Abstract
Our purpose was to examine the effects of pubertal status on generalized joint laxity in a population of male and female athletes. We hypothesized that females would show higher generalized joint laxity after the onset of puberty while males would not. This cross-sectional cohort study included 275 female and 143 male middle school and high school basketball and soccer athletes. Joint laxity was assessed using the Beighton and Horan Joint Mobility Index. BHJMI scores were averaged and female and male athletes were compared by pubertal stage. Females demonstrated increased joint laxity scores between pre-pubertal and post-pubertal groups (P=0.042), while males did not. Pre-pubertal male and female athletes were not different in cumulative joint laxity scores (female pre-puberty Mean=2.00; male pre-pubertal Mean=1.66). However, following the onset of puberty females (pubertal Mean=2.96; post-pubertal Mean= 3.03) demonstrated a greater joint laxity score compared to males (pubertal Mean=1.24; post-pubertal Mean=1.30). Gender differences in BHJMI score was found at puberty and post-puberty (P<0.001). In contrast to males, females may have greater generalized joint laxity following the onset of puberty. Structural and physiological changes that occur during puberty such as alterations in passive joint restraints, may affect the type, severity and incidence of injuries in the maturing adolescent population.
Keywords: Generalized Joint Laxity, ACL injury, Joint Hypermobility, Puberty
Introduction
Epidemiological studies indicate that adolescents experience an increased rate of ligament sprains and sports injuries as they mature [1, 2]. Both males and females experience ligament sprains at similar rates prior to puberty; however, after 12 years of age females demonstrate higher ligament sprain rates compared to males [3, 4]. The anterior cruciate ligament (ACL) injury incidence is 2–8 fold higher in adolescent female athletes compared to males.[5] Prior to puberty, however, there does not appear to be a significant difference in ACL injury rates between the genders.[6, 7] Cumulatively, these studies suggest a potential association between changes that occur during the onset of puberty and increased ACL injury risk in female athletes.
While males and females exhibit similar changes in relative limb segment growth and development associated with puberty, there is divergence in other anatomical [8], hormonal [9] and neuromuscular measures.[10–13] Identification of anatomical, hormonal or neuromuscular gender differences related to puberty may help to delineate the mechanisms underlying the increased risk of ACL injuries in females compared to males. One potential measure that may be related to higher risk of knee and ACL injury is increased generalized joint laxity and knee joint laxity.[14, 15] Increased generalized joint laxity (hypermobility) is defined as increased joint range of motion relative to the normal population. Joint stabilization provided by both active and passive restraints help provide protection against joint injury. Ligaments are passive joint restraints, while muscles contribute to both passive and active joint restraints. Ligaments, joint capsules, joint surface contacts, passive or reflexive muscle tension and soft tissues all contribute to joint stability and end-range of motion. [16] Therefore, alterations in any of these structures during puberty may compromise joint stability and could potentially lead to joint injury and loss of function.
While the effects of chronological age, race, and gender on generalized joint laxity and the prevalence of hyperlaxity are relatively well defined in a non-athletic population [17–19], the effects of pubertal status on laxity measures in an athletic population are not established in the literature. Although chronological age is a common covariate employed in gender studies, comparison of males and females by chronological age during the adolescent period may not be optimal.[20] The average onset of puberty differs between the genders, with females beginning puberty at an earlier age; hence comparisons of puberty dependent variables in males and females by age during this pubertal period may not be valid. The purpose of the present study was to examine the effects of pubertal status on generalized joint laxity in a population of male and female athletes. We hypothesized that females would show an increase in generalized joint laxity after the onset of puberty while males would not demonstrate a comparable increase.
Materials and Methods
Subjects
The present study employed a cross-sectional cohort experimental design. Subjects for this study were 275 female and 143 male (ages 11–18) healthy preadolescent and adolescent athletes from local middle school and high schools within the same school district. Data was collected just prior to the start of the athletes’ competitive soccer or basketball seasons. Informed written consent was obtained from a guardian of each subject and approved by the Institutional Review Board prior to screening. After the informed consent was obtained, height and mass were recorded (Table 1). Subjects who reported prior knee injuries were excluded from the data analysis.
Table 1.
Subject demographics and anthropometric data.
| Male | Female | |||||||
|---|---|---|---|---|---|---|---|---|
| Subjects | Age (y) | Height (cm) | Weight (kg) | Subjects | Age (y) | Height (cm) | Weight (kg) | |
| Prepubertal | 32 | 12.3 ± 0.8 | 152.8 ± 6.6 | 43.8 ± 8.7 | 31 | 12.1 ± 0.6 | 148.6 ± 7.2 | 40.4 ± 11.0 |
| Pubertal | 45 | 13.7 ± 1.0 | 167.1 ± 7.7 | 56.4 ± 9.4 | 68 | 12.7 ± 1.1 | 157.6 ± 6.0 | 50.8 ± 9.5 |
| Postpubertal | 66 | 16.1 ± 1.2 | 177.0 ± 7.1 | 68.7 ± 9.1 | 176 | 14.9 ± 1.5 | 164.2 ± 6.9 | 60.8 ± 9.9 |
Subjects were classified into three pubertal categories (pre-pubertal, pubertal, and post-pubertal) using the modified Pubertal Maturational Observational Scale (PMOS). The PMOS utilizes parental questionnaires and investigator observations to classify subjects into the pubertal categories, pre-pubertal (equivalent to Tanner Stage I), pubertal (equivalent to Tanner Stage 2 and 3) or post-pubertal (equivalent to Tanner Stages 4 and 5).[21] The PMOS scale has shown high reliability and can be used to differentiate between pubertal stages based on indicators of adolescent growth, breast development, menstruation status, axillary and leg hair growth, muscular development, presence of acne and evidence of sweating during physical activities.[10, 11, 21, 22]
Procedures
Each subject was tested by the same licensed physical therapist for generalized joint laxity using the Beighton and Horan Joint Mobility Index (BHJMI).[23] Generalized joint laxity tests consisted of fifth-finger hyperextension greater than 90 degrees (Figure 1A), elbow hyperextension greater than 10 degrees (Figure 1B), wrist and thumb to forearm opposition (Figure 1C), knee hyperextension greater than 10 degrees (Figure 1D) and palms to floor with knees fully extended (Figure 1E). All tests were performed bilaterally, except for trunk flexion (Figure 1E), with goniometric measurements following placement guidelines by Norkin and White [24] and procedures as previously described.[23] A point value of 1 was given each time an athlete surpassed the designated laxity measure at each of the 9 joints tested (right thumb, left thumb, right knee, left knee, right fifth-finger, left fifth-finger, right elbow, left elbow, and palms to floor). An injury allowance point was given to a joint that did not surpass the designated laxity measure if a subject had a history of a significant injury to that joint that limited mobility (e.g., elbow or thumb fracture) and the subject displayed a positive test on the corresponding contralateral joint.[25] Points were summed to give an overall BHJMI score (0–9). Subject composite scores were categorized into 3 categories (0–2, 3–4,5–9), with the last category representing the most hypermobile subjects.[23] A reliability study of the collected measurements was also performed. Six subjects participated in a three-session between day reliability assessment of the testing procedure. The sessions were held at approximately the same time of day. The intrarater reliability of the measurement of joint laxity score was high (ICC=0.972).
Figure 1.
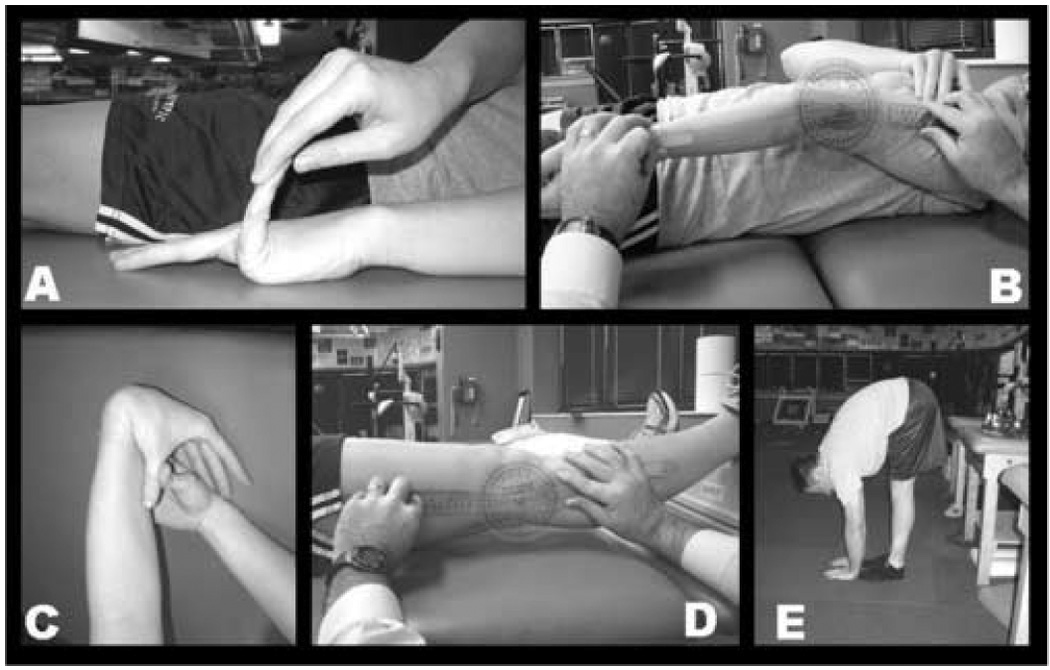
Figure 1A) Fifth finger hyperextension test. Each subject’s forearm, wrist, and fingers were stabilized on a flat table. The tester passively extended the subjects fifth finger as far as possible without pain. Hyperextension of 90 degrees or greater resulted in a score of 1. Hyperextension less than 90 degrees resulted in a score of 0.
Figure 1B) Elbow hyperextension test. Each subject’s shoulder was abducted to approximately 80 degrees, with the forearm supinated. The axis of the goniometer was placed over the lateral epicondyle of the humerus, with the distal end aligned with the radial styloid process and the proximal arm aligned along the lateral midline of the subject’s humerus. If elbow hyperextension was 10 degrees or greater a score of 1 was given. Hyperextension of less than 10 degrees resulted in a score of 0.
Figure 1C) Thumb opposition test. Each subject flexed the wrist and pulled the thumb towards the forearm using the opposite hand. If the thumb could be abducted to touch the forearm the score of 1 was given. Inability to touch the forearm resulted in a score of zero.
Figure 1D) Knee hyperextension test. Each subject was placed in a supine position with a box placed under both ankles. The axis of the goniometer was aligned with the lateral epicondyle of the femur. The distal arm was positioned with the lateral malleolus and the proximal arm was aligned with the greater trochanter. Hyperextension of the knee 10 degrees or greater resulted in a score of 1, anything less than 10 degrees resulted in a score of 0.
Figure 1E) Palms to floor test. Each subject was instructed to keep both knees extended and attempt to touch the floor with the palms flat to the floor. The ability to touch both palms flat on the floor resulted in a score of 1. If the subject was unable to place both palms flat on the floor a score of 0 was given.
Statistical Analysis
Statistical means and standard deviations were calculated to compare gender and pubertal stages (pre-pubertal, pubertal, and post-pubertal). Nonparametric analyses were utilized based on evidence that the data were not normally distributed (Kolmogorov-Smirnov normality test P < 0.001) and consisted of Kruskal-Wallis and Mann-Whitney (P < 0.05). Statistical analyses were conducted in SPSS for Windows, release 12.0 (SPSS Inc., Chicago, Illinois).
Results
Figure 2 displays the BHJMI Joint Laxity scores for the male and female athletes at the three different pubertal stages: pre-pubertal, pubertal and post-pubertal. Female athletes demonstrated a significant difference in generalized joint laxity, between pubertal groups (P=0.042), as measured by the BHJMI. In contrast, there was not a BHJMI score difference between pubertal groups in the male athletes (P=0.582). Specifically, females had significantly greater BHJMI scores post-pubertal, (P=0.042), while males did not.
Figure 2.
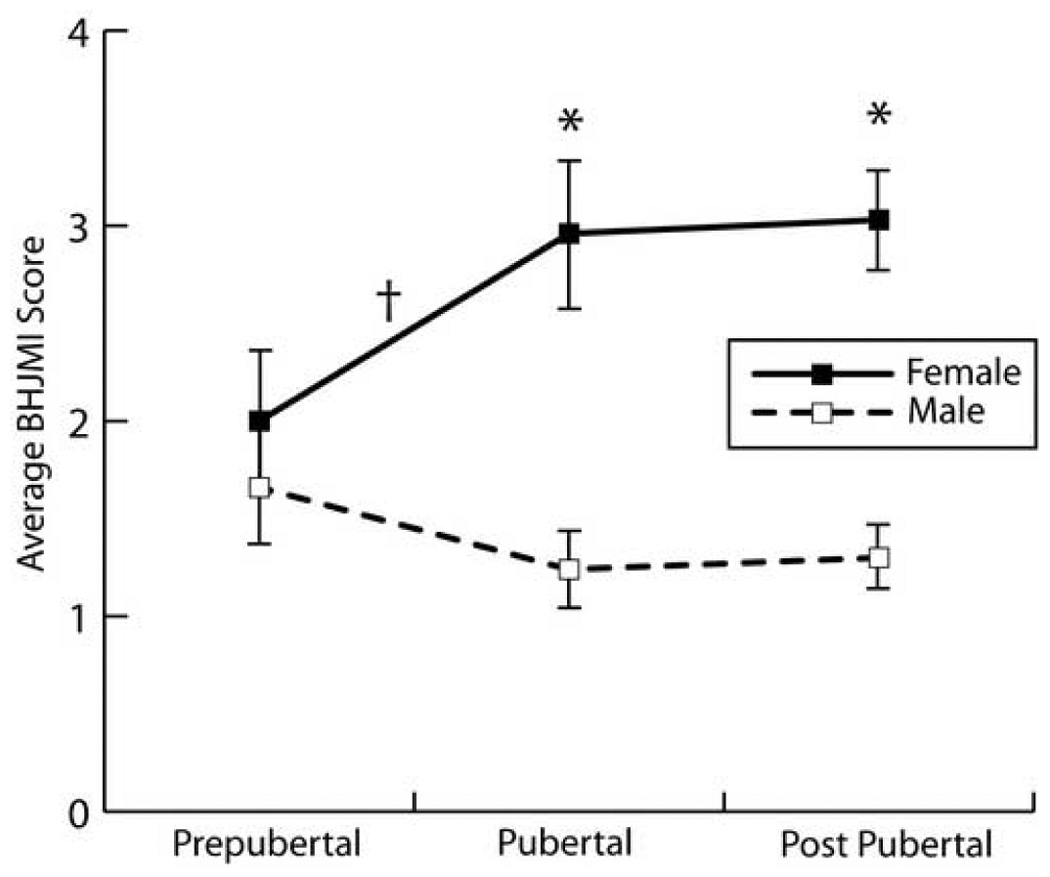
Gender and pubertal comparison (measured by the Pubertal Maturational Observational Scale) of the mean (± 1 SEM) Beighton and Horan Joint Mobility Index scores. *Females demonstrated higher generalized joint laxity scores compared to males at the pubertal and post-pubertal stages (P < 0.001).†Pubertal females demonstrated higher generalized joint laxity scores when compared to pre-pubertal females (P < 0.05).
When gender differences within each pubertal group were examined separately, there were no significant differences between males and females in cumulative joint laxity scores prior to puberty (P=0.385; female pre-pubertal =2.0; male pre-pubertal 1.7). Table 2 shows hypermobility prevalence (Beighton and Horan Joint Mobility Index score of greater than or equal to 5) in the subject population. The prevalence of hypermobility was not different between the genders in the pre-pubertal group of athletes (female 10.7%, male 3.2%, P = 0.286). However, following puberty, females (pubertal =3.0; post-pubertal = 3.0) demonstrated significantly greater (P<0.001) joint laxity scores compared to males (pubertal =1.2; post-pubertal =1.3). The prevalence of hypermobility (score ≥ 5) was 10.7%, 33.3% and 28.5% in females throughout the pubertal stages, respectively. In comparison, the prevalence of hypermobility in males was 3.2%, 2.3% and 6.5% (Table 2). Table 3 presents the gender specific percentage of athletes who demonstrated positive laxity scores at each of the tested joints.
Table 2.
Hypermobility prevalence (Beighton and Horan Joint Mobility Index score of greater than or equal to 5).
| Male < 5 | Male ≥ 5 | Male Prevalence | Female < 5 | Female ≥ 5 | Female Prevalence | |
|---|---|---|---|---|---|---|
| Pre-Pubertal | 31 | 1 | 3.2% | 28 | 3 | 10.7% |
| Pubertal | 44 | 1 | 2.3% | 51 | 17 | 33.3% |
| Post-Pubertal | 62 | 4 | 6.5% | 137 | 39 | 28.5% |
Table 3.
Gender specific percentage of athletes who demonstrated positive laxity scores at each of the tested joints.
| Pre-Pubertal | Pubertal | Post-Pubertal | ||||
|---|---|---|---|---|---|---|
| Male (%) | Female (%) | Male (%) | Female (%) | Male (%) | Female (%) | |
| Finger | 12.5 | 33.9 | 11.1 | 27.2 | 6.1 | 34.9 |
| Elbow | 15.6 | 8.1 | 10.0 | 23.5 | 5.3 | 21.3 |
| Thumb | 53.1 | 40.3 | 28.9 | 59.6 | 32.6 | 57.1 |
| Knee | 0.0 | 14.5 | 10.0 | 27.9 | 15.2 | 24.1 |
| Palms to Floor | 3.1 | 6.5 | 4.4 | 19.1 | 12.1 | 26.7 |
Discussion
The findings of the current study support the hypothesis that generalized joint laxity increases in female, but not male, athletes during puberty. Prior to puberty, males and females demonstrated similar BHJMI scores. The study findings indicate that following the onset of puberty, females develop greater generalized joint laxity, while no similar changes in joint laxity are observed in male athletes. This potentially leads to decreased static stability in female athletes and indicates that generalized laxity changes that occur during puberty are likely to underlie decreased passive joint stability in females.
Several prior studies have examined joint laxity in children, adolescents and adults with somewhat conflicting results. [17–19, 25–29] The reported prevalence of increased joint laxity in studied populations has varied widely, ranging from 5% to 34% and appears to be influenced by age, gender, and race. [17–19, 25–29] In general, traditional theories relating laxity to maturation speculate that children exhibit "looser" joints but as they age they tend to "tighten" and to demonstrate a decrease in joint range of motion.[18, 28, 30] Although numerous studies report that laxity decreases with age, an extensive review of the literature failed to produce any studies that examined the association between puberty and generalized joint laxity.[19, 25] In order to compare females and males during adolescence, pubertal stage rather than chronological age is a superior measure for comparing adolescent children during growth and development. Though previous studies have examined laxity differences between males and females, they report conflicting results; some report that females tend to show more ligament laxity than males, while others report no significant difference between genders.[25, 29–31] The purpose of this study was to examine the effects of both gender and pubertal status in an adolescent population, specifically, an athletic population.
In the current study, approximately 33% of the female athletes tested after the start of puberty were classified as hypermobile according to the BHJMI criteria. Prior investigations indicate that post-pubertal females tend to demonstrate higher incidence of increased knee laxity [14, 32] and increased generalized joint laxity [18, 19, 25, 27] compared to their male counterparts. The increase in laxity in females after the onset of puberty may be related to gender differences in hormonal or anatomical changes that occur during puberty. Specifically, the relationship between sex hormones and their possible effects on joint laxity and ACL injury risk have been studied extensively. Hormones may affect ligament synthesis, degradation, and loading properties; however, the effects of sex hormones on ligament biology and ACL injury risk are currently not well understood[9, 33]. Future research should focus on establishing the role of sex hormones in generalized joint laxity and ACL injury risk.
Although hypermobility may be an asset for dancers and musicians [34, 35] and may act as a positive selective factor for these activities, the benefits of hypermobility for athletes have yet to be determined and are likely to be highly specific to the sport. In fact, several studies relate joint hypermobility in athletes to a higher risk for injury, including ACL rupture, in jumping and cutting sports.[14, 15, 36] Uhorchak et al. reported that increased (greater than one standard deviation above the mean) generalized joint laxity increased the risk of ACL injury.[15] Similarly, Ramesh et al. found ACL injury was more prevalent in those with increased joint laxity, specifically those with increased knee joint laxity.[36] Rozzi et al. reported that compared to males, females demonstrate greater knee joint laxity and longer time intervals needed to detect knee joint movement into extension. Similarly, Shultz et al. showed that female collegiate athletes with increased knee joint laxity also had delayed reflex time and increased reflex amplitude in biceps femoris muscle activity following a perturbation.[37] These findings indicate that increased joint laxity may partially underlie neuromuscular deficits that may increase risk for sports injury, specifically knee injury and ACL injury, when compared to those with more normal passive joint stability. [14, 15, 36]
Female athletes demonstrate measurable neuromuscular deficits compared to males. Hewett et al. demonstrated that compared to males, females display greater maximum lower extremity valgus angles and greater total medial motion of the knees during a jump task following the onset of puberty.[11] These decreases in dynamic knee stability are coupled with decreased knee flexor torques in female athletes after puberty, compared to males.[11] In addition, while males demonstrate a neuromuscular spurt during puberty, demonstrated by increased vertical jump height and increased ability to attenuate landing forces, females do not experience a similar increase in neuromuscular performance.[10] Poor dynamic knee control and the absence of a neuromuscular spurt, coupled with increased generalized joint laxity in maturing females, may be linked to the increased dynamic coronal plane knee motion, which may increase ACL injury risk following the onset of puberty. [11, 38] Neuromuscular interventions may help to compensate for decreased passive joint stability by increasing dynamic joint stability since resistance training, in addition to increasing muscle strength and recruitment, may also result in advantageous adaptations in bones, tendons and ligaments and help reduce injuries.[39, 40] Therefore, increased joint awareness and improved active joint motion restraints may provide joint protection for an athlete that demonstrates looser passive joint restraints as evidenced by ligament laxity.
There are several limitations to the present study. Tanner Staging, the "Gold Standard" measurement for determining pubertal status, was not used for this study. However, the current study utilized one experienced rater to quality control the reported variables for each athlete at the time of testing and to determine accurate pubertal categorizations. In addition, the PMOS has been used successfully in prior investigations with similar populations and is reported to be a reliable assessment tool to determine pubertal status. Another limitation to this study is the reliability of generalized joint laxity testing. Although generalized joint laxity testing has been used in many studies, the reproducibility and reliability can be relatively low, as evidenced by studies that show conflicting results regarding joint laxity prevalence. [17–19, 25–30] This limitation was minimized in the current study by using one rater with over 12 years experience in the physical therapy setting and with multiple years of experience screening hundreds of athletes using the BHJMI. Intrarater reliability tested for this individual was high. An additional concern is that this study was cross-sectional in nature and therefore the study design could not be used to determine changes over time within the subjects tested. Future studies should address this question with longitudinal study designs in order to determine changes in individuals and groups of individuals over time. Despite these limitations, this study accurately measured the effects of puberty and gender on generalized joint laxity in a large mixed age group of male and female athletes.
Summary and Conclusions
In conclusion, the current findings support the hypothesis that female athletes experience an increase in generalized joint laxity following the onset of puberty, while males do not demonstrate a significant change in laxity with puberty. This increase in joint laxity associated with the onset of puberty in females may be related to their concomitant increase in knee and ACL injury incidence in female athletes.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Adirim TA, Cheng TL. Overview of injuries in the young athlete. Sports Med. 2003;33(1):75–81. doi: 10.2165/00007256-200333010-00006. [DOI] [PubMed] [Google Scholar]
- 2.Michaud PA, Renaud A, Narring F. Sports activities related to injuries? A survey among 9–19 year olds in Switzerland. Inj Prev. 2001;7(1):41–45. doi: 10.1136/ip.7.1.41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Tursz A, Crost M. Sports-related injuries in children. A study of their characteristics, frequency, and severity, with comparison to other types of accidental injuries. Am J Sports Med. 1986;14(4):294–299. doi: 10.1177/036354658601400409. [DOI] [PubMed] [Google Scholar]
- 4.Shea KG, et al. Anterior cruciate ligament injury in pediatric and adolescent soccer players: an analysis of insurance data. J Pediatr Orthop. 2004;24(6):623–628. doi: 10.1097/00004694-200411000-00005. [DOI] [PubMed] [Google Scholar]
- 5.Agel J, Arendt EA, Bershadsky B. Anterior cruciate ligament injury in national collegiate athletic association basketball and soccer: a 13-year review. Am J Sports Med. 2005;33(4):524–530. doi: 10.1177/0363546504269937. [DOI] [PubMed] [Google Scholar]
- 6.Gallagher SS, et al. The incidence of injuries among 87,000 Massachusetts children and adolescents: results of the 1980–81 Statewide Childhood Injury Prevention Program Surveillance System. Am J Public Health. 1984;74(12):1340–1347. doi: 10.2105/ajph.74.12.1340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Andrish JT. Anterior cruciate ligament injuries in the skeletally immature patient. Am J Orthop. 2001;30(2):103–110. [PubMed] [Google Scholar]
- 8.Griffin LY, et al. Noncontact anterior cruciate ligament injuries: risk factors and prevention strategies. J Am Acad Orthop Surg. 2000;8(3):141–150. doi: 10.5435/00124635-200005000-00001. [DOI] [PubMed] [Google Scholar]
- 9.Zazulak BT, et al. A Systematic Review of the Effects of the Menstrual Cycle on Anterior Knee Laxity in Females. Sports Med. 2006;36(10):847–862. doi: 10.2165/00007256-200636100-00004. [DOI] [PubMed] [Google Scholar]
- 10.Quatman CE, et al. Maturation Leads to Gender Differences in Landing Force and Vertical Jump Performance: A Longitudinal Study. Am J Sports Med. 2006;34(5):806–813. doi: 10.1177/0363546505281916. [DOI] [PubMed] [Google Scholar]
- 11.Hewett TE, Myer GD, Ford KR. Decrease in neuromuscular control about the knee with maturation in female athletes. J Bone Joint Surg Am. 2004;86-A(8):1601–1608. doi: 10.2106/00004623-200408000-00001. [DOI] [PubMed] [Google Scholar]
- 12.Ford KR, et al. Gender differences in the kinematics of unanticipated cutting in young athletes. Medicine & Science in Sports. 2005;37(1):124–129. [PubMed] [Google Scholar]
- 13.Ford KR, Myer GD, Hewett TE. Valgus knee motion during landing in high school female and male basketball players. Med Sci Sports Exerc. 2003;35(10):1745–1750. doi: 10.1249/01.MSS.0000089346.85744.D9. [DOI] [PubMed] [Google Scholar]
- 14.Rozzi SL, et al. Knee joint laxity and neuromuscular characteristics of male and female soccer and basketball players. Am J Sports Med. 1999;27(3):312–319. doi: 10.1177/03635465990270030801. [DOI] [PubMed] [Google Scholar]
- 15.Uhorchak JM, et al. Risk factors associated with noncontact injury of the anterior cruciate ligament: a prospective four-year evaluation of 859 West Point cadets. Am J Sports Med. 2003;31(6):831–842. doi: 10.1177/03635465030310061801. [DOI] [PubMed] [Google Scholar]
- 16.Solomonow M, Krogsgaard M. Sensorimotor control of knee stability. A review. Scand J Med Sci Sports. 2001;11(2):64–80. doi: 10.1034/j.1600-0838.2001.011002064.x. [DOI] [PubMed] [Google Scholar]
- 17.Larsson LG, et al. Hypermobility: prevalence and features in a Swedish population. Br J Rheumatol. 1993;32(2):116–119. doi: 10.1093/rheumatology/32.2.116. [DOI] [PubMed] [Google Scholar]
- 18.Jansson A, et al. General joint laxity in 1845 Swedish school children of different ages: age- and gender-specific distributions. Acta Paediatr. 2004;93(9):1202–1206. doi: 10.1080/08035250410023971. [DOI] [PubMed] [Google Scholar]
- 19.Larsson LG, Baum J, Mudholkar GS. Hypermobility: features and differential incidence between the sexes. Arthritis Rheum. 1987;30(12):1426–1430. doi: 10.1002/art.1780301216. [DOI] [PubMed] [Google Scholar]
- 20.Malina RM, Bouchard C. Growth, maturation, and physical activity. Champaign, Il: Human Kinetics. 1991:501. [Google Scholar]
- 21.Davies PL, Rose JD. Motor skills of typically developing adolescents: awkwardness or improvement? Phys Occup Ther Pediatr. 2000;20(1):19–42. [PubMed] [Google Scholar]
- 22.Davies PS. Dept. of Psychology. Laramie: University of Wyoming; 1995. Assessment of cognitive development in adolescents by means of neuropsychological tasks; p. 144. [Google Scholar]
- 23.Boyle KL, Witt P, Riegger-Krugh C. Intrarater and Interrater Reliability of the Beighton and Horan Joint Mobility Index. J Athl Train. 2003;38(4):281–285. [PMC free article] [PubMed] [Google Scholar]
- 24.Norkin CC, White DJ. Meausurement of Joint Motion: A guide to Goniometry. Philladelphia: F. A. Davis Company; 1985. [Google Scholar]
- 25.Decoster LC, et al. Prevalence and features of joint hypermobility among adolescent athletes. Arch Pediatr Adolesc Med. 1997;151(10):989–992. doi: 10.1001/archpedi.1997.02170470023005. [DOI] [PubMed] [Google Scholar]
- 26.Biro F, Gewanter HL, Baum J. The hypermobility syndrome. Pediatrics. 1983;72(5):701–706. [PubMed] [Google Scholar]
- 27.Seckin U, et al. The prevalence of joint hypermobility among high school students. Rheumatol Int. 2005;25(4):260–263. doi: 10.1007/s00296-003-0434-9. [DOI] [PubMed] [Google Scholar]
- 28.Hakim AJ, et al. The genetic epidemiology of joint hypermobility: a population study of female twins. Arthritis Rheum. 2004;50(8):2640–2644. doi: 10.1002/art.20376. [DOI] [PubMed] [Google Scholar]
- 29.Arroyo IL, Brewer EJ, Giannini EH. Arthritis/arthralgia and hypermobility of the joints in schoolchildren. J Rheumatol. 1988;15(6):978–980. [PubMed] [Google Scholar]
- 30.Cheng JC, Chan PS, Hui PW. Joint laxity in children. J Pediatr Orthop. 1991;11(6):752–756. doi: 10.1097/01241398-199111000-00010. [DOI] [PubMed] [Google Scholar]
- 31.Gedalia A, et al. Hypermobility of the joints in juvenile episodic arthritis/arthralgia. J Pediatr. 1985;107(6):873–876. doi: 10.1016/s0022-3476(85)80178-5. [DOI] [PubMed] [Google Scholar]
- 32.Huston LJ, Wojtys EM. Neuromuscular performance characteristics in elite female athletes. Am J Sports Med. 1996;24(4):427–436. doi: 10.1177/036354659602400405. [DOI] [PubMed] [Google Scholar]
- 33.Hewett TE, Zazulak BT, Myer GD. Effects of the Menstrual Cycle on Anterior Cruciate Ligament Injury Risk: A Systematic Review. Am J Sports Med. 2007 doi: 10.1177/0363546506295699. [DOI] [PubMed] [Google Scholar]
- 34.McCormack M, et al. Joint laxity and the benign joint hypermobility syndrome in student and professional ballet dancers. J Rheumatol. 2004;31(1):173–178. [PubMed] [Google Scholar]
- 35.Larsson LG, et al. Benefits and disadvantages of joint hypermobility among musicians. N Engl J Med. 1993;329(15):1079–1082. doi: 10.1056/NEJM199310073291504. [DOI] [PubMed] [Google Scholar]
- 36.Ramesh R, et al. The risk of anterior cruciate ligament rupture with generalised joint laxity. J Bone Joint Surg Br. 2005;87(6):800–803. doi: 10.1302/0301-620X.87B6.15833. [DOI] [PubMed] [Google Scholar]
- 37.Shultz SJ, Carcia CR, Perrin DH. Knee joint laxity affects muscle activation patterns in the healthy knee. J Electromyogr Kinesiol. 2004;14(4):475–483. doi: 10.1016/j.jelekin.2003.11.001. [DOI] [PubMed] [Google Scholar]
- 38.Hewett TE, et al. Biomechanical Measures of Neuromuscular Control and Valgus Loading of the Knee Predict Anterior Cruciate Ligament Injury Risk in Female Athletes: A Prospective Study. Am J Sports Med. 2005;33(4):492–501. doi: 10.1177/0363546504269591. [DOI] [PubMed] [Google Scholar]
- 39.Kraemer WJ, Duncan ND, Volek JS. Resistance training and elite athletes: adaptations and program considerations. J Orthop Sports Phys Ther. 1998;28(2):110–119. doi: 10.2519/jospt.1998.28.2.110. [DOI] [PubMed] [Google Scholar]
- 40.Fleck SJ, Falkel JE. Value of resistance training for the reduction of sports injuries. Sports Med. 1986;3(1):61–68. doi: 10.2165/00007256-198603010-00006. [DOI] [PubMed] [Google Scholar]


