Abstract
Estrogens, acting through the estrogen receptors (ERs), play crucial roles in regulating the function of reproductive and other systems under physiological and pathological conditions. ER activity in regulating target genes is modulated by the binding of both steroidal and synthetic nonsteroidal ligands, with ligand binding inducing ERs to adopt various conformations that control their interactions with transcriptional coregulators. Previously, we developed an intramolecular folding sensor with a mutant form of ERα (ERG521T) that proved to be essentially unresponsive to the endogenous ligand 17β-estradiol, yet responded very well to certain synthetic ligands. In this study, we have characterized this G521T-ER mutation in terms of the potency and efficacy of receptor response toward several steroidal and nonsteroidal ligands in two different ways: directly, by ligand effects on mutant ER conformation (by the split-luciferase complementation system), and indirectly, by ligand effects on mutant ER transactivation. Full-length G521T-ER shows no affinity for estradiol and does not activate an estrogen-responsive reporter gene. The synthetic pyrazole agonist ligand propyl-pyrazole-triol is approximately 100-fold more potent than estradiol in inducing intramolecular folding and reporter gene transactivation with the mutant ER, whereas both ligands have high potency on wild-type ER. This estradiol-unresponsive mutant ER can also specifically highlight the agonistic property of the selective ER modulator, 4-hydroxytamoxifen, by reporter gene transactivation, even in the presence of estradiol, and it can exert a dominant-negative effect on estrogen-stimulated wild-type ER. This system provides a model for ER-mutants that show differential ligand responsiveness to gene activation to gain insight into the phenomenon of hormone resistance observed in endocrine therapies of ER-positive breast cancers.
ESTROGEN RECEPTORS (ERα and ERβ) regulate the expression of a number of gene products required for the growth of cells in response to the endogenous estrogen 17β-estradiol (E2) (1). In pathological conditions, estrogen can play a critical role in the progression of ER-positive tumors (2), and ER is one of the main therapeutic targets in the prevention and treatment of estrogen-sensitive carcinomas (3). In addition, hormone replacement therapy remains an important option to protect postmenopausal women against osteoporosis and heart disease and to mitigate hot flushes. Hence, drug discovery efforts have focused on identifying new ER ligands with mixed agonist/antagonist or pure antagonist properties, both from natural sources (phytoestrogens), as well as by the synthesis of nonsteroidal estrogens (4,5), some of the latter being termed “SERMs” (selective ER modulators) and exemplified by tamoxifen and raloxifene (RAL).
The crystal structures of ER ligand-binding domains (ER-LBDs) complexed with different ER ligands (6) provide useful insight into the design and synthesis of new ligands. Although computer modeling and structure-based design can help in predicting molecular interactions and structure-activity relationships, the pharmacological actions of these ligands are unpredictable and require further biological evaluation. Thus, it remains important to fully characterize nuclear hormone receptor ligands in cells and in animal models before considering their use in humans.
Several assay systems are currently available to characterize ER ligands for their biological activity through ER in vitro and in cell-based assays, but only a few can be directly extended for use in animals. We approached this issue by using bioluminescence imaging to study estrogen biology in living animals (7). Our system involves monitoring luminescence that derives from intramolecular complementation of a split luciferase gene that is activated by ligand-induced folding of the ER-LBD, and the responsiveness of various versions of this ligand sensor system to selected ER ligands was validated in cellular systems (7).
In adapting this bioluminescence ER ligand sensor system for in vivo use, we developed a version that contained a carefully developed single amino acid mutation in the ERα-LBD, G521T. This ER mutant was selected to be essentially unresponsive to E2, so that it could be used in mice without interference from the endogenous ligand, while being responsive to certain nonsteroidal estrogens, such as diethylstilbestrol (DES), and SERMs, so that their activity could be studied in vivo by bioluminescence.
In this study, we have characterized the ER-intramolecular folding sensor with this mutant form of ER for its responsiveness toward a broad range of ER ligands, and we have also examined the ability of full-length ERα with this G521T mutation to regulate downstream gene transactivation. Curiously, the G521T mutant has essentially no binding affinity for, nor responsiveness to, E2 but appears to have an approximately 100-fold enhanced affinity and responsiveness for ligands with a pyrazole core structure. We also find that the presence of this mutated receptor can decrease the transcriptional output of the wild-type receptor in the presence of E2, demonstrating that unoccupied G521T ER has a dominant-negative effect on agonist-occupied wild-type ER.
Our studies offer insights into the biology of ER and also provide some rationalization for the impact that naturally occurring mutations can have on the therapeutic efficacy of endocrine therapies in breast cancer and the development of endocrine resistance.
RESULTS
Monitoring the Specificity of Ligand-Induced ER Intramolecular Folding with Split Renilla Luciferase Complementation by Wild-Type (ER-LBD-FWT) and Mutant (ER-LBD-FG521S and ER-LBD-FG521T) Receptors
As reported previously (7), the ER ligand conformational sensor is constituted using ER-LBD (in some cases also with the F domain, denoted “ER-LBD-F”) that is flanked by split portions of Renilla luciferase at its N and C terminus. The various versions of this sensor system, as well as the other plasmids used in this study, are shown in Fig. 1A. In the absence of ligand, no luciferase signal is evident. When ligands bind to the intervening ER-LBD sequence, however, its N and C termini become approximated. This allows the two split components of the luciferase protein to fold together, generating the luciferase signal by protein complementation.
Figure 1.
Evaluation of ER-Intramolecular Sensor with Wild-Type and Mutant ER-Ligand Binding Domains
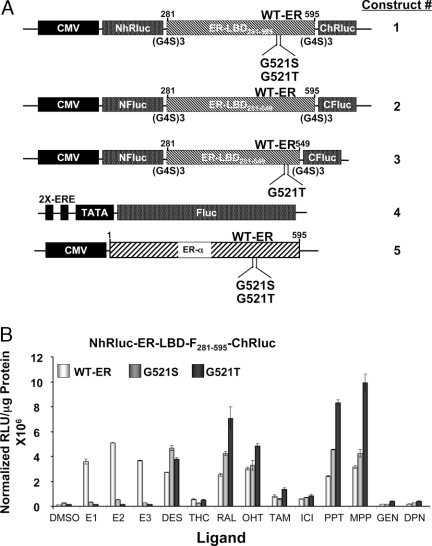
A, Schematic representation of the different expression vector constructs used in this study. The intramolecular folding sensor vectors are designed to express fusion proteins from a split luciferase gene flanking a portion of the ERα gene. Construct 1 has an N-terminal fragment of Renilla luciferase (NhRluc) at the N terminus of a portion of ERα containing amino acids 281–595 and a C-terminal fragment of Renilla luciferase (ChRluc) at the C terminus. (The 281–595 sequence encompasses the ligand-binding and the F domains and is termed ERα-LBD-F.) Construct 1 was also prepared with the ERα-LBD-F point mutants, G521S and G521T. Constructs 2 and 3 are of similar design, but have split firefly luciferase fragments flanking wild-type and mutant (G521T) ERα-LBD-F (amino acids 281–595; construct 2) or ERα-LBD (amino acids 281–549; construct 3). Construct 4 is a reporter gene having two EREs preceding a minimal promoter (TATA) followed by firefly luciferase gene, reported previously (32). Construct 5 comprises vectors expressing full-length wild-type ERα and the mutant ERs (G521S and G521T). All of the ER expression vectors are under the control of a cytomegalovirus promoter. B, Split Renilla luciferase ER-intramolecular folding sensor with wild-type and mutant ER-LBD-F (281–595) studied for their response to various ER ligands. The 293T cells transfected with wild-type and mutant (G521S and G521T) ER-intramolecular folding sensors (panel A, construct 1) with split Renilla luciferase fragments were assayed for Renilla luciferase activity 24 h after exposure to 1 μm of several ER ligands. Error bars are sems of triplicate determinations. CMV, Cytomegalovirus; DMSO, dimethylsulfoxide; TAM, tamoxifen; WT-ER, wild-type ER; ICI, Faslodex.
We previously identified mutant forms of ER (G521S and G521T) that are less responsive or fully unresponsive to E2, by preparing all possible mutants at the G521 site and assaying them against seven different ligands (7). These systems were studied in transiently transfected ER-negative human embryonic kidney (HEK) 293T cells. The cells were assayed for complemented Renilla luciferase activity after 24 h of exposure to natural and synthetic agonist, mixed agonist/antagonist, and pure antagonist ligands (Fig. 1B). The results show luciferase signal output from ligand-induced intramolecular folding of wild-type and single mutant receptor ER-LBD-F constructs (G521S and G521T).
As expected from our previous work (7), the mutant sensors show no complementation for the endogenous ligand E2. In addition, the other two common endogenous steroidal estrogens, estriol (E3) and estrone (E1), also exhibit a dramatic loss in induction of luciferase complementation in the two mutant ERs. Interestingly, despite loss of their responsiveness to steroidal agonists (E1, E2, E3), these two mutant ERs retained good, and at times enhanced, responsiveness to other classes of ER ligands, the nonsteroidal estrogen DES, the SERMs [raloxifene (RAL) and hydroxytamoxifen (OHT)], and the pyrazole-based ligands [propyl-pyrazole-triol (PPT) and methyl-piperidino-pyrazole (MPP)] (Fig. 1B). The response of the G521T mutant sensor to RAL, PPT, and MPP was significantly (P < 0.05) greater than to DES.
Diarylpropionitrile (DPN) and genistein (GEN), which are ligands highly selective for ERβ, showed no complementation of the Renilla luciferase signal (Fig. 1B); because our sensor system is based on ERα, these results confirm the ER subtype specificity of our system. R,R-tetrahydrochrysene is an interesting ER ligand that behaves as an agonist on ERα and an antagonist on ERβ and was previously shown not able to stabilize ERα-LBD dimers, unlike most other ER ligands (8,9,10,11,12). It is not surprising to see a low signal output from this ligand with our receptor intramolecular folding sensor because of its unique binding mode and character; these results confirm the sensitivity of our system to identify ligands of distinctive pharmacological character.
The split Renilla luciferase complementation system described above, and reported previously (7), is based on a portion of ERα that embodies the C-terminal F-domain, as well as the LBD (hence the designation ER-LBD-F281–595). This system responds to ER ligands of a different biocharacter, including ER agonists, mixed agonist/antagonists, and pure antagonist. Using a shorter ER-LBD construct (ER-LBD281–549; Fig. 1A, construct 2, that lacks the F domain), we were able to distinguish among these different classes. As described below, we have also made G521T mutant versions of this shorter, ligand bioactivity-discriminating sensor system (Fig. 1A, construct 2), as well as the G521S and G521T mutants of full-length ERα (Fig. 1A, construct 5) to study ligand specificity in gene transactivation assays.
Expanding the ER-Intramolecular Folding Sensor from Split Renilla to Firefly Luciferase with Enhanced Signal and Retention of Ligand Biocharacter Profiles for ERα-LBD and ERα-LBD-F Constructs
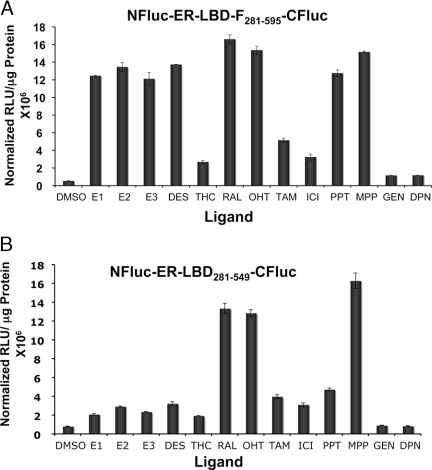
To further develop this system, we optimized the ER-intramolecular folding system with split firefly luciferase complementation, using the newly identified fragments (NFluc-398 and CFLuc-394) (13). Firefly luciferase offers the potential of higher signal output in animal imaging (compare signal levels, Figs. 1B vs. 2A) and also has a much longer signal lifetime than the Renilla luciferase system.
Figure 2.
Comparison of Split Firefly Luciferase-ER Intramolecular Folding Sensor with ER-LBD-F (Amino Acids 281–595) or ER-LBD (Amino Acids 281–549)
A (amino acids 281–595), The 293T cells transfected with the ER-intramolecular folding sensor construct of ER amino acids 281–595 and the split firefly luciferase fragments (Fig. 1A, construct 2) were assayed for firefly luciferase activity after exposure to 1 μm of several ER ligands. Error bars are sems of triplicate determinations. B (amino acids 281–549), The 293T cells transfected with the ER-intramolecular folding sensor construct of ER amino acids 281–549 and the split firefly luciferase fragments (Fig. 1A, construct 3) were assayed firefly luciferase activity after exposure to 1 μm of several ER ligands. Error bars are sems of triplicate determinations. DMSO, Dimethylsulfoxide; TAM, tamoxifen; ICI, Faslodex.
The split firefly luciferase system was studied using 12 different ER ligands, including those that bind very preferentially to ERβ (DPN and GEN). The results show significant ligand-induced complementation for all ERα-binding ligands by the system that has the extended ER-LBD-F core comprising amino acids 281–595 (Fig. 2A). By contrast, as noted before (7), the shorter construct, comprised of amino acids 281–549, discriminated among these ligands based on their biocharacter, giving higher signal levels for mixed agonist/antagonists (i.e. SERMs) and lower signal levels for agonists (Fig. 2B). As expected, the pyrazole mixed agonist/antagonist (MPP) shows a higher signal compared with the pyrazole agonist (PPT) within the ER-LBD intramolecular folding system (7). No complementation was seen for ERβ-specific ligands DPN and GEN with or without the F domain.
ER with the G521T Mutation Shows Essentially No Response to or Affinity for E2 as Measured by Ligand-Induced Intramolecular Folding, Estrogen-Responsive Reporter Gene Activation, and [3H]E2 Uptake
We used three different approaches to further evaluate the binding affinity and responsiveness of the G521T-mutant ER to the endogenous ligand E2. A proteomic approach involved ligand-induced ER-LBD intramolecular folding using the split firefly luciferase sensor system. The other two approaches used full-length wild-type or G521T mutant ERα and involved a genomic transcriptional output method (ERE-luciferase reporter gene-based gene transactivation system) and a ligand-based method (17β-[3H]E2 uptake by cells transiently transfected with various ER constructs) (Figs. 3-7 and supplemental Figs. 1 and 2 published as supplemental data on The Endocrine Society’s Journals Online web site at http://mend.endojournals.org).
Figure 3.
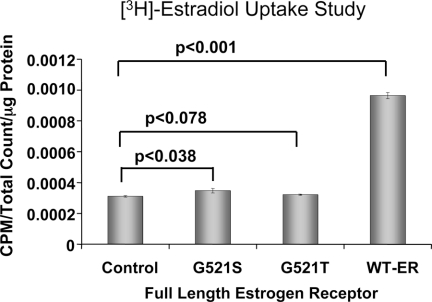
Receptor Binding Assay in Transient 293T Cells
Whole-cell receptor binding assay in 293T cells transfected with full-length wild-type and mutant ERs (G521S and G521T) (Fig. 1A, construct 5). Cell uptake was measured after exposure of cells to [3H]E2. WT-ER, Wild-type ER.
Figure 4.
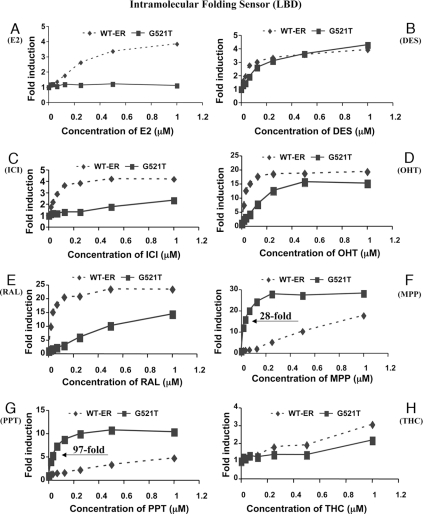
The 293T Cells Stably Expressing ER-Intramolecular Folding Sensors with Wild-Type and Mutant (G521T) ER-LBD Studied by Titrating with Different ER Ligands
The 293T cells stably transfected with the split firefly luciferase ER-intramolecular folding sensor (Fig. 1A, construct 3) were studied by titrating (0–1 μm) with several ER ligands (panels A–H, as indicated), including the pyrazole derivatives PPT and MPP. WT-ER, Wild-type ER; ICI, Faslodex.
Figure 5.
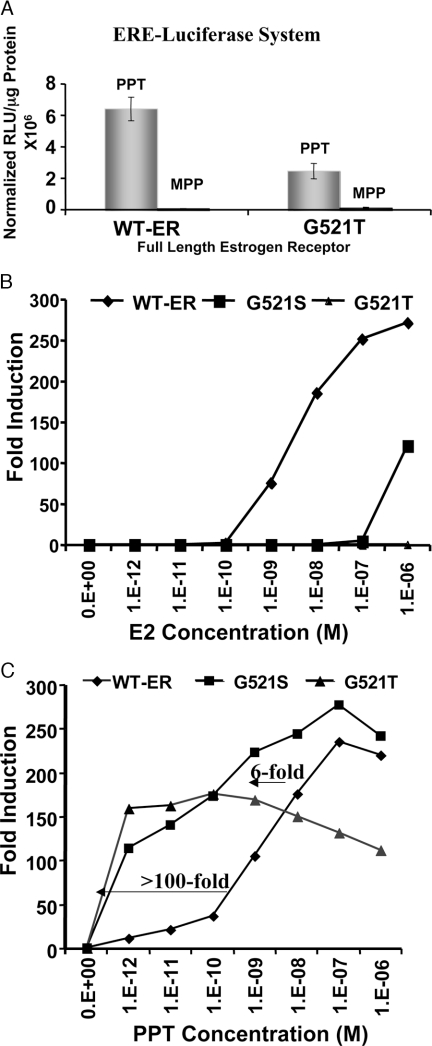
The Wild-Type (G521G) and Mutant (G521T) Full-Length ER with ERE-Luciferase Gene Activation System
A, The 293T cells were cotransfected with either wild-type or mutant ER and an estrogen-responsive reporter gene and were studied for reporter gene expression 24 h after exposure to 1 μm of agonist ligand PPT and antagonist ligand MPP. Both cells show reporter gene activation only when exposed to the agonist PPT, not the antagonist MPP. B and C, Comparison of full-length wild-type and mutant ERs (G521S and G521T) in an estrogen-responsive reporter gene system titrated with E2 and PPT. The 293T cells cotransfected with the estrogen-responsive reporter gene and either wild-type or mutant ERs (Fig. 1A, constructs 4 and 5) were assayed for luciferase activity 24 h after exposure to different concentrations (1 pm to 1 μm) of E2 and PPT. WT-ER, Wild-type ER.
Figure 6.
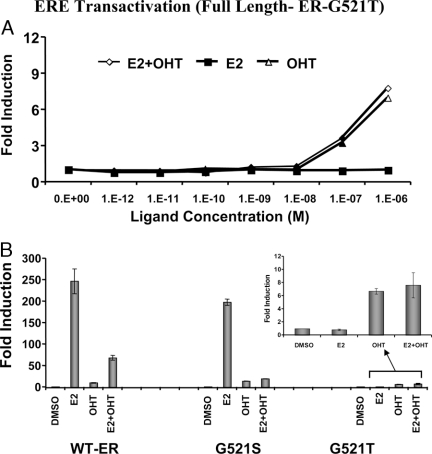
Use of Mutant ERs to Assess SERM Agonist Activity
A, The 293T cells were cotransfected with an estrogen-responsive reporter gene and full-length G521T mutant ER (Fig. 1A, constructs 4 and 5) were assayed for luciferase activity 24 h after exposure to different concentrations of E2 or OHT or by both together. B, The 293T cells were cotransfected with an estrogen-responsive reporter gene and full-length wild-type or mutant (G521S or G521T) ER (Fig. 1A, constructs 4 and 5) and were assayed for luciferase activity 24 h after exposure to 1 μm of E2, OHT, or both together. DMSO, Dimethylsulfoxide; RLU, relative light unit; WT-ER, wild-type ER.
Figure 7.
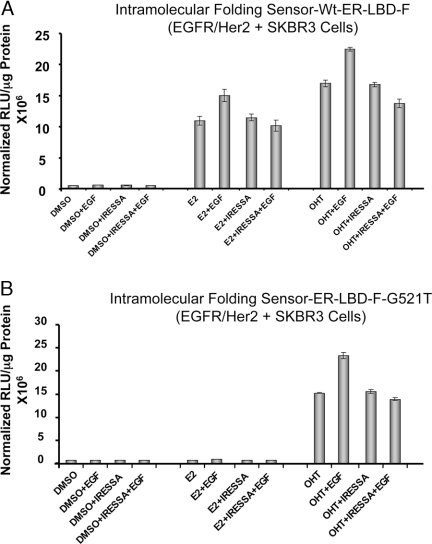
Monitoring EGF-Induced Conformational Changes in ER-LBD-F with Renilla Luciferase Complementation within EGFR/Her2+ SKBR3 Cells
Cells were transfected with wild-type or mutant ER conformational sensor (Fig. 1A, construct 1) and were exposed to 1 μm ligand ± 50 ng/ml EGF (panels A and B). The cells were also studied by blocking the EGFR with the EGFR inhibitor IRESSA, along with the ligands (E2 or OHT) before exposure to EGF. The enhanced luciferase activity specifically in EGFR un-blocked cells (without IRESSA) in the presence of ligands (E2 or OHT) are indicative of a conformational change in receptor upon EGF addition. DMSO, Dimethylsulfoxide; RLU, relative light unit.
For the proteomic analysis, 293T (ER negative) cells, transiently transfected with ER-LBD intramolecular folding systems (Fig. 1A, construct 3), both the ER-LBDG521T and ER-LBDwt for comparison were assayed for firefly luciferase complementation activity 24 h after exposure to 1 μm E2 (supplemental Fig. 1). E2 induced very marked complementation, but only in cells transfected with the intramolecular folding sensor based on wild-type ER; the mutant ER showed no complementation, even with this saturating concentration of E2 (supplemental Fig. 1).
We next compared the response of wild-type (ERWT) and mutant (ERG521T) ERs to increasing concentrations of E2 (0–1 μm), using both the intramolecular folding (Fig. 1A, construct 3) and estrogen response element (ERE) reporter gene transactivation (Fig. 1A, constructs 4 and 5) assays (supplemental Fig. 2). The results show E2 concentration-dependent activation only by the wild-type and not by the mutant ER in both the intramolecular folding assay (supplemental Fig. 2A) and the reporter gene activation assay systems (supplemental Fig. 2B). Thus, there is an excellent correlation between our split luciferase intramolecular folding assay and the gold-standard ERE-transactivation assay: both assays demonstrate, in parallel fashion, the dramatic loss of E2 responsiveness of the G521T mutant ER constructs (LBD and full length, respectively) (supplemental Fig. 2).
To confirm that the differential responsiveness of mutant ERs is based on differences in their ligand binding affinities, we measured [3H]E2 uptake in cells transfected with either full-length ERα or mutant ERs. Markedly higher ligand uptake (P < 0.001) was noted in cells transfected with wild-type ER than the mutant receptors (ERG521S and ERG521T) (Fig. 3). Notably, cells transfected with the ERG521S mutant show a modest but significantly higher level of ligand uptake compared with mock-transfected cells (P < 0.05), whereas uptake in cells with the ERG521T mutant was not significantly different from control (Fig. 3).
Selective Enhanced Responsiveness of ERG521T for Pyrazole Ligands PPT and MPP, Demonstrated by Both Receptor Intramolecular Folding and Reporter Gene Transactivation Approaches
Assays of our Renilla luciferase ER-LBD-F (WT, G521S, G521T) intramolecular folding sensors with an expanded panel of ligands (Fig. 1B) showed not only a decrease in the response level of the two mutant ERs to E2, but also a substantially higher signal output from our synthetic pyrazole core ligands, PPT and MPP (Fig. 1B). We confirmed and further examined this phenomenon with our firefly luciferase ER-LBDWT and ER-LBDG521T mutant intramolecular folding sensors by using titration assays (1 pm to 1 μm) with various ligands (see supplemental Table 1) to determine whether the increased responsiveness of the G521T ER sensor resulted from a novel intramolecular fold that gave enhanced complementation or from an enhanced potency in the response of these constructs to pyrazole ligands, likely reflecting an enhanced affinity. These results are shown in Fig. 4, A–H.
Compared with wild-type ER, the ER-intramolecular folding sensor based on the mutant ER-LBDG521T (Fig. 1A, construct 3) shows dramatically enhanced differential potency in response to the synthetic pyrazole derivatives (PPT and MPP), which is estimated to be 28-fold greater for MPP and 97-fold greater for PPT (Fig. 4, F and G). Once again, the mutant ER-LBD (ER-LBDG521T) intramolecular folding sensor based on firefly luciferase shows no luciferase complementation with E2 at any of the concentrations studied (Fig. 4A). Other ligands show either no significant differences between the wild-type and mutant ER sensors (DES, Fig. 4B; THC, Fig. 4H) or differences in favor of the wild-type ER sensor (ICI, OHT, and RAL, Fig. 4, C–E). These titration studies clearly demonstrate that the pyrazole core ligands (MPP and PPT) are the only class of ER ligands with a highly selective increase in response potency for the G521T receptor construct.
To further validate our ER-LBD intramolecular folding results with pyrazole ligands, we also used a genomic transcriptional activation approach with full-length ER constructs to monitor gene transactivation. The 293T cells cotransfected either with and ERE-luciferase reporter gene and full-length wild-type (ERWT) or with mutant (ERG521T) receptors (Fig. 1A, constructs 4 and 5) were assayed for luciferase activity after exposure to ligand agonist PPT and antagonist MPP at 1 μm concentrations (Fig. 5A). The results show a significant level (P < 0.001) of activated luciferase activity only from transiently transfected cells exposed to the agonist (PPT) and not the antagonist (MPP) pyrazole ligand. Although the response was greater with wild-type ER, this result confirms preservation of the downstream gene activation property by the mutant ER (G521T) (Fig. 5A).
We also titrated increasing levels of E2 or PPT to characterize the potency and efficacy selectivity of these ligands for wild-type ER vs. the two mutant ER constructs (G521S and G521T) (Fig. 5, B and C). As before, the two mutants were marginally (G541S) or fully unresponsive (G521T) to E2 (Fig. 5B). On the other hand, PPT shows markedly enhanced potency in reporter gene transactivation through the mutant ERs vs. wild-type ER. Compared with wild-type ER, PPT shows a 6-fold increase in potency with G521S and more than 100-fold increase in potency with the G521T receptor (Fig. 5C). Interestingly, the efficacy (total transactivation signal output) of PPT through the G521T mutant receptor is somewhat less than with the other two, suggesting decreased agonistic property of this ligand through this mutant receptor (Fig. 5, A–C).
A Structural Rationale for the Loss of E2 Binding and Enhanced Pyrazole Ligand Binding to the G521T Mutant ER
Elegant crystallographic studies with the ERα-LBD complexed with various ligand classes have given much insight into ligand-receptor-coregulator binding interactions at the molecular level. The x-ray structure of ERα-LBD bound with E2 shows that G521 is in helix 11 (supplemental Fig. 3A). A closer look at the ligand-binding pocket surrounding E2 with a space-filling model shows that this glycine residue is in close proximity with carbons 15 and 16, looming above the edge of the D-ring of the steroidal core of E2 (supplemental Fig. 3B, left). Using this crystallographic structure, we can model in a threonine residue at position 521, and it is evident (supplemental Fig. 3B, right) that the larger residue clashes sterically with both the D-ring atoms and even the angular methyl group, which is consistent with the reduced potency of the steroidal core ligands E1, E2, and E3, as we have observed in our experiment (Fig. 4).
Similar analysis of the ERα-LBD complexed with RAL shows that there is not a steric clash with either the G521 residue (supplemental Fig. 3C, left) or a T521 residue when it is inserted by modeling (supplemental Fig. 3C, right). Although no crystal structure exists for the ERα-LBD bound with pyrazole core ligands, we have previously reported molecular modeling and structure-activity relationship data with a proposed binding mode of pyrazole agonist and antagonist ligands to ERα-LBD and their selective affinity for ER α- over β-receptor (11,14,15,16,17). Using our model of the ERα-LBD bound with PPT, we can again see that, as with RAL, neither G521 nor T521 encounter steric clashes with the ligand (supplemental Fig. 3D, leftand right, respectively). This lack of steric clash of the ERα G521T mutant with both RAL and PPT is consistent with the high potency of these ligands with the G521T ER mutant (Fig. 5). With both RAL and PPT, the rotational flexibility of the appended phenol ring, which is closest to the 521 position, enables steric interaction with the 521T residue to be avoided through simple single-bond rotation; the rigidity of E2 does not enable the ligand to escape steric interaction with 521T.
Full-Length G521T ERα that Is Specifically Unresponsive to E2 Is a Good Candidate for Evaluating the Agonist Activity of SERMs and Growth Factor-Induced Changes in ER Conformation
We also evaluated whether mutant ER constructs unresponsive to E2 can be used to highlight the mixed agonist/antagonist properties exerted by SERM ligands such as OHT. Using the genomic transcriptional output approach, 293T cells were cotransfected with the ERE-luciferase reporter gene along with the vector expressing full-length mutant ERG521T (Fig. 1A, constructs 4 and 5) and assayed for luciferase activity after 24 h of exposure to increasing concentrations (1 pm to 1 μm) of E2 or OHT, or equimolar concentrations of both ligands together. Exposure to E2 gave no increase in luciferase signal (Fig. 6A), whereas cells exposed to OHT alone or in combination with E2 show significantly (P < 0.05) higher luciferase signals at the higher concentrations.
A similar study was performed using full-length wild-type ER and the ER mutants ERG521S and ERG521T at saturating ligand concentrations (1 μm) each of E2 or OHT, or both together (Fig. 6B). The stimulatory activity of E2 decreases progressively from wild-type ER to the G521S and G521T mutants, yet the stimulatory effect of OHT, although small, remains constant in these three systems (Fig. 6B). These results suggest that G521T mutant ER is a good candidate for sensing the agonistic property associated with SERMs (Fig. 6A). It is of note that Danielian et al. (18) made a similar observation using a mouse ER mutant at residue 525 (mouse G525R, which corresponds to 521 in human ERα), which has a differential affinity for OHT compared with E2.
We find that a major limiting factor in this proposed transcriptional output approach to evaluating the agonistic property of OHT is the endogenous wild-type receptor found in various cell lines that binds E2 and activates ERE reporters, thereby masking the specific OHT agonist signal (Paulmurugan, R., unpublished observations).
We used our Renilla luciferase ERα-LBD-F intramolecular folding sensor (Fig. 1A, construct 2) the signal output of which is independent of endogenous wild-type receptor to overcome this obstacle. As a model study, we monitored the intramolecular folding patterns of wild-type and mutant (G521S and G521T) ERα-LBD-F in a EGFR/Her2-positive cell line (SKBR3) in the presence of E2 or OHT with and without 50 ng/ml epidermal growth factor (EGF) (Fig. 7, A and B; and supplemental Fig. 4). As noted before (Figs. 1B and 2A), this ERα-LBD-F system with the mutant ERs shows less response to the steroidal estrogen compared with SERMs.
Notably, there is a substantial increase in intramolecular folding signal in the presence of EGF, most evident with OHT, which suggests that downstream phosphorylation cascades affect receptor conformation. When the cells were blocked with IRESSA (also known as Geftinib) (25 μm), a specific inhibitor of EGFR, before inducing with EGF, no significant (P < 0.05) EGF-specific increase in the luciferase complementation by ligands E2 or OHT was observed (Fig. 7, A and B).
The SKBR3 cells expressing the sensors either with wild-type ER or G521T mutant ER-LBD-F were also studied by exposure to several other ligands both in the presence and the absence of EGF. The results shows EGF-specific increase in the luciferase complementation by ligands MPP and PPT in addition to E2 and OHT, but ligand RAL shows no specific EGF-mediated induction (supplemental Fig. 5).
Although this result needs to be confirmed with various ligands in several cell lines of different origin, it serves as a unique and promising approach to study the cross talk between EGFR/Her2 signaling and conformational changes in tamoxifen-bound ER. We had previously shown growth factor-specific ER phosphorylation patterns (19) but, to our knowledge, this is the first reported evidence of a novel receptor conformational change secondary to upstream kinase activity. Further experiments are required to determine the manner in which EGF signaling is able to affect the conformation of the ERα LBD.
The ER Mutant Unresponsive to E2 Can Be Used to Study the Biology of Receptor Dimerization and Downstream Interaction with Coregulators within an Estrogen-Responsive Transactivation System
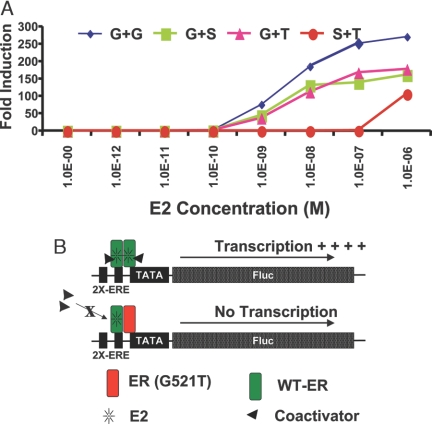
We also characterized the role of full-length mutant ERs (G521S and G521T) in cotransfection studies where they are combined with the full-length wild-type ER using an estrogen-responsive reporter gene system. The cells, cotransfected with a combination of vectors as shown in Fig. 8A, were assayed for luciferase activity 24 h after exposure to different concentrations of E2. The results show that the presence of ER mutants unresponsive to E2 results in a significant reduction (P < 0.001) in both the potency and efficacy of E2 in ERE-regulated gene transactivation through the wild-type receptor. These findings suggest that the mutant ERs, which would not bind to or be activated by E2, have a dominant-negative character. One possible mechanism is the formation of heterodimers with ligand-bound wild-type ERα and these half-occupied heterodimers being less effective in reporter gene activation (Fig. 8B).
Figure 8.
Dominant-Negative Effect of G521T Mutant ERα on the Transcriptional Activity of Wild-Type ERα
A, The 293T cells were cotransfected with an estrogen-responsive reporter gene and either full-length wild-type ER alone or in combination with equimolar amounts of one mutant ER (WT + G521S or WT + G521T), or both the mutants (G521S + G521T) and were assayed for luciferase activity 24 h after exposure to different concentrations of E2. B, Schematic model of wild-type ER dimers and wild-type ER and G521T mutant ER heterodimers. Results from reporter gene transcription assays suggest that an agonist ligand needs to be bound to both monomers within an ER dimer to elicit coregulator binding and activation of ERE-regulated gene transcription.
DISCUSSION
In this study, we have characterized the mutant ERs (ERG521T and ERG521S) generated in our laboratory as E2-resistant constructs for imaging estrogen action in live animals by ligand-induced intramolecular folding changes in ER (7). These mutant ERs can be used to monitor the agonist activity of SERMs and the downstream effects of growth factor stimulation on ER conformation, and to probe the function of ER dimers. We have also optimized a split firefly luciferase complementation system, which has a persistent bioluminescence signal better suited for in vivo animal imaging studies, and shown that its differential ligand responsiveness is the same as that of the split Renilla luciferase complementation system studied previously (7).
The ERα G521T Mutant Is Unresponsive to Steroidal Estrogens but Responds to a Variety of Nonsteroidal ER Ligands
We profiled the ERα G521T mutant against a large set of ligands using both ligand-induced intramolecular folding systems (proteomic approach) and the ERE-luciferase reporter gene activation systems (genomic approach). The G521T mutant ER (ERG521T) showed no activity to the steroidal estrogens, E1, E2, and E3, in these assays (Figs. 3–5 and supplemental Figs. 1 and 2), and it failed to mediate [3H]E2 uptake in ERα-transfected cells. The related G521S mutant ER (ERG521S) showed only very low activity in these assays.
Previous studies have shown that mouse ER with a mutation from glycine to arginine at this same site (mERG525R) exhibits low affinity for E2 (1000-fold less) (20). It has also been shown before that methionine and serine at positions 521/522 of human ER completely abolish its responsiveness to E2, as measured by gene transactivation (18).
Interestingly, we found that ERG521T showed differential enhanced responsiveness, both in terms of potency and efficacy, to pyrazole compounds (MPP and PPT), both in terms of intramolecular folding and ERE-luciferase gene activation (Figs. 4, F and G, and 5C). Compared with wild-type ERα, PPT with ERG521T shows an approximately 100-fold higher potency in both the folding and the reporter gene assays (Figs. 4G and 5C). These results demonstrate that there is an excellent correlation between our split firefly luciferase intramolecular folding system and the standard ERE-responsive gene transactivation system.
Although it was known through our alanine scanning studies that a G521A mutation reduced E2 binding and transactivation potency without affecting the activity of some nonsteroidal ligands (21,22), to our knowledge, ERαG521T is the first single-residue human ER mutant that has lost, essentially completely, responsiveness to the steroidal estrogens. Yet, despite being unresponsive to E1, E2, and E3, ERαG521T remains responsive to nonsteroidal ER ligands (DES, RAL, and OHT) and even has enhanced responsiveness to the pyrazole-core ER ligands (PPT and MPP). Most of the ER mutants reported earlier either retained some level of affinity for E2 or lost their binding for most other ER ligands (23).
The Structural Basis of the Differential Ligand Response to the G521 Mutation in Luciferase Complementation and Transactivation
It is of note that the other mutant (ERG521S) demonstrates an intermediate level of loss of responsiveness to E2, and gain of responsiveness to PPT when compared with the G521T mutant receptor (Fig. 5, B and C, and Fig. 6B), a result that is reminiscent of the activity of the ERαG521A mutant mentioned above (21,22). Although the results of the our prior alanine scanning study, which predated the x-ray structure of the ERα-LBD, was interpreted as being consistent with the 521 site being near the D-ring of steroidal estrogens, we now know from structural studies (6), and models based on them (supplemental Fig. 3), that any residue more bulky than the hydrogen of glycine will encounter steric clashes with carbons 15, 16, and 18 of steroidal estrogens. Curiously, those nonsteroidal ligands that have a second phenol group in this region (e.g. DES, OHT, PPT, and MPP) can avoid this contact by a simple rotation of this phenyl ring.
The ERα G521T Mutant Is a Powerful Probe to Study Basic Questions in the Mechanism of ER Action
After characterizing ERαG521T for its ligand response profile and biocharacter signal output from our protein folding and reporter gene assays, we used this novel receptor construct as a tool to study estrogen biology. Clinically it has been shown that breast cancer cells that are both Her2 positive and ER positive tend to be more resistant to tamoxifen therapy. The mechanism of tamoxifen resistance, especially in cell lines with enhanced kinase activity, has been a very active area of research and, in some cases, tamoxifen has been found to become an agonist, stimulating rather than inhibiting cell proliferation (24,25). Although a cross talk between kinase signaling and genomic ER signaling has been suggested (24,25), the molecular mechanism causing this reversal in tamoxifen biocharacter in Her2-overexpressing cells remains to be elucidated.
Detecting SERM Agonism
In this study, we showed that the E2-unresponsive G521T mutant ER can selectively highlight the agonistic properties of OHT on ER genomic signaling in ER-negative 293T cells (Fig. 6, A and B); the activated signal is significantly higher (10 ± 2-fold) than control and E2-treated cells. It is of note that both mouse and human ER mutants have been previously reported by other groups with similar goals of abolishing E2 responsiveness and sensing the agonistic property of SERMs by simple transfection assays (18,23). The major limitation with these genomic approaches using ERE-dependent transactivation is that these studies remain restricted to ER-negative cell lines. Fortuitously, our split luciferase proteomic ER sensor detects ligand-induced intramolecular changes in ER-LBD conformation, producing highly specific bioluminescence signals upon luciferase complementation that works in various cell types, even in the presence of endogenous wild-type ER (7).
Detecting Growth Factor-ER Cross Talk
Our intramolecular folding results in EGFR/Her2-positive cells (SKBR3) ± EGF suggest that we can monitor a unique receptor conformational change in ER (more so with OHT than E2) with the addition of a growth factor that stimulates EGFR kinase activity (Fig. 7, A and B and supplemental Figs. 4 and 5). Although we and others have previously shown ER phosphorylation downstream of various kinase signaling (19), to our knowledge this is the first documented evidence of a unique receptor conformational change secondary to upstream EGF stimulation. The specific growth factor receptor blocking studies further support our results.
Probing Ligand Occupancy Effects on ER Dimer Activity and Downstream Target Gene Activation
ER heterodimerization remains an incompletely understood but crucial element in the originally described tripartite ER signaling mechanism (26). There have been several studies demonstrating ligand-induced dimerization and the sequel of gene activation in vitro (cell lysates) and in vivo (intact yeast cells) by using estrogen and estrogen-related receptors (27,28). To our knowledge, however, no one has thus far reported the need for double occupancy of both monomers with agonist ligands within a heterodimer for downstream target gene activation.
In this study, we used the ERE-reporter gene system to show that cotransfection of the E2-unresponsive mutant ERs reduced the level of E2-induced gene transactivation from the wild-type receptor (Fig. 8A). Thus, the ERWT/ERG521T heterodimer can presumably form and bind to the ERE, but with only the ERWT monomer able to bind E2, it is likely unable to recruit coactivators and thus shows decreased transactivation. These results indicate that ER dimers with ligand agonist bound to both monomers are required for effective activation of ERE-based downstream target genes (Fig. 8B).
These results correlate very well with our previous protein microarray findings using coactivator-coated protein slides to recruit differential fluorophore-labeled ERα or ERβ in the presence of ER subtype-selective ligands (29). We found that ERα/ERβ heterodimers, with only a single monomer occupied by an agonist ligand and with the other monomer unliganded, were not able to interact effectively with coactivator proteins (29). In this study, the suppressive effects of unliganded ERαG521T on liganded wild-type ER are reminiscent of the dominant-negative effect of certain other ER mutants, e.g. ERα L540Q (30,31). In contrast to ERαG521T, however, ERαL540Q retains affinity for estrogens.
Although we are still early in the development and optimization phase of our estrogen-imaging sensors, current findings suggest that our constructs can be used as tools to study various aspects of estrogen biology. In addition, the activity of our novel ER sensors can be monitored through imaging of intact cells and stably transfected cells implanted in live animals (7), further studies of which are ongoing.
MATERIALS AND METHODS
Chemicals, Enzymes, and Reagents
The plasmid pcDNA-FLUC encoding the full-length firefly luciferase (FLUC) was constructed by cloning the PCR-amplified fragment of FLUC from pG5-LUC plasmid (Promega Corp., Madison, WI) into the NheI and XhoI restriction sites pcDNA3.1 (+) eukaryotic expression vector. LARII substrate for firefly luciferase assay and 5× passive lysis buffer was purchased from Promega Corp. Restriction enzymes, modification enzymes, and T4-DNA ligase were purchased from New England Biolabs (Beverly, MA). Triple-Master mix Taq-DNA polymerase for PCR amplification was purchased from Brinkmann Eppendorf (Hamburg, Germany). Site-directed mutagenesis was performed using the Stratagene kit (La Jolla, CA). Ampicillin for bacterial culture and dimethyl sulfoxide were purchased from Sigma (St. Louis, MO). Bacterial culture media were purchased from BD Diagnostic Systems (Sparks, MD). Plasmid extraction kits and DNA gel extraction kits were purchased from QIAGEN (Valencia, CA). Coelenterazine was purchased from Nanolight technology (Pinetop, AZ). All of the animal cell culture media, fetal bovine serum, the antibiotics streptomycin and penicillin, and plastic wares for cell cultures and Lipofectamine 2000 transfection reagents and pcDNA3.1(+) eukaryotic expression vector for constructing different plasmid vectors were purchased from Invitrogen (Carlsbad, CA). The EGFR inhibitor IRESSA was purchased from LC Laboratories (Woburn, MA).
Construction of Plasmids
The NH2-terminal portion (NLUC, amino acids 1–398) and COOH-terminal portion (CLUC, amino acids 394–550) of the Fluc gene was PCR amplified using the forward primer designed with NheI and the start codon, and the reverse primer designed with BamHI with a peptide linker sequence (GGGGSGGGGSGGGGS) and BamHI forward primer and XhoI reverse primer with stop codon, respectively, for N- and C-terminal firefly luciferase fragments (NFluc and CFluc) using the pcDNA-FLUC plasmid constructed above as a template (primer sequences available upon request). Similarly, the Renilla luciferase fragments (NRluc and CRluc) were amplified using the forward primer designed with BamHI, and the reverse primer was designed with XhoI and a stop codon. The restriction enzyme-digested fragments were cloned into a corresponding enzyme-digested vector backbone of pcDNA3.1(+) (Invitrogen, Carlsbad, CA) modified to express the puromycin resistance gene for stable cells selection. The full-length ER expressing under cytomegalovirus promoter was used for creating full-length ER with G521S and G521T mutants by using the Stratagene site-directed mutagenesis kit. The 2ERE-pS2-luciferase reporter gene (ERE-luciferase) was used for all transactivation experiments (32).
Cell Culture
All cell lines used in this study were purchased from American Type Culture Collection (Manassas, VA). HEK 293T cells were grown in MEM supplemented with 5% charcoal-treated fetal bovine serum and 1% penicillin/streptomycin solutions.
Cell Transfection, Firefly, and Renilla Luciferase Assays
Transfections were performed in 80% confluent 24-h-old cultures. For transient transfection, 200 ng/well of DNA was used. Lipofectamine 2000 was used for transfection following the manufacturer’s instructions. Unless otherwise specified 5 ng/well of other optical reporters was used for transfection normalization in all the transient transfection studies. To make stable cells, puromycin was used as selection marker. The cells were assayed after 24-h incubation at 37 C at 5% CO2 with specific conditions mentioned in each specific methods. The luminometer assays for firefly luciferase and Renilla luciferase activity were performed as previously described (33,34,35). In brief, transfected cells were lysed in 200 ml of ice-cold 1× passive lysis buffer supplied by Promega and were shaken for 15 min on ice. The cell lysates were centrifuged for 5 min at 1.3 × 104 g at 4 C to remove cell debris. To determine Renilla luciferase activity, 20 ml of supernatant was assayed by addition of 0.5 mg of coelenterazine in 100 ml of 0.05 m sodium PBS at pH 7.0 (PBS), followed by photon counting in the luminometer (model T 20/20; Turner Designs, Sunnyvale, CA) for 10 sec. Firefly luciferase activity was determined as described for Renilla luciferase activity, except 100 ml of LARII substrate from Promega was used. Protein concentrations in cell lysates were determined by Bradford Assay (Bio-Rad Laboratories, Hercules, CA). Renilla luciferase activities were normalized for protein content and for transfection efficiency using firefly luciferase activity and expressed as relative light units (RLU) per microgram protein per minute of counting.
ER-Intramolecular Folding Sensors with Wild and Mutant ER-LBDs with Split Renilla and Firefly Luciferase Complementation Systems Studied by Inducing with Different ER Ligands
The 80% confluent 24-h-old cultures of 293T cells in 12-well culture plates were used for transfection. Each well 200 ng of constructed experimental DNA along with 5 ng of transfection normalization control plasmid were used. (The experiments conducted with split-Renilla luciferase sensors, full-length firefly luciferase reporter served as normalization control. Similarly in the case of experiments with split-firefly luciferase sensors, full-length Renilla luciferase served as normalization control.) The cells were assayed for Fluc and Rluc assays as mentioned above after 24 h of induction with 1 μm of different ER ligands mentioned in the figures. Triplicates of each sample were performed in all experiments.
ERE-Luciferase-Based Gene Activation System with Full-Length Wild and Mutant ER Studied by Activating with Different ER Ligands
The 80% confluent 24-h-old cultures of 293T cells in 12-well culture plates were cotransfected with 200 ng each of ERE-Luciferase and full-length ER (either wild-type or mutants) along with 5 ng of Rluc plasmid for transfection normalization control per well. The cells were assayed for firefly and Renilla luciferases as mentioned above after 24 h of induction with 1 μm of ligands in the case of fixed concentrations or different concentrations in the case of titrations. Triplicates were performed for each samples, and each experiment was repeated at least three times.
Receptor Binding Assay by [3H]E2 Uptake
The 293T cells were plated in 12-well culture plate at a concentration of 2.5 × 103 cells per well. The cells were transfected with 500 ng/well of full-length wild-type or mutant ERs. After 24 h of incubation at 37 C with 5% CO2, the cells were used for [3H]E2 uptake. In brief, the cells were exposed to 1 μCi/ml of [3H]E2 for 3 h, washed three times with PBS, and lysed in 1 ml of 1% sodium dodecyl sulfate. The lysates were used for measuring the protein, and intracellular accumulated [3H]E2 was measured by scintillation counting. The results were represented as counts per min/total count/μg protein.
Competitive Dimerization by Full-Length Wild and Mutant ERs in a ERE-Luciferase-Based Gene Activation System
The 293T cells were plated 24 h before at a concentration of 2.5 × 103 cells per well in a 12-well culture plates used for transfection. The cells were cotransfected with 500 ng ERE-luciferase with 250 ng each of full-length wild-type and mutant ER (WT + G521S and WT + G521T) or mutant ER with another mutant ER (G521S + G521T). The cells were assayed for luciferase activity after 24 h of exposure to different concentrations of E2 (0–1 μm). The results were normalized with cotransfected Rluc activity and also by measuring the protein.
Full-Length Mutant ER (G521T) Coactivated by Ligand E2 with OHT in ERE-Luciferase-Based Gene Activation System
The 293T cells plated 24 h before in a concentration of 2.5 × 103 cells per well in a 12-well culture plates used for transfection. The cells were cotransfected with 500 ng ERE-luciferase with 500 ng of full-length mutant ER (G521T). The cells were assayed for luciferase activity after 24 h of exposure to different concentrations of ligand E2 and OHT (0–1 μm), separately, and also by an equimolar mixture of both. The results were normalized with cotransfected Rluc activity and also by measuring protein.
Data Analysis
Each experiment was repeated at least three times, and results were expressed as mean ± sd or sems. Statistical differences were determined by Student’s t test using P < 0.05 as cut-off point.
Supplementary Material
Acknowledgments
We thank John Comninos for the molecular modeling studies.
Footnotes
This work was supported by National Institutes of Health Grants NCI ICMIC P50 CA114747, NCI SAIRP R24 CA92865, and R01 CA82214 (to S.S.G.); R37 DK15556 (to J.A.K.); and R01 CA18119 (to B.S.K.).
Disclosure Statement: The authors have nothing to disclose.
First Published Online May 1, 2008
Abbreviations: DES, Diethylstilbestrol; DPN, diarylpropionitrile; E1, estrone; E2, 17β-estradiol; E3, estriol; EGF, epidermal growth factor; EGFR, EGF receptor; ER, estrogen receptor; ERE, estrogen response element; GEN, genistein; HEK, human embryonic kidney; IRESSA, Geftinib; LBD, ligand-binding domain; LBD-F, ligand binding domain with F domain; MPP, methyl-piperidino-pyrazole; OHT, hydroxytamoxifen; PPT, propyl-pyrazole-triol; RAL, raloxifen; SERM, selective ER modulator.
References
- Shao W, Brown M 2004 Advances in estrogen receptor biology: prospects for improvements in targeted breast cancer therapy. Breast Cancer Res 6:39–52 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holst F, Stahl PR, Ruiz C, Hellwinkel O, Jehan Z, Wendland M, Lebeau A, Terracciano L, Al-Kuraya K, Janicke F, Sauter G, Simon R 2007 Estrogen receptor α (ESR1) gene amplification is frequent in breast cancer. Nat Genet 39:655–660 [DOI] [PubMed] [Google Scholar]
- Carroll JS, Meyer CA, Song J, Li W, Geistlinger TR, Eeckhoute J, Brodsky AS, Keeton EK, Fertuck KC, Hall GF, Wang Q, Bekiranov S, Sementchenko V, Fox EA, Silver PA, Gingeras TR, Liu XS, Brown M 2006 Genome-wide analysis of estrogen receptor binding sites. Nat Genet 38:1289–1297 [DOI] [PubMed] [Google Scholar]
- Ho SM, Leung YK, Chung I 2006 Estrogens and antiestrogens as etiological factors and therapeutics for prostate cancer. Ann NY Acad Sci 1089:177–193 [DOI] [PubMed] [Google Scholar]
- Kousteni S, Bellido T, Plotkin LI, O'Brien CA, Bodenner DL, Han L, Han K, DiGregorio GB, Katzenellenbogen JA, Katzenellenbogen BS, Roberson PK, Weinstein RS, Jilka RL, Manolagas SC 2001 Nongenotropic, sex-nonspecific signaling through the estrogen or androgen receptors: dissociation from transcriptional activity. Cell 104:719–730 [PubMed] [Google Scholar]
- Brzozowski AM, Pike AC, Dauter Z, Hubbard RE, Bonn T, Engstrom O, Ohman L, Greene GL, Gustafsson JA, Carlquist M 1997 Molecular basis of agonism and antagonism in the oestrogen receptor. Nature 389:753–758 [DOI] [PubMed] [Google Scholar]
- Paulmurugan R, Gambhir SS 2006 An intramolecular folding sensor for imaging estrogen receptor-ligand interactions. Proc Natl Acad Sci USA 103:15883–15888 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harrington WR, Sheng S, Barnett DH, Petz LN, Katzenellenbogen JA, Katzenellenbogen BS 2003 Activities of estrogen receptor α- and β-selective ligands at diverse estrogen responsive gene sites mediating transactivation or transrepression. Mol Cell Endocrinol 206:13–22 [DOI] [PubMed] [Google Scholar]
- Kraichely DM, Sun J, Katzenellenbogen JA, Katzenellenbogen BS 2000 Conformational changes and coactivator recruitment by novel ligands for estrogen receptor-α and estrogen receptor-β: correlations with biological character and distinct differences among SRC coactivator family members. Endocrinology 141:3534–3545 [DOI] [PubMed] [Google Scholar]
- Shiau AK, Barstad D, Radek JT, Meyers MJ, Nettles KW, Katzenellenbogen BS, Katzenellenbogen JA, Agard DA, Greene GL 2002 Structural characterization of a subtype-selective ligand reveals a novel mode of estrogen receptor antagonism. Nat Struct Biol 9:359–364 [DOI] [PubMed] [Google Scholar]
- Sun J, Meyers MJ, Fink BE, Rajendran R, Katzenellenbogen JA, Katzenellenbogen BS 1999 Novel ligands that function as selective estrogens or antiestrogens for estrogen receptor-α or estrogen receptor-β. Endocrinology 140:800–804 [DOI] [PubMed] [Google Scholar]
- Tamrazi A, Carlson KE, Daniels JR, Hurth KM, Katzenellenbogen JA 2002 Estrogen receptor dimerization: ligand binding regulates dimer affinity and dimer dissociation rate. Mol Endocrinol 16:2706–2719 [DOI] [PubMed] [Google Scholar]
- Paulmurugan R, Gambhir SS 2007 Combinatorial library screening for developing an improved split-firefly luciferase fragment-assisted complementation system for studying protein-protein interactions. Anal Chem 79:2346–2353 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stauffer SR, Coletta CJ, Tedesco R, Nishiguchi G, Carlson K, Sun J, Katzenellenbogen BS, Katzenellenbogen JA 2000 Pyrazole ligands: structure-affinity/activity relationships and estrogen receptor-α-selective agonists. J Med Chem 43:4934–4947 [DOI] [PubMed] [Google Scholar]
- Stauffer SR, Huang Y, Coletta CJ, Tedesco R, Katzenellenbogen JA 2001 Estrogen pyrazoles: defining the pyrazole core structure and the orientation of substituents in the ligand binding pocket of the estrogen receptor. Bioorg Med Chem 9:141–150 [DOI] [PubMed] [Google Scholar]
- Stauffer SR, Huang YR, Aron ZD, Coletta CJ, Sun J, Katzenellenbogen BS, Katzenellenbogen JA 2001 Triarylpyrazoles with basic side chains: development of pyrazole-based estrogen receptor antagonists. Bioorg Med Chem 9:151–161 [DOI] [PubMed] [Google Scholar]
- Sun J, Huang YR, Harrington WR, Sheng S, Katzenellenbogen JA, Katzenellenbogen BS 2002 Antagonists selective for estrogen receptor α. Endocrinology 143:941–947 [DOI] [PubMed] [Google Scholar]
- Danielian PS, White R, Hoare SA, Fawell SE, Parker MG 1993 Identification of residues in the estrogen receptor that confer differential sensitivity to estrogen and hydroxytamoxifen. Mol Endocrinol 7:232–240 [DOI] [PubMed] [Google Scholar]
- Likhite VS, Stossi F, Kim K, Katzenellenbogen BS, Katzenellenbogen JA 2006 Kinase-specific phosphorylation of the estrogen receptor changes receptor interactions with ligand, deoxyribonucleic acid, and coregulators associated with alterations in estrogen and tamoxifen activity. Mol Endocrinol 20:3120–3132 [DOI] [PubMed] [Google Scholar]
- Littlewood TD, Hancock DC, Danielian PS, Parker MG, Evan GI 1995 A modified oestrogen receptor ligand-binding domain as an improved switch for the regulation of heterologous proteins. Nucleic Acids Res 23:1686–1690 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ekena K, Weis KE, Katzenellenbogen JA, Katzenellenbogen BS 1996 Identification of amino acids in the hormone binding domain of the human estrogen receptor important in estrogen binding. J Biol Chem 271:20053–20059 [DOI] [PubMed] [Google Scholar]
- Ekena K, Weis KE, Katzenellenbogen JA, Katzenellenbogen BS 1997 Different residues of the human estrogen receptor are involved in the recognition of structurally diverse estrogens and antiestrogens. J Biol Chem 272:5069–5075 [DOI] [PubMed] [Google Scholar]
- Logie C, Nichols M, Myles K, Funder JW, Stewart AF 1998 Positive and negative discrimination of estrogen receptor agonists and antagonists using site-specific DNA recombinase fusion proteins. Mol Endocrinol 12:1120–1132 [DOI] [PubMed] [Google Scholar]
- Osborne CK, Shou J, Massarweh S, Schiff R 2005 Crosstalk between estrogen receptor and growth factor receptor pathways as a cause for endocrine therapy resistance in breast cancer. Clin Cancer Res 11:865s–870s [PubMed] [Google Scholar]
- Schiff R, Osborne CK 2005 Endocrinology and hormone therapy in breast cancer: new insight into estrogen receptor-α function and its implication for endocrine therapy resistance in breast cancer. Breast Cancer Res 7:205–211 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katzenellenbogen JA, O'Malley BW, Katzenellenbogen BS 1996 Tripartite steroid hormone receptor pharmacology: interaction with multiple effector sites as a basis for the cell- and promoter-specific action of these hormones. Mol Endocrinol 10:119–131 [DOI] [PubMed] [Google Scholar]
- Horard B, Castet A, Bardet PL, Laudet V, Cavailles V, Vanacker JM 2004 Dimerization is required for transactivation by estrogen-receptor-related (ERR) orphan receptors: evidence from amphioxus ERR. J Mol Endocrinol 33:493–509 [DOI] [PubMed] [Google Scholar]
- Wang H, Peters GA, Zeng X, Tang M, Ip W, Khan SA 1995 Yeast two-hybrid system demonstrates that estrogen receptor dimerization is ligand-dependent in vivo. J Biol Chem 270:23322–23329 [DOI] [PubMed] [Google Scholar]
- Kim SH, Tamrazi A, Carlson KE, Katzenellenbogen JA 2005 A proteomic microarray approach for exploring ligand-initiated nuclear hormone receptor pharmacology, receptor selectivity, and heterodimer functionality. Mol Cell Proteomics 4:267–277 [DOI] [PubMed] [Google Scholar]
- Ince BA, Zhuang Y, Wrenn CK, Shapiro DJ, Katzenellenbogen BS 1993 Powerful dominant negative mutants of the human estrogen receptor. J Biol Chem 268:14026–14032 [PubMed] [Google Scholar]
- Schodin DJ, Zhuang Y, Shapiro DJ, Katzenellenbogen BS 1995 Analysis of mechanisms that determine dominant negative estrogen receptor effectiveness. J Biol Chem 270:31163–31171 [DOI] [PubMed] [Google Scholar]
- de Haan G, Chusacultanachai S, Mao C, Katzenellenbogen BS, Shapiro DJ 2000 Estrogen receptor-KRAB chimeras are potent ligand-dependent repressors of estrogen-regulated gene expression. J Biol Chem 275:13493–13501 [DOI] [PubMed] [Google Scholar]
- Paulmurugan R, Umezawa Y, Gambhir SS 2002 Noninvasive imaging of protein-protein interactions in living subjects by using reporter protein complementation and reconstitution strategies. Proc Natl Acad Sci USA 99:15608–15613 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paulmurugan R, Massoud TF, Huang J, Gambhir SS 2004 Molecular imaging of drug-modulated protein-protein interactions in living subjects. Cancer Res 64:2113–2119 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paulmurugan R, Gambhir SS 2003 Monitoring protein-protein interactions using split synthetic renilla luciferase protein-fragment-assisted complementation. Anal Chem 75:1584–1589 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.