Abstract
The transcriptional coactivator peroxisome-proliferator-activated receptor-γ coactivator-1α (PGC-1α) is induced in the liver in response to fasting and coordinates the activation of targets necessary for increasing energy production for gluconeogenesis and ketogenesis. After partial hepatectomy, the liver must restore its mass while maintaining metabolic homeostasis to ensure survival. Here we report that PGC-1α is rapidly and dramatically induced after hepatectomy, with an amplitude of induction that exceeds the fasting response. Maximal activation of PGC-1α after hepatectomy is dependent on the basic leucine zipper transcription factor, CCAAT/enhancer binding protein-β (C/EBPβ), a critical factor in hepatocyte proliferation. We demonstrate in vivo C/EBPβ binding to C/EBP and cAMP response element sites in the PGC-1α promoter and show that the C/EBP site is essential for PGC-1α activation. Expression of the PGC-1α target, carnitine palmitoyl transferase 1a, the rate-limiting enzyme in fatty acid β-oxidation, and of long-chain acyl-coenzyme A dehydrogenase, an enzyme involved in β-oxidation of long chain fatty acids, was significantly reduced in C/EBPβ−/− livers after hepatectomy. These findings identify C/EBPβ as a direct activator of PGC-1α in the regenerating liver. The demonstration of a functional link between C/EBPβ and PGC-1α activation provides a likely mechanism for how upstream signaling pathways in the regenerating liver can enable the adaptation to the changed metabolic status.
TRANSCRIPTIONAL COACTIVATOR proteins are recruited to target gene promoters in response to cellular signals and mediate the adaptation to changes in tissue homeostasis. Although some coactivators like cAMP response element-binding protein (CREB)-binding protein and p300 have intrinsic histone acetyltransferase activity to promote chromatin access for RNA polymerase, others activate transcription through their ability to recruit chromatin-remodeling proteins and/or to act as a scaffold for tissue-specific transcription factors (1). The latter category is represented by peroxisome-proliferator-activated receptor-γ coactivator-1α (PGC-1α), an essential regulator of processes that provide energy in settings of increased metabolic requirements including thermal regulation and gluconeogenesis (2). PGC-1α is highly expressed in tissues that exhibit substantial metabolic demands including skeletal muscle, heart, and kidney and is induced in response to physiological perturbations that signal increased metabolic needs including exercise, caloric restriction, and oxidative stress (3,4,5,6).
In the fed state, PGC-1α is expressed at low levels in the liver (7,8). However, PGC-1α is markedly induced in response to fasting and in diabetes, conditions associated with increased rates of fatty acid oxidation and gluconeogenesis (8,9). Gain- and loss-of-function studies have suggested critical roles of PGC-1α in augmenting energy production in the liver via multiple mechanisms including activation of the gluconeogenic enzymes phosphoenolpyruvate carboxykinase (PEPCK) and glucose-6-phosphatase (G6Pase) (10,11,12,13), regulation of fatty acid β-oxidation, tricarboxylic acid cycle flux (14,15), mitochondrial respiration (16), and heme biosynthesis (17). Thus, PGC-1α is critical for the activation of a program of genes that augment energy production in response to increased metabolic demands in the liver.
PGC-1α activity is mediated by transcriptional and posttranscriptional mechanisms (18). The identification of signaling pathways that activate PGC-1α transcription in the liver has primarily focused on the response to fasting. In this context, PGC-1α activation is mediated by the cAMP regulated transcription factor CREB (11) and the glucocorticoid receptor (19), and by FoxO1 in a hepatoma cell line (20). In fact, current models hold that the major effects of cAMP and glucocorticoids on hepatic gluconeogenesis are mediated by PGC-1α.
After partial hepatectomy, the liver must restore its mass and maintain homeostasis to ensure the survival of the organism. This highly synchronized proliferative, growth, and homeostatic response is dependent in part on the coordinated transcriptional activation of groups of genes responsible for each of these biological programs (21). Similar to the fasted response, after partial hepatectomy the liver increases expression of genes encoding gluconeogenic enzymes, uncoupling proteins (22), and nuclear hormone receptors including PPARα (23), all of which are known to be regulated by PGC-1α in other systems. The potential role of PGC-1α in response to increased hepatic metabolic stress after partial hepatectomy has not been investigated.
We hypothesized that PGC-1α could be involved in coordinating the transcriptional activation of genes important for homeostasis, growth, and/or proliferative responses in the regenerating liver. Here we report that PGC-1α is rapidly and dramatically induced during compensatory hepatic growth and show that the leucine zipper transcription factor CCAAT/enhancer binding protein-β (C/EBPβ) is a transcriptional regulator of PGC-1α during this response.
RESULTS
PGC-1α Is Induced during the Hepatic Growth Response in C/EBPβ-Dependent Fashion
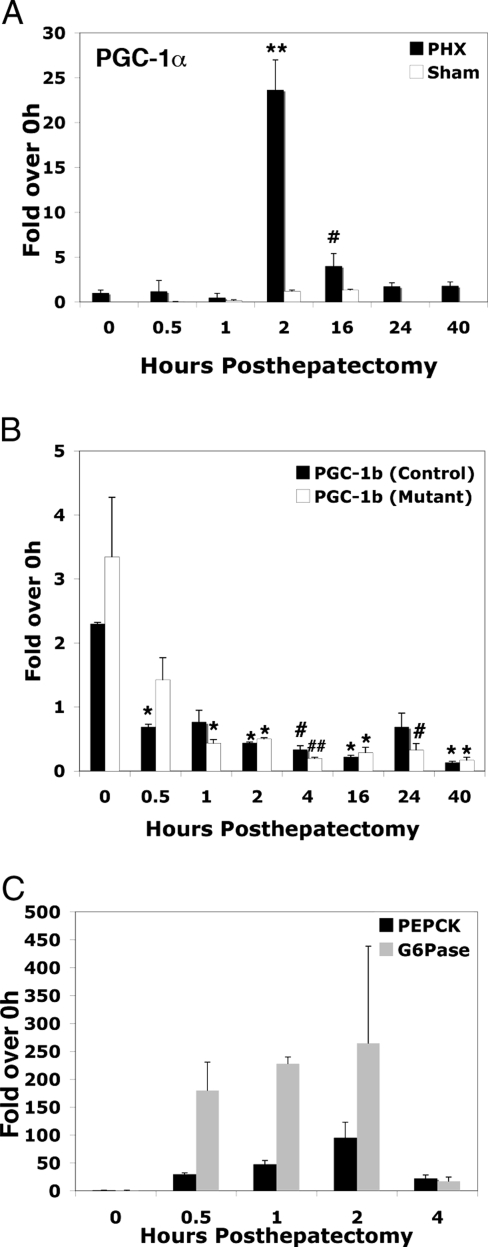
Reasoning that liver regeneration is a condition of high metabolic demand, we first assessed whether the metabolic coactivator PGC-1α was induced after partial hepatectomy. PGC-1α expression was dramatically induced, with maximal mRNA levels and protein levels occurring 2 and 4 h after hepatectomy, respectively (Figs. 1A and 2, A and B). In contrast, we detected minimal PGC-1α elevation in the 0.5-, 1-, 2-, and 16-h sham-operated animals (Fig. 1A), indicating that neither short-term fasting nor the stress of surgery was sufficient to induce PGC-1α. In contrast, expression of the closely related PGC-1β gene decreased after hepatectomy (Fig. 1B). These data suggest that PGC-1α is an important coactivator of transcriptional targets in the regenerating liver. Interestingly, the peak induction of PGC-1α after hepatectomy occurs later than the initial activation of the gluconeogenic enzymes G6Pase and PEPCK (Fig. 1C) (24,25), suggesting that PGC-1α activates genes necessary for functions other than gluconeogenesis during the period of compensatory hepatic growth.
Figure 1.
PGC-1α Is Dramatically Induced after Partial Hepatectomy
Total RNA was isolated and reverse transcribed from livers of control mice that were sham-operated or had undergone partial hepatectomy (A) from control and C/EBPβ−/− mice that had undergone partial hepatectomy (B). cDNA was quantified using real-time PCR and normalized to TBP cDNA levels. The levels of PGC-1α detected in 0.5-, 1-, 2-, 16-, 24-, and 40-h posthepatectomy (PHX) and 0.5-, 1-, 2-, and 16-h sham PHX samples are expressed relative to quiescent liver (0 h). C, G6Pase and PEPCK cDNA were quantified using real-time PCR as in A and B in 0.5-, 1-, 2-, and 4-h PHX samples expressed relative to quiescent liver (0 h). Error bars indicate sem. N = 2–4. A, PGC-1α. **, P value < 0.001 0 h vs. 2 h, #, P value < 0.05 0 h vs. 16 h. B, PGC-1β. *, P value < 0.01, 0-h control vs. 0.5 h control, 0-h control vs. 1-h mutant, 0-h control vs. 2-h control, 0-h control vs. 2-h mutant, 0 h control vs. 16-h control, 0-h control vs. 16-h mutant, 0-h control vs. 40-h control, 0-h control vs. 40-h mutant; #, P value < 0.05 0-h control vs. 24-h mutant.
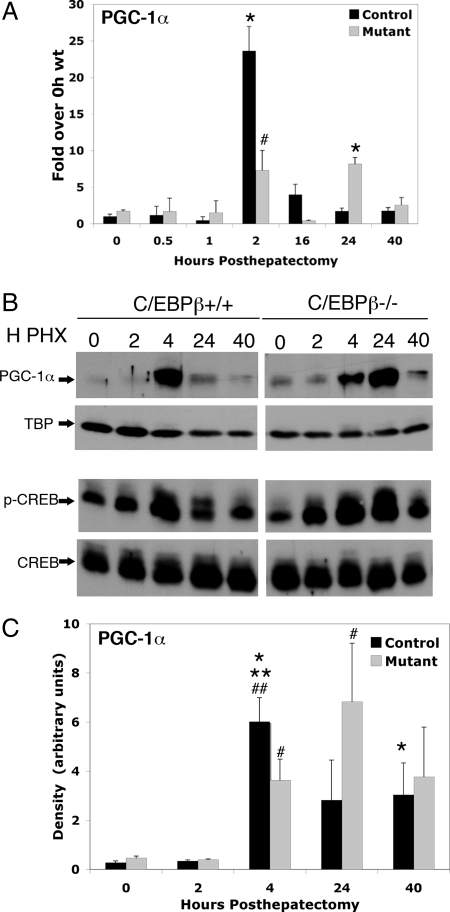
Figure 2.
C/EBPβ Regulates PGC-1α Expression in the Regenerating Liver
A, Total RNA was isolated and reverse transcribed from wild-type and C/EBPβ−/− livers at the indicated time points after partial hepatectomy. cDNA was quantified using real-time PCR and normalized to TBP cDNA levels. All data are shown as fold over 0-h wild-type expression levels. Error bars indicate sem. N = 2–4. *, P value < 0.001, 2-h control vs. 0-h control, 24-h mutant vs. 0-h control; #, P value < 0.05, 2-h control vs. 2-h mutant. B, Nuclear protein was isolated from wild-type and C/EBPβ−/− livers at indicated time points after partial hepatectomy, separated by SDS-PAGE, and immunoblotted with either α-PGC-1α, α-TBP, α-CREB, or α-phospho-CREB. Immunoblot of PGC-1α is representative of composite data from two to four samples for each time point. C, Quantification of nuclear PGC-1α protein expression. Band intensities for PGC-1α and TBP shown in B were quantified by densitometry, and PGC-1α protein levels normalized to TBP are shown. **, P value <0.001, 4-h control vs. 0-h control; ##, P value < 0.05, 4-h control vs. 0-h control; *, P value < 0.05, 4-h control vs. 24-h control; 40-h control vs. 24-h control; #, P value < 0.05, 4-h mutant vs. 0-h mutant, 24-h mutant vs. 0-h control.
Transcriptional activation of PGC-1α in the liver during a prolonged fast is mediated by the transcription factor CREB in response to glucagon (11) and by glucocorticoids (19). The increase in the glucagon to insulin ratio that occurs after partial hepatectomy is similar to what occurs in the fasted state, suggesting that PGC-1α transcription is likely to be regulated, at least in part, via the cAMP/CREB pathway after hepatectomy (26). Maximal activation of PGC-1α in response to fasting occurs 24 h or later after food deprivation (10). Therefore, the rapid and massive induction of PGC-1α mRNA detected after partial hepatectomy indicates that factors in addition to CREB are likely to contribute to PGC-1α activation in this model.
The basic leucine zipper transcription factor C/EBPβ is significantly induced after partial hepatectomy at time points corresponding to the peak of PGC-1α transcriptional activation, and C/EBPβ activity can be increased by the cAMP signaling pathway (24,27,28). Moreover, C/EBPβ mediates cAMP-inducible expression of PGC-1α in adipocytes (29). These previous findings suggested that C/EBPβ could be involved in the transcriptional activation of PGC-1α in the regenerating liver. To investigate this possibility, we quantified expression of PGC-1α mRNA in C/EBPβ−/− livers after hepatectomy. As shown in Fig. 2, A and C, the peak induction of PGC-1α mRNA was reduced approximately 2-fold in C/EBPβ−/− livers relative to wild-type controls 2 h after hepatectomy. PGC-1α protein was nearly 2-fold lower at the 4-h time point relative to wild-type controls. In contrast to wild-type livers, PGC-1α protein remained elevated in the mutant livers 24 h after hepatectomy. These data taken together suggest that maximal induction of PGC-1α is C/EBPβ dependent but that other factors may be responsible for the increased expression of PGC-1α at the 24-h time point in the mutant livers. We did not detect any compensatory up-regulation of PGC-1β in the C/EBPβ−/− livers after hepatectomy (Fig. 1B).
One possibility for the reduced activation of PGC-1α in C/EBPβ−/− mice is reduced expression and/or phosphorylation of the known PGC-1α activator, CREB. Therefore, we determined both total and nuclear phospho-CREB levels by immunoblot analysis. As shown in Fig. 2B, total CREB protein expression and phospho-CREB were similar in control and C/EBPβ−/− livers after hepatectomy, thus suggesting that C/EBPβ does not regulate PGC-1α indirectly via CREB. However, it is conceivable that CREB contributes to the increased transcription of PGC-1α in the mutants at 24 h after hepatectomy. The level of nuclear transducer of regulated CREB activity (TORC2), an enhancer of CREB activity (30,31,32,33), was not altered in the absence of C/EBPβ (data not shown).
C/EBPβ Occupies the PGC-1α Promoter in Both Quiescent and Regenerating Liver
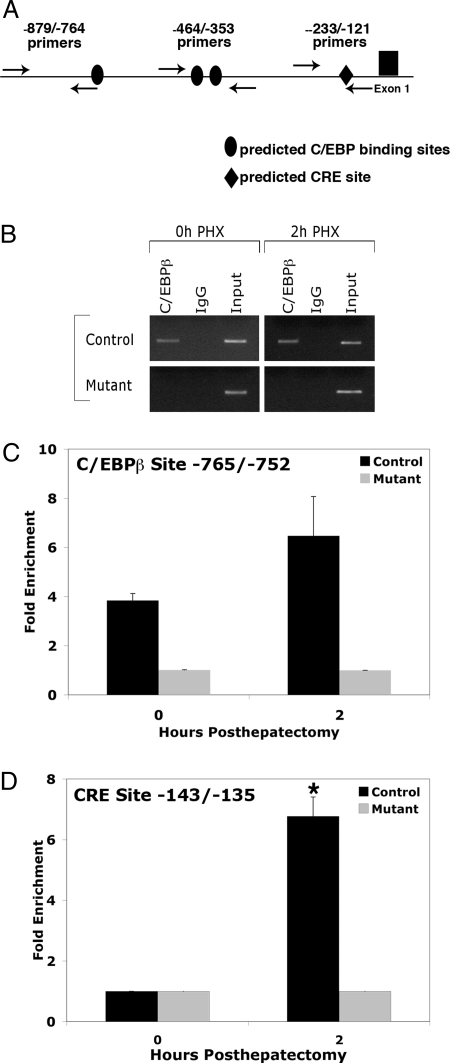
To investigate whether C/EBPβ regulates PGC-1α directly, we used computational tools to identify C/EBPβ consensus sites in the PGC-1α promoter. We identified several potential binding sites for C/EBPβ in the PGC-1α promoter at nucleotide positions −765 to −752, −438 to 425, and −421 to −408. To further evaluate C/EBPβ binding to the PGC-1α promoter in the liver, chromatin immunoprecipitation (ChIP) assays were performed using primers spanning each of these putative binding sites. Quantitative assessment of C/EBPβ occupancy on the PGC-1α promoter using primers near the −765/−752 element demonstrated that C/EBPβ binds directly to the PGC-1α promoter in vivo in both quiescent liver and 2 h after hepatectomy (Fig. 3, B and C). In contrast, C/EBPβ was not enriched on the PGC-1α promoter using primers spanning the two downstream putative C/EBPβ binding sites (data not shown). These ChIP data support the notion that C/EBPβ occupies the predicted C/EBP binding site located at −765/−752 in the PGC-1α promoter.
Figure 3.
C/EBPβ Occupancy on the PGC-1α Promoter after Partial Hepatectomy
A, Chromatin extracted from control and C/EBPβ−/− livers 0 and 2 h after hepatectomy was immunoprecipitated with antibodies specific to C/EBPβ or rabbit IgG. Primers surrounding the C/EBPβ site −765/−752 or CRE site −143/−135 in the PGC-1α promoter were used for PCR to assess the enrichment of these sites in immunoprecipitated DNA. B, Immunoprecipitated chromatin was analyzed by PCR with −879/−764 primers for the C/EBPβ site and −233/−121 primers for the CRE site. C and D, Quantification of C/EBPβ occupancy on the C/EBPβ site (C) and CRE site in PGC-1α promoter (D). *, P value < 0.05, 0-h control vs. 2-h control (n = 2–4).
During adipocyte differentiation, C/EBPβ occupancy in the CRE region of the PGC-1α promoter increases in response to forskolin treatment, which increases intracellular cAMP levels (29). We therefore investigated whether C/EBPβ also occupied the CRE site after hepatectomy and could contribute to PGC-1α induction in the regenerating liver. In contrast to the −765/−752 C/EBPβ site, where some C/EBPβ binding was evident even in quiescent liver (Fig. 3, B and C), there was no enrichment of C/EBPβ to the CRE region in quiescent liver (Fig. 3D). Strikingly, C/EBPβ occupancy on the CRE site increased more than 6-fold 2 h after hepatectomy. These data taken together suggest that C/EBPβ regulates PGC-1α transcription via binding to both C/EBPβ and CRE sites in the PGC-1α promoter.
C/EBPβ Directly Activates PGC-1α Transcription
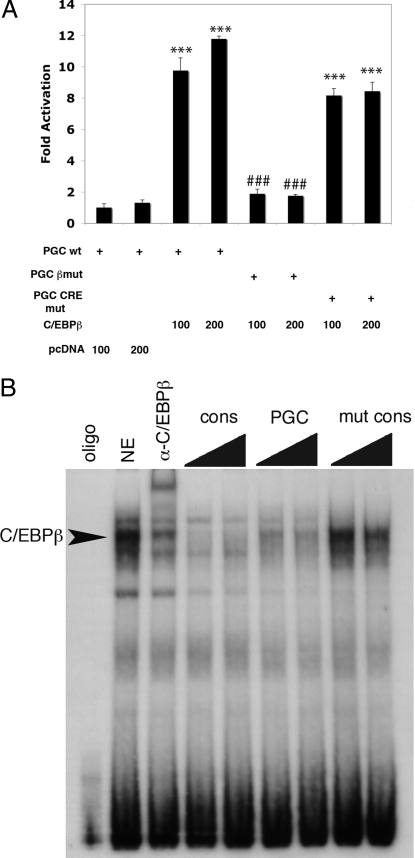
To assess the contribution of the C/EBPβ binding site located at −765/−752 in the PGC-1α to the transcriptional activation of PGC-1α by C/EBPβ, we used a PGC-1α-luciferase reporter plasmid encoding 3.1 kb of promoter sequence upstream of the PGC-1α transcriptional start site (34). Cotransfection of a C/EBPβ expression plasmid with the PGC-1α-luciferase reporter plasmid was associated with a greater than 7-fold activation of luciferase expression (Fig. 4A). Mutation of the C/EBPβ binding site located at −765/−752 markedly decreased activation of the PGC-1α promoter. In contrast, mutation of the CRE site located at −143/−135 did not significantly reduce activation of the PGC-1α promoter by C/EBPβ. These results suggest that C/EBPβ activates the PGC-1α promoter primarily via the C/EBP binding site. ChIP assays do not indicate the precise location of transcription factor binding to chromatin. Therefore, EMSA of liver nuclear extracts was employed to confirm that C/EBPβ is able to bind to this site in the PGC-1α promoter (Fig. 4B). Our finding that mutation of the C/EBPβ binding site abolishes PGC-1α activation provides functional confirmation of the importance of the −765/−752 C/EBPβ site identified as an in vivo C/EBPβ binding site on the PGC-1α promoter after partial hepatectomy and further strengthens the notion that C/EBPβ is a direct transcriptional activator of PGC-1α during compensatory hepatic growth.
Figure 4.
C/EBPβ Directly Activates PGC-1α Transcription
A, Cotransfection of baby hamster kidney cells with C/EBPβ and pGL3-PGC-1α containing 3.1-kb region of the PGC-1α promoter results in dose-dependent increase in luciferase activity. Mutations of the C/EBPβ binding site at −765/−752 (C/EBPβ mut) decreases C/EBPβ-dependent transcriptional activation. Mutation of the CRE site in the PGC-1α at −143/−135 (PGC CRE mut) did not block C/EBPβ activation of the PGC-1α promoter. ***, P value < 0.001 compared with pcDNA control; ###, not significantly different from pcDNA control (n =3). B, EMSA demonstrates that C/EBPβ contained in mouse liver nuclear extract (NE) binds to a 32P-labeled oligonucleotide encoding the C/EBP site mutated in A. In supershift experiments, α-C/EBPβ shifts the band identified by arrow. Increasing amounts of an unlabeled consensus (cons) C/EBP sequence or the PGC-1α C/EBP site compete with the labeled probe. Mutated PGC-1α C/EBP (mut cons) site does not compete with labeled probe.
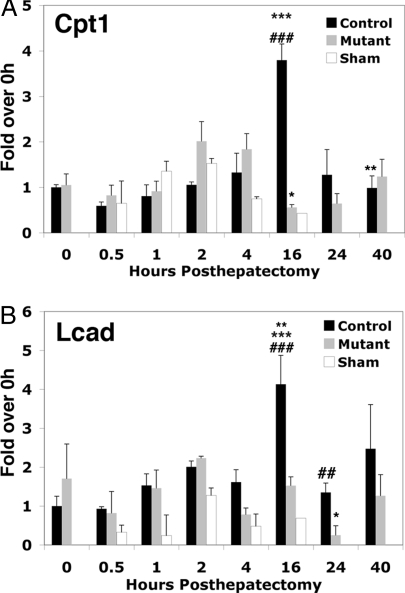
We had previously reported that hepatocyte proliferation is impaired in C/EBPβ−/− mice after hepatectomy (24). C/EBPβ−/− mice also exhibited a prolonged period of hypoglycemia that was not associated with decreased expression of either PEPCK or G6Pase (24). We therefore investigated whether other PGC-1α target genes important for glucose homeostasis were reduced in the C/EBPβ−/− livers. The PGC-1α target gene, carnitine palmitoyltransferase 1A (cpt1a), which encodes the rate-limiting enzyme in β-oxidation of fatty acids was significantly reduced 16 h after hepatectomy in C/EBPβ mutant livers (Fig. 5A). Activation of cpt1a by PGC-1α may therefore be important for generating gluconeogenic substrates during the period of rapid cell growth in the regenerating liver. Long-chain acyl-coenzyme A dehydrogenase (LCAD, encoded by the acadl locus), the enzyme responsible for α-β dehydrogenation of long-chain fatty acid substrates was also significantly induced 16 h after hepatectomy, consistent with the time course of PGC-1α induction. LCAD was not induced in the C/EBPβ mutant livers after hepatectomy, suggesting that expression of this fatty acid oxidation gene is also C/EBPβ dependent.
Figure 5.
Cpt1a and LCAD Expression Are Reduced in C/EBPβ−/− Livers after Hepatectomy
Total RNA was isolated and reverse transcribed from livers of control mice that had undergone Sham surgery and from control and C/EBPβ−/− mice that had undergone partial hepatectomy. cDNA was quantified using real-time PCR and normalized to TBP cDNA levels. The levels of cpt1a (A) and LCAD (B) detected in 0.5-, 1-, 2-, 16-, 24-, and 40-h posthepatectomy (PHX) and 0.5-, 1-, 2-, 4-, and 16-h Sham PHX samples are expressed relative to quiescent liver (0 h). Error bars indicate sem; n = 2–4. A: ***, P value < 0.01, 16-h control vs. 16-h mutant PHX; ###, P value < 0.05, 16-h control vs. 0-h control; *, P value < 0.05, 4-h mutant vs. 16-h mutant; **, P < 0.05 16-h control vs. 40-h control. B: ###, P value < 0.01, 16-h control vs. 0-h control; ***, P value < 0.01, 16-h control vs. 1-h mutant; **, P value < 0.05, 16-h control vs. 24-h control; ##, P value < 0.05, 24-h control vs. 24-h mutant; *, P value = 0.01, 16-h mutant vs. 24-h mutant.
DISCUSSION
The coactivator protein PGC-1α is a master regulator of energy metabolism in multiple tissues (18). Previous studies in the liver have focused on pathways that are responsible for PGC-1α activation in response to fasting. Here we demonstrate for the first time that PGC-1α is dramatically induced during the first few hours after partial hepatectomy, a model characterized by energy requiring cellular processes necessary for compensatory hepatic growth and maintenance of glucose homeostasis. In fact, the amplitude of induction after partial hepatectomy exceeds that seen in fasting, possibly reflecting the more rapid change in metabolic demand in this model.
Multiple groups have shown that ectopic PGC-1α in the liver or hepatocytes can induce expression of PEPCK and G6Pase (1,10,12,35). However, the relevance of PGC-1α for PEPCK and G6Pase activation in the fasting liver is controversial. Recent studies using the PGC-1α null mouse have shown that activation of PEPCK and G6Pase in response to fasting occurs normally in the absence of PGC-1α (13,14). Our data provide additional evidence for PGC-1α-independent activation of these gluconeogenic genes. We have found that PGC-1α mRNA and protein levels are induced 2 and 4 h after hepatectomy, respectively. In contrast, expression of PEPCK and G6Pase is induced already 30–60 min after the surgery and must thus occur without the aid of PGC-1α.
PGC-1α activates genes involved in fatty acid oxidation in the liver in response to fasting, a process that is essential for generation of substrates required for ATP production, ketogenesis, and gluconeogenesis (8,15,36,37). We now provide evidence that cpt1a, the rate-limiting enzyme for β-oxidation of fatty acids and a known PGC-1α target, and LCAD, the enzyme responsible for the initial step in the oxidation of long-chain fatty acids, are both significantly reduced in the C/EBPβ−/− livers 16 h after hepatectomy, a time point at which we had previously detected dramatic increases in the expression of genes involved in cytoskeletal organization and ribosome biogenesis, energy-dependent processes that are critical for hepatocyte growth and proliferation in the regenerating liver (21). Activation of cpt1a by PGC-1α may therefore be important for generating ATP during this period of rapid cell growth in the regenerating liver. Although we cannot establish whether activation of LCAD is due to PGC-1α or C/EBPβ directly, the kinetics of LCAD activation in the regenerating liver are consistent with the potential for direct regulation by PGC-1α. Future studies will be necessary to distinguish between these possibilities.
We reported previously that C/EBPβ−/− mice exhibit prolonged hypoglycemia associated with reduced hepatocyte proliferation that was not associated with differences in either PEPCK or G6Pase expression (24). The markedly reduced expression of cpt1a and LCAD in the C/EBPβ−/− livers after hepatectomy provides a possible explanation for this sustained period of hypoglycemia and impaired hepatocyte proliferation. Another PGC-1α target gene involved in glucose homeostasis, pyruvate dehydrogenase kinase, isozyme 4 (PDK4), was highly induced 4 h after hepatectomy at the time when PGC-1α protein expression was maximal (38). PDK4 would be predicted to increase pyruvate availability for gluconeogenesis, an essential component of metabolic homeostasis during the period of compensatory growth after partial hepatectomy. We did not detect any differences in PDK4 activation in the C/EBPβ−/− livers, suggesting that other pathways may compensate for the reduction in PGC-1α protein in C/EBPβ−/− mice. Future studies will investigate the direct contribution of PGC-1α for PDK4 function in this model of hepatic growth.
The principal regulators of PGC-1α transcription in the liver identified thus far include CREB (11) and glucocorticoid receptor (19). The potential for C/EBPβ to directly regulate PGC-1α in the liver had not been appreciated previously. Here we have shown that in the absence of C/EBPβ, PGC-1α activation is significantly reduced in an in vivo model of compensatory hepatic growth. Using ChIP assays, we show that C/EBPβ occupancy on the PGC-1α promoter in vivo correlates with maximal induction of PGC-1α. We have provided functional confirmation of the importance of the C/EBPβ site at position −765/−752 in the PGC-1α promoter, further strengthening the argument that C/EBPβ is a direct transcriptional activator of PGC-1α. The finding that mutation of the C/EBPβ binding site in the PGC-1α promoter completely abolishes activation of the PGC-1α promoter suggests that there may be a cooperative effect in which C/EBPβ binding to the C/EBP promoter element is required for C/EBPβ recruitment to the CRE. Our in vivo ChIP data support this model, because C/EBPβ occupancy is only detected on the C/EBP site in quiescent liver and occupancy on the CRE site is not detected until 2 h after hepatectomy. However, mutation of the CRE site did not decrease PGC-1α promoter activation by C/EBPβ in the transfection assay system. This finding suggests that C/EBPβ activation of the PGC-1α promoter is mediated primarily via the C/EBP site and that the CRE site is not required. However, we cannot exclude the possibility that the CRE site contributes to PGC-1α activation in vivo.
The absence of PGC-1α transcriptional activation in the quiescent liver despite occupancy by C/EBPβ and the dynamic recruitment of C/EBPβ to the CRE region after hepatectomy suggests that posttranslational modification of C/EBPβ and/or recruitment of coactivator proteins are necessary for C/EBPβ to activate PGC-1α transcription in vivo. C/EBPβ can be rapidly activated by posttranslational modifications induced downstream of mitogenic, metabolic, and cytokine signaling pathways (39). As a result, C/EBPβ represents a likely integrator of stimulus-specific activation of PGC-1α during hepatic growth and for the maintenance of glucose homeostasis. Future studies will investigate the signaling pathways responsible for activation of PGC-1α by C/EBPβ and the downstream targets of PGC-1α during hepatic growth.
MATERIALS AND METHODS
Mice
The derivation of mice homozygous for the C/EBPβ mutation has been reported previously (24). Partial hepatectomies were performed as described (24). Sham surgeries included similar duration of anesthesia exposure and incision of skin and body wall without manipulation of the liver. Animals were given access to food and water before and after the surgery. All animal studies were performed in accordance with the University of Pennsylvania Institutional Animal Care and Use Committee guidelines.
Quantitative RNA Analysis
Total liver RNA was prepared from wild-type and C/EBPβ−/− mice 0, 0.5, 1, 2, 4, 16, 24, and 40 h after partial hepatectomy and 0.5, 1, 2, 4, and 16 h after sham surgeries using the RNAeasy kit (QIAGEN, Valencia, CA). RNA (5 μg) was used as template to synthesize cDNA using Superscript II reverse transcriptase (Invitrogen, Carlsbad, CA) with priming by oligo deoxythymidine18. The resulting cDNA was dissolved in 300 μl H2O. Diluted cDNA (1 μl) was employed as template in quantitative real-time PCR analysis using the Brilliant SYBR Green QPCR Master Mix (Stratagene, La Jolla, CA), 10 μm primers, and the reference dye at a 1:200 dilution in the Mx4000 PCR System (Stratagene). Cycling parameters were 95 C for 10 min and 40 cycles of 95 C (30 sec), 60 C (50 sec), and 72 C (30 sec) followed by a melting-curve analysis. All reactions were performed in triplicate with reference dye normalization, and the median cycle threshold (Ct) value was used for analysis. The specificity of the PCR amplification was confirmed by dissociation curve analysis. Expression of all genes was normalized to that of TATA-box binding protein (TBP) and shown as fold induction over the wild-type 0-h expression level. Primer sequences (Operon Biotechnologies, Menlo Park, CA) are as follows (designated from 5′ to 3′): PGC-1α forward, TGCGTGTGTGTATGTGTGTGTG; PGC-1α reverse, CCTTGTTCGTTCTGTTCAGGTG; PGC-1β forward, GAGGAGTCCCTTCCCTCATC; PGC-1β reverse, TCCTCGAAGGTTAAGGCTGA; PEPCK forward, ATGCCTGTCTGTCCCATTGT; PEPCK reverse, GGTAAGGAAGG-GCGGTGTAT; G6Pase forward, TGCTGTGTCTGGTAGGCAAC; G6Pase reverse, AACATCGGAGTGACCTTTGG; Cpt1a forward, CTTCCAAGGCAGAAGAGTGG; Cpt1a reverse, CCTTGGCTGCGGTAGACTA; LCAD forward, CATATTCCCCCAGGACATTG; LCAD reverse, CACAATTGCCTCTATGTGCATT; TBP forward, CCCCTTGTACCCTTCAC-CAAT; and TBP reverse, GAAGCTGCGGTACAATTCCAG.
Transcription Factor Binding Sites
PCR and real-time PCR primers for ChIP assay analysis were designed to flank C/EBPβ binding sites within the PGC-1α gene promoter. These C/EBPβ binding sites were found by analyzing the mouse PGC-1α promoter sequences obtained from the UCSC Genome Browser (40), using the transcription factor search program TFSEARCH (41). Binding site matches with scores less than 85 (of 100) were ignored.
Plasmid Constructs
pGL3-PGC-1 and pcDNA-LAP encoding full-length rat C/EBPβ protein were gifts from Dr. Erik Olson (New York University School of Medicine, New York, NY) and Dr. David Kurtz (Medical University of South Carolina), respectively. A 3.1-kb region from the mouse PGC-1 5′ flanking region was inserted upstream of the pPGL3 firefly luciferase coding sequence to construct PGL3-PGC-1 (34). A mutated version of the predicted C/EBPβ binding site located at −765/−752 was generated by overlap PCR (mutated sequence, gggGagaAaaattt). The CRE site at −143/−135 was mutated in the PGL3-PGC-1 promoter (mutated sequence, AgGACtAC) using the QuikChange mutagenesis system (Invitrogen).
Transient Transfections and Luciferase Reporter Assays
Baby hamster kidney cells were seeded 24 h before transfection in 60-mm plates at 1 × 105 per plate and cultured in medium containing 10% FBS, l-glutamine, penicillin, and streptomycin. Effectene transfection reagent (QIAGEN) was used as directed by the manufacturer to perform transient transfections. Each plate received 300 ng of the appropriate luciferase reporter construct. To monitor transfection efficiency, 60 ng Renilla luciferase was cotransfected as an internal control, and 40 h after transfection, cells were harvested using 1× PLB buffer (Promega, Madison, WI). The dual luciferase reporter assay (Promega) was used to measure the luciferase activity according to the manufacturer’s recommendations, and the activity was normalized for transfection efficiency to the Renilla luciferase activity. Each transfection was performed in triplicate.
ChIP Assays
ChIP assays were performed as previously described (42) with the following modifications: 25 μg chromatin DNA was used in each immunoprecipitation as determined by analyzing an aliquot of purified DNA from each sample using 260-nm UV absorption. To ensure that salt concentrations were identical across all samples, the volume of each 25-μg DNA chromatin sample was adjusted to a total volume of 250 μl in nuclear lysis buffer and then diluted 1:1 in ChIP dilution buffer. Chromatin samples were precleared, immunoprecipitated with 2 μg antibody, and washed as previously described (43). Anti-C/EBPβ (sc-50) was purchased from Santa Cruz Biotechnology (Santa Cruz, CA), and antirabbit IgG (100-NC) antibody was purchased from Lab Vision (Thermo-Scientific, Fremont, CA). Immunoprecipitated DNA was purified using a QiaQuick PCR purification kit (QIAGEN) in a final elution volume of 60 μl. Purified total input DNA from each chromatin preparation was diluted to a concentration of 5 ng/ml to be used as control template in quantitative PCR analyses. Quantitative PCR analysis was performed using the Brilliant SYBR Green QPCR Master Mix (Stratagene), 10 μm primers, and the reference dye at a 1:200 dilution according to manufacturer’s instructions using the Mx4000 PCR System (Stratagene). One microliter of immunoprecipitated DNA or diluted total input DNA was used in each reaction. All reactions were performed with three to five biological replicates and three technical replicates each with reference dye normalization. The median cycle threshold (Ct) value was used for analysis. ChIP quantitative PCRs were normalized to total input by calculating the fold enrichment in the ChIP sample relative to input using the 28S ribosomal locus as a reference control. The following formula was used to make this calculation: IP signal = 2[(28S gene − test gene) − (input 28S gene − input test gene)].
Primers used for ChIP qPCRs are as follows (designated from 5′ to 3′): C/EBPβ site −765 to −752: −879/−863 PGC-1α promoter forward, GGGGAACCCAAGAGTCT, and −784/−764 PGC-1α promoter reverse, CCCAAATCAGCTG-TCTCCT; C/EBPβ sites at −438 to −425 and −421 to –408: −464/−445 PGC-1α promoter forward, CTGAGTCTGGGGCTACTTGG, and −372/−353 PGC-1α promoter reverse, TCCATCCAAAACAGGCAAAT; CRE site at −143/−135: −233/−214 PGC-1α promoter forward, CAAAGGCCAAGTGTTTCCTT, and −138/−121 PGC-1α promoter reverse, TTGCTGCACAAACTCCTGA.
Immunoblot Assays
Nuclear extracts were prepared from mouse liver as described previously (5), and 10 μg protein was separated on 10% sodium dodecyl sulfate-polyacrylamide gels and transferred to a polyvinylidene difluoride membrane [Millipore (Bedford, MA) catalog no. IPVH00010]. Membranes were blocked for 2 h in 5% milk in TBS-0.1% Tween 20 before incubation with either anti-PGC-1α (Santa Cruz Biotechnology; sc-13067) 1:500, anti-CREB-1 (Santa Cruz; sc-58) 1:500, anti-TORC2 [Calbiochem (La Jolla, CA) ST1099) 1:2000 or anti-TBP [Abcam (Cambridge, MA) no. ab818] 1:2000 overnight at 4 C in 5% milk in TBS-0.1% Tween 20. For detection of phosphorylated CREB, membranes were blocked and incubated in 5% BSA/TBS-0.1% Tween 20 with anti-phospho-CREB [Cell Signaling (Beverly, MA); catalog no. 9198] 1:1000. Horseradish peroxidase-labeled goat antirabbit (Invitrogen catalog no. 811620) or mouse (Invitrogen catalog no. 626510) was used as the secondary antibody at a 1:10000 dilution. Blots were developed using the ECL plus Western blotting detection system (Amersham Biosciences, Piscataway, NJ) as described by the manufacturer.
Densitometry
The Chemidoc program (Bio-Rad Quantity One Software, Hercules, CA) was used to determine volume of PGC-1α immunoblot signal. PGC-1α volume was normalized to the volume of TBP loading control.
EMSAs
Nuclear extracts were prepared from mouse livers as described previously (24). Single-stranded oligonucleotides were annealed and radiolabeled using Klenow polymerase and α-dCTP. All experiments were performed using an excess of probe. Nuclear extract (2 μg) was incubated with radiolabeled oligonucleotide for 30 min at room temperature in binding buffer [10 mm Tris-HCl (pH 7.5), 50 mm NaCl, 1 mm dithiothreitol, 1 mm EDTA, 5% glycerol] and electrophoresed on nondenaturing 5% polyacrylamide gels in 0.5× Tris-borate-EDTA buffer at 250 V at 4 C. The gels were dried and exposed to x-ray film. Poly-deoxyinosine-deoxycytosine (1 μg) was used as a nonspecific DNA competitor in each reaction. C/EBPβ supershift experiments were performed by incubating 1 μl primary antibody (Santa Cruz Biotechnology; sc-50) with the nuclear extract and radiolabeled probe. For competition experiments, 10- or 40-fold excess cold oligonucleotides encoding C/EBP consensus, PGC-1α-C/EBPβ, or PGC-1α-mut C/EBPβ sequences were incubated with nuclear extract and radiolabeled PGC-1α C/EBPβ site probe: C/EBP consensus sequence forward, GCTGATTTGGGGTTGCGCGCAATTTGTTAG; C/EBP consensus sequence reverse, AGGTCTAACAAATTGCGCGCAACCCCAAAT; PGC-1α promoter C/EBPβ site forward, AGCTGATTTGGGGTAGAGAAATTTGT; PGC-1α promoter C/EBPβ site reverse, AGGTACAAATTTCTCTACCCCAAATC; PGC-1α promoter C/EBPβ mutant site forward, AGCTGATTTGGGGGAGAAAAATTTGT; PGC-1α promoter C/EBPβ mutant site reverse, AGGTACAAATTTTTCTCCCCCAAATC.
Statistical Analysis
Student’s t test with equal variance and two-tailed distribution was used to determine the significance of differences between groups [Microsoft (Redmond, WA) Excel statistical analysis software]. Results where indicated are expressed as mean ± se.
Acknowledgments
We thank Akivaga Tsingalia for expert technical assistance and Dr. Klaus Kaestner for helpful discussions and critical reading of the manuscript.
Footnotes
This work was supported by National Institutes of Health/National Institute of Diabetes and Digestive and Kidney Diseases Grant R01 DK-056669 (to L.E.G.).
Disclosure Summary: The authors have nothing to declare.
First Published Online May 8, 2008
Abbreviations: ChIP, Chromatin immunoprecipitation; cpt1a, carnitine palmitoyltransferase 1A; CREB, cAMP response element-binding protein; G6Pase, glucose-6-phosphatase; LCAD, long-chain acyl-coenzyme A dehydrogenase; PDK4, pyruvate dehydrogenase kinase, isozyme 4; PEPCK, phosphoenolpyruvate carboxykinase; PGC-1α, peroxisome-proliferator-activated receptor-γ coactivator-1α; TBP, TATA-box binding protein.
References
- Spiegelman BM, Heinrich R 2004 Biological control through regulated transcriptional coactivators. Cell 119:157–167 [DOI] [PubMed] [Google Scholar]
- Puigserver P, Rhee J, Donovan J, Walkey CJ, Yoon JC, Oriente F, Kutamura Y, Altomonte J, Dong H, Accili D, Spiegelman BM 2003 Insulin-regulated hepatic gluconeogenesis through FOXO1-PGC-1α interaction. Nature 423:550–555 [DOI] [PubMed] [Google Scholar]
- Weinberg JM, Venkatachalam MA, Roeser NF, Nissim I 2000 Mitochondrial dysfunction during hypoxia/reoxygenation and its correction by anaerobic metabolism of citric acid cycle intermediates. Proc Natl Acad Sci USA 97:2826–2831 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weinberg JM, Roeser NF, Davis JA, Venkatachalam MA 1997 Glycine-protected, hypoxic, proximal tubules develop severely compromised energetic function. Kidney Int 52:140–151 [DOI] [PubMed] [Google Scholar]
- Feldkamp T, Kribben A, Weinberg JM 2005 Assessment of mitochondrial membrane potential in proximal tubules after hypoxia-reoxygenation. Am J Physiol Renal Physiol 288:F1092–F1102 [DOI] [PubMed] [Google Scholar]
- Bonventre JV, Weinberg JM 2003 Recent advances in the pathophysiology of ischemic acute renal failure. J Am Soc Nephrol 14:2199–2210 [DOI] [PubMed] [Google Scholar]
- Rosen ED, Spiegelman BM 2000 Molecular regulation of adipogenesis. Annu Rev Cell Dev Biol 16:145–171 [DOI] [PubMed] [Google Scholar]
- Nisoli E, Clementi E, Paolucci C, Cozzi V, Tonello C, Sciorati C, Bracale R, Valerio A, Francolini M, Moncada S, Carruba MO 2003 Mitochondrial biogenesis in mammals: the role of endogenous nitric oxide. Science 299:896–899 [DOI] [PubMed] [Google Scholar]
- Rodgers JT, Lerin C, Haas W, Gygi SP, Spiegelman BM, Puigserver P 2005 Nutrient control of glucose homeostasis through a complex of PGC-1α and SIRT1. Nature 434:113–118 [DOI] [PubMed] [Google Scholar]
- Yoon JC, Puigserver P, Chen G, Donovan J, Wu Z, Rhee J, Adelmant G, Stafford J, Kahn CR, Granner DK, Newgard CB, Spiegelman BM 2001 Control of hepatic gluconeogenesis through the transcriptional coactivator PGC-1. Nature 413:131–138 [DOI] [PubMed] [Google Scholar]
- Herzig S, Long F, Jhala US, Hedrick S, Quinn R, Bauer A, Rudolph D, Schutz G, Yoon C, Puigserver P, Spiegelman B, Montminy M 2001 CREB regulates hepatic gluconeogenesis through the coactivator PGC-1. Nature 413:179–183 [DOI] [PubMed] [Google Scholar]
- Rhee J, Inoue Y, Yoon JC, Puigserver P, Fan M, Gonzalez FJ, Spiegelman BM 2003 Regulation of hepatic fasting response by PPARγ coactivator-1α (PGC-1): requirement for hepatocyte nuclear factor 4α in gluconeogenesis. Proc Natl Acad Sci USA 100:4012–4017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin J, Wu PH, Tarr PT, Lindenberg KS, St-Pierre J, Zhang CY, Mootha VK, Jager S, Vianna CR, Reznick RM, Cui L, Manieri M, Donovan MX, Wu Z, Cooper MP, Fan MC, Rohas LM, Zavacki AM, Cinti S, Shulman GI, Lowell BB, Krainc D, Spiegelman BM 2004 Defects in adaptive energy metabolism with CNS-linked hyperactivity in PGC-1α null mice. Cell 119:121–135 [DOI] [PubMed] [Google Scholar]
- Burgess SC, Leone TC, Wende AR, Croce MA, Chen Z, Sherry AD, Malloy CR, Finck B 2006 Diminished hepatic gluconeogenesis via defects in tricarboxylic acid cycle flux in peroxisome proliferator-activated receptor γ coactivator-1α (PGC-1α)-deficient mice. J Biol Chem 281:19002–19008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mootha VK, Lindgren CM, Eriksson KF, Subramanian A, Sihag S, Lehar J, Puigserver P, Carlsson E, Ridderstrale M, Laurila E, Houstis N, Daly MJ, Patterson N, Mesirov JP, Golub TR, Tamayo P, Spiegelman B, Lander ES, Hirschhorn JN, Altshuler D, Groop LC 2003 PGC-1α-responsive genes involved in oxidative phosphorylation are coordinately downregulated in human diabetes. Nat Genet 34:267–273 [DOI] [PubMed] [Google Scholar]
- Leone TC, Lehman JJ, Finck BN, Schaeffer PJ, Wende AR, Boudina S, Courtois M, Wozniak DF, Sambandam N, Bernal-Mizrachi C, Chen Z, Holloszy JO, Medeiros DM, Schmidt RE, Saffitz JE, Abel ED, Semenkovich CF, Kelly DP 2005 PGC-1α deficiency causes multi-system energy metabolic derangements: muscle dysfunction, abnormal weight control and hepatic steatosis. PLoS Biol 3:e101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Handschin C, Lin J, Rhee J, Peyer AK, Chin S, Wu PH, Meyer UA, Spiegelman BM 2005 Nutritional regulation of hepatic heme biosynthesis and porphyria through PGC-1α. Cell 122:505–515 [DOI] [PubMed] [Google Scholar]
- Finck BN, Kelly DP 2006 PGC-1 coactivators: inducible regulators of energy metabolism in health and disease. J Clin Invest 116:615–622 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herzog B, Hall RK, Wang XL, Waltner-Law M, Granner DK 2004 Peroxisome proliferator-activated receptor γ coactivator-1α, as a transcription amplifier, is not essential for basal and hormone-induced phosphoenolpyruvate carboxykinase gene expression. Mol Endocrinol 18:807–819 [DOI] [PubMed] [Google Scholar]
- Daitoku H, Yamagata K, Matsuzaki H, Hatta M, Fukamizu A 2003 Regulation of PGC-1 promoter activity by protein kinase B and the forkhead transcription factor FKHR. Diabetes 52:642–649 [DOI] [PubMed] [Google Scholar]
- White P, Brestelli JE, Kaestner KH, Greenbaum LE 2005 Identification of transcriptional networks during liver regeneration. J Biol Chem 280:3715–3722 [DOI] [PubMed] [Google Scholar]
- Horimoto M, Fulop P, Derdak Z, Wands JR, Baffy G 2004 Uncoupling protein-2 deficiency promotes oxidant stress and delays liver regeneration in mice. Hepatology 39:386–392 [DOI] [PubMed] [Google Scholar]
- Anderson SP, Yoon L, Richard EB, Dunn CS, Cattley RC, Corton JC 2002 Delayed liver regeneration in peroxisome proliferator-activated receptor-α-null mice. Hepatology 36:544–554 [DOI] [PubMed] [Google Scholar]
- Greenbaum LE, Li W, Cressman D, Peng Y, Ciliberto G, Poli V, Taub R 1998 CCAAT enhancer binding protein β is required for normal hepatocyte proliferation in mice after partial hepatectomy. J Clin Invest 102:996–1007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mohn KL, Laz TM, Hsu JC, Melby AE, Bravo R, Taub R 1991 The immediate-early growth response in regenerating liver and insulin-stimulated H-35 cells: comparison to serum-stimulated 3T3 cells and identification of 41 novel immediate-early genes. Mol Cell Biol 11:381–390 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bucher NL, Swaffield MN 1975 Regulation of hepatic regeneration in rats by synergistic action of insulin and glucagon. Proc Natl Acad Sci USA 72:1157–1160 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park EA, Song S, Vinson C, Roesler WJ 1999 Role of CCAAT enhancer-binding protein β in the thyroid hormone and cAMP induction of phosphoenolpyruvate carboxykinase gene transcription. J Biol Chem 274:211–217 [DOI] [PubMed] [Google Scholar]
- Greenbaum LE, Cressman DE, Haber BA, Taub R 1995 Coexistence of C/EBPα, β, growth-induced proteins and DNA synthesis in hepatocytes during liver regeneration. J Clin Invest 96:1351–1365 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karamanlidis G, Karamitri A, Docherty K, Hazlerigg DG, Lomax MA 2007 C/EBPβ reprograms white 3T3-L1 preadipocytes to a brown adipocyte pattern of gene expression. J Biol Chem 282:24660–24669 [DOI] [PubMed] [Google Scholar]
- Conkright MD, Canettieri G, Screaton R, Guzman E, Miraglia L, Hogenesch JB, Montminy M 2003 TORCs: transducers of regulated CREB activity. Mol Cell 12:413–423 [DOI] [PubMed] [Google Scholar]
- Bittinger MS, McWhinnie E, Meltzer J, Kourgenko V, Lataro B, Liu X, Chen CH, Song C, Garza D, Labow M 2004 Activation of cAMP response element-mediated gene expression by regulated nuclear transport of TORC proteins. Curr Biol 14:2156–2161 [DOI] [PubMed] [Google Scholar]
- Screaton RA, Conkright MD, Katoh Y, Best JL, Canettieri G, Jeffries S, Guzman E, Niessen S, JR YI, Takemori H, Okamoto M, Momtminy M 2004 The CREB coactivator TORC2 functions as a calcium- and cAMP-sensitive coincidence detector. Cell 119:61–74 [DOI] [PubMed] [Google Scholar]
- Iourgenko V, Zhang W, Mickanin C, Daly I, Jiang C, Hexham JM, Orth AP, Miraglia L, Meltzer J, Garza D, Chirn GW, McWhinnie E, Cohen D, Skelton J, Terry R, Yu Y, Bodian D, Buxton FP, Zhu J, Song C, Labow MA 2003 Identification of a family of cAMP response element-binding protein coactivators by genome-scale functional analysis in mammalian cells. Proc Natl Acad Sci USA 100:12147–12152 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Czubryt MP, McAnally J, Fishman GI, Olson EN 2003 Regulation of peroxisome proliferator-activated receptor γ coactivator 1α (PGC-1α) and mitochondrial function by MEF2 and HDAC5. Proc Natl Acad Sci USA 100:1711–1716 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelly DP, Scarpulla RC 2004 Transcriptional regulatory circuits controlling mitochondrial biogenesis and function. Genes Dev 18:357–368 [DOI] [PubMed] [Google Scholar]
- Moore ML, Park EA, McMillin JB 2003 Upstream stimulatory factor represses the induction of carnitine palmitoyltransferase-Iβ expression by PGC-1. J Biol Chem 278:17263–17268 [DOI] [PubMed] [Google Scholar]
- Patti ME, Butte AJ, Crunkhorn S, Cusi K, Berria R, Kashyap S, Miyazaki Y, Kohane I, Costello M, Saccone R, Landaker EJ, Goldfine AB, Mun E, DeFronzo R, Finlayson J, Kahn CR, Mandarino LJ 2003 Coordinated reduction of genes of oxidative metabolism in humans with insulin resistance and diabetes: potential role of PGC1 and NRF1. Proc Natl Acad Sci USA 100:8466–8471 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma K, Zhang Y, Elam MB, Cook GA, Park EA 2005 Cloning of the rat pyruvate dehydrogenase kinase 4 gene promoter: activation of pyruvate dehydrogenase kinase 4 by the peroxisome proliferator-activated receptor γ coactivator. J Biol Chem 280:29525–29532 [DOI] [PubMed] [Google Scholar]
- Buck M, Chojkier M 2003 Signal transduction in the liver: C/EBPβ modulates cell proliferation and survival. Hepatology 37:731–738 [DOI] [PubMed] [Google Scholar]
- Heinemeyer T, Wingender E, Reuter I, Hermjakov H, Kel AE, Kel OV, Ignatieva EV, Ananko EA, Podkolodnaya OA, Kolpakov FA, Podkolodny NL, Kolchanov NA 1998 Databases on transcriptional regulation: TRANSFAC, TRRD, and COMPEL. Nucleic Acids Res 26:364–370 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kent WJ, Sugnet CW, Furey TS, Roskin KM, Pringle TH, Zahler AM, Haussler D 2002 The human genome browser at UCSC. Genome Res 12:996–1006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friedman JR, Larris B, Le PP, Peiris TH, Arsenlis A, Schug J, Tobias JW, Kaestner KH, Greenbaum LE 2004 Orthogonal analysis of C/EBPβ targets in vivo during liver proliferation. Proc Natl Acad Sci USA 101:12986–12991 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wells J, Graveel CR, Bartley SM, Madore SJ, Farnham PJ 2002 The identification of E2F1-specific target genes. Proc Natl Acad Sci USA 99:3890–3895 [DOI] [PMC free article] [PubMed] [Google Scholar]