Abstract
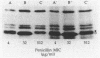
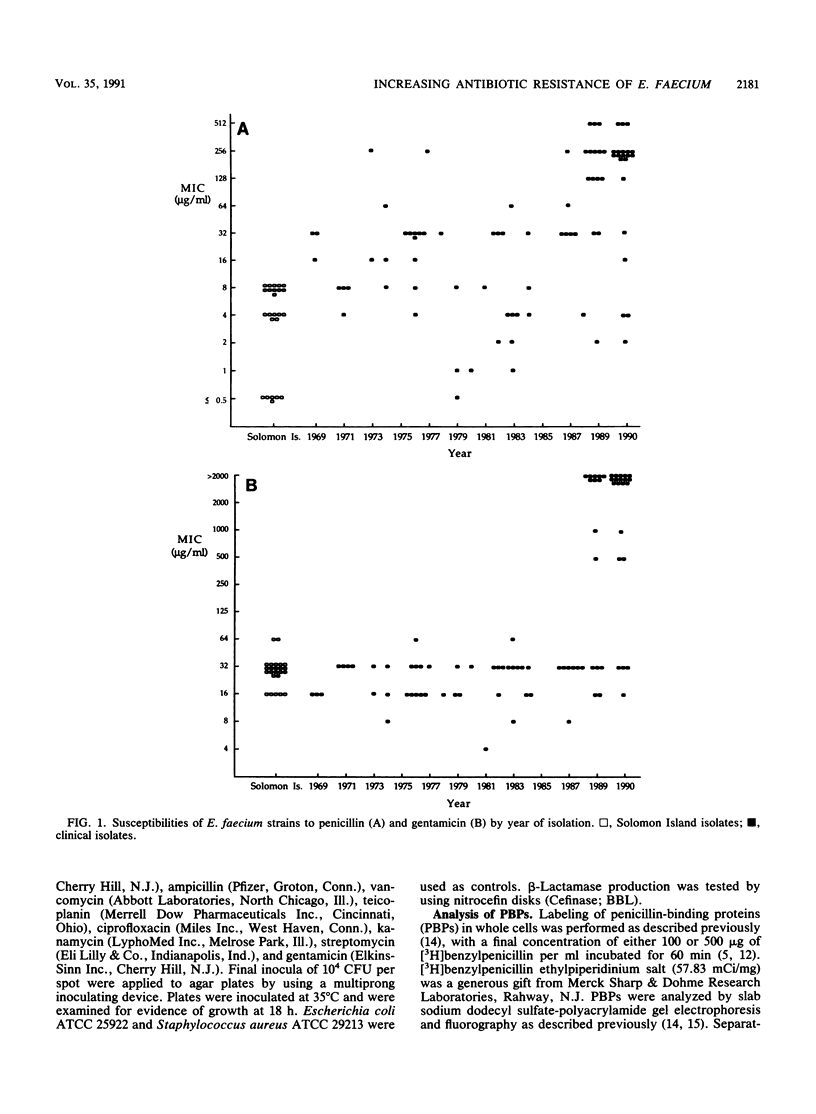
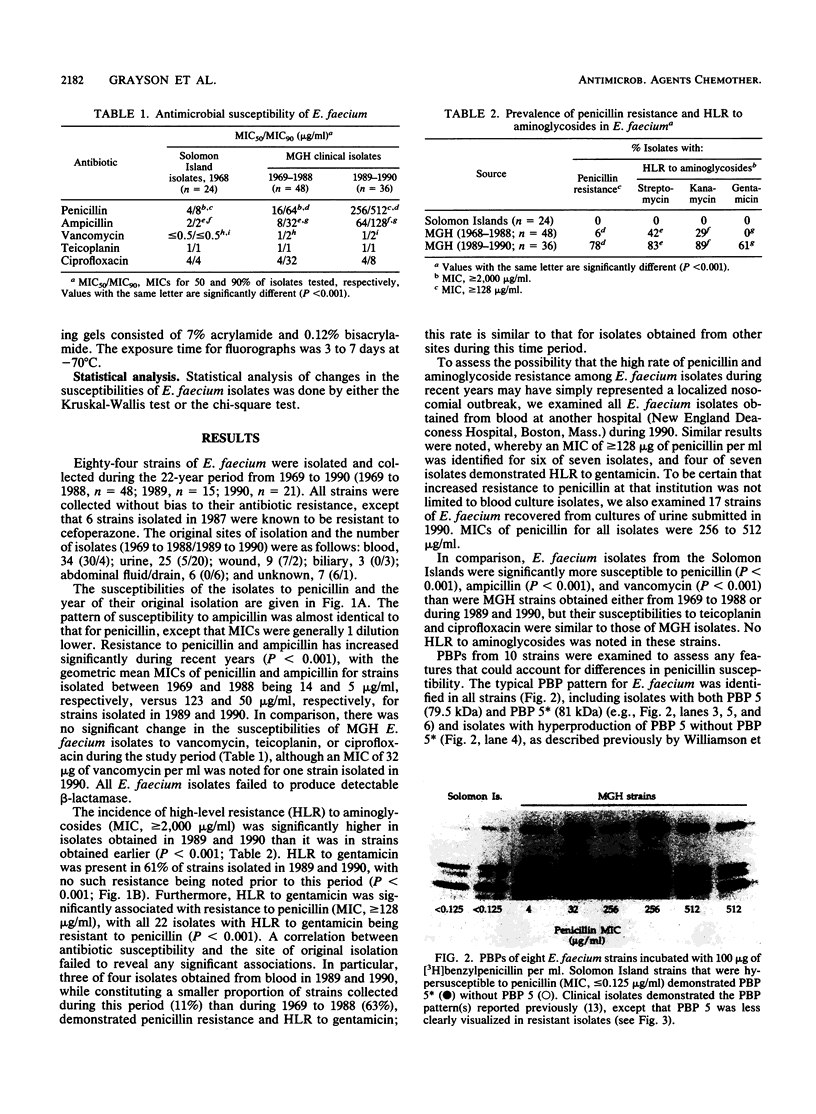
To identify any change in the antibiotic resistance of Enterococcus faecium, we examined the antibiotic susceptibilities of clinical strains (n = 84) isolated at one institution during the 22 years since 1968. A significant increase in resistance to penicillin was observed during the study period: the MICs of penicillin for 50 and 90% of isolates tested were 16 and 64 micrograms/ml, respectively, from 1969 to 1988 (n = 48; geometric mean MIC, 14 micrograms/ml) , whereas they were 256 and 512 micrograms/ml, respectively, from 1989 to 1990 (n = 36; geometric mean MIC, 123 micrograms/ml) (P less than 0.001). A comparable increase in resistance to ampicillin was also noted (P less than 0.001). No strains produced detectable beta-lactamase. In contrast, susceptibilities to vancomycin, teicoplanin, and ciprofloxacin remained stable. High-level resistance to gentamicin was observed in none of 48 isolates from 1969 to 1988, but was present in 22 of 36 strains (61%) from 1989 to 1990 (P less than 0.001) and was significantly associated with resistance (MIC, greater than or equal to 128 micrograms/ml) to penicillin (P less than 0.001). To assess the potential evolution of antibiotic resistance in this species, clinical isolates (n = 24) were compared with strains isolated in 1968 from a human population in the Solomon Islands that was never exposed to antibiotics. Solomon Island isolates were significantly more susceptible than all clinical strains to penicillin, ampicillin, and vancomycin (P less than 0.001 for each), but they exhibited no differences in susceptibility to teicoplanin or ciprofloxacin. The penicillin-binding affinity of penicillin-binding protein 5 (PBP 5) in penicillin-resistant clinical strains (MIC, 512 micrograms/ml) was notably lower than that in strains with more typical susceptibilities, suggesting an alteration in this PBP as a possible mechanism for increased penicillin resistance. Solomon Island strains most susceptible to penicillin demonstrated a prominent PBP 5* and the absence of PBP 5. These changes in the antibiotic resistance of E. faecium emphasize the importance of identifying this species in patients with serious enterococcal infections and the necessity of assessing its susceptibility to both beta-lactams and aminoglycosides if effective therapy is to be identified.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bush L. M., Calmon J., Cherney C. L., Wendeler M., Pitsakis P., Poupard J., Levison M. E., Johnson C. C. High-level penicillin resistance among isolates of enterococci. Implications for treatment of enterococcal infections. Ann Intern Med. 1989 Apr 1;110(7):515–520. doi: 10.7326/0003-4819-110-7-515. [DOI] [PubMed] [Google Scholar]
- Courvalin P. Resistance of enterococci to glycopeptides. Antimicrob Agents Chemother. 1990 Dec;34(12):2291–2296. doi: 10.1128/aac.34.12.2291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eliopoulos G. M., Wennersten C., Moellering R. C., Jr Resistance to beta-lactam antibiotics in Streptococcus faecium. Antimicrob Agents Chemother. 1982 Aug;22(2):295–301. doi: 10.1128/aac.22.2.295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Facklam R. R., Collins M. D. Identification of Enterococcus species isolated from human infections by a conventional test scheme. J Clin Microbiol. 1989 Apr;27(4):731–734. doi: 10.1128/jcm.27.4.731-734.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fontana R., Cerini R., Longoni P., Grossato A., Canepari P. Identification of a streptococcal penicillin-binding protein that reacts very slowly with penicillin. J Bacteriol. 1983 Sep;155(3):1343–1350. doi: 10.1128/jb.155.3.1343-1350.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gardner P., Smith D. H., Beer H., Moellering R. C., Jr Recovery of resistance (R) factors from a drug-free community. Lancet. 1969 Oct 11;2(7624):774–776. doi: 10.1016/s0140-6736(69)90482-6. [DOI] [PubMed] [Google Scholar]
- Oster S. E., Chirurgi V. A., Goldberg A. A., Aiken S., McCabe R. E. Ampicillin-resistant enterococcal species in an acute-care hospital. Antimicrob Agents Chemother. 1990 Sep;34(9):1821–1823. doi: 10.1128/aac.34.9.1821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sapico F. L., Canawati H. N., Ginunas V. J., Gilmore D. S., Montgomerie J. Z., Tuddenham W. J., Facklam R. R. Enterococci highly resistant to penicillin and ampicillin: an emerging clinical problem? J Clin Microbiol. 1989 Sep;27(9):2091–2095. doi: 10.1128/jcm.27.9.2091-2095.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watanakunakorn C. Enterococci from blood cultures during 1980-1989: susceptibility to ampicillin, penicillin and vancomycin. J Antimicrob Chemother. 1990 Oct;26(4):602–604. doi: 10.1093/jac/26.4.602. [DOI] [PubMed] [Google Scholar]
- Williamson R., Gutmann L., Horaud T., Delbos F., Acar J. F. Use of penicillin-binding proteins for the identification of enterococci. J Gen Microbiol. 1986 Jul;132(7):1929–1937. doi: 10.1099/00221287-132-7-1929. [DOI] [PubMed] [Google Scholar]
- Zighelboim S., Tomasz A. Penicillin-binding proteins of multiply antibiotic-resistant South African strains of Streptococcus pneumoniae. Antimicrob Agents Chemother. 1980 Mar;17(3):434–442. doi: 10.1128/aac.17.3.434. [DOI] [PMC free article] [PubMed] [Google Scholar]