 | 847 | 8.975 | 47.025 | n, o | |
| 8 | Lej da Champfèr | 11 | n.d. | n.d. | 9 | 33 | 1 | 1791 | 9.806 | 46.469 | f, o | |
| 9 | Lej da S. Murezzan | 43 | n.d. | n.d. | 20 | 44 | 1 | 1768 | 9.849 | 46.495 | f | |
| 10 | Lej da Segel | 11 | n.d. | n.d. | 137 | 71 | 4 | 1797 | 9.732 | 46.418 | f | |
| 11 | Lej da Silvaplauna | 13 | n.d. | n.d. | 127 | 77 | 3 | 1791 | 9.796 | 46.449 | f | |
| 12 | Lungenersee | 10 | n.d. | n.d. | 66 | 68 | 2 | 689 | 8.162 | 46.804 | l, o | |
| 13 | Murtensee | X | 27 | 155 | 23 | 550 | 46 | 23 | 429 | 7.105 | 46.940 | a, d |
| 14 | Neuenburgersee | X | 14 | 59 | 23 | 13 980 | 152 | 218 | 429 | 6.736 | 46.835 | a, c, d |
| 15 | Pfäffikersee | X | 22 | 379 | 32 | 59 | 35 | 3 | 537 | 8.783 | 47.352 | a, d, g |
| 16 | Sarnersee | X | 5 | 21 | 25 | 244 | 52 | 8 | 469 | 8.221 | 46.871 | a, d |
| 17 | Sempachersee | X | 34 | 165 | 19 | 639 | 87 | 14 | 504 | 8.158 | 47.143 | a, c, d |
| 18 | Sihlsee | 13 | n.d. | n.d. | 97 | 23 | 11 | 889 | 8.788 | 47.122 | l, o | |
| 19 | Thunersee | X | 3 | 23 | 22 | 6470 | 217 | 48 | 558 | 7.673 | 46.713 | a, d |
| 20 | Türlersee | X | 15 | 157 | 28 | 6 | 22 | 0 | 643 | 8.503 | 47.270 | g, l |
| 21 | Vierwaldstättersee | X | 7 | 32 | 27 | 11 907 | 214 | 114 | 434 | 8.364 | 47.013 | a, c, d |
| 22 | Wägitalersee | 19 | n.d. | n.d. | 149 | 65 | 4 | 900 | 8.922 | 47.095 | m, o | |
| 23 | Walensee | X | 2 | 30 | 27 | 3180 | 145 | 24 | 419 | 9.211 | 47.123 | a, d |
| 24 | Zugersee | X | 114 | 210 | 20 | 3174 | 198 | 38 | 413 | 8.483 | 47.161 | a, d, l |
| 25 | Zürichsee, Obersee | X | 14 | 41 | 31 | 467 | 48 | 20 | 406 | 8.845 | 47.203 | a, d |
| 26 | Zürichsee, Untersee | X | 25 | 130 | 33 | 3300 | 136 | 68 | 406 | 8.577 | 47.293 | a, c, d |
| lakes south of the Alps | ||||||||||||
| 27 | Lago di Alserio | X | 26 | 280 | 18 | 7 | 8 | 1 | 260 | 9.217 | 45.785 | e, h |
| 28 | Lago di Comabbio | X | 72 | 200 | 28 | 17 | 8 | 4 | 243 | 8.692 | 46.763 | b, e, h |
| 29 | Lago di Como | X | 35 | 74 | 24 | 22 500 | 410 | 145 | 198 | 9.267 | 46.000 | e, h |
| 30 | Lago d'Endine | X | 17 | 35 | 19 | 12 | 9 | 2 | 334 | 9.938 | 45.778 | e, h, i |
| 31 | Lago d'Idro | 24 | n.d. | n.d. | 684 | 122 | 11 | 370 | 10.517 | 45.767 | b, e, h | |
| 32 | Lago d'Iseo | X | 17 | 41 | 23 | 7600 | 251 | 61 | 186 | 10.067 | 45.733 | e, h |
| 33 | Lago di Lugano | X | 47 | 176 | 23 | 5860 | 288 | 49 | 271 | 8.971 | 45.994 | d, e, h |
| 34 | Lago Maggiore | X | 11 | 35 | 25 | 37 500 | 370 | 213 | 194 | 8.654 | 45.967 | a, c, e, h |
| 35 | Lago di Mergozzo | 1 | n.d. | n.d. | 83 | 73 | 2 | 194 | 8.466 | 45.956 | b, h | |
| 36 | Lago di Monate | 5 | n.d. | n.d. | 45 | 34 | 3 | 266 | 8.664 | 45.786 | e, h | |
| 37 | Lago di Montorfano | X | 8 | 15 | 4 | 2 | 7 | 0 | 397 | 9.138 | 45.783 | e, h |
| 38 | Lago Moro | 8 | n.d. | n.d. | 4 | 42 | 0 | 389 | 10.158 | 45.863 | b, e, h | |
| 39 | Lago d' Orta | 4 | n.d. | n.d. | 1300 | 143 | 18 | 290 | 8.400 | 45.817 | b, h | |
| 40 | Lago di Pusiano | X | 74 | 200 | 12 | 69 | 24 | 5 | 259 | 9.273 | 45.802 | e, h |
| 41 | Lago del Segrino | 12 | n.d. | n.d. | 1 | 9 | 0 | 374 | 9.267 | 45.829 | b, h | |
| 42 | Lago di Sirio | 24 | n.d. | n.d. | 5 | 44 | 0 | 271 | 8.929 | 45.451 | b, h | |
| 43 | Lago di Varese | X | 82 | 400 | 28 | 160 | 26 | 15 | 238 | 8.750 | 45.800 | e, h |
(b) Hybrid class identification
All the individuals were assayed at four polymorphic allozyme loci (AAT, AO, PGI, PGM) using cellulose acetate electrophoresis (see Keller & Spaak 2004). These loci were used to identify hybrid classes with NewHybrids (Anderson & Thompson 2002) that uses Bayesian statistics to calculate a posterior probability that reflects the level of certainty of an individual belonging to a specific hybrid category. Probability threshold for allocation was set to 95% or more; the remaining individuals could not be attributed with confidence to a specific hybrid class (Fx) and therefore were excluded from subsequent statistical analyses. Since NewHybrids can only assign individuals to two hybridizing taxa, each lake population was divided into taxa pairs based on AAT and AO loci that are diagnostic for D. galeata, D. hyalina and D. cucullata (Wolf & Mort 1986; Gießler 1997). Individuals incorporating alleles from all three parental taxa (Fxhgc) were excluded from the analysis with NewHybrids. In this way, we discriminated between D. galeata (Pgal), D. hyalina (Phyl), D. cucullata (Pcuc), D. galeata×D. hyalina F1-hybrids (F1hg), D. hyalina×D. cucullata F1-hybrids (F1hc), and D. galeata×D. hyalina×D. cucullata Fx-hybrids (Fxhgc).
(c) Abiotic lake descriptors, trophic state and lake history
To analyse geographical distribution of the studied Daphnia communities, we used latitude (LA) and longitude (LO) as environmental descriptors. In addition the terms for a cubic surface regression (xy, x2, y2, x2y, xy2, x3 and y3) were applied because spatial species responses do not need to be linear (Borcard et al. 1992). Abiotic environmental descriptors were lake volume (VO), DM, surface area (SU), elevation (EL), temperature (TE) and current total phosphorus loads (PT; table 1). Total phosphorus values representing the trophic lake state are mean spring circulation measures, and were obtained from long-term water quality monitoring projects in Italy and Switzerland or from other published sources (table 1). For five lakes with no published PT, values were determined from integrative water samples of the upper 20 m. A comparison of field samples with literature data was done for 14 lakes; they did not differ significantly (Wilcoxon matched pairs test, p=0.42). As proxy for TE, we used the annual mean air temperature Climate Normals (1961–1990) from the Swiss Meteorological Institute (MeteoSwiss) corrected for sea level, which can be taken as surrogate for water temperature (Livingstone et al. 1999). Further, we were eager to know whether abiotic environmental and spatial parameters or trophic lake history might explain single taxon dominance. Therefore, we included in the analysis the highest total phosphorus load (PM) measured in the course of eutrophication (since 1900; obtained from the same sources as PT), the number of years since PM had occurred (YE) and the rate of phosphorus load change (ΔP) calculated as [log(PM/PT)]/Δt. This analysis was applied to a subset of 28 lakes (hereafter referred as ‘historic dataset’; table 1).
(d) Statistical analyses
Environmental parameters were log-transformed if needed. Since the Daphnia taxon dataset contains many zeros, we chose canonical correspondence analysis (CCA), a multivariate technique that considers only proportional relationships between variables and removes all differences between any data formats (see Jackson 1997). The CCAs as well as follow-up statistical tests (e.g. classical variation partitioning) were performed with the Canoco package, v. 4.5 (ter Braak & Šmilauer 2002). Variation partitioning with adjustments was performed using the program of Peres-Neto et al. (2006). Discriminant function analysis (DFA) and subsequent testing of selected variables with a Mann–Whitney U-test were performed with Statistica for Windows, release v. 7.1 (StatSoft, Inc.). Details of analyses are given below.
(i) Influence of EV and SV
CCA (Borcard et al. 1992) was used to partition the variance in the Daphnia taxa dataset into pure spatial, pure environmental and spatially structured environmental fractions. This was done in order to disentangle explanatory power attributed to each of these three fractions and to evaluate gradients and patterns found in the CCA. For this analysis, we discriminated between spatial (SV-variables: LA, LO, xy, x2, y2, x2y, xy2 and x3) and EV (VO, DM, SU, EL, TE and PT) variables. Data reduction was obtained with forward selection (ter Braak & Šmilauer 2002). A permutation test with 999 Monte Carlo permutations and α≤0.05 was used for the forward selection procedure and further to test the significance of variables in the full model CCA. Variance inflation factors (VIFs; a measure for cross-correlation of explanatory variables) were checked and eliminated if VIFs were more than 20 (threshold criterion for exclusion given by ter Braak & Šmilauer 2002). A partial CCA with selected EV- and SV-variables as covariables (covariable CCA) was performed to test the robustness of the pure EV fraction in explaining Daphnia taxon patterns. In addition, the explanatory significance of each of the two datasets (EV and SV) was tested with a new, unbiased variance partitioning method proposed by Peres-Neto et al. (2006).
(ii) Daphnia community description
A CCA including all selected EV- and SV-variables (full model CCA) was used to disentangle whether differences in Daphnia taxon composition can be attributed to environmental and spatial gradients. Statistical significance of the full model as well as the first two canonical axes was determined by a randomization test (999 permutations, Lepš & Šmilauer 2003).
(iii) F1hg-hybrid and parental success
To understand which factors determine taxon dominance, we discriminated between lakes where single taxon abundance was more than 50% (hereafter referred to as ‘dominated’) and the other lakes (‘not dominated’). Stepwise DFA (α=0.05, tolerance greater than 0.01) was performed to test which set of variables is able to discriminate between dominated and not dominated communities. An additional DFA was performed for F1hg-hybrids, to determine whether the variables of the trophic lake history (historic dataset; table 1) were powerful to discriminate between dominated and not dominated lakes. The ability of the forward selection procedure in DFA to find the best explanatory variable has been criticized (James & McCulloch 1990); therefore we tested the discriminatory power of the selected significant variables with a Mann–Whitney U-test.
3. Results
(a) Influence of EV and SV
Variance partitioning was used to separate pure EV, pure SV and spatially structured EV variation in the Daphnia communities. With forward selection, PT, TE, SU and VO entered in the EV-CCA, whereas in the SV-CCA only LA was included (table 2). EV and SV-variables together (full model CCA) explained 34.8% of the variation in the Daphnia taxa dataset. This variance could be attributed with the method of Borcard et al. (1992) to 21.4% as pure EV, 4.3% as pure SV and 9.1% as spatially structured EV (table 2). In fact LA was the greatest single predictor of species occurrence. However, 65.2% of the variation remained unexplained. The covariable CCA showed that the pure EV-fraction was significant (F=3.04; p=0.002), with PT as the only significant variable (table 2). With the adjusted method of Peres-Neto et al. (2006), 9.9% of the variation could be attributed to pure EV, 2.4% to pure SV and 5.1% to spatially structured EV. Only the pure EV-fraction explained a significant portion of the variation in the dataset (pEV=0.001 and pSV=0.56).
Table 2.
Explanatory contributions of EV and SV variables in canonical correspondence analyses (CCAs), determined by permutation test (999 Monte Carlo permutations and α≤0.05). (Full model CCA with all selected EV and SV variables; EV-CCA (pure EV- and spatially-structured EV-fraction); SV-CCA (pure SV- and spatially-structured EV-fraction), and covariable CCA (pure EV-fraction). Abbreviations: λ, explained variance; %, explained variance in percentages; LA, latitude; VO, lake volume; SU, lake surface; TE, temperature; PT, total phosphorus load. Marginal effects explain the variation in the species data singly, whereas conditional effects show the amount of extra variation each variable contributed when it was added to the models. For details see text.)
| model type | variable | marginal (independent) effects | conditional (partial) effects | ||||||
|---|---|---|---|---|---|---|---|---|---|
| λ | p | F | % | λ | p | F | % | ||
| full model CCA | LA | 0.30 | 0.001 | 6.53 | 13.3 | 0.30 | 0.001 | 6.35 | 13.3 |
| PT | 0.14 | 0.022 | 2.80 | 6.2 | 0.15 | 0.008 | 3.28 | 6.6 | |
| SU | 0.11 | 0.075 | 2.16 | 4.9 | 0.09 | 0.059 | 2.24 | 4.0 | |
| TE | 0.27 | 0.002 | 5.52 | 12.0 | 0.12 | 0.026 | 2.69 | 5.3 | |
| VO | 0.13 | 0.051 | 2.44 | 5.8 | 0.13 | 0.011 | 3.13 | 5.8 | |
| EV-CCA | PT | 0.14 | 0.023 | 2.80 | 6.2 | 0.12 | 0.024 | 2.85 | 5.3 |
| SU | 0.11 | 0.073 | 2.16 | 4.9 | 0.18 | 0.002 | 4.14 | 8.0 | |
| TE | 0.27 | 0.001 | 5.52 | 12.0 | 0.27 | 0.001 | 5.52 | 12.0 | |
| VO | 0.13 | 0.038 | 2.44 | 5.8 | 0.12 | 0.017 | 2.68 | 5.3 | |
| SV-CCA | LA | 0.30 | 0.001 | 6.35 | 13.3 | 0.30 | 0.001 | 6.35 | 13.3 |
| covariable CCA | PT | 0.15 | 0.012 | 3.24 | 6.6 | 0.15 | 0.012 | 3.28 | 6.6 |
| SU | 0.09 | 0.111 | 1.84 | 4.0 | 0.10 | 0.053 | 2.24 | 4.4 | |
| TE | 0.10 | 0.085 | 2.11 | 4.4 | 0.11 | 0.035 | 2.69 | 4.9 | |
| VO | 0.08 | 0.143 | 1.74 | 3.5 | 0.12 | 0.009 | 3.13 | 5.3 | |
(b) Daphnia community description
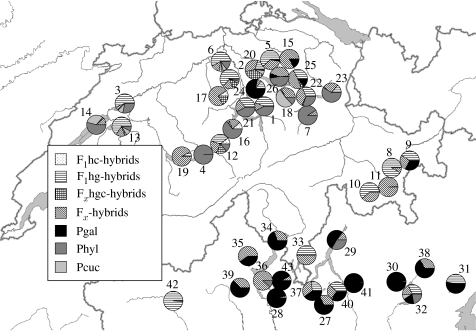
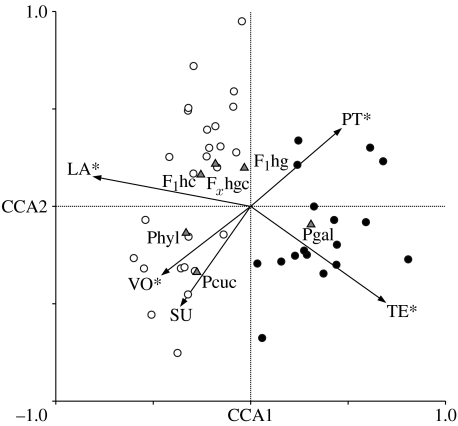
Species occurrence and responses to gradients in the EV and SV were used for a description of Daphnia community patterns and to detect putative taxa-specific habitat preferences. In most lakes, hybrids co-occurred with one or two parental taxa (figure 1). Coexistence of hybrids with all three parental species was found in Lago di Iseo (no. 32). F1hg-hybrids were found in 25 lakes, F1hc-hybrids in 3 lakes, Fxhgc-hybrids in 15 lakes and Fx-hybrids in all lakes excluding Brienzersee (no. 4; figure 1). Twenty-six lakes were dominated by a single taxon (Pgal, 11 lakes; Phyl, 5 lakes; Pcuc, one lake and F1hg-hybrids, 9 lakes). Both canonical axes (CCA1: F=9.00, p=0.001 and CCA2: F=4.36, p=0.03) and the entire CCA (F=3.96, p=0.001) explained a significant amount of the weighted variance in the Daphnia taxon composition (figure 2). The randomization test indicated that PT, VO, TE and LA explain a significant amount of the variation in the species data (table 2). None of the final variables that entered the model had VIF larger than 20. Based on the full model CCA, we could discriminate between lakes north and south of the Alps. Phyl and Pcuc occurred more in larger, low phosphorus lakes and Pgal occurred more in warmer, more southern lakes. All hybrid classes clustered together, but not in an intermediate position to their parental species (figure 2).
Figure 1.
Taxon composition of Daphnia asexual females across 43 analysed lakes north and south of the Alps (2003 and 2004). Pie charts represent relative frequencies of three parental taxa (Pgal, D. galeata; Phyl, D. hyalina; and Pcuc, D. cucullata) and different hybrid classes (F1hg, D. galeata×D. hyalina; F1hc, D. hyalina×D. cucullata; Fxhgc, D. galeata×D. hyalina×D. cucullata; and Fx, later generation hybrids). For lake numbers see table 1.
Figure 2.
Full model CCA results of EV and SV on Daphnia communities in all 43 lakes north (open circles) and south (filled circles) of the Alps. Vectors represent environmental (VO, lake volume; PT, total phosphorus load; SU, lake surface; and TE, temperature) and spatial (LA, latitude) parameters that point in the direction of increasing importance for the respective variables. Arrow angles relative to axis and EV- or SV-variables indicate correlation strengths. Solid triangles symbolize relative proportions of various Daphnia taxa (Pgal, D. galeata; Phyl, D. hyalina; Pcuc, D. cucullata; F1hg, D. galeata×D. hyalina; F1hc, D. hyalina×D. cucullata; and Fxhgc, D. galeata×D. hyalina×D. cucullata). Asterisks indicate variable explaining a significant amount of variation in the Daphnia taxa dataset (see table 2).
(c) F1hg-hybrid and parental success
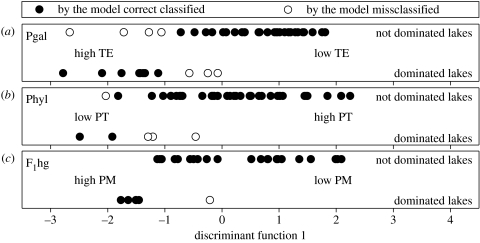
To answer the question about which variables are responsible for single taxon dominance, we performed stepwise discriminant function analyses (DFAs). Only for Pgal and Phyl the DFA was significant. For Pgal dominance, TE was the only significant variable, whereas for Phyl dominance, PT was significant (table 3). With the fitted models several lakes were misclassified (Pgal: seven lakes; Phyl: four lakes; figure 3). F1hg dominance was not explainable with the recent dataset (DFA: Wilks' λ=0.806, F4,38=2.28, p=0.078) but with trophic lake history, in particular with PM (table 3). Only one lake was misclassified in this case (figure 3). The discriminatory power of all selected significant DFA variables was approved using Mann–Whitney U tests (PgalTE, p<0.001; PhylPT, p=0.021 and F1hgPM, p=0.019).
Table 3.
Variables that contribute to the discrimination of single taxon dominated Daphnia communities. (Variables were identified using a stepwise DFA with forward selection. The lower the Wilks' λ the higher is the discriminatory power of the entire model. The lower the partial λ the higher is the contribution of the variable to the overall discrimination. Discrimination for D. galeata and D. hyalina dominance was based on all 43 lakes and for F1hg dominance, it was based on 28 lakes with information about the trophic lake history (historic dataset; table 1). Pgal, D. galeata; Phyl, D. hyalina; F1hg, D. galeata×D. hyalina; LA, latitude; LO, longitude; VO, lake volume; SU, lake surface; TE, temperature; PT, total phosphorus load; PM, maximal phosphorus load.)
| dominant Daphnia taxon | variable | Wilks' λ | partial λ | d.f. | F | p |
|---|---|---|---|---|---|---|
| Pgal | full model | 0.623 | — | 3,39 | 7.88 | <0.001 |
| TE | — | 0.722 | — | 15.03 | <0.001 | |
| LO | — | 0.866 | — | 6.03 | 0.019 | |
| PT | — | 0.975 | — | 1.00 | 0.322 | |
| Phyl | full model | 0.769 | — | 3,39 | 3.91 | 0.016 |
| PT | — | 0.831 | — | 7.93 | 0.008 | |
| LA | — | 0.943 | — | 2.37 | 0.132 | |
| SU | — | 0.966 | — | 1.37 | 0.248 | |
| F1hg | full model | 0.652 | — | 2,25 | 6.68 | <0.005 |
| PM | — | 0.737 | — | 8.92 | 0.006 | |
| LO | — | 0.874 | — | 3.62 | 0.069 |
Figure 3.
Distribution of ‘single taxon dominated’ (50% or more) and not dominated Daphnia communities along extracted first discriminant function: (a) D. galeata dominance (Pgal), (b) D. hyalina dominance (Phyl) and (c) F1hg dominance (F1hg). Discrimination for D. galeata and D. hyalina dominance was based on all 43 lakes and for F1hg dominance was based on 28 lakes with information about the trophic lake history (historic dataset; table 1). PT, total phosphorus load; TE, temperature; and PM, maximal phosphorus load.
4. Discussion
Differences in the taxa composition of the studied Daphnia communities was more attributed to environmental variation than to spatial variation, using the classical method of Borcard et al. (1992) and the adjusted method of Peres-Neto et al. (2006). Taxon occurrence patterns were explained by EV (PT, SU, TE and VO). Daphnia hyalina dominance was attributed to present total phosphorus loads (PT) and D. galeata dominance was attributed to TE. F1hg dominance, however, was only explainable with historic phosphorus loads (PM; figure 3). This finding indicates that although lakes have recovered from past eutrophication, they are still affected by previous pollution.
Separation of D. hyalina and D. galeata along environmental gradients is consistent with biogeographic patterns (figure 1). For example, D. hyalina is more likely to be found in large lakes with low total phosphorus loads (figure 2) as described by Flößner & Kraus (1986). The total phosphorus load additionally explains the D. hyalina dominance, pronouncing the susceptibility of this taxon to eutrophication (table 3; figure 3). Daphnia galeata occurrence was associated with warm temperatures and low altitude (figure 2), whereof temperature was more important as it explains D. galeata dominance (table 3; figure 3). We did not find a significant effect of total phosphorus load on D. galeata dominance (table 3), although Flößner & Kraus (1986) stated that this species is promoted by eutrophication.
In contrast to D. galeata, F1hg-hybrids responded to the trophic conditions. Although present EV provided no satisfactory explanation of F1hg success, historic high trophic conditions significantly explained the present F1hg dominance (figure 3). Strong trophy changes can disturb entire lake ecosystems, by influencing the biomass, species richness and community composition of phytoplankton (e.g. Seip & Reynolds 1995). Disturbance leads to habitat changes or is the origin of novel habitat types (e.g. deep, cold eutrophic lakes), which hybrids then might occupy. Our study indeed suggests that Daphnia hybrids have novel ecological preferences: all hybrid classes clustered together in the multivariate space but not in the intermediate way to their parental species (figure 2). A similar pattern is reported from a recent experimental study: Daphnia hybrids' reaction norms were not intermediate to the ones of parental species (Wolinska et al. 2007b). Another factor that may facilitate hybrid persistence and success is asexual reproduction (e.g. Moody & Les 2002). Asexually reproducing hybrids can avoid or at least delay hybrid breakdown (the break up of co-adapted gene complexes), which allows fit genotypes to reach high abundances. High fitness of asexual Daphnia hybrid clones (Keller et al. 2007) may alter competition among taxa and hence may accentuate the influence of environmental factors on Daphnia populations.
Changes in ecosystems may alter the biology of species and thus enable hybridization by breaking down phenological barriers (Lamont et al. 2003). On the other hand, disturbance might lead to ill-adapted populations, increasing the chance for successful invasion and colonization (Allendorf et al. 2001; Levin 2004). In hybridizing species, the presence of hybrids in a specific habitat can be explained in two ways. Either hybrid Daphnia taxa are locally produced (Spaak 1997; Jankowski & Straile 2003; Keller & Spaak 2004; Keller et al. 2007), which requires coexistence of both parental species during certain time periods, or hybrids are produced elsewhere and subsequently colonize new biotopes. The first mechanism relies on dispersal of parentals, whereas the latter one relies on dispersal of hybrids. Dispersal of species from the D. galeata–hyalina–cucullata species complex is coupled with sexually produced diapausing eggs. Hybrids of this species complex, however, have a reduced sexual fitness (Keller & Spaak 2004; Keller et al. 2007), indicating a reduced dispersal capacity. By contrast, D. galeata invests more in sexual reproduction than hybrids (Keller et al. 2007) or D. hyalina (Jankowski & Straile 2004). This suggests that D. galeata may have invaded lakes that were formerly inhabited by other species and that hybrids were locally produced. This scenario is likely as D. galeata hybridizes with almost every species in the D. longispina complex (Taylor et al. 2005). Moreover, there is genetic evidence for multiple hybridization events in natural Daphnia populations (e.g. Spaak 1997).
Geographical barriers that have traditionally been considered as natural boundaries for dispersal, such as the Alps, have become porous due to human activity during the last century. Human transportation across Europe and the Alps was suspected, for example, to cause the spread and colonization of new biotopes by D. parvula (reviewed by Panov et al. 2004). When a species reaches a new water body, its capacity for colonization depends on a competitive advantage under given environmental conditions (De Meester et al. 2002). Abiotic and biotic conditions that promote coexistence of parentals and hybrids in natural Daphnia populations sometimes enable one taxon to dominate entire lake systems (figure 3). In addition to the conditions that we have tested in the present study, there are other important biotic factors able to maintain the coexistence of Daphnia taxa, for example, dynamic frequency-dependent host–parasite interactions (Wolinska et al. 2006) or delayed hybrid breakdown due to low sexual reproduction (Keller et al. 2007).
So far we have discussed species composition shifts based on trophic state changes, dispersal capacity of Daphnia and hybrid production. Other processes such as stochastic space–time fluctuations, and the noise introduced by unmeasured EV might interfere with EV and SV used to describe community structures in this study. Uncertainty factors may result in a large fraction of unexplained variations, which is indeed not uncommon in ecological surveys (e.g. Borcard et al. 1992; Lepš & Šmilauer 2003). But even in cases with explained variability less than 10%, well-interpretable structures can be detected (Lepš & Šmilauer 2003). In the deep oligotrophic Lago Maggiore (no. 34), for instance, a recent change in the Daphnia community composition from D. hyalina to D. galeata (Manca 2004) was proposed to have been caused by a water temperature increase (of approximately 1°C in the upper 50 m over the last three decades), and by the shift to an earlier and longer thermal stratification (Manca et al. 2007). This example demonstrates the sensitivity of lake ecosystems to climate change as suggested by Schindler (2006). However, the rough proxy we used for water temperature (weather station data) is accurate enough to discover such long-term processes. We found that lakes with D. galeata dominance experienced higher temperature than not dominated lakes, confirming that D. galeata might be favoured by climate change.
Based on the analysis of 43 lake populations, we conclude that the present taxon composition of the D. galeata–hyalina–cucullata species complex can be attributed to disturbance (eutrophication) and abiotic habitat conditions. Our results show that contemporary F1hg dominance is mainly the result of the magnitude of phosphorus load in the past.
Acknowledgments
We thank Christian Rellstab for providing us with zooplankton samples from Brienzersee, and Esther Keller and Andrea Ferrari for collecting zooplankton samples from all other lakes. Colleen Durkin and Piotr Madej helped run allozyme electrophoresis and Rosi Sieber provided us with GIS data of studied lakes. Christoph Tellenbach, Nelson Hairston Jr, Chris Robinson and Ryan Thum made useful comments on an earlier version of the manuscript, and provided statistical and linguistic help. We thank two anonymous reviewers for their valuable comments that helped us to improve this manuscript. This work was partly supported by grant 31-65003.01 from the Swiss National Science Foundation.
Footnotes
One contribution of 16 to a Theme Issue ‘Hybridization in animals: extent, processes and evolutionary impact’.
References
- Allendorf F.W, Leary R.F, Spruell P, Wenburg J.K. The problems with hybrids: setting conservation guidelines. Trends Ecol. Evol. 2001;16:613–622. doi:10.1016/S0169-5347(01)02290-X [Google Scholar]
- Anderson E.C, Thompson E.A. A model-based method for identifying species hybrids using multilocus genetic data. Genetics. 2002;160:1217–1229. doi: 10.1093/genetics/160.3.1217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arnold, M. L. 1997 Natural hybridization and evolution Oxford Series in Ecology and Evolution. New York, NY; Oxford, UK: Oxford University Press.
- Arnold M.L, Hodges S.A. Are natural hybrids fit or unfit relative to their parents? Trends Ecol. Evol. 1995;10:67–71. doi: 10.1016/S0169-5347(00)88979-X. doi:10.1016/S0169-5347(00)88979-X [DOI] [PubMed] [Google Scholar]
- Barton N.H. The role of hybridization in evolution. Mol. Ecol. 2001;10:551–568. doi: 10.1046/j.1365-294x.2001.01216.x. doi:10.1046/j.1365-294x.2001.01216.x [DOI] [PubMed] [Google Scholar]
- Barton N.H, Hewitt G.M. Analysis of hybrid zones. Annu. Rev. Ecol. Syst. 1985;16:113–148. doi:10.1146/annurev.es.16.110185.000553 [Google Scholar]
- Borcard D, Legendre P, Drapeau P. Partialling out the spatial component of ecological variation. Ecology. 1992;73:1045–1055. doi:10.2307/1940179 [Google Scholar]
- Burke J.M, Arnold M.L. Genetics and the fitness of hybrids. Annu. Rev. Genet. 2001;35:31–52. doi: 10.1146/annurev.genet.35.102401.085719. doi:10.1146/annurev.genet.35.102401.085719 [DOI] [PubMed] [Google Scholar]
- BUWAL 2002 Die genutzte Umwelt—Fischerei: Lieber natürliche als gedüngte Fische. In Umwelt Schweiz 2002, pp. 221–226. Bern, Switzerland: BUWAL, Bundesamt für Statistik.
- De Meester L, Gomez A, Okamura B, Schwenk K. The monopolization hypothesis and the dispersal-gene flow paradox in aquatic organisms. Acta Oecol. 2002;23:121–135. doi:10.1016/S1146-609X(02)01145-1 [Google Scholar]
- De Meester L, Vanoverbeke J, De Gelas K, Ortells R, Spaak P. Genetic structure of cyclic parthenogenetic zooplankton populations—a conceptual framework. Arch. Hydrobiol. 2006;167:217–244. doi:10.1127/0003-9136/2006/0167-0217 [Google Scholar]
- DeMott W.R, Gulati R.D, Van Donk E. Daphnia food limitation in three hypereutrophic Dutch lakes: evidence for exclusion of large-bodied species by interfering filaments of cyanobacteria. Limnol. Oceanogr. 2001;46:2054–2060. [Google Scholar]
- Dowling T.E, Secor C.L. The role of hybridization and introgression in the diversification of animals. Annu. Rev. Ecol. Syst. 1997;28:593–619. doi:10.1146/annurev.ecolsys.28.1.593 [Google Scholar]
- Elber F, Hürlimann J, Niederberger K. Entwicklung des Gesamtphosphors im Türlersee anhand der im Sediment eingelagerten Kieselalgen. Baudirektion Kanton Zürich, Amt für Abfall, Wasser, Energie und Luft; Zürich: 2001. p. 38. [Google Scholar]
- Emms S.K, Arnold M.L. The effect of habitat on parental and hybrid fitness: transplant experiments with Louisiana irises. Evolution. 1997;51:1112–1119. doi: 10.1111/j.1558-5646.1997.tb03958.x. doi:10.2307/2411040 [DOI] [PubMed] [Google Scholar]
- Flößner D, Kraus K. On the taxonomy of the Daphnia hyalina–galeata complex (Crustacea: Cladocera) Hydrobiologia. 1986;137:97–115. doi:10.1007/BF00004206 [Google Scholar]
- Garibaldi L, Brizzio M.C, Varallo A, Mosello R. The improving trophic conditions of Lake Endine (Northern Italy) Mem. Ist. Ital. Idrobiol. 1997;56:23–36. [Google Scholar]
- Gießler S. Analysis of reticulate relationships within the Daphnia longispina species complex. Allozyme phenotype and morphology. J. Evol. Biol. 1997;10:87–105. doi:10.1046/j.1420-9101.1997.10010087.x [Google Scholar]
- Hairston N.G, Lampert W, Cáceres C.E, Holtmeier C.L, Weider L.J, Gaedke U, Fischer J.M, Fox J.A, Post D.M. Rapid evolution revealed by dormant eggs. Nature. 1999;401:446. doi:10.1038/46731 [Google Scholar]
- Harrison R.G. Pattern and process in a narrow hybrid zone. Heredity. 1986;56:337–349. doi:10.1038/hdy.1986.55 [Google Scholar]
- Hobæk A, Skage M, Schwenk K. Daphnia galeata×D. longispina hybrids in western Norway. Hydrobiologia. 2004;526:55–62. doi:10.1023/B:HYDR.0000041614.68315.ec [Google Scholar]
- Hubbell S.P. Princeton University Press; Princeton, NJ: 2001. The unified neutral theory of biodiversity and biogeography. [DOI] [PubMed] [Google Scholar]
- Jackson D.A. Compositional data in community ecology: the paradigm or peril of proportions? Ecology. 1997;78:929–940. doi:10.1890/0012-9658(1997)078[0929:CDICET]2.0.CO;2 [Google Scholar]
- James F.C, McCulloch C.E. Multivariate-analysis in ecology and systematics—panacea or pandora box. Annu. Rev. Ecol. Syst. 1990;21:129–166. doi:10.1146/annurev.es.21.110190.001021 [Google Scholar]
- Jankowski T, Straile D. A comparison of egg-bank and long-term plankton dynamics of two Daphnia species, D. hyalina and D. galeata: potentials and limits of reconstruction. Limnol. Oceanogr. 2003;48:1948–1955. [Google Scholar]
- Jankowski T, Straile D. Allochronic differentiation among Daphnia species, hybrids and backcrosses: the importance of sexual reproduction for population dynamics and genetic architecture. J. Evol. Biol. 2004;17:312–321. doi:10.1046/j.1420-9101.2003.00666.x [PubMed] [Google Scholar]
- Keller P. Die Wasserqualität in den Zuger Gewässern von 1997 bis 2000. Umwelt Blickpunkt. 2003;20:4–17. [Google Scholar]
- Keller B, Spaak P. Nonrandom sexual reproduction and diapausing egg production in a Daphnia hybrid species complex. Limnol. Oceanogr. 2004;49:1393–1400. [Google Scholar]
- Keller B, Bürgi H.R, Sturm M, Spaak P. Ephippia and Daphnia abundances under changing trophic conditions. Verh. Int. Ver. Theor. Angew. Limnol. 2002;28:851–855. [Google Scholar]
- Keller B, Wolinska J, Tellenbach C, Spaak P. Reproductive isolation keeps hybridizing Daphnia species distinct. Limnol. Oceanogr. 2007;52:984–991. [Google Scholar]
- Kerfoot W.C, Weider L.J. Experimental paleoecology (resurrection ecology): chasing Van Valen's Red Queen hypothesis. Limnol. Oceanogr. 2004;49:1300–1316. [Google Scholar]
- Kiefer B. Universität Zürich; Zürich, Switzerland: 1987. Untersuchungen zum Einfluss des Wasserregimes eines voralpinen Pumpspeicher-Sees (Wägitaler See) auf die Nährstoffversorgung der Phytoplanktonpopulation. [Google Scholar]
- Lamont B.B, He T, Enright N.J, Krauss S.L, Miller B.P. Anthropogenic disturbance promotes hybridization between Banksia species by altering their biology. J. Evol. Biol. 2003;16:551–557. doi: 10.1046/j.1420-9101.2003.00548.x. doi:10.1046/j.1420-9101.2003.00548.x [DOI] [PubMed] [Google Scholar]
- Lepš J, Šmilauer P. Cambridge University Press; Cambridge, UK: 2003. Multivariate analysis of ecological data using Canoco. [Google Scholar]
- Levin D.A. The ecological transition in speciation. New Phytol. 2004;161:91–96. doi:10.1046/j.1469-8137.2003.00921.x [Google Scholar]
- Liechti P. Der Zustand der Seen in der Schweiz. Schriftenreihe Umwelt. 1994;237:162. [Google Scholar]
- Livingstone D.M, Lotter A.F, Walker I.R. The decrease in summer surface water temperature with altitude in Swiss Alpine lakes: a comparison with air temperature lapse rates. Arct. Antarct. Alp. Res. 1999;31:341–352. doi:10.2307/1552583 [Google Scholar]
- Ludovisi A, Pandolfi P, Taticchi M.I. A proposed framework for the identification of habitat utilisation patterns of macrophytes in River Po catchment basin lakes (Italy) Hydrobiologia. 2004;523:87–101. doi:10.1023/B:HYDR.0000033097.58497.fe [Google Scholar]
- Manca, M. 2004 Indagini sull'ambiente pelagico. Popolamenti planctonici. Analisi morfotipica e genetica delle popolazioni di Daphnia In C.N.R.-I.S.E. Sezione di Idrobiologia ed Ecologia delle Acque Interne-Ricerche sull'evoluzione del Lago Maggiore. Aspetti limnologici. Programma quinquennale 2003–2007. Campagna 2003 (eds Commissione Internazionale per la protezione delle acque italo-svizzere), p. 68.
- Manca M.M, Portogallo M, Brown M.E. Shifts in phenology of Bythotrephes longimanus and its modern success in Lake Maggiore as a result of changes in climate and trophy. J. Plankton Res. 2007;29:515–525. doi:10.1093/plankt/fbm033 [Google Scholar]
- Moody M.L, Les D.H. Evidence of hybridity in invasive watermilfoil (Myriophyllum) populations. Proc. Natl Acad. Sci. USA. 2002;99:14 867–14 871. doi: 10.1073/pnas.172391499. doi:10.1073/pnas.172391499 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore W.S. An evaluation of narrow hybrid zones in vertebrates. Q. Rev. Biol. 1977;52:263–277. doi:10.1086/409995 [Google Scholar]
- Ohlendorf C. ETH-Zürich; Zürich, Switzerland: 1998. High Alpine lake sediments as chronicles for regional glacier and climate history in the Upper Engadine, southeastern Switzerland. [Google Scholar]
- Osservatorio dei Laghi Lombardi 2005 Qualità delle acque lacustri in Lombardia—1° Rapporto OLL 2004 Regione Lombardia, ARPA Lombardia: Fondazione Lombardia per l'Ambiente e IRSA/CNR.
- Panov V.E, Krylov P.I, Riccardi N. Role of diapause in dispersal and invasion success by aquatic invertebrates. J. Limnol. 2004;63:56–69. [Google Scholar]
- Peres-Neto P.R, Legendre P, Dray S, Borcard D. Variation partitioning of species data matrices: estimation and comparison of fractions. Ecology. 2006;87:2614–2625. doi: 10.1890/0012-9658(2006)87[2614:vposdm]2.0.co;2. doi:10.1890/0012-9658(2006)87[2614:VPOSDM]2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Ribi B, Bührer H, Ambühl H. Eawag; Dübendorf, Switzerland: 2001. Hypsographische Daten von Seen. [Google Scholar]
- Schindler D.W. Recent advances in the understanding and management of eutrophication. Limnol. Oceanogr. 2006;51:356–363. [Google Scholar]
- Schwenk K, Spaak P. Ecological and genetics of interspecific hybridization in Daphnia. In: Streit B, Städler T, Lively C.M, editors. Evolutionary ecology of freshwater animals. Birkhäuser Verlag; Basel, Switzerland: 1997. pp. 199–229. [Google Scholar]
- Seip K.L, Reynolds C.S. Phytoplankton functional attributes along trophic gradient and season. Limnol. Oceanogr. 1995;40:589–597. [Google Scholar]
- Spaak P. Temporal changes in the genetic structure of the Daphnia species complex in Tjeukemeer, with evidence for backcrossing. Heredity. 1996;76:539–548. doi:10.1038/hdy.1996.77 [Google Scholar]
- Spaak P. Hybridization in the Daphnia galeata complex: are hybrids locally produced? Hydrobiologia. 1997;360:127–133. doi:10.1023/A:1003157117667 [Google Scholar]
- Spaak P, Hoekstra J.R. Life history variation and the coexistence of a Daphnia hybrid with its parental species. Ecology. 1995;76:553–564. doi:10.2307/1941213 [Google Scholar]
- Taylor D.J, Hebert P.D.N. Daphnia galeata mendotae as a cryptic species complex with interspecific hybrids. Limnol. Oceanogr. 1992;37:658–665. [Google Scholar]
- Taylor D.J, Sprenger H.L, Ishida S. Geographic and phylogenetic evidence for dispersed nuclear introgression in a daphniid with sexual propagules. Mol. Ecol. 2005;14:525–537. doi: 10.1111/j.1365-294X.2005.02415.x. doi:10.1111/j.1365-294X.2005.02415.x [DOI] [PubMed] [Google Scholar]
- ter Braak, C. J. F. & Šmilauer, P. 2002 Canocoreference manual andCanoDrawfor Windows user's guide: software for Canonical Community Ordination, version 4.5. Ithaca, NY: Microcomputer Power.
- Weider L.J. Niche breadth and life history variation in a hybrid Daphnia complex. Ecology. 1993;74:935–943. doi:10.2307/1940817 [Google Scholar]
- Wolf H.G, Mort M.A. Interspecific hybridization underlies phenotypic variability in Daphnia populations. Oecologia. 1986;68:507–511. doi: 10.1007/BF00378763. doi:10.1007/BF00378763 [DOI] [PubMed] [Google Scholar]
- Wolinska J, Bittner K, Ebert D, Spaak P. The coexistence of hybrid and parental Daphnia: the role of parasites. Proc. R. Soc. B. 2006;273:1977–1983. doi: 10.1098/rspb.2006.3523. doi:10.1098/rspb.2006.3523 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolinska J, Keller B, Manca M, Spaak P. Parasite survey of a Daphnia hybrid complex: host-specificity and environment determine infection. J. Anim. Ecol. 2007a;76:191–200. doi: 10.1111/j.1365-2656.2006.01177.x. doi:10.1111/j.1365-2656.2006.01177.x [DOI] [PubMed] [Google Scholar]
- Wolinska J, Löffler A, Spaak P. Taxon-specific reaction norms to predator cues in a hybrid Daphnia complex. Freshw. Biol. 2007b;52:1198–1209. doi:10.1111/j.1365-2427.2007.01757.x [Google Scholar]