Abstract
In coming decades, global climate changes are expected to produce large shifts in vegetation distributions at unprecedented rates. These shifts are expected to be most rapid and extreme at ecotones, the boundaries between ecosystems, particularly those in semiarid landscapes. However, current models do not adequately provide for such rapid effects—particularly those caused by mortality—largely because of the lack of data from field studies. Here we report the most rapid landscape-scale shift of a woody ecotone ever documented: in northern New Mexico in the 1950s, the ecotone between semiarid ponderosa pine forest and piñon–juniper woodland shifted extensively (2 km or more) and rapidly (<5 years) through mortality of ponderosa pines in response to a severe drought. This shift has persisted for 40 years. Forest patches within the shift zone became much more fragmented, and soil erosion greatly accelerated. The rapidity and the complex dynamics of the persistent shift point to the need to represent more accurately these dynamics, especially the mortality factor, in assessments of the effects of climate change.
Distributions of vegetation across landscapes depend on climate, perhaps best illustrated by the wholesale movement of plant species across geographic and topographic gradients during the last deglaciation (1). The responses of vegetation to variations in climate are expected to be most rapid and extreme at ecotones, the boundaries between ecosystems (2–5), with semiarid ecotones considered to be among the most sensitive (6). Persistent vegetation shifts are most clearly detected in the distributions and abundances of long-lived woody plants, namely, trees and shrubs (7). Previous studies of woody ecotones document landscape-scale shifts in vegetation as occurring only over relatively long (decades to millennia) periods (8–12). Moreover, most field studies and model-based assessments of vegetation responses to climate have focused on changes associated with natality and growth, which are inherently slow processes for woody plants—even though the most rapid changes in vegetation are caused by mortality rather than natality (13). In coming decades, climate changes are expected to produce major shifts in vegetation distributions at unprecedented rates, in large part due to mortality (14); however, largely because of the lack of field data on vegetation mortality, current models do not represent adequately such rapid effects (14, 15). Furthermore, as woody vegetation contains 80% of the world’s terrestrial carbon (16), an improved understanding of mortality-induced responses of woody vegetation to climate is essential for addressing the environmental and policy implications of climate variability and global change (17, 18).
We wish to highlight how rapidly shifts in vegetation can take place in response to climate. Here we demonstrate that the ecotone between semiarid ponderosa pine forest and piñon–juniper woodland shifted extensively (2 km or more) and rapidly (<5 years) through mortality in response to a severe drought. Remnant forest patches became much more fragmented, and high soil erosion rates were initiated in the ecotone shift zone. Moreover, the shift has been a persistent one. The rapidity and the complex dynamics of this persistent shift specifically point to the need to represent more accurately the mortality dynamics of woody vegetation in assessments of the effects of global climate change.
The focus of our study was an ecotone between ponderosa pine forest (Pinus ponderosa) and piñon–juniper woodland (Pinus edulis and Juniperus monosperma). The site we selected, a 2,378-ha (1 ha = 104 m2) portion of Frijolito Mesa, Bandelier Wilderness (35° 51′ N, 106° 19′ E), in the Jemez Mountains of northern New Mexico, offered strong evidence of a recent ecotone shift in response to climate. The site ranges in elevation from 1,800 to 2,200 m and spans a corresponding climatic gradient; precipitation varies around a mean of 41 cm/yr at 2,010 m (19). Other factors in our selection of this site as a study area were its upland topography, with only one major elevation gradient; its designated wilderness status, with minimal history of human disturbance; and an extensively documented fire history (20). These conditions allowed us to isolate and focus more accurately on the effects of climate variations on vegetation.
We quantified changes in the ecotone over a 40-yr period on the basis of Geographic Information System (GIS) analyses of a sequence of aerial photographs taken between 1935 and 1975—a period encompassing a severe regional drought in the mid-1950s (21, 22). Full-coverage photographs of the area, which existed for 1935, 1954, 1963, and 1975, and partial coverage photographs from 1951 were used to map vegetation patches in terms of ponderosa pine cover: areas with more than 10% cover were classified as forest, and the remainder of the area as piñon–juniper woodland. Higher resolution (scale = 1:5,000), partial-coverage photographs from 1958 allowed us to sharpen our estimate of the timing of ecotone changes; written documents on file at Bandelier National Monument further confirmed this estimate. We verified our mapping results with field observations of the persistence and mortality of ponderosa pines, which included documenting the presence of live ponderosa trees and the remains of dead trees as a function of elevation and topographic position. In addition, we confirmed the relationship between the local elevation/moisture gradient and water stress for ponderosa pine, as measured by the changes in stem diameter of 30 trees monitored continuously since 1991 (10 trees at each of three sites along this gradient).
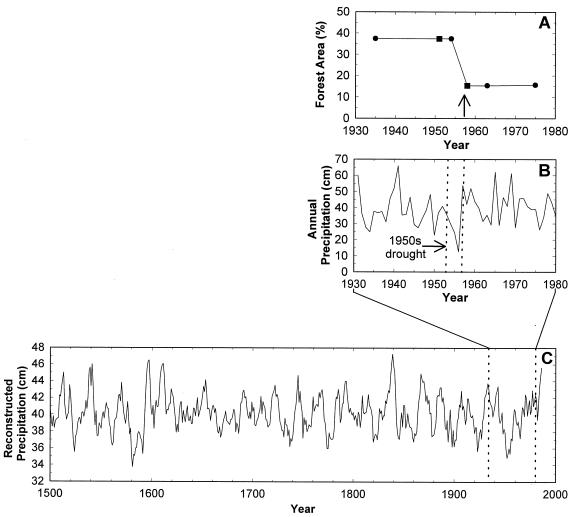
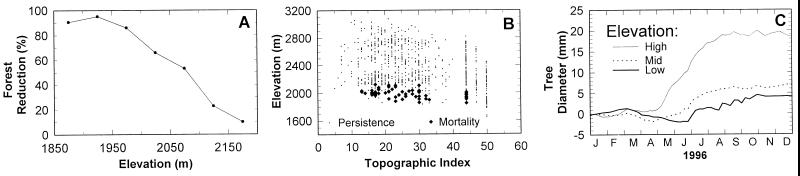
Most striking is how extensively (Fig. 1) and how rapidly (Fig. 2A) the ecotone shifted in conjunction with the 1950s drought. The extensive mortality of ponderosa pine at drier, lower elevations caused a landscape-scale shift in <5 yr (1954–1958; Fig. 2A). The shift coincided with the culmination of the drought (Fig. 2B), which was one of the most severe of the past 500 yr (refs. 23–25; Fig. 2C). That the mortality of the ponderosa pines was primarily a result of this brief but extreme variation in climate is supported by data from the GIS analyses (Fig. 3A) and from ground-based field observations (Fig. 3B), which show that lower-elevation sites, which are drier, suffered higher loss of trees. Further, recent dendrometer measurements of ponderosa pines along an elevational gradient in the same landscape reveal that the trees at lower elevations exhibit greater water stress and slower growth (Fig. 3C).
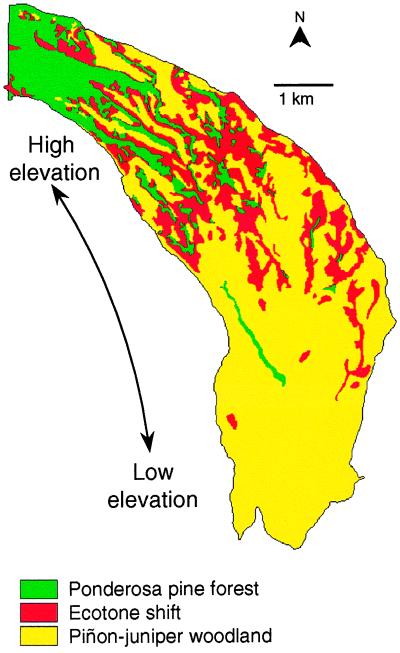
Figure 1.
Changes in vegetation cover between 1954 and 1963 in the study area, showing persistent ponderosa pine forest (365 ha), persistent piñon–juniper woodland (1527 ha), and the ecotone shift zone (486 ha) where forest changed to woodland.
Figure 2.
(A) Changes in percent forest area between 1935 and 1975. Circles represent GIS estimates derived from mapped data; squares represent estimates for years with only partial aerial photograph coverage. The arrow indicates the time of historical observations of extensive tree mortality (22). (B) Annual precipitation at Bandelier National Monument, 1930–1980, highlighting the period of extreme drought (dashed vertical lies). (C) Annual precipitation (5-yr running average), reconstructed from dendrochronological records for the Jemez Mountains, encompassing the study site (Unpublished data from J. S. Dean and G. S. Funkhouser, personal communication). The magnitude of the 1950s drought (within the dashed vertical lines) was exceeded only by the drought of the late 1500s. Other precipitation reconstructions in the region show similar patterns (23–25).
Figure 3.
(A) Forest reduction between 1954 and 1963, estimated from GIS analyses. Forest reduction was greatest at lower elevations. (B) Field observations of the persistence (⋅) and mortality (⧫) of ponderosa pines, determined from remains of dead trees as a function of elevation and topographic position. Topographic position indices ranged from small values for exposed ridgetops to large values for sheltered valley bottoms (22, 26). Across most topographic positions, mortality was greatest at lower elevations. (C) Mean changes in stem diameter during calendar year 1996 for ponderosa pine along an elevation/moisture gradient (10 trees were measured at each of three sites: 2,010 m elevation (bold line) with 41 cm/year precipitation; 2,320 m (dashed line) with 51 cm/year; 2,780 m (thin line) with 89 cm/year). Annual stem diameter increment (tree growth) was greater at the mesic, high-elevation site than at the xeric, low-elevation site. In addition, at the end of a dry winter/spring period in 1995–1996, stem diameter actually decreased because of water stress at the low-elevation site between April and mid-June, normally the time of most rapid growth.
Other factors amplified the climate-induced ecotone shift. Human suppression of fires since the 1880s had allowed piñon and juniper to become more densely established beneath or close to low-elevation ponderosa pines by the time of the 1950s drought (22). The novel association between species thus created—which had been precluded in the past by frequent fires—meant that during this drought many piñon and juniper trees were competing with ponderosa for available water. Because both piñon and juniper are very efficient at using shallow water (27), water stress on the ponderosa pines was probably exacerbated. In addition, infestations of bark beetles (Dendroctonus sp. and Ips sp.) accompanied the drought, as indicated by historic documents (22). These infestations certainly contributed to the mortality of the ponderosa pines, as drought-stressed ponderosa pines are commonly unable to defend themselves from beetle attacks (28). Although we cannot segregate the intertwined effects of the beetles and of the drought, it is clear that the drought was the underlying cause of the mortality. As shown in Fig. 3, the spatial pattern of ponderosa pine mortality corresponds directly to elevation/moisture gradients. Furthermore, mortality of ponderosa pine in the 1950s was apparently widespread on drier, low-elevation sites across the drought-affected regions of the southwestern United States (29, 30). It is noteworthy that piñon mortality also occurred at drier, lower-elevation sites in the Bandelier area (22), as well as elsewhere in New Mexico (25, 31), even though piñon are not attacked by the same species of bark beetles that affect ponderosa pine (28). Also, when wet conditions beginning in 1957 broke the 1950s drought (Fig. 2B), widespread ponderosa pine mortality reportedly ceased within about a year (22). Collectively, these observations indicate that the timing and spatial pattern of ponderosa pine mortality was driven by the drought rather than by bark beetles. Hence, we assert that the mortality of the ponderosa pines was caused by the drought and was amplified by historic fire suppression, increased density of piñon and juniper, and drought-triggered infestations of bark beetles.
As a result of the ecotone shift, the patches of ponderosa pine forest became more fragmented, as theory suggests should occur with climate-induced ecotone shifts (32, 33). Between 1954 and 1963, the number of forest patches in our study area increased from 20 to 42, and their perimeter-to-area ratio increased from 0.012 m−1 to 0.020 m−1. This fragmentation is important because many ecosystem properties are a function of patch size and pattern (34).
Moreover, the effects of the drought in the ecotone shift zone have been persistent (Fig. 2A). There is still little evidence of ponderosa pine reestablishment in spite of favorable climatic conditions in recent decades, and hence the shift in the ponderosa pine forest has endured for 40 yr. Furthermore, the 1950s drought apparently initiated other persistent changes in ecosystem properties, particularly soil erosion (35). Large reductions in herbaceous cover were widely observed elsewhere in the southwestern United States during the 1950s (36, 37). At our site, herbaceous cover was not quantified in the 1950s, but low values of herbaceous groundcover and high soil-erosion rates have been documented since at least the early 1970s (22). Currently herbaceous cover remains very sparse (≈2%) and erosion rates remain extremely high [≈4 mm/yr (38)]. Reductions in herbaceous cover, such as those caused by drought, can trigger a shift across a threshold to high erosion rates (35) like those we currently observe. Thus, a short-duration climatic event not only brought about persistent changes in the ecotone but might also have altered ecosystem properties.
Mortality-induced vegetation shifts take place more rapidly than do natality-induced shifts associated with plant establishment and migration (13, 39). We suggest that mortality-induced shifts as rapid as the one we report (i.e., <5 yr) have occurred frequently and extensively in the past, but have not been documented previously at high temporal or spatial resolution because of constraints inherent in most paleoecological methods [whereas shifts over periods of decades to millenia certainly have been well documented (8–12, 40)]. It is now becoming increasingly possible to delineate such landscape-scale ecotone shifts given the development of ever-lengthening time series of remote sensing data (e.g., satellite imagery and aerial photography) that explicitly record patterns of landscape change.
Most of the previously developed models that predict how vegetation distributions may shift in response to future climate have focused on the slower changes associated with natality and growth rather than the more rapid changes resulting from mortality (15, 41). Although assessments based on current models do include the expectation that global warming will accelerate the mortality of woody plants and thereby produce changes in vegetation distributions worldwide (14), the models generally assume an equilibrium between climate and vegetation. In contrast, our findings show that even brief climatic events can have profound and persistent ecosystem effects, reinforcing the importance of more accurately incorporating vegetation mortality and the complexity of associated ecosystem responses (e.g., increased forest fragmentation, soil erosion, insect outbreaks, and fire) into models that predict vegetation dynamics (17, 42–44).
The vegetation changes we report have some site-specific characteristics that could limit the application of our findings to other ecosystems: (i) the ecotone shift occurred in conjunction with the mortality of a single dominant species (ponderosa pine); (ii) piñon and juniper had already become established before the drought; and (iii) bark beetles amplified the mortality effects of the drought. Nonetheless, we propose that the unprecedentedly rapid climate changes expected in coming decades (45–47) will produce rapid and extensive contractions in the geographic distributions of long-lived woody species and shifts in associated ecotones such as the one we document. These shifts are very likely to occur globally because semiarid forests and woodlands and their associated ecotones are widespread and considered to be among the most sensitive to changes in climate (6). Because regional droughts of even greater magnitude and longer duration than the 1950s drought are expected as global warming progresses (45, 48), the ecological effects of droughts associated with global climate change are likely to be even greater than those documented here.
Acknowledgments
We thank J. L. Betancourt, M. H. Ebinger, V. T. Hriscu, W. K. Lauenroth, S. N. Martens, T. J. O’Shea, T. W. Swetnam, and two anonymous reviewers for comments, J. S. Dean and G. S. Funkhouser for use of unpublished data (National Science Foundation Grant DBS-9205968), and K. L. Beeley, S. B. Bracker, and C. W. Meyer for assistance with data analysis and graphics. This work was supported by the U.S. Geological Survey (Midcontinent Ecological Science Center), Bandelier National Monument, and by Laboratory Directed Research and Development funding from Los Alamos National Laboratory. Erosion data were collected with support from the Environmental Restoration Project at Los Alamos National Laboratory.
ABBREVIATION
- GIS
geographic information system
Footnotes
This paper was submitted directly (Track II) to the Proceedings Office.
References
- 1.Betancourt J L, Van Devender T R, editors. Packrat Middens: The Last 40,000 Years of Biotic Change. Tucson, AZ: Univ. of Arizona Press; 1990. [DOI] [PubMed] [Google Scholar]
- 2.Gosz J R. Ecol Appl. 1992;2:248–261. doi: 10.2307/1941859. [DOI] [PubMed] [Google Scholar]
- 3.Risser P G. Bioscience. 1995;45:318–325. [Google Scholar]
- 4.Pitelka L F the Plant Migration Workshop Group. Am Sci. 1997;85:464–473. [Google Scholar]
- 5.Holland M M, Risser P G, Naiman R J, editors. Ecotones: The Role of Landscape Boundaries in the Management and Restoration of Changing Environments. New York: Chapman & Hall; 1991. [Google Scholar]
- 6.Intergovernmental Panel on Climate Change. Climate Change 1995, Impacts, Adaptations and Mitigation of Climate Change: Scientific-Technical Analyses. Cambridge, U.K.: Cambridge Univ. Press; 1996. pp. 171–189. [Google Scholar]
- 7.Schlesinger W H, Gramenopoulos N. Global Change Biol. 1996;2:137–141. [Google Scholar]
- 8.Davis M B. Forest Succession: Concepts and Applications. New York: Springer; 1981. pp. 132–177. [Google Scholar]
- 9.Delcourt P A, Delcourt H R. In: Landscape Boundaries. Hansen A J, di Castri F, editors. New York: Springer; 1992. pp. 19–54. [Google Scholar]
- 10.MacDonald G M, Edwards T W D, Moser K A, Pienitz R, Smol J P. Nature (London) 1993;361:243–246. [Google Scholar]
- 11.Lloyd A H, Graumlich L J. Ecology. 1997;78:1199–1210. [Google Scholar]
- 12.Whitlock C, Bartlein P J. Nature (London) 1997;388:57–61. [Google Scholar]
- 13.Mueller-Dombois D. Bioscience. 1987;37:575–583. [Google Scholar]
- 14.Intergovernmental Panel on Climate Change. The Regional Impacts of Climate Change. Cambridge, U.K.: Cambridge Univ. Press; 1998. pp. 439–456. [Google Scholar]
- 15.Shugart H H. Terrestrial Ecosystems in Changing Environments. Cambridge, U.K.: Cambridge Univ. Press; 1998. [Google Scholar]
- 16.Dixon R K, Brown S, Houghton R A, Solomon A M, Trexler M C, Wisneiwski J. Science. 1994;263:185–190. doi: 10.1126/science.263.5144.185. [DOI] [PubMed] [Google Scholar]
- 17.Kirilenko A P, Solomon A M. Clim Change. 1998;38:15–49. [Google Scholar]
- 18.Condit R. Clim Change. 1998;39:413–427. [Google Scholar]
- 19.Bowen B M. Los Alamos Climatology. Los Alamos, NM: Los Alamos National Laboratory LA-11735-MS; 1990. [Google Scholar]
- 20.Touchan R, Allen C D, Swetnam T W. Fire Effects in Southwestern Forests, tech. ed. Allen, C. D. Fort Collins, CO: USDA For. Serv. Gen. Tech. Report RM-GTR-286; 1996. pp. 33–46. [Google Scholar]
- 21.Thomas H E. General Summary of the Effects of the Drought in the Southwest, Geological Survey Prof. Paper 372-H. Washington D.C.: Gov. Printing Office; 1963. [Google Scholar]
- 22.Allen C D. Ph.D. dissertation. Berkeley: Univ. of California; 1989. [Google Scholar]
- 23.Grissino-Mayer H D. Tree Rings, Environment, and Humanity, eds. Dean, J. S., Meko, D. M. & Swetnam, T. W [Radiocarbon (special issue)] 1996. pp. 191–204. [Google Scholar]
- 24.Meko D, Stockton C W, Boggess W R. Water Resourc Bull. 1995;31:789–801. [Google Scholar]
- 25.Swetnam T W, Betancourt J L. J Clim. 1998;11:3128–3147. [Google Scholar]
- 26.Parker A J. Southwest Nat. 1980;25:9–22. [Google Scholar]
- 27.Breshears D D, Myers O B, Johnson S R, Meyer C W, Martens S N. J Ecol. 1997;85:289–299. [Google Scholar]
- 28.Furniss R L, Carolin V M. Western Forest Insects. USDA For. Serv. Misc. Publ. No. 1339. Washington, D.C.: Gov. Printing Office; 1977. [Google Scholar]
- 29.Hillerman, T. (June 23, 1957) Santa Fe New Mexican; pp. 1, 3.
- 30.Malde H E. Science. 1964;145:123–129. doi: 10.1126/science.145.3628.123. [DOI] [PubMed] [Google Scholar]
- 31.Betancourt J L, Pierson E A, Rylander K A, Fairchild-Parks J A, Dean J S. Managing Piñon–juniper Ecosystems for Sustainability and Social Needs, tech. coord. Aldon, A. F. & Shaw, D. W. Fort Collins, CO: USDS For. Serv. Gen. Tech. Report RM-236; 1993. pp. 42–62. [Google Scholar]
- 32.Gosz J R. Ecol Appl. 1993;3:369–376. doi: 10.2307/1941905. [DOI] [PubMed] [Google Scholar]
- 33.Neilson R P. Ecol Appl. 1993;3:385–395. doi: 10.2307/1941907. [DOI] [PubMed] [Google Scholar]
- 34.Pickett S T A, Cadenasso M L. Science. 1995;269:331–334. doi: 10.1126/science.269.5222.331. [DOI] [PubMed] [Google Scholar]
- 35.Davenport D W, Breshears D D, Wilcox B P, Allen C D. J Range Manage. 1998;51:231–240. [Google Scholar]
- 36.Young V A. J Range Manage. 1956;9:139–142. [Google Scholar]
- 37.Herbel C H, Ares F N, Wright R A. Ecology. 1972;53:1084–1093. [Google Scholar]
- 38.Wilcox B P, Pitlick J, Allen C D, Davenport D W. In: Advances in Hillslope Processes. Anderson M G, Brooks S M, editors. Vol. 1. New York, 1996: Wiley; 1996. pp. 61–77. [Google Scholar]
- 39.Davis M B. Clim Change. 1989;15:75–82. [Google Scholar]
- 40.Davis M B, Botkin D B. Quat Res. 1985;23:327–340. [Google Scholar]
- 41.Solomon A M, Kirilenko A P. Global Ecol Biogeog Lett. 1997;6:139–148. [Google Scholar]
- 42.Steffen W L, Cramer W, Plöchl M, Bugmann H. J Veg Sci. 1997;7:321–328. [Google Scholar]
- 43.Chapin F S, III, Starfield A M. Clim Change. 1997;35:449–461. [Google Scholar]
- 44.Neilson R P, Drapek R J. Global Change Biol. 1998;4:505–521. [Google Scholar]
- 45.Intergovernmental Panel on Climate Change. Climate Change 1995: The Science of Climate Change. Cambridge, U.K.: Cambridge Univ. Press; 1996. [Google Scholar]
- 46.Broecker W S. Science. 1997;278:1582–1588. doi: 10.1126/science.278.5343.1582. [DOI] [PubMed] [Google Scholar]
- 47.Mann M E, Bradley R S, Hughes M K. Nature (London) 1998;392:779–787. [Google Scholar]
- 48.Overpeck J T. Science. 1998;271:1820–1821. [Google Scholar]