Abstract
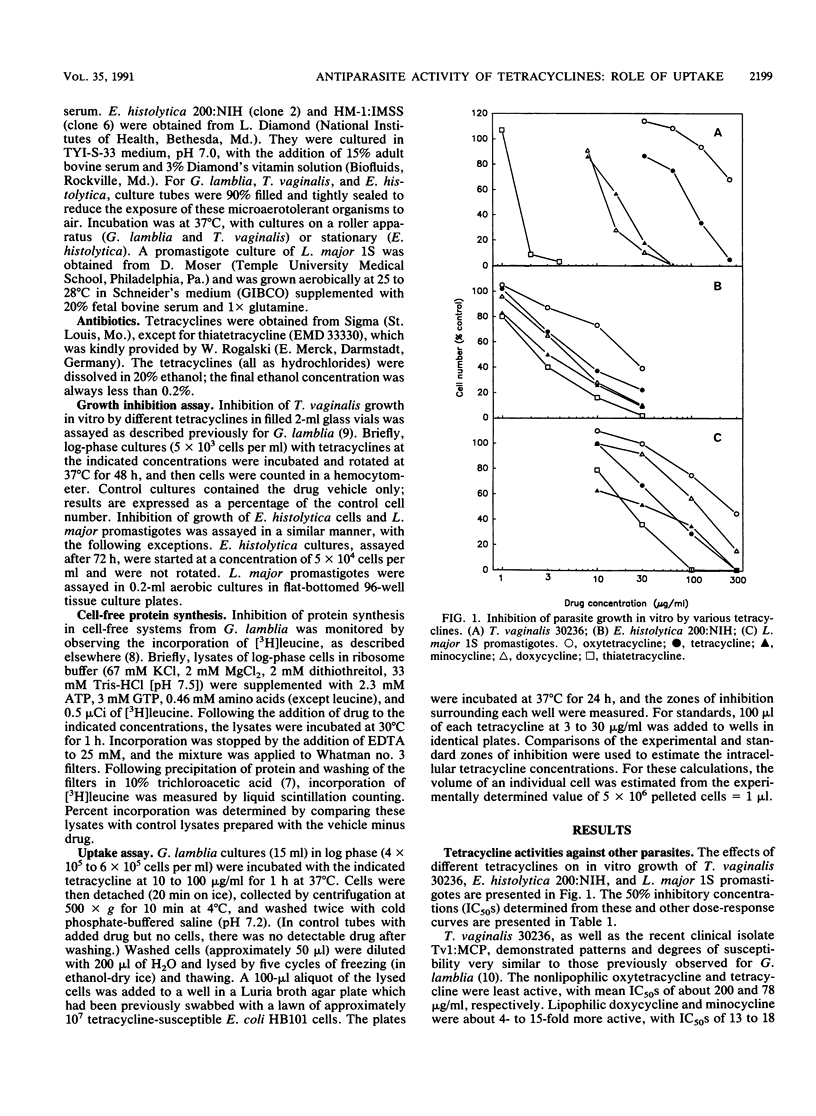
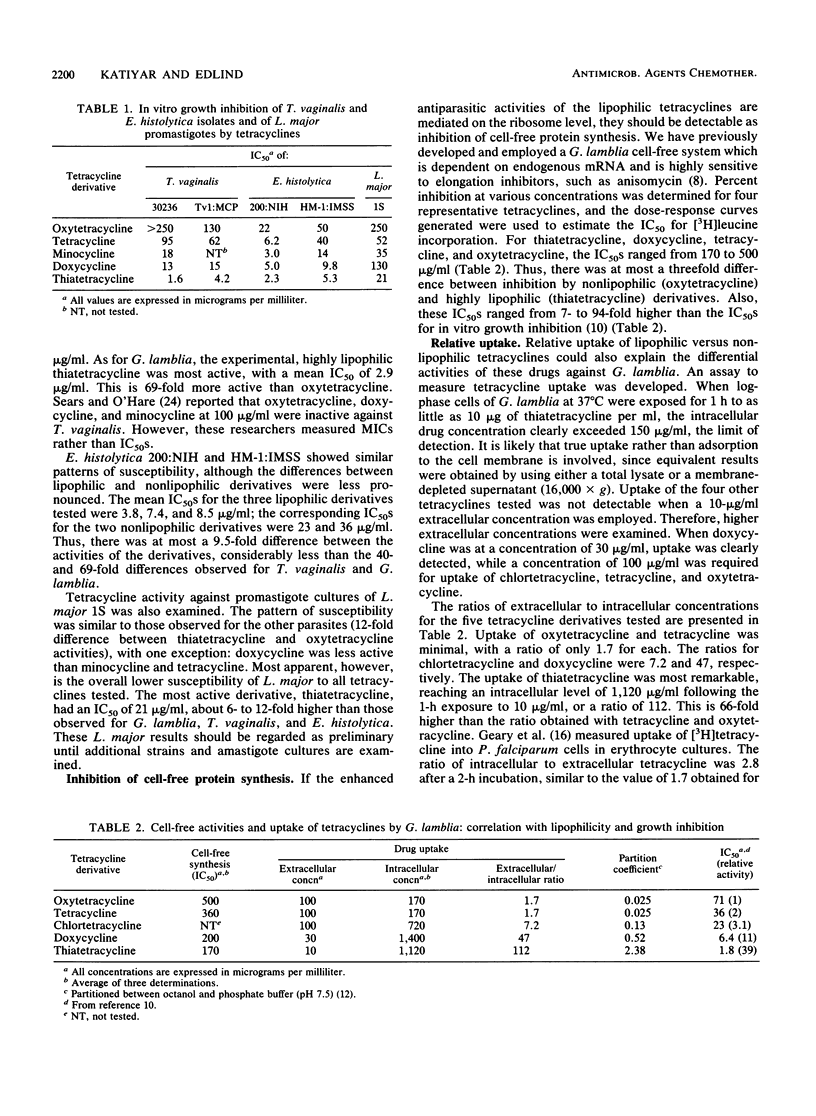
It was previously noted that the inhibitory activities of lipophilic tetracyclines against the growth of Giardia lamblia in vitro were up to 40-fold greater than those of nonlipophilic tetracyclines (50% inhibitory concentration [IC50] = 1.8 to 71 micrograms/ml) (T. D. Edlind, Antimicrob. Agents Chemother. 33:2144-2145, 1989). We have now extended this observation to Trichomonas vaginalis (IC50 = 2.9 to 200 micrograms/ml), Entaoeba histolytica (IC50 = 3.8 to 36 micrograms/ml), and Leishmania major promastigotes (IC50 = 21 to 250 micrograms/ml; one strain only). The basis for these differential tetracycline activities was investigated with G. lamblia. In a cell-free protein synthesis system, lipophilic and nonlipophilic tetracyclines had similar, relatively low activities (IC50 = 170 to 500 micrograms/ml). On the other hand, tetracycline uptake into intact cells after a 1-h incubation varied dramatically: the ratios of intracellular to extracellular drug concentrations were 1.7 to 7.2 for nonlipophilic tetracyclines and 47 to 112 for lipophilic derivatives. Thus, the variable effects of tetracyclines on the growth of G. lamblia can be fully accounted for by differences in uptake. Passive diffusion probably plays a more important role than active transport in uptake of lipophilic tetracyclines, since similar results were obtained with cells rendered nonviable by metronidazole treatment.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ball P. R., Shales S. W., Chopra I. Plasmid-mediated tetracycline resistance in Escherichia coli involves increased efflux of the antibiotic. Biochem Biophys Res Commun. 1980 Mar 13;93(1):74–81. doi: 10.1016/s0006-291x(80)80247-6. [DOI] [PubMed] [Google Scholar]
- Boothroyd J. C., Wang A., Campbell D. A., Wang C. C. An unusually compact ribosomal DNA repeat in the protozoan Giardia lamblia. Nucleic Acids Res. 1987 May 26;15(10):4065–4084. doi: 10.1093/nar/15.10.4065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen C. J., Chin J. E., Ueda K., Clark D. P., Pastan I., Gottesman M. M., Roninson I. B. Internal duplication and homology with bacterial transport proteins in the mdr1 (P-glycoprotein) gene from multidrug-resistant human cells. Cell. 1986 Nov 7;47(3):381–389. doi: 10.1016/0092-8674(86)90595-7. [DOI] [PubMed] [Google Scholar]
- Dams E., Hendriks L., Van de Peer Y., Neefs J. M., Smits G., Vandenbempt I., De Wachter R. Compilation of small ribosomal subunit RNA sequences. Nucleic Acids Res. 1988;16 (Suppl):r87–173. doi: 10.1093/nar/16.suppl.r87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edlind T. D., Chakraborty P. R. Unusual ribosomal RNA of the intestinal parasite Giardia lamblia. Nucleic Acids Res. 1987 Oct 12;15(19):7889–7901. doi: 10.1093/nar/15.19.7889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edlind T. D., Hang T. L., Chakraborty P. R. Activity of the anthelmintic benzimidazoles against Giardia lamblia in vitro. J Infect Dis. 1990 Dec;162(6):1408–1411. doi: 10.1093/infdis/162.6.1408. [DOI] [PubMed] [Google Scholar]
- Edlind T. D. Susceptibility of Giardia lamblia to aminoglycoside protein synthesis inhibitors: correlation with rRNA structure. Antimicrob Agents Chemother. 1989 Apr;33(4):484–488. doi: 10.1128/aac.33.4.484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edlind T. D. Tetracyclines as antiparasitic agents: lipophilic derivatives are highly active against Giardia lamblia in vitro. Antimicrob Agents Chemother. 1989 Dec;33(12):2144–2145. doi: 10.1128/aac.33.12.2144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FRANKLIN T. J. The inhibition of incorporation of leucine into protein of cell-free systems from rat liver and Escherichia coli by chlortetracycline. Biochem J. 1963 Jun;87:449–453. doi: 10.1042/bj0870449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franklin T. J., Higginson B. Active accumulation of tetracycline by Escherichia coli. Biochem J. 1970 Jan;116(2):287–297. doi: 10.1042/bj1160287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geary T. G., Divo A. A., Jensen J. B. Uptake of antibiotics by Plasmodium falciparum in culture. Am J Trop Med Hyg. 1988 May;38(3):466–469. doi: 10.4269/ajtmh.1988.38.466. [DOI] [PubMed] [Google Scholar]
- Herwaldt B. L., Krogstad D. J., Schlesinger P. H. Antimalarial agents: specific chemoprophylaxis regimens. Antimicrob Agents Chemother. 1988 Jul;32(7):953–956. doi: 10.1128/aac.32.7.953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keister D. B. Axenic culture of Giardia lamblia in TYI-S-33 medium supplemented with bile. Trans R Soc Trop Med Hyg. 1983;77(4):487–488. doi: 10.1016/0035-9203(83)90120-7. [DOI] [PubMed] [Google Scholar]
- Moazed D., Noller H. F. Interaction of antibiotics with functional sites in 16S ribosomal RNA. Nature. 1987 Jun 4;327(6121):389–394. doi: 10.1038/327389a0. [DOI] [PubMed] [Google Scholar]
- Pang L., Limsomwong N., Singharaj P. Prophylactic treatment of vivax and falciparum malaria with low-dose doxycycline. J Infect Dis. 1988 Nov;158(5):1124–1127. doi: 10.1093/infdis/158.5.1124. [DOI] [PubMed] [Google Scholar]
- Sears S. D., O'Hare J. In vitro susceptibility of Trichomonas vaginalis to 50 antimicrobial agents. Antimicrob Agents Chemother. 1988 Jan;32(1):144–146. doi: 10.1128/aac.32.1.144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sherman I. W. The ribosomes of the simian malaria Plasmodium knowlesi--II. A cell-free protein synthesizing system. Comp Biochem Physiol B. 1976;53(4):447–450. doi: 10.1016/0305-0491(76)90196-6. [DOI] [PubMed] [Google Scholar]
- Sogin M. L., Gunderson J. H., Elwood H. J., Alonso R. A., Peattie D. A. Phylogenetic meaning of the kingdom concept: an unusual ribosomal RNA from Giardia lamblia. Science. 1989 Jan 6;243(4887):75–77. doi: 10.1126/science.2911720. [DOI] [PubMed] [Google Scholar]
- Wilson C. M., Serrano A. E., Wasley A., Bogenschutz M. P., Shankar A. H., Wirth D. F. Amplification of a gene related to mammalian mdr genes in drug-resistant Plasmodium falciparum. Science. 1989 Jun 9;244(4909):1184–1186. doi: 10.1126/science.2658061. [DOI] [PubMed] [Google Scholar]



