Abstract
The actions of LH to induce ovulation and luteinization of preovulatory follicles are mediated principally by activation of cAMP-dependent protein kinase (PKA) in granulosa cells. PKA activity is targeted to specific locations in many cells by A kinase-anchoring proteins (AKAPs). We previously showed that FSH induces expression of microtubule-associated protein (MAP) 2D, an 80-kDa AKAP, in rat granulosa cells, and that MAP2D coimmunoprecipitates with PKA-regulatory subunits in these cells. Here we report a rapid and targeted dephosphorylation of MAP2D at Thr256/Thr259 after treatment with human chorionic gonadotropin, an LH receptor agonist. This event is mimicked by treatment with forskolin or a cAMP analog and is blocked by the PKA inhibitor myristoylated-PKI, indicating a role for cAMP and PKA signaling in phosphoregulation of granulosa cell MAP2D. Furthermore, we show that Thr256/Thr259 dephosphorylation is blocked by the protein phosphatase 2A (PP2A) inhibitor, okadaic acid, and demonstrate interactions between MAP2D and PP2A by coimmunoprecipitation and microcystin-agarose pull-down. We also show that MAP2D interacts with glycogen synthase kinase (GSK) 3β and is phosphorylated at Thr256/Thr259 by this kinase in the basal state. Increased phosphorylation of GSK3β at Ser9 and the PP2A B56δ subunit at Ser566 is observed after treatment with human chorionic gonadotropin and appears to result in LH receptor-mediated inhibition of GSK3β and activation of PP2A, respectively. Taken together, these results show that the phosphorylation status of the AKAP MAP2D is acutely regulated by LH receptor-mediated modulation of kinase and phosphatase activities via PKA.
GRANULOSA CELLS of mature preovulatory (PO) ovarian follicles respond to a midcycle surge of pituitary LH by altering gene expression to promote luteinization and ovulation. These responses are initiated through binding of LH to surface LH receptors. The LH receptor is a seven-transmembrane protein that couples to the stimulatory guanine nucleotide-binding protein Gs and signals to adenylyl cyclase (1). Activation of adenylyl cyclase rapidly increases the local concentration of the second-messenger cAMP resulting in activation of protein kinase A (PKA) (2). cAMP and PKA are largely responsible for early events of LH signaling that drive granulosa cell differentiation and steroid synthesis and culminate in ovulation of the oocyte (3,4,5,6,7).
PKA is a tetrameric holoenzyme consisting of a dimeric regulatory (R) subunit and two catalytic (C) subunits. Binding of cAMP to the R subunits causes the release of the active C subunits and allows phosphorylation of PKA substrates. PKA is often targeted to specific cellular locations by A kinase-anchoring proteins (AKAPs), a family of proteins functionally identified by their binding affinity for PKA R subunits (reviewed in Refs. 8 and 9). Cellular localization of AKAPs and associated PKA holoenzyme is achieved by interaction between an AKAP targeting domain and a cellular organelle or structure, such as a component of the cytoskeleton. Targeting of PKA activity by AKAPs increases the specificity of PKA action by controlling its access to various substrates. However, AKAPs also bind to a variety of signaling molecules, including other kinases, phosphatases, phosphodiesterases, and PKA substrates (8,9). Thus, the scaffolding function of AKAPs may allow for spatial regulation of numerous cellular signaling events.
We previously showed that FSH induces the expression of an 80-kDa AKAP in granulosa cells as they mature to a PO phenotype (10). We identified this AKAP as microtubule-associated protein (MAP)2D, a low-molecular weight splice variant of the MAP2 family of proteins (11). MAP2 family members are well characterized as neuronal MAPs and AKAPs and are expressed as splice-variants from a single gene (12,13,14). They contain an N-terminal R subunit-binding domain that allows for binding to PKA (15,16). MAP2 isoforms also bind to numerous cytoskeletal elements including microtubules, via three or four C-terminal microtubule-binding domains (MTBDs); microfilaments, also via MTBDs; and, perhaps, intermediate filaments (reviewed in Ref. 17) (18,19,20,21,22,23).
The high-molecular weight MAP2A and MAP2B isoforms appear to be highly phosphorylated at serine/threonine residues in vivo, and at least 15 of the 46 phosphorylatable residues of MAP2A/2B are conserved in the low-molecular weight MAP2D isoform (reviewed in Ref. 17). Two residues, Thr256/Thr259 (equivalent to Thr1620/Thr1623 in MAP2A/2B), are present in a proline-rich domain that lies immediately N terminal to the first MTBD. These residues are phosphoregulated in vitro by a variety of proline-directed kinases and by protein phosphatase 1 (PP1) and/or protein phophatase 2A (PP2A) (24). Similar regulation has been observed in cultured neurons (25) and rat brain tissue (26). Furthermore, phosphorylation of these residues was observed in COS-1 cells overexpressing both GSK3 and the highly homologous MAP2C isoform (lacking only the fourth MTBD present in MAP2D) and resulted in regulation of microtubule binding affinity and microtubule polymerization dynamics (27).
Recently we showed that MAP2D in granulosa cells is phosphorylated at Thr256/Thr259 coincident with its FSH-induced expression (11). Based on the physiological expression of this predominately neuronal protein in the ovary at a time when the organ is exposed to the midcycle surge of LH, we sought to investigate the phosphorylation state of MAP2D in PO ovarian granulosa cells, to identify the kinase(s) that promote phosphorylation at Thr256/Thr259, and to determine the effects of LH on the phosphorylation state at these sites. Our results show that cAMP/PKA-dependent LH receptor signaling promotes a rapid, transient decrease in phosphorylation of MAP2D at Thr256/Thr259. This dephosphorylation of MAP2D at Thr256/Thr259 appears to be mediated by a simultaneous activation of PP2A activity, which we identify as a MAP2D-binding partner, and inhibition of glycogen synthase kinase 3 (GSK3), another MAP2D binding partner and the predominant Thr256/Thr259 kinase in granulosa cells.
RESULTS
Ovarian Granulosa Cell MAP2D Exists in a Phosphorylated State and Is Susceptible to an Endogenous Phosphatase Activity
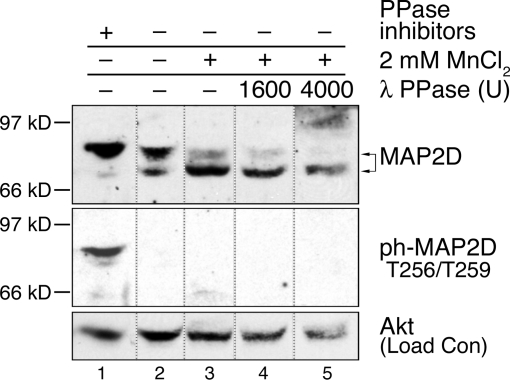
Based on previous reports that phosphorylation of MAP2 family proteins at multiple sites may result in changes in electrophoretic mobility (12,28,29,30), the electrophoretic mobility of MAP2D in rat PO granulosa cell lysates was analyzed by Western blot. MAP2D protein migrated through polyacrylamide gel as at least two distinct bands (Fig. 1). When cell extracts were incubated (30 min at 30 C) in buffer containing standard phosphatase inhibitors, the majority (89%) of MAP2D protein was detected as a slower migrating band at approximately 80 kDa (Fig. 1, lane 1). The remaining MAP2D protein (11%) was detected as a faster migrating band at approximately 70 kDa.
Figure 1.
Ovarian Granulosa Cell MAP2D Exists in a Phosphorylated State and Is Susceptible to an Endogenous Phosphatase Activity
Rat PO granulosa cells were collected and lysed by sonication in buffer with the phosphatase inhibitors EDTA, sodium orthovanadate, sodium fluoride, and sodium pyrophosphate (lane 1; Complete Buffer A), buffer without phosphatase inhibitors (lane 2; Minimal Buffer A), or buffer with MnCl2 (lanes 3–5; MnCl2 Phosphatase Reaction Buffer). For further details, see Materials and Methods. Clarified lysates were incubated at 30 C for 30 min after addition of no phosphatase (lanes 1–3) or 1600 U (lane 4) or 4000 U (lane 5) of λ protein phosphatase (PPase). Arrows indicate faster (∼70 kDa) and slower (∼80 kDa) migrating MAP2D bands. Position of Mr standards are indicated. Western blots of lysates were probed with the indicated antibodies. Total Akt levels were analyzed by Western blotting and used as a loading control (Load Con). Lines between lanes indicate cropped images. Results are representative of three separate experiments.
To verify that the presence of two bands resulted from distinct phosphorylation states of MAP2D, lysates were incubated (30 min at 30 C) in buffers with variable permissiveness toward phosphatase activity or in the presence of exogenous phosphatase activity. When phosphatase inhibitors were removed from the incubating buffer, 37% of MAP2D was now detected as the faster migrating 70-kDa band (Fig. 1, lane 2). The addition of 2 mm MnCl2, which can activate phosphatases including PP2A under certain conditions (31,32), further increased the proportion of MAP2D protein in the faster band to 70% (lane 3). Finally, the addition of λ-phosphatase increased the proportion of MAP2D protein in the faster 70-kDa band to up to 78% (lanes 4 and 5). These results indicate that fully dephosphorylated MAP2D migrates at approximately 70 kDa and that MAP2D phosphorylated on one or more sites migrates about 80 kDa. These results also confirm that the majority of MAP2D in freshly isolated ovarian granulosa cells exists in a phosphorylated state (Fig. 1, lane 1).
We also evaluated the migration position of MAP2D phosphorylated on Thr256/Thr259 in the proline-rich domain using a phospho-specific antibody developed against a synthetic peptide containing these sites (sequence RpT256PGpT259PGTPSY) (Cell Signaling Technology, Beverly, MA).a MAP2D phosphorylated at Thr256/Thr259 was only detected at the size comparable to the slower migrating MAP2D band (80 kDa, lane 1) and was only detected in the presence of phosphatase inhibitors. Because 63% of total MAP2D continued to migrate with the 80-kDa band (lane 2) even with dephosphorylation of Thr256/Thr259 sites, we can also conclude that one or more sites in addition to Thr256/Thr259 must be phosphorylated on MAP2D and the phosphorylation of these additional sites must be responsible for the migration of MAP2D at 80 kDa.
Phosphorylation of MAP2D at Thr256/Thr259 Is Rapidly Decreased upon LH Receptor Stimulation
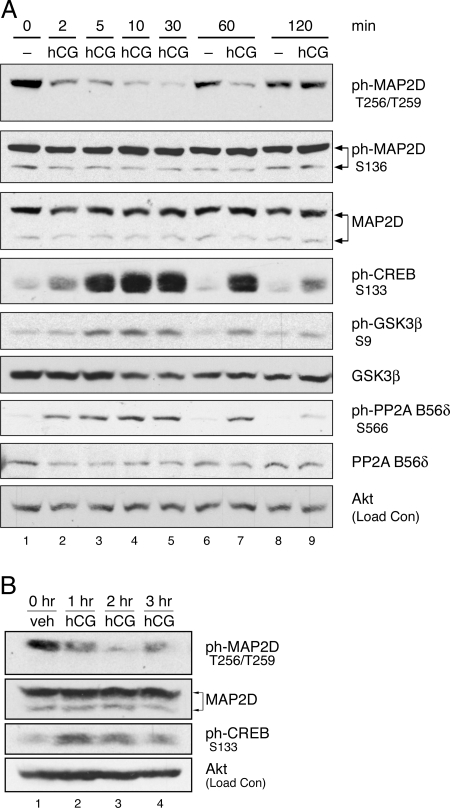
Based on the ability of LH receptor signaling to modulate many critical functions in PO granulosa cells (3,4,5,6,7), we analyzed the phosphoregulation of MAP2D Thr256/Thr259 in PO granulosa cells isolated from pregnant mare serum gonadotropin (PMSG)-primed rats upon activation of the LH receptor by the LH receptor agonist hCG. Cultured cells were treated with or without hCG for various times, and Western blots of total cell lysates were probed with phospho-specific antibody. MAP2D phosphorylation at Thr256/Thr259 was readily detected in untreated cells (Fig. 2A, lanes 1, 6, and 8). Treatment with hCG (lanes 2–5 and 7) caused a rapid decrease in Thr256/Thr259 phosphorylation, with a significant decrease to 18 ± 3% (n = 28; P < 0.01) of untreated control by 10 min. The timing of this decrease in phosphorylation occurred as early as 2 min and lasted as long as 1 h, corresponding with activation of PKA as demonstrated by increased phosphorylation of cAMP response element-binding protein (CREB) at Ser133, a direct PKA target in granulosa cells (3,5). Changes in phosphorylation of MAP2D at a different site, Ser136, were not observed after hCG treatment (Fig. 2A). We also probed blots with 12E8 (provided by Dr. P. Seubert, Elan Pharmaceuticals, South San Francisco, CA), an antibody that recognizes phosphoserine in the KXGS motifs present in each MTBD (33,34,35). The serine residue in the KXGS motif can be phosphorylated by PKA (21). Whereas phosphorylation of recombinant MAP2D with recombinant PKA yielded a readily detectable signal using 12E8, no signal was detected in granulosa cells treated with hCG (data not shown). These results suggest that LH receptor signaling is not promoting MAP2D phosphorylation at these recognized PKA sites on MAP2D.b Taken together, these results indicate that MAP2D undergoes a rapid and specific decrease in Thr256/Thr259 phosphorylation upon activation of LH receptor signaling.
Figure 2.
Phosphorylation of MAP2D at Thr256/Thr259 Is Rapidly Decreased upon LH Receptor Stimulation
In panel A, PO granulosa cells from PMSG-primed rats were isolated and plated overnight on fibronectin. Cells were then left untreated (−) or treated with 1 IU/ml hCG for the indicated times. Western blot results are representative of three separate experiments. In panel B, PMSG-primed rats were injected with 50 IU hCG or saline (veh) for the indicated times before ovaries were harvested and whole-ovary extracts were prepared in homogenization buffer, as described in Materials and Methods. Clarified homogenates were used for Western blot. Results are representative of two separate experiments. ph-, Phosphorylated.
As described above, MAP2D phosphorylated on Thr256/Thr259 migrates at approximately 80 kDa (Fig. 1). We occasionally detected a modest shift in the migration position of total MAP2D correlating with the dephosphorylation at Thr256/Thr259 sites manifested as a tightening of the 80-kDa MAP2D band after hCG treatment (for example, see Fig. 4A and Fig. 6, A–C). However, the overall migration position of MAP2D does not appear to be affected by phosphoregulation of Thr256/Thr259 sites because LH receptor-stimulated dephosphorylation of Thr256/Thr259 does not alter the distribution of total MAP2D between the 70- and 80-kDa bands (Fig. 2A). Moreover, the presence of signal for phosphorylated Ser136 in both the 70- and 80-kDa bands indicates that phosphorylation at this site also does not affect the migration position of MAP2D on SDS-PAGE and is thus not responsible for the molecular mass shift of this protein. The 70-kDa band thus represents an apparent hypophosphorylated MAP2D, whereas the 80-kDa band represents a hyperphosphorylated protein.
Figure 4.
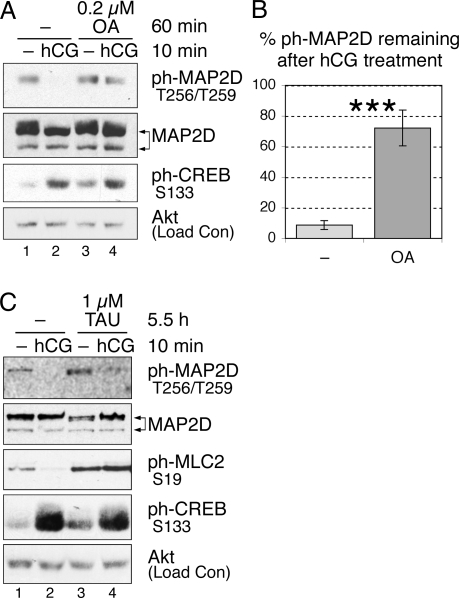
PP2A Preferential Treatment with the Ser/Thr Phosphatase Inhibitor, Okadaic Acid, Blocks the hCG-Induced Decrease in MAP2D Phosphorylation at Thr256/Thr259
A, PO granulosa cells were left untreated (−) or pretreated with 0.2 μm okadaic acid (OA) for 60 min and then left untreated (−) or treated with 1 IU/ml hCG for 10 min, as indicated. Western blot results are representative of four separate experiments. B, Thr256/Thr259 phosphorylated MAP2D levels were quantified by densitometric analysis of Western blot results, as stated in Materials and Methods, and normalized to total MAP2D protein levels. Values are for hCG-treated samples expressed as the percentage of the corresponding untreated (−) controls (100%), with no inhibitor pretreatment (−) or 60 min OA pretreatment, as indicated. Values are the mean ± se from four separate experiments. ***, P < 0.01. In C, PO granulosa cells were left untreated (−) or pretreated with 1 μm tautomycin (TAU) for 5.5 h and then left untreated (−) or treated with 1 IU/ml hCG for 10 min, as indicated. ph-, Phosphorylated.
Figure 6.
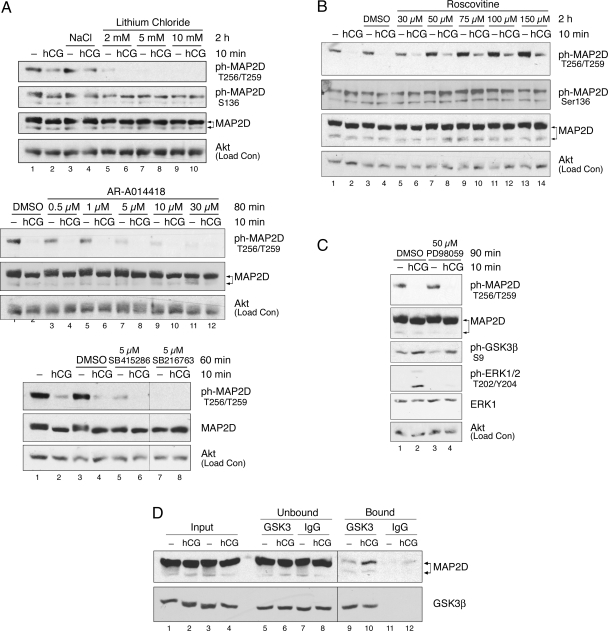
GSK3 Inhibitors Block Basal State Phosphorylation of MAP2D at Thr256/Thr259
A, PO granulosa cells were pretreated with indicated concentrations of the GSK3 inhibitors lithium chloride, AR-A014418, SB415286, SB216763, or control (50 mm NaCl or dimethylsulfoxide) for the indicated times and then left untreated (−) or treated with 1 IU/ml hCG for 10 min. Results of lithium chloride and AR-A014418 pretreatments are representative of at least three separate experiments. B, PO granulosa cells were pretreated with the indicated concentrations of the CDK inhibitor roscovitine or vehicle control (dimethylsulfoxide) for 2 h and then left untreated (−) or treated with 1 IU/ml hCG for 10 min. Results are representative of two separate experiments. C, PO granulosa cells were pretreated with 50 μm of the MEK inhibitor PD98059 or vehicle control (dimethylsulfoxide) for 90 min and then left untreated (−) or treated with 1 IU/ml hCG for 10 min. Western blot results are representative of three separate experiments. D, Detergent-soluble PO granulosa cell extracts from cells left untreated or treated with 1 IU/ml hCG for 10 min were subjected to coimmunoprecipitation, as described in Materials and Methods, with agarose-conjugated GSK3α/β mAb (Santa Cruz) or control agarose-conjugated mouse IgG (Santa Cruz). Input, unbound, and bound/immunoprecipitated proteins were probed by Western blot with the indicated antibodies. To avoid overexposure, input and unbound images shown for the MAP2 Western are from shorter exposures than bound/immunoprecipitated images. Immunoprecipitation results are representative of three separate experiments. Input and unbound fractions reflect 6.25% of total lysate and bound reflects 100% of total lysate. DMSO, Dimethylsulfoxide; ph-, phosphorylated.
To verify that the decrease in MAP2D Thr256/Thr259 phosphorylation is a physiological response to hormone in vivo, PMSG-primed rats were treated with 50 IU hCG by ip injection for various times before preparation of whole ovarian extracts. Results show that phosphorylation of MAP2D at Thr256/Thr259 is readily detected in ovarian extracts from saline-injected rats (Fig. 2B, lane 1) and decreased to approximately 20% of vehicle control by 2 h after hCG injection (compare lanes 1 and 3). The in vivo timing of MAP2D dephosphorylation corresponded with activation of PKA as demonstrated by increased phosphorylation of CREB at Ser133. These results confirm that a decrease in MAP2D phosphorylation at Thr256/Thr259 occurs in vivo as well as in cultured cells in response to activation of LH receptor signaling. These results also show that the preponderance of MAP2D in the hyperphosphorylated state in cultured granulosa cells, as seen in Fig. 2A in total MAP2D blot, is duplicated in these in vivo results.
LH-Dependent Decrease in MAP2D Phosphorylation at Thr256/Thr259 Occurs via cAMP and PKA Signaling
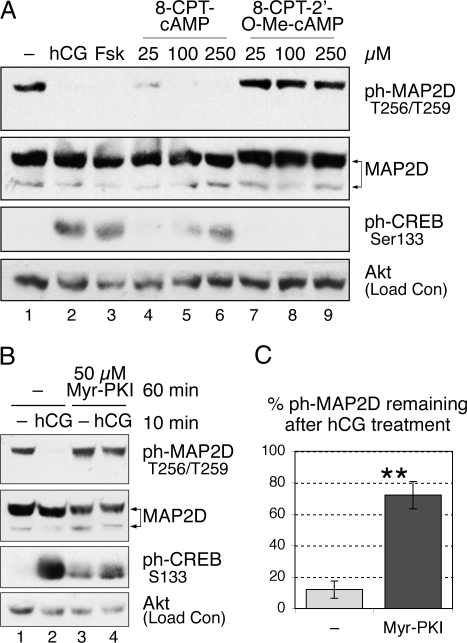
Because cAMP is recognized as the predominant second messenger downstream of LH receptor signaling in granulosa cells, we determined the role of cAMP in the LH-dependent decrease in MAP2D phosphorylation at Thr256/Thr259 using the direct pharmacological adenylyl cyclase activator, forskolin, and the cell-permeable cAMP analog, 8-(4-chlorophenylthio) cAMP (8-CPT-cAMP). Treatment of granulosa cells for 10 min with forskolin or 8-CPT-cAMP activated PKA signaling, as evidenced by increased phosphorylation of CREB at Ser133 (Fig. 3A, compare lane 1 vs. lanes 3 and 6, respectively). Similarly forskolin (Fig. 3A, lane 3) or 8-CPT-cAMP (lanes 4–6) mimicked the effect of hCG and induced decreases in phosphorylation of MAP2D at Thr256/Thr259 to undetectable levels, compared with untreated cells (lane 1). These results indicate that increased cAMP signaling is sufficient to cause a decrease in MAP2D phosphorylation at Thr256/Thr259.
Figure 3.
cAMP/PKA-Dependent Signaling Participates in the LH Receptor-Mediated Decrease in Phosphorylation of MAP2D Thr256/Thr259
A, PO granulosa cells were left untreated (−) or treated with 1 IU/ml hCG, 10 μm forskolin (Fsk), or the indicated concentrations of 8-CPT-cAMP or 8-pCPT-2′-O-Me-cAMP for 10 min, as indicated. B, PO granulosa cells were left untreated (−) or pretreated with 50 μm Myr-PKI for 60 min and then left untreated (−) or treated with 1 IU/ml hCG for 10 min, as indicated. Western blot results are representative of four separate experiments. C, Thr256/Thr259-phosphorylated MAP2D levels were quantified by densitometric analysis of Western blot results, as stated in Materials and Methods, and normalized to total MAP2D protein levels. Values for hCG-treated samples are expressed as the percentage of the corresponding untreated (−) controls (100%), with no inhibitor pretreatment (−) or 60 min Myr-PKI pretreatment, as indicated. Values are the mean ± se from four separate experiments. **, P < 0.05. ph-, Phosphorylated.
Increased production of cAMP leads to activation of a number of cAMP-dependent signaling pathways. Most commonly, cAMP activates PKA. In some cells, cAMP has also been shown to activate signaling independently of PKA by binding to the Rap1 guanine nucleotide exchange factor, Epac (exchange protein activated by cAMP) (36,37). To investigate whether the LH-dependent decrease in MAP2D phosphorylation at Thr256/Thr259 is PKA dependent, granulosa cells were treated for 10 min with 8-(4-chlorophenylthio)-2′-O-methyl cAMP (8-pCPT-2′-O-Me-cAMP), a cell-permeable cAMP analog capable of activating Epac but not PKA (38). As shown in Fig. 3A, no decrease in phosphorylation of MAP2D at Thr256/Thr259 was observed in 8-pCPT-2′-O-Me-cAMP-treated cells compared with untreated cells (lanes 7–9 vs. lane 1). These results suggest that cAMP activation of PKA, not Epac, is necessary for dephosphorylation of MAP2D at Thr256/Thr259.
To confirm the role for PKA, granulosa cells were pretreated for 1 h with the heat-stable, cell-permeable PKA inhibitor Myr-PKI (myristoylated PKA inhibitor) before treatment for 10 min with or without hCG. Myr-PKI pretreatment inhibited PKA activation under hCG treatment, as evidenced by decreased phosphorylation of CREB at Ser133 (Fig. 3B, compare lanes 1 and 2 vs. lanes 3 and 4). PKA inhibition by Myr-PKI significantly prevented the LH-dependent decrease in MAP2D phosphorylation at Thr256/Thr259 [Fig. 3C; 12 ± 5.5% of phosphorylated MAP2D (ph-MAP2D) remains after hCG treatment with no inhibitor present vs. 72 ± 8.6% of ph-MAP2D in the presence of Myr-PKI; n = 4, P < 0.05]. These results indicate that PKA activation is necessary for the observed decrease in MAP2D Thr256/Thr259 phosphorylation.
LH-Dependent Decrease in MAP2D Phosphorylation at Thr256/Thr259 Is Blocked by Pretreatment with a PP2A-Selective Concentration of the Ser/Thr-Phosphatase Inhibitor Okadaic Acid
Both PP2A and PP1 are capable of dephosphorylating synthetic MAP2A/2B peptides at Thr1620/Thr1623 (equivalent to Thr256/Thr259 in MAP2D) in in vitro reactions (24), and a Ser/Thr phosphatase appears to be involved in phosphoregulation of this site in cultured neurons (25) and rat brain tissue (26). In the following experiments, the involvement of PP2A and/or PP1 in the LH receptor-mediated phosphoregulation of MAP2D at Thr256/Thr259 was evaluated. At appropriate doses, okadaic acid and tautomycin have been reported to be preferential inhibitors of PP2A and PP1, respectively (39,40,41). Granulosa cells were pretreated with a PP2A-preferential dose (0.2 μm) of okadaic acid before treatment with or without hCG. Western blot analysis of cell lysates showed that okadaic acid pretreatment reduced the LH-dependent decrease in MAP2D phosphorylation at Thr256/Thr259 (Fig. 4A, compare lanes 1 and 2 vs. lanes 3 and 4). This reduction was found to be significant (Fig. 4B; 8.7 ± 2.8% of ph-MAP2D remains after hCG treatment with no inhibitor vs. 72 ± 12% in the presence of okadaic acid; n = 4; P < 0.01).
Pretreatment of granulosa cells with a PP1-preferential dose (1 μm) of tautomycin did not abolish the LH-dependent decrease in MAP2D phosphorylation at Thr256/Thr259 (Fig. 4C; 5% of ph-MAP2D remains after hCG treatment with no inhibitor vs. 29% in the presence of tautomycin). Tautomycin, however, fully blocked the hCG-stimulated dephosphorylation of myosin light chain 2 at Ser19, a known target of PP1 (42). These results suggest that the decrease in phosphorylation of MAP2D Thr256/Thr259 after LH receptor activation is mediated, at least in part, by PP2A activity but not by PP1 activity.
Coimmunoprecipitation and Affinity-Pull-Down Analyses from PO Granulosa Cells Reveal Interactions between MAP2D and PP2A, but not PP1 Catalytic Subunits
Based on our indirect evidence for the involvement of PP2A in MAP2D phosphoregulation, we determined whether there is an interaction between MAP2D and PP2A in ovarian granulosa cells. Affinity-pull-down analysis was performed from clarified granulosa cell lysates using microcystin-agarose, an affinity reagent that binds both PP2A and PP1. As expected, microcystin-agarose precipitated both PP2A and PP1 (Fig. 5, lane 8). The microcystin-agarose pull-down assay also isolated MAP2D from granulosa cell lysates, suggesting that MAP2D forms interactions with one or both of these Ser/Thr phosphatases. Interestingly, both the 70- and 80-kDa forms of MAP2D were pulled down by microcystin-agarose conjugate, suggesting that both hyperphosphorylated (80 kDa) and hypophosphorylated (70 kDa) forms of MAP2D bind to PP2A and/or PP1. However, our results suggest that microcystin-agarose stably binds a relatively small pool of MAP2D as well as PP1 and PP2A, based on the absence of detectable depletion in unbound fractions (Fig. 5, compare lanes 4 and 5). Immunoprecipitation analyses were also performed using monoclonal antibodies specifically recognizing either PP2A catalytic subunit (PP2A-c), MAP2 protein, or an irrelevant epitope [antihemagglutinin (HA)-Tag monoclonal antibody (mAb)] as a control. Immunoprecipitation of PP2A-c coimmunoprecipitated MAP2D (Fig. 5, lane 7). As with microcystin-agarose, both the 70- and 80-kDa forms of MAP2D were immunoprecipitated, although the hypophosphorylated 70-kDa protein predominated. Immunoprecipitation with the PP2A-c antibody resulted in marked depletion of PP2A-c (Fig. 5, lane 3) but not of MAP2D, suggesting that PP2A-c stably binds a relatively small pool of MAP2D. Conversely, MAP2D immunoprecipitation coimmunoprecipitated PP2A-c but did not coimmunoprecipitate PP1 catalytic subunit (lane 6). Taken together, these results indicate that MAP2D forms a complex in granulosa cells with PP2A but not with PP1. These results also suggest that PP2A complexes with a relatively small pool of MAP2D seemingly independent at least of Thr256/Thr259 MAP2D phosphorylation sites.
Figure 5.
Coimmunoprecipitation and Affinity-Pull-Down Analyses Reveal Interactions between MAP2D and PP2A, But Not PP1Catalytic Subunits
Detergent-soluble PO granulosa cell extracts were subjected to coimmunoprecipitation (IP) and affinity-pull-down analyses as described in Materials and Methods. IPs were performed with MAP2 (HM-2) mAb (Sigma), PP2A-c (1D6) mAb (Upstate Biotechnology/Millipore), or control (Con) antibody against an irrelevant epitope (HA-Tag mAb, Cell Signaling Technology). Pull-down analysis was performed with microcystin-agarose (MC, Upstate Biotechnology/Millipore), a PP2A/PP1 affinity-pull-down reagent. Input, unbound, and bound immunoprecipitated proteins were probed by Western blot with the indicated rabbit polyclonal antibodies. Input reflects 5% of total lysate, unbound reflects 13.3% of total unbound fraction, and bound reflects 100% of immunoprecipitated/pulled-down protein. Immunoprecipitation results are representative of three separate experiments.
LH Receptor Signaling Modulates Phosphorylation of MAP2D at Thr256/Thr259 through Regulation of Both GSK3β and PP2A
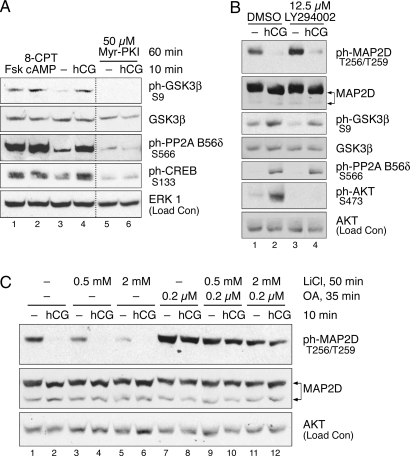
LH receptor-regulated dephosphorylation of MAP2D at Thr256/Thr259 could be mediated not only by enhanced PP2A activity toward MAP2D but also by reduced activity of the kinase that phosphorylates MAP2D Thr256/Thr259. We initially investigated the kinase(s) responsible for basal phosphorylation of MAP2D Thr256/Thr259 in granulosa cells. In vitro phosphorylation of high-molecular weight MAP2A/2B by proline-directed kinases, such as GSK3, cyclin-dependent kinases (CDKs), and MAPK superfamily members, has been demonstrated (24). PO granulosa cells were pretreated with a panel of GSK3 inhibitors, the CDK inhibitor roscovitine, or the MAPK ERK pathway inhibitor PD98059, at a variety of doses before 10 min treatment with or without hCG. Western blot analysis of cell lysates indicated that inhibition of GSK3 activity by a 2-h pretreatment with lithium chloride reduced basal state phosphorylation of MAP2D Thr256/Thr259 in the absence of hCG to undetectable levels (Fig. 6A, top panel, compare odd-numbered lanes). Basal state phosphorylation of Ser136, a recognized phosphorylation site on MAP2A/2B/2C for proline-directed kinases (43), was not affected by the 2-h pretreatment with lithium chloride (Fig. 6A, top panel, odd-numbered lanes). Basal state phosphorylation at Thr256/Thr259 on MAP2D was similarly lost upon pretreatment of granulosa cells with the GSK3 inhibitors AR-A014418, SB415286, and SB216763 (Fig. 6A, middle and bottom panels). Inhibition of roscovitine-sensitive CDK activity by a 2-h roscovitine pretreatment not only failed to reduce MAP2D phosphorylation but also appeared to enhance phosphorylation at this site (Fig. 6B, compare odd-numbered lanes). Basal state phosphorylation of MAP2D at Ser136 was also not reduced by roscovitine pretreatment. Inhibition of ERK pathway activity by the MAPK kinase (MEK) inhibitor PD98059 reduced ERK activation (Fig. 6C, compare lanes 2 and 4) but failed to reduce MAP2D Thr256/Thr259 phosphorylation (compare lanes 1 and 3). Taken together, these results suggest that GSK3 is necessary for basal phosphorylation of MAP2D at Thr256/Thr259. Given that GSK3 inhibition reduced MAP2D phosphorylation at Thr256/Thr259 to undetectable levels, GSK3 appears to be responsible for most of the phosphorylation at these sites in granulosa cells. Consistent with these results, MAP2D coimmunoprecipitated with GSK3β from granulosa cells left untreated or treated with hCG (Fig. 6D, lanes 9 and 10) but was not pulled down with control IgG (lanes 11 and 12). The apparent increased association of GSK3β and MAP2D in hCG-treated cells was unexpected and is not understood.
GSK3β is recognized to be basally active in most cells and to be inhibited by phosphorylation at Ser9 by a number of kinases, including Akt, p90RSK, and PKA (reviewed in Ref. 44). Because the dephosphorylation of Thr256/Thr259 is both rapid and PKA dependent, we determined whether LH receptor activation led to phosphorylation of GSK3β at Ser9 in granulosa cells. GSK3β phosphorylation at Ser9 was detected at low levels in untreated cells (Fig. 2A, lanes 1, 6, and 8). Treatment with hCG (lanes 2–5 and 7) caused a rapid increase in Ser9 phosphorylation, comparable in timing to the dephosphorylation of MAP2D Thr256/Thr259. The timing of this increased phosphorylation also corresponded with activation of PKA, as demonstrated by increased phosphorylation of CREB at Ser133. Indeed, it was found that cAMP/PKA signaling regulates GSK3β phosphorylation, because treatment with forskolin or 8-CPT-cAMP was sufficient to induce Ser9 phosphorylation of GSK3β (Fig. 7A) and pretreatment with the PKA inhibitor Myr-PKI blocked the hCG-induced increase in Ser9 phosphorylation (Fig. 7A, compare lanes 4 and 6). We next determined whether the effect of PKA on phosphorylation of GSK3β Ser9 is direct or is mediated by signaling through Akt or p90RSK. LH receptor activation by hCG induced Akt phosphorylation at Ser473 (Fig. 7B, compare lanes 1 and 2), and this activating phosphorylation was abrogated by pretreatment with the phosphatidylinositol (PI)-3 kinase inhibitor LY294002 (lanes 3 and 4). However, PI-3 kinase inhibition did not block phosphorylation of GSK3β at Ser9. Ser9 phosphorylation of GSK3β was also unaffected by the MEK inhibitor PD98059 (Fig. 6C). These results suggest that GSK3β is rapidly inhibited upon activation of LH receptor signaling by PKA-catalyzed phosphorylation of Ser9 independent of PI-3 kinase/Akt signaling or MEK/ERK/p90RSK signaling.
Figure 7.
Modulation of MAP2D at Thr256/Thr259 through LH-Mediated Regulation of Both GSK3β and PP2A
A, PO granulosa cells were left untreated or pretreated with 50 μm myristoylated-PKI (Myr-PKI) for 60 min and then left untreated (−) or treated with 10 μm forskolin (Fsk), 500 μm of 8-CPT-cAMP, or 1 IU/ml hCG for 10 min, as indicated. Western blot results are representative of two separate experiments. Lines between lanes indicate cropped images. B, PO granulosa cells were pretreated with 12.5 μm of the PI-3 kinase inhibitor LY294002 in dimethylsulfoxide or an equivalent concentration of dimethylsulfoxide for 60 min and then left untreated (−) or treated with 1 IU/ml hCG for 10 min, as indicated. Western blot results are representative of three separate experiments. C, PO granulosa cells were first left untreated (−) or pretreated with the indicated concentrations of the GSK3 inhibitor lithium chloride (LiCl) for 50 min; cells were then left untreated (−) or pretreated with the PP2A inhibitor, okadaic acid (OA), for an additional 35 min; cells were then left untreated (−) or treated with 1 IU/ml hCG for 10 min. Similar results were obtained using okadaic acid combined with the GSK3 inhibitor AR-A014418 (not shown). DMSO, Dimethylsulfoxide; ph-, phosphorylated.
We next investigated the mechanism by which PP2A activity could be regulated. The PP2A holoenzyme consists of a catalytic C subunit, a scaffolding A subunit, and one of a large variety of possible regulatory B subunits (45). Although PP2A activity can be regulated by a variety of mechanisms targeting any one of these subunits (reviewed in Ref. 45), the rapid timing and PKA dependence of MAP2D dephosphorylation suggests that phosphoregulation is a likely mechanism. Though regulation of PP2A activity by cAMP and/or PKA has been reported under a variety of conditions (46,47,48,49), the mechanism of this regulation was until recently unknown. However, we have recently shown that PKA phosphorylates the B56δ-regulatory subunit of PP2A in vitro and in human embryonic kidney 293 cells and, in particular, PKA phosphorylation of Ser566 of the B56δ subunit was found to be necessary and sufficient for activation of PP2A (50). To observe possible LH receptor-dependent phosphoregulation of the B56δ subunit, cultured granulosa cells were treated with or without hCG for various times, and Western blots of total cell lysates were probed with a phospho-specific antibody against Ser566 of the B56δ subunit of PP2A. Phosphorylation at Ser566 was detected at low levels in untreated cells (Fig. 2A, lanes 1, 6, and 8). hCG caused a rapid increase in phosphorylation of B56δ Ser566 (lanes 2–5 and 7), comparable in timing to the dephosphorylation of MAP2D Thr256/Thr259 and with the activation of PKA. Indeed, treatment with forskolin or 8-CPT-cAMP was sufficient to induce Ser566 phosphorylation of the B56δ subunit (Fig. 7A), and inhibition of PKA by Myr-PKI blocked Ser566 phosphorylation by LH receptor signaling (compare lanes 4 and 6). Pretreatment with the PI 3-kinase inhibitor LY294002 had no effect on B56δ subunit phosphorylation at Ser566 (Fig. 7B, compare lanes 2 and 4). These results demonstrate that the B56δ subunit of PP2A is rapidly phosphorylated in a PKA-dependent manner upon activation of LH receptor signaling, suggesting a mechanism for hormonal regulation of PP2A activity.
It could be argued that the role of PP2A in MAP2D phosphoregulation, as demonstrated by inhibition with okadaic acid, is a static one and that active regulation of Thr256/Thr259 occurs exclusively through inhibitory phosphorylation of GSK3β. To determine whether PP2A activity against Thr256/Thr259 is actively regulated by LH receptor signaling, cultured granulosa cells were pretreated with a combination of inhibitors: first cells were pretreated without or with the GSK3β inhibitor lithium chloride for 50 min; next, cells were further pretreated without or with the PP2A inhibitor okadaic acid for an additional 35 min; and finally, cells were treated for 10 min without or with hCG. In agreement with results shown in Fig. 6A, lithium chloride pretreatment alone inhibited basal phosphorylation of MAP2D Thr256/Thr259 (Fig. 7C, lanes 1, 3, and 5), but even with substantial inhibition of basal phosphorylation (by 82% relative to control; compare lanes 1 and 5), hCG treatment resulted in a loss of the remaining phosphorylation at this site (compare lanes 5 and 6). However, even when combined with the same inhibitory concentrations of lithium chloride, PP2A inhibition by okadaic acid pretreatment was capable of potently inhibiting this hCG-induced decrease in phosphorylation (compare lanes 11 and 12). Similar results were observed using okadaic acid combined with the GSK3 inhibitor AR-A014418 (data not shown). Taken together, these results demonstrate that active regulation of both the kinase and the phosphatase is necessary for phosphoregulation of Thr256/Thr259.
DISCUSSION
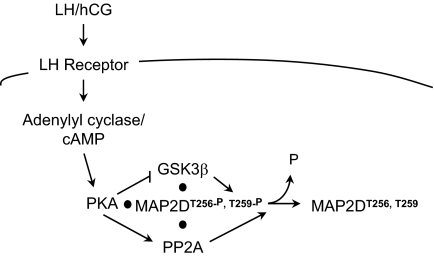
Activation of the LH receptor in PO granulosa cells triggers gene expression leading to ovulation, oocyte maturation, and differentiation of granulosa to luteal cells to radically remodel the structure and function of ovarian follicles. Whereas many downstream transcriptional targets of LH have been identified (51), little is known about how LH signals to effect this dramatic transformation in granulosa cell structure or function. Preovulatory granulosa cells selectively express MAP2D, a lower molecular weight splice variant of the neuronal protein MAP2 that binds microtubules and microfilaments and regulates cytoskeletal dynamics in neurons (23,52,53,54,55). We have identified a novel LH receptor-signaling mechanism in granulosa cells by which LH orchestrates a coordinated increase of phosphatase activity and decrease of kinase activity against MAP2D to promote selective dephosphorylation of Thr256/Thr259, as modeled in Fig. 8. Specifically, the LH analog hCG stimulates PKA-mediated phosphorylation of GSK3β on Ser9 to inhibit GSK3β activity and of the B56δ-regulatory subunit of PP2A on Ser566 to increase phosphatase activity toward Thr256/Thr259 on MAP2D.
Figure 8.
Schematic Model of LH-Mediated Regulation of the Dephosphorylation of MAP2D on Thr256/Thr259
Results described are consistent with the model in which LH/hCG activates the LH receptor and adenylyl cyclase, raising intracellular cAMP concentrations to activate PKA. PKA bound to MAP2D (11) phosphorylates/activates the B56δ subunit of the PP2A holoenzyme to promote dephosphorylation of MAP2D Thr256/Thr259. PKA also phosphorylates/inactivates the MAP2D Thr256/Thr250 kinase GSK3β also complexed to MAP2D.
MAP2 proteins are highly phosphorylated, particularly on Ser and Thr residues in the C-terminal proline-rich and microtubule-binding domains. The larger MAP2A/2B proteins may be phosphorylated in vitro or in neuronal cells at up to 46 sites by a wide variety of kinases, including PKA (16), Ca2+/calmodulin-dependent protein kinase II (56), Ca2+/phospholipid-dependent protein kinase C (57,58), microtubule affinity-regulating kinases (59), Src-family kinases (60), and proline-directed kinases, including GSK3, CDKs, and MAPK superfamily kinases (24,61,62). Phosphorylation by one or more kinases is generally believed to alter the association between MAP2 and microtubules as well as to alter kinetics of microtubule polymerization. For example, PKA is reported to phosphorylate MAP2A/2B at eight serine/threonine residues in the projection domain (that is deleted from MAP2C/2D) and at the serine contained within the KXGS motifs located in each of the three MTBDs, resulting in decreased binding of MAP2 to tubulin (reviewed in Ref. 17). PKA phosphorylates MAP2C only at the three KXGS sites in the MTBDs, resulting in relocation of MAP2C from microtubules to peripheral actin-enriched membrane ruffles (21). MAP2D is identical to MAP2C but contains a fourth MTBD and thus a fourth PKA phosphorylation site (17).
MAP2D in granulosa cells also appears to be highly phosphorylated (see Fig. 1 and Ref. 11). We focused on the phosphorylation state of two residues, Thr256/Thr259, located in the proline-rich domain adjacent to the first MTBD. These residues are 100% conserved in mouse, rat, and human species and are also highly conserved in the corresponding domain of the related MAP, τ (63,64). The majority of the MAP2A/2B present in growth cones of hippocampal neurons is phosphorylated at these residues (25). These residues are in vitro targets for the proline-directed kinases GSK3, CDKs, and MAPK superfamily kinases (24) as are the corresponding residues in τ (Ser202/Thr205c) (64). Moreover, phosphorylation of Thr256/Thr259 in COS-1 cells overexpressing both MAP2C and GSK3β inhibits microtubule bundling consistent with reduced binding of MAP2C to microtubules (27). Similarly, phosphorylation of τ at Ser202/Thr205 impairs binding of τ to microtubules (65). However, the functional significance of the phosphorylation of Thr256/Thr259 in MAP2C/2D or the corresponding Thr1620/Thr1623 in MAP2A/2B in a physiological context is not known.
Phosphorylation of MAP2D on Thr256/Thr259 in granulosa cells is coincident with FSH-induced increases in MAP2D expression (11). Our results using a collection of cell-permeable inhibitors for GSK3, CDKs, and the ERK pathway indicate that GSK3 is required for most, if not all, of this basal phosphorylation of MAP2D at Thr256/Thr259 in granulosa cells. Moreover, GSK3β is present in a protein complex with MAP2D, based on coimmunoprecipitation of GSK3β with MAP2D in granulosa cells. GSK3β exists in an active state in cells (66) consistent with the seemingly coincident expression and phosphorylation of MAP2D at these residues in granulosa cells (11). Also, GSK3 has been shown to phosphorylate other MAP2 isoforms at sites equivalent to Thr256/Thr259 (25). Whereas GSK3 phosphorylation of substrate proteins often occurs at residues that have been primed by prior phosphorylation, we found no indication that priming by CDK5, ERK, or PKA was necessary for phosphorylation of MAP2D at Thr256/Thr259 in intact cultured granulosa cells because inhibition of these kinases did not have an inhibitory effect on basal phosphorylation of this site (see Fig. 6). However, priming by another kinase at one or more sites must be necessary for GSK3β-dependent phosphorylation at MAP2D Thr256/Thr259 (61,62,67).
The LH receptor agonist hCG promotes a rapid yet transient dephosphorylation of MAP2D at Thr256/Thr259 in granulosa cells in vitro and in vivo. Our results suggest that rather than promoting a global dephosphorylation of MAP2D, LH receptor signaling appears to be targeted specifically to Thr256/Thr259. Whereas MAP2D in granulosa cells is also phosphorylated at Ser136, a site located near the N-terminal RII-binding domain (43), phosphorylation at this site is not regulated by LH receptor signaling. MAP2D dephosphorylated at Thr256/Thr259 continues to migrate with the hyperphosphorylated 80-kDa band and not with the hypophosphorylated MAP2D detected at 70 kDa (see Figs. 1 and 2), suggesting that the large shift from the 80- to 70-kDa migration position is determined by the phosphorylation states of other sites that are not regulated by LH receptor signaling. The migration shift that does accompany MAP2D Thr256/Thr259 dephosphorylation is quite modest; we occasionally visualized this very small shift as a tightening of the profile of the upper 80-kDa MAP2D band (see total MAP2D in Figs. 4A and 6). Finally, consistent with the conclusion that LH receptor activation does not promote global dephosphorylation of MAP2D, we previously reported that total levels of MAP2D phosphorylation measured by 32Pi incorporation are not decreased by hCG treatment (11).
Whereas the LH receptor couples to Gs, Gi, and Gq/11 in granulosa cells (68), the majority of LH receptor signaling appears to be mediated by cAMP/PKA (reviewed in Refs. 69 and 70). Indeed, LH is recognized to activate PKA (2) and to signal via PKA to activate ERK (3) and reported ERK-regulated target genes such as the low-density lipoprotein receptor, early-growth response factor 1, and steroidogenic acute regulatory protein, thus contributing to enhanced steroidogenesis and luteinization of granulosa cells (reviewed in Ref. 70). LH also signals via cAMP to promote expression of epidermal growth factor-like growth factors to activate the epidermal growth factor receptor and ERK, among other possible targets, leading to expression of cyclooxygenase-2 and resulting cumulus expansion and oocyte maturation (71,72) by a pathway dependent on PKA activation (73). However, not all of LH receptor signaling is PKA dependent. LH signals via cAMP to activate p38 MAPK by an apparently PKA-independent pathway (3).
Our results show that dephosphorylation of MAP2D Thr256/Thr259 in response to LH receptor signaling is also regulated by PKA, based on inhibition by Myr-PKI and ineffectiveness of the cAMP analog that serves as an agonist for the Rap1 guanine nucleotide exchange factor Epac but not for PKA (38). We reasoned that PKA-dependent dephosphorylation of MAP2D at Thr256/Thr259 could be mediated by either reduced activity of GSK3β, by activation of a phosphatase, or by a combination of both. Our data show that, indeed, both kinase and phosphatase activities are transiently modulated by LH receptor signaling, resulting in rapid and potent dephosphorylation of MAP2D at Thr256/Thr259. First we showed that phosphorylation of GSK3β at Ser9 increased rapidly upon activation of LH receptor signaling and decreased by 2 h after hCG treatment coincident with rephosphorylation of MAP2D at Thr256/Thr259. LH receptor-stimulated phosphorylation of Ser9 on GSK3β was not inhibited by the PI-3 kinase inhibitor LY294002 or by the MEK inhibitor PD98059 but was inhibited by Myr-PKI, suggesting that Ser9 on GSK3β is a direct PKA target. This result is consistent with previous reports that PKA directly phosphorylates GSK3β on Ser9 (reviewed in Ref. 74). Second, our results support a role for PP2A in actively dephosphorylating MAP2D at Thr256/Thr259. This conclusion is based in part on the ability of the preferential PP2A inhibitor okadaic acid, at nanomolar concentrations, to prevent dephosphorylation of MAP2D at Thr256/Thr259 compared with the ineffectiveness of the preferential PP1 inhibitor tautomycin (39,40,41,75). Using a combination of PP2A and GSK3β inhibitors, we demonstrate that, even in the absence of most GSK3 activity, PP2A is necessary for the hCG-induced decrease in phosphorylation. This result supports the hypothesis that both a decrease in kinase activity as well as an increase in phosphatase activity against MAP2D are responsible for regulation of phosphorylated Thr256/Thr259.
Furthermore, immunoprecipitation and microcystin-agarose pull-down analyses demonstrated interactions between MAP2D and PP2A-c in granulosa cells. Our results thus suggest that PP2A binds to MAP2D and, upon stimulation by LH receptor signaling through PKA, actively dephosphorylates Thr256/Thr259. Our results also suggest that PP2A-c appears to bind MAP2D independent of its phosphorylation status at least at Thr256/Thr259, based on its ability to bind both the hypophosphorylated 70-kDa and hyperphosphorylated 80-kDa forms of MAP2D. Our results also suggest that PP2A-c binds stably to a relatively small pool of MAP2D, based on depletion of PP2A-c but not of MAP2D (see Fig. 5, unbound lanes) in PP2A-c immunoprecipitation experiments.
The PP2A holoenzyme consists of a catalytic C subunit, a scaffolding A subunit, and one of a large variety of possible regulatory B subunits (reviewed in Ref. 45). LH-dependent regulation of PP2A activity could occur by regulation of subunit expression, acute regulation of intrinsic C subunit activity by changes in Leu309 methylation (76,77) and/or Tyr307 phosphorylation (78,79), or regulation of the substrate specificity of C subunit activity through modification of regulatory B subunits (45). In granulosa cells, the rapid timing and PKA dependence of PP2A activation suggests phosphoregulation is a likely mechanism. Activation of PP2A activity by cAMP and/or PKA has been reported previously under a variety of conditions (46,47,48). Earlier studies indicated that the B56δ-regulatory subunit (originally called B″δ) can be phosphorylated in vitro by PKA, leading to changes in substrate specificity of the PP2A holoenzyme (80). Expanding upon this report, we have recently shown that PKA phosphorylates Ser566 on B56δ in vitro and that phosphorylation of this residue by PKA is necessary and sufficient to activate PP2A in transfected human embryonic kidney 293 cells as well as in striatal neurons (50). Our results here show that the B56δ-regulatory subunit of PP2A in granulosa cells is rapidly phosphorylated at Ser566 by LH receptor signaling, and that this phosphorylation is inhibited by Myr-PKI. Moreover, phosphorylation of Ser566 on B56δ declines coincident with rephosphorylation of MAP2D at Thr256/Thr259 2 h after LH receptor activation (see Fig. 2A). Taken together, these results suggest that the B56δ-regulatory subunit of PP2A is a direct PKA target in granulosa cells that upon phosphorylation accelerates the dephosphorylation of Thr256/Thr259 on MAP2D.
MAP2D was originally identified in granulosa cells based on its ability to bind to PKA RII subunits (10). Indeed, the entire soluble pool of granulosa cell MAP2D, isolated in the absence of detergents in buffers that do not stabilize microtubules, binds PKA R subunits (Hunzicker-Dunn, M., personal observation). We hypothesize, based on our coimmunoprecipitation results, that a multiprotein complex exists in granulosa cells, in which a relatively small pool of MAP2D phosphorylated on Thr256/Thr259 binds not only PKA but also the PP2A holoenzyme and GSK3β, as depicted schematically in Fig. 8. In this signaling complex, both B56δ PP2A-regulatory subunit and GSK3β appear to be direct PKA targets that selectively regulate the phosphorylation of MAP2D at Thr256/Thr259. We hypothesize that the convergence of PKA, GSK3β, and PP2A with the pool of MAP2D phosphorylated at Thr256/Thr259 facilitates the ability of the LH receptor to signal to MAP2D to promote its dephosphorylation at these specific sites.
In summary, MAP2D in granulosa cells is basally phosphorylated (in the absence of LH receptor signaling) on Thr256/Thr259 primarily by GSK3β. LH receptor signaling in PO granulosa cells promotes rapid dephosphorylation of MAP2D Thr256/Thr259 via PKA-dependent phosphorylation of Ser566 on the B56δ-regulatory subunit of PP2A and PKA-dependent phosphorylation of Ser9 on GSK3β. These phosphorylation events are recognized to promote activation of PP2A (50) and inactivation of GSK3β (66), respectively. Moreover, these proteins appear to be present in a complex consisting of the AKAP MAP2D, PKA, GSK3β, and PP2A. This is the first report, to our knowledge, of the regulation of the phosphorylation status of MAP2D Thr256/Thr259, sites that are conserved in MAP2C and the larger MAP2A/2B isoforms, by a PKA-signaling pathway that both stimulates protein phosphatase activity and inhibits protein kinase activity. This mechanism might also contribute to regulation of these MAP2 phosphorylation sites, as well as corresponding sites in τ, in neuronal cells, where these MAPs are especially abundant and are believed to contribute to neurite outgrowth as well as microtubule dynamics.
MATERIALS AND METHODS
Materials
The following were purchased: hCG from Abraxis Pharmaceutical Products (Schaumburg, IL); PMSG, forskolin, pepstatin A, leupeptin, benzamidine, phenylmethylsulfonyl fluoride, soybean trypsin inhibitor, SB415286, and SB216763 from Sigma-Aldrich (St. Louis, MO); antipain dihydrochloride, calpain inhibitor II, E-64, and aprotinin from Roche (Indianapolis, IN); protein A/G PLUS-agarose from Santa Cruz Biotechnology, Inc. (Santa Cruz, CA); λ Protein Phosphatase and Reaction Buffer from New England Biolabs (Ipswich, MA); adenosine 3′,5′-cyclic monophosphate, 8-(4-chlorophenylthio) cAMP (8-CPT-cAMP), myristoylated PKA inhibitor amide 14–22 (Myr-PKI), lithium chloride, AR-A014418 (GSK-3β Inhibitor VIII), roscovitine, and PD98059, from EMD Biosciences/Calbiochem (La Jolla, CA); 8-pCPT-2′-O-Me-cAMP from Biolog Life Science Institute (Bremen, Germany); okadaic acid and tautomycin from Alexis Biochemicals (Lausen, Switzerland); human fibronectin from BD Biosciences (San Jose, CA); and ECL reagents, Rainbow molecular weight markers, and Hybond-C Extra nitrocellulose membranes from Amersham Biosciences/GE Healthcare (Buckinghamshire, UK).
Antibodies
Antiphospho-PP2A-B56δ (Ser566) and total PP2A-B56δ polyclonal antibodies were generated against the phosphopeptide LRRKpSELPQC or purified rat B56δ, respectively (50). Antiphospho-MAP2 (Thr1620/Thr1623) antibody (see footnote 1) was purchased from Cell Signaling Technology (Danvers, MA). This antibody was generated against a synthetic peptide that includes RpTPGpTPGTPSY, purified using nonphospho- and phosphopeptide affinity columns, and tested for phospho-specific reactivity using phospho- and nonphosphopeptide ELISAs by Cell Signaling Technology. This antibody was used for Western blot detection of MAP2D phosphorylation at residues Thr256/Thr259 (equivalent to Thr1620/Thr1623 in MAP2A/2B). The following were also purchased: anti-MAP2 (HM-2) mouse mAb from Sigma-Aldrich (St. Louis, MO); anti-MAP2, anti-Akt, antiphospho-MAP2 (Ser136), antiphospho-myosin light chain 2 (Ser19), antiphospho-p44/42 MAPK (Thr202/Tyr204) (ph-ERK1/2), antiphospho-Akt (Ser473), antiphospho-PKA substrate, and antiphospho-GSK3α/β (Ser21/9) rabbit polyclonal antibodies, anti-GSK3β (27C10) rabbit mAb, and anti-HA tag (6E2) mouse mAb from Cell Signaling Technology; antiphospho-CREB (Ser133) (10E9) and anti-PP2A-c (1D6) mouse mAbs, anti-PP1 and anti-PP2A-c rabbit polyclonal antibodies, and microcystin-agarose from Upstate Biotechnology/Millipore (Lake Placid, NY); anti-MAPK (Zymed ERK-798; Zymed Laboratories, South San Francisco, CA) mouse mAb from Invitrogen (Carlsbad, CA); and anti-GSK3α/β mouse monoclonal-agarose conjugate and normal mouse IgG-agarose conjugate from Santa Cruz Biotechnology (Santa Cruz, CA). An antibody that detects serine phosphorylation in the KXGS motif of the MTBD, 12E8, was kindly provided Dr. P. Seubert (35), Elan Pharmaceuticals (South San Francisco, CA).
Animals
Immature female Sprague Dawley rats (Charles River Laboratories, Inc., Portage, MI) were obtained at 17 d of age, housed at Northwestern University animal care facilities, and maintained in accordance with the Guidelines for the Care and Use of Laboratory Animals by protocols approved by the Northwestern University Animal Care and Use committee.
PO Granulosa Cell Culture
Granulosa cells were mechanically isolated from ovaries of 24-d-old rats primed by sc injections of 10 IU PMSG in 0.1 ml PBS on d 22 to promote maturation of follicles to the PO phenotype. Collected cells were used immediately or plated overnight on fibronectin (BD Biosciences, San Jose, CA)-coated plastic dishes in DMEM/F12 serum-free medium supplemented with 10 nm 17β-estradiol, 100 U/ml penicillin, and 100 μg/ml streptomycin, and treated with indicated additions approximately 20 h after plating (81).
Protease Inhibition
Protease Inhibitor Cocktail was prepared and added to various buffers such that final concentrations included 10 μg/ml pepstatin A, 10 μg/ml aprotinin, 10 μg/ml leupeptin, 50 μg/ml soybean trypsin inhibitor, 25 mm benzamidine, 10 μg/ml E-64, 1 mm phenylmethyl sulfonylfluoride, 7 μg/ml calpain inhibitor II, and 50 μg/ml antipain dihydrochloride.
Whole Ovarian Extracts
Rats (24 d old), primed by sc injections with 10 IU PMSG 48 h prior, were given ip injections of 50 IU hCG or saline. Ovaries were harvested at various time points after injections; dissected free of bursa, fat, and oviducts; weighed; and homogenized at 4 C in homogenization buffer [10 mm Tris (pH 7.0), 5 mm EDTA, 1 mm EGTA, 0.32 m sucrose, 1 mm sodium orthovanadate, 20 mm sodium fluoride, 2.5 mm sodium pyrophosphate and Protease Inhibitor Cocktail] using 12 strokes with a ground-glass homogenizer. Homogenates were clarified by centrifugation at 10,000 × g at 4 C for 20 min. Supernatants were added to 0.5× volume sodium dodecyl sulfate-sample buffer and denatured by boiling. Protein concentrations were controlled by homogenization at a 10:1 ratio of homogenization buffer (ml)/wet tissue weight (g) followed by loading equal volumes for each SDS-PAGE gel lane.
Electrophoresis and Western Blot Analysis
For plated cells, treatments were terminated by aspirating medium and rinsing cells once with PBS. Total cell extracts were collected by scraping cells in sodium dodecyl sulfate-sample buffer (2) and denatured by boiling. Protein concentrations were controlled by plating identical cell numbers per plate in each experiment followed by loading equal volumes for each SDS-PAGE gel lane. Separation of ovarian lysate protein was by SDS-PAGE using 10% or 13% separating gels (82). Separated protein was electrophoretically transferred to Hybond-C Extra nitrocellulose membrane (Amersham Pharmacia Biotech, Piscataway, NJ). Membranes were incubated with primary antibody overnight at 4 C, and protein-antibody complexes were detected using horseradish peroxidase-linked anti-IgG (Cell Signaling Technology) and enhanced chemiluminescence (Amersham Biosciences/GE Healthcare). Films were scanned with an Epson 1640SU scanner and Adobe Photoshop version 7.0 software (Adobe Systems, Inc., San Jose, CA) with minimal processing. Relative protein quantities were calculated from densitometric measurements of Western band intensities using Molecular Analyst software (Bio-Rad Laboratories, Inc., Hercules, CA). Statistical analyses are presented as mean ± se and were performed by paired Student’s t test; P < 0.05 was accepted as significant (83).
In Vitro Phosphatase Assay for Electrophoretic Migration Analysis
PO rat granulosa cells in suspension were collected and lysed by sonication in Minimal Buffer A [50 mm piperazine-N,N′-bis(ethanesulfonic acid), pH 6.6; 100 mm NaCl; 0.5% Nonidet P-40; 2 mm EGTA; and Protease Inhibitor Cocktail], Complete Buffer A (Minimal Buffer A plus 1 mm EDTA, 1 mm sodium orthovanadate, 20 mm sodium fluoride, and 2.5 mm sodium pyrophosphate), or MnCl2 Phosphatase Reaction Buffer (50 mm Tris HCl, pH 7.5; 100 mm dithiothreitol; 0.1 mm EGTA; 0.01% Brij 35; 2 mm MnCl2; 0.5% Nonidet P-40; 2 mm EGTA; and Protease Inhibitor Cocktail). Lysates were clarified by centrifugation at 10,000 × g for 5 min, and then incubated at 30 C for 30 min, with or without the addition of exogenous λ protein phosphatase (New England Biolabs).
Coimmunoprecipitation and Microcystin Pull-Down Analysis
Primary rat PO granulosa cells in suspension were collected and lysed by sonication in Complete Buffer A. Lysates were clarified by centrifugation at 10,000 × g for 5 min, after which a fraction was removed as input. For immunoprecipitation, detergent-soluble cell extracts were precleared by incubation with protein A/G PLUS-agarose for 30 min at 4 C on a rotator. Extracts were then incubated overnight at 4 C on a rotator in the presence of 60 μl microcystin-agarose, protein A/G PLUS-agarose and 10 μl mouse monoclonal antibodies against MAP2, PP2A catalytic subunit, or an irrelevant epitope (HA-Tag mAb), or 40 μl GSK3β mAb-agarose or IgG-agarose conjugates. Unbound protein in the flow through was collected and denatured in SDS-PAGE sample buffer. Agarose beads were washed in Complete Buffer A with 10% glycerol added. Bound proteins were eluted and denatured in SDS-PAGE sample buffer.
Footnotes
This work was supported by National Institutes of Health (NIH) Grants HD-046955 (to M.H.D.), DA-10044 and MH074866 (to A.C.N.), NIH Training Grant Award T32 DK007169, and NIH Medical Scientist Training Grant T32-GM08152.
Current address for A.B.K. and M.H.-D.: School of Molecular Biosciences, Washington State University, Pullman, Washington 83843.
Disclosure statement: The authors of this manuscript have nothing to disclose.
First Published Online May 8, 2008
Abbreviations: AKAP, A-kinase anchoring protein; C, PKA catalytic subunit; CDK, cyclin-dependent kinase; 8-CPT-cAMP, 8-(4-chlorophenylthio) cAMP; 8-pCPT-2′-O-Me-cAMP, 8-(4-chlorophenylthio)-2′-O-methyl cAMP; CREB, cAMP response element-binding protein; Epac, exchange protein activated by cAMP; GSK, glycogen synthase kinase; HA, hemagglutinin; hCG, human chorionic gonadotropin; mAb, monoclonal antibody; MAP, microtubule-associated protein; MEK, MAPK kinase; MTBD, microtubule-binding domain; Myr-PKI, myristoylated PKA inhibitor; ph-MAP2D, phosphorylated MAP2D; PI, phosphatidylinositol; PKA, cAMP-dependent protein kinase; PMSG, pregnant mare serum gonadotropin; PO, preovulatory; PP1, protein phosphatase 1; PP2A, protein phosphatase 2; PP2A-c, PP2A catalytic subunit; R, PKA regulatory subunit.
This antibody, designated antiphospho-MAP2 (Thr1620/Thr1623) for the higher molecular weight MAP2A/2B isoforms, preferentially recognizes phosphorylation of both Thr1620 and Thr1623 on MAP2A/2B and Thr256 and Thr259 on MAP2D, but may also recognize the single phosphorylation of either threonine (Cell Signaling Technology). See Materials and Methods for additional information.
Antiphospho-PKA substrate antibody (Cell Signaling Technology) similarly failed to detect signal at 80 kDa in MAP2 immunoprecipitations from granulosa cells (n = 5; data not shown).
Numbering based on the largest isoform of human brain τ.
References
- Marsh JM 1970 The stimulatory effect of luteinizing hormone on adenylylcyclase in the bovine corpus luteum. J Biol Chem 245:1596–1603 [PubMed] [Google Scholar]
- Hunzicker-Dunn M 1981 Selective activation of rabbit ovarian protein kinase isozymes in rabbit ovarian follicles and corpora lutea. J Biol Chem 256:12185–12193 [PubMed] [Google Scholar]
- Salvador LM, Maizels E, Hales DB, Miyamoto E, Yamamoto H, Hunzicker-Dunn M 2002 Acute signaling by the LH receptor is independent of protein kinase C activation. Endocrinology 143:2986–2994 [DOI] [PubMed] [Google Scholar]
- Richards JS 1994 Hormonal control of gene expression in the ovary. Endocr Rev 15:725–751 [DOI] [PubMed] [Google Scholar]
- Mukherjee A, Park-Sarge OK, Mayo KE 1996 Gonadotropins induce rapid phosphorylation of the 3′,5′-cyclic adenosine monophosphate response element binding protein in ovarian granulosa cells. Endocrinology 137:3234–3245 [DOI] [PubMed] [Google Scholar]
- Park-Sarge O-K, Mayo KE 1994 Regulation of the progesterone receptor gene by gonadotropins and cyclic adenosine 3′,5′-monophosphate in rat granulosa cells. Endocrinology 134:709–718 [DOI] [PubMed] [Google Scholar]
- Richards JS, Fitzpatrick SL, Clemens JW, Morris JK, Alliston T, Sirois J 1995 Ovarian cell differentiation: a cascade of multiple hormones, cellular signals, and regulated genes. Recent Prog Horm Res 50:223–254 [DOI] [PubMed] [Google Scholar]
- Feliciello A, Gottesman ME, Avvedimento EV 2001 The biological functions of A-kinase anchor proteins. J Mol Biol 308:99–114 [DOI] [PubMed] [Google Scholar]
- Smith FD, Langeberg LK, Scott JD 2006 The where's and when's of kinase anchoring. Trends Biochem Sci 31:316–323 [DOI] [PubMed] [Google Scholar]
- Carr DW, DeManno DA, Atwood A, Hunzicker-Dunn M, Scott JD 1993 Follicle-stimulating hormone regulation of A-kinase anchoring proteins in granulosa cells. J Biol Chem 268:20729–20732 [PubMed] [Google Scholar]
- Salvador LM, Flynn MP, Avila J, Reierstad S, Maizels ET, Alam H, Park Y, Scott JD, Carr DW, Hunzicker-Dunn M 2004 Neuronal microtubule-associated protein 2D is a dual A-kinase anchoring protein expressed in rat ovarian granulosa cells. J Biol Chem 279:27621–27632 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garner CC, Matus A 1988 Different forms of microtubule-associated protein 2 are encoded by separate mRNA transcripts. J Cell Biol 106:779–783 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferhat L, Ben-Ari Y, Khrestchatisky M 1994 Complete sequence of rat MAP2D, a novel MAP2 isoform. C R Acad Sci III 317:304–309 [PubMed] [Google Scholar]
- Kalcheva N, Albala J, O'Guin K, Rubino H, Garner C, Shafit-Zagardo B 1995 Genomic structure of human microtubule-associated protein 2 (MAP-2) and characterization of additional MAP-2 isoforms. Proc Natl Acad Sci USA 92:10894–10898 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Theurkauf WE, Vallee RB 1982 Molecular characterization of the cAMP-dependent protein kinase bound to microtubule-associated protein 2. J Biol Chem 257:3284–3290 [PubMed] [Google Scholar]
- Sloboda RD, Rudolph SA, Rosenbaum JL, Greengard P 1975 Cyclic AMP-dependent endogenous phosphorylation of a microtubule-associated protein. Proc Natl Acad Sci USA 72:177–181 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanchez C, Diaz-Nido J, Avila J 2000 Phosphorylation of microtubule-associated protein 2 (MAP2) and its relevance for the regulation of the neuronal cytoskeleton function. Prog Neurobiol 61:133–168 [DOI] [PubMed] [Google Scholar]
- Falconer MM, Vielkind U, Brown DL 1989 Association of acetylated microtubules, vimentin intermediate filaments, and MAP 2 during early neural differentiation in EC cell culture. Biochem Cell Biol 67:537–544 [DOI] [PubMed] [Google Scholar]
- Heimann R, Shelanski ML, Liem RK 1985 Microtubule-associated proteins bind specifically to the 70-kDa neurofilament protein. J Biol Chem 260:12160–12166 [PubMed] [Google Scholar]
- Bloom GS, Luca FC, Vallee RB 1985 Cross-linking of intermediate filaments to microtubules by microtubule-associated protein 2. Ann NY Acad Sci 455:18–31 [DOI] [PubMed] [Google Scholar]
- Ozer RS, Halpain S 2000 Phosphorylation-dependent localization of microtubule-associated MAP2c to the actin cytoskeleton. Mol Biol Cell 11:3573–3587 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bloom GS, Vallee RB 1983 Association of microtubule-associated protein 2 (MAP 2) with microtubules and intermediate filaments in cultured brain cells. J Cell Biol 96:1523–1531 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roger B, Al-Bassam J, Dehmelt L, Milligan RA, Halpain S 2004 MAP2c, but not τ, binds and bundles F-actin via its microtubule binding domain. Curr Biol 14:363–371 [DOI] [PubMed] [Google Scholar]
- Sanchez C, Tompa P, Szucs K, Friedrich P, Avila J 1996 Phosphorylation and dephosphorylation in the proline-rich C-terminal domain of microtubule-associated protein 2. Eur J Biochem 241:765–771 [DOI] [PubMed] [Google Scholar]
- Sanchez MC, Diaz-Nido J, Avila J 1998 Regulation of a site-specific phosphorylation of the microtubule-associated protein 2 during the development of cultured neurons. Neuroscience 87:861–870 [DOI] [PubMed] [Google Scholar]
- Gong CX, Wegiel J, Lidsky T, Zuck L, Avila J, Wisniewski HM, Grundke-Iqbal I, Iqbal K 2000 Regulation of phosphorylation of neuronal microtubule-associated proteins MAP1b and MAP2 by protein phosphatase-2A and -2B in rat brain. Brain Res 853:299–309 [DOI] [PubMed] [Google Scholar]
- Sanchez C, Perez M, Avila J 2000 GSK3β-mediated phosphorylation of the microtubule-associated protein 2C (MAP2C) prevents microtubule bundling. Eur J Cell Biol 79:252–260 [DOI] [PubMed] [Google Scholar]
- Olesen OF 1994 Expression of low molecular weight isoforms of microtubule-associated protein 2. Phosphorylation and induction of microtubule assembly in vitro. J Biol Chem 269:32904–32908 [PubMed] [Google Scholar]
- Arias C, Sharma N, Davies P, Shafit-Zagardo B 1993 Okadaic acid induces early changes in microtubule-associated protein 2 and τ phosphorylation prior to neurodegeneration in cultured cortical neurons. J Neurochem 61:673–682 [DOI] [PubMed] [Google Scholar]
- Sanchez C, az-Nido J, Avila J 1995 Variations in in vivo phosphorylation at the proline-rich domain of the microtubule-associated protein 2 (MAP2) during rat brain development. Biochem J 306:481–487 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cai L, Chu Y, Wilson SE, Schlender KK 1995 A metal-dependent form of protein phosphatase 2A. Biochem Biophys Res Commun 208:274–279 [DOI] [PubMed] [Google Scholar]
- Longin S, Jordens J, Martens E, Stevens I, Janssens V, Rondelez E, De Baere I, Derua R, Waelkens E, Goris J, Van HC 2004 An inactive protein phosphatase 2A population is associated with methylesterase and can be re-activated by the phosphotyrosyl phosphatase activator. Biochem J 380:111–119 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biernat J, Wu YZ, Timm T, Zheng-Fischhofer Q, Mandelkow E, Meijer L, Mandelkow EM 2002 Protein kinase MARK/PAR-1 is required for neurite outgrowth and establishment of neuronal polarity. Mol Biol Cell 13:4013–4028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mandelkow EM, Thies E, Trinczek B, Biernat J, Mandelkow E 2004 MARK/PAR1 kinase is a regulator of microtubule-dependent transport in axons. J Cell Biol 167:99–110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seubert P, Mawal-Dewan M, Barbour R, Jakes R, Goedert M, Johnson GV, Litersky JM, Schenk D, Lieberburg I, Trojanowski JQ 1995 Detection of phosphorylated Ser262 in fetal τ, adult τ, and paired helical filament τ. J Biol Chem 270:18917–18922 [DOI] [PubMed] [Google Scholar]
- Kawasaki H, Springett GM, Mochizuki N, Toki S, Nakaya M, Matsuda M, Housman DE, Grabiel AM 1998 A family of cAMP-binding proteins that directly activate Rap1. Science 282:2275–2279 [DOI] [PubMed] [Google Scholar]
- de Rooij J, Zwartkruis FJ, Verheijen MH, Cool RH, Nijman SM, Wittinghofer A, Bos JL 1998 Epac is a Rap1 guanine-nucleotide-exchange factor directly activated by cyclic AMP. Nature 396:474–477 [DOI] [PubMed] [Google Scholar]
- Enserink JM, Christensen AE, de Rooij J, van Triest M, Schwede F, Genieser HG, Doskeland SO, Blank JL, Bos JL 2002 A novel Epac-specific cAMP analogue demonstrates independent regulation of Rap1 and ERK. Nat Cell Biol 4:901–906 [DOI] [PubMed] [Google Scholar]
- Favre B, Turowski P, Hemmings BA 1997 Differential inhibition and posttranslational modification of protein phosphatase 1 and 2A in MCF7 cells treated with calyculin-A, okadaic acid, and tautomycin. J Biol Chem 272:13856–13863 [DOI] [PubMed] [Google Scholar]
- Resjo S, Oknianska A, Zolnierowicz S, Manganiello V, Degerman E 1999 Phosphorylation and activation of phosphodiesterase type 3B (PDE3B) in adipocytes in response to serine/threonine phosphatase inhibitors: deactivation of PDE3B in vitro by protein phosphatase type 2A. Biochem J 341:839–845 [PMC free article] [PubMed] [Google Scholar]
- Yan Y, Mumby MC 1999 Distinct roles for PP1 and PP2A in phosphorylation of the retinoblastoma protein. PP2a regulates the activities of G(1) cyclin-dependent kinases. J Biol Chem 274:31917–31924 [DOI] [PubMed] [Google Scholar]
- Cohen P, Holmes CFB, Tsukitani Y 1990 Okadaic acid: a new probe for the study of cellular regulation. Trends Biochem Sci 15:98–102 [DOI] [PubMed] [Google Scholar]
- Berling B, Wille H, Roll B, Mandelkow EM, Garner C, Mandelkow E 1994 Phosphorylation of microtubule-associated proteins MAP2a,b and MAP2c at Ser136 by proline-directed kinases in vivo and in vitro. Eur J Cell Biol 64:120–130 [PubMed] [Google Scholar]
- Jope RS, Johnson GV 2004 The glamour and gloom of glycogen synthase kinase-3. Trends Biochem Sci 29:95–102 [DOI] [PubMed] [Google Scholar]
- Sontag E 2001 Protein phosphatase 2A: the Trojan horse of cellular signaling. Cell Signal 13:7–16 [DOI] [PubMed] [Google Scholar]
- Feschenko MS, Stevenson E, Nairn AC, Sweadner KJ 2002 A novel cAMP-stimulated pathway in protein phosphatase 2A activation. J Pharmacol Exp Ther 302:111–118 [DOI] [PubMed] [Google Scholar]
- Floer M, Stock J 1994 Carboxyl methylation of protein phosphatase 2A from Xenopus eggs is stimulated by cAMP and inhibited by okadaic acid. Biochem Biophys Res Commun 198:372–379 [DOI] [PubMed] [Google Scholar]
- Nishi A, Bibb JA, Snyder GL, Higashi H, Nairn AC, Greengard P 2000 Amplification of dopaminergic signaling by a positive feedback loop. Proc Natl Acad Sci USA 97:12840–12845 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim Y, Sung JY, Ceglia I, Lee KW, Ahn JH, Halford JM, Kim AM, Kwak SP, Park JB, Ho RS, Schenck A, Bardoni B, Scott JD, Nairn AC, Greengard P 2006 Phosphorylation of WAVE1 regulates actin polymerization and dendritic spine morphology. Nature 442:814–817 [DOI] [PubMed] [Google Scholar]
- Ahn JH, McAvoy T, Rakhilin SV, Nishi A, Greengard P, Nairn AC 2007 Protein kinase A activates protein phosphatase 2A by phosphorylation of the B56δ subunit. Proc Natl Acad Sci USA 104:2979–2984 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Espey LL, Richards JS 2002 Temporal and spatial patterns of ovarian gene transcription following an ovulatory dose of gonadotropin in the rat. Biol Reprod 67:1662–1670 [DOI] [PubMed] [Google Scholar]
- Felgner H, Frank R, Biernat J, Mandelkow EM, Mandelkow E, Ludin B, Matus A, Schliwa M 1997 Domains of neuronal microtubule-associated proteins and flexural rigidity of microtubules. J Cell Biol 138:1067–1075 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weisshaar B, Doll T, Matus A 1992 Reorganisation of the microtubular cytoskeleton by embryonic microtubule-associated protein 2 (MAP2c). Development 116:1151–1161 [DOI] [PubMed] [Google Scholar]
- Weisshaar B, Matus A 1993 Microtubule-associated protein 2 and the organization of cellular microtubules. J Neurocytol 22:727–734 [DOI] [PubMed] [Google Scholar]
- Gamblin TC, Nachmanoff K, Halpain S, Williams Jr RC 1996 Recombinant microtubule-associated protein 2c reduces the dynamic instability of individual microtubules. Biochemistry 35:12576–12586 [DOI] [PubMed] [Google Scholar]
- Schulman H 1984 Phosphorylation of microtubule-associated proteins by a Ca2+/calmodulin-dependent protein kinase. J Cell Biol 99:11–19 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akiyama t, Nishida E, Ishida J, Saji N, Ogawara H, Hoshi M, Miyata Y, Sakai H 1986 Purified protein kinase C phosphorylates microtubule-associated protein 2. J Biol Chem 261:15648–15651 [PubMed] [Google Scholar]
- Tsuyama S, Bramblett GT, Huang KP, Flavin M 1986 Calcium/phospholipid-dependent kinase recognizes sites in microtubule-associated protein 2 which are phosphorylated in living brain and are not accessible to other kinases. J Biol Chem 261:4110–4116 [PubMed] [Google Scholar]
- Illenberger S, Drewes G, Trinczek B, Biernat J, Meyer HE, Olmsted JB, Mandelkow EM, Mandelkow E 1996 Phosphorylation of microtubule-associated proteins MAP2 and MAP4 by the protein kinase p110mark. Phosphorylation sites and regulation of microtubule dynamics. J Biol Chem 271:10834–10843 [DOI] [PubMed] [Google Scholar]
- Zamora-Leon SP, Bresnick A, Backer JM, Shafit-Zagardo B 2005 Fyn phosphorylates human MAP-2c on tyrosine 67. J Biol Chem 280:1962–1970 [DOI] [PubMed] [Google Scholar]
- Chang L, Jones Y, Ellisman MH, Goldstein LS, Karin M 2003 JNK1 is required for maintenance of neuronal microtubules and controls phosphorylation of microtubule-associated proteins. Dev Cell 4:521–533 [DOI] [PubMed] [Google Scholar]
- Bjorkblom B, Ostman N, Hongisto V, Komarovski V, Filen JJ, Nyman TA, Kallunki T, Courtney MJ, Coffey ET 2005 Constitutively active cytoplasmic c-Jun N-terminal kinase 1 is a dominant regulator of dendritic architecture: role of microtubule-associated protein 2 as an effector. J Neurosci 25:6350–6361 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chapin SJ, Bulinski JC 1992 Microtubule stabilization by assembly-promoting microtubule-associated proteins: a repeat performance. Cell Motil Cytoskeleton 23:236–243 [DOI] [PubMed] [Google Scholar]
- Goedert M, Jakes R, Vanmechelen E 1995 Monoclonal antibody AT8 recognises τ protein phosphorylated at both serine 202 and threonine 205. Neurosci Lett 189:167–169 [DOI] [PubMed] [Google Scholar]
- Takahashi S, Saito T, Hisanaga S, Pant HC, Kulkarni AB 2003 τ Phosphorylation by cyclin-dependent kinase 5/p39 during brain development reduces its affinity for microtubules. J Biol Chem 278:10506–10515 [DOI] [PubMed] [Google Scholar]
- Dajani R, Fraser E, Roe SM, Young N, Good V, Dale TC, Pearl LH 2001 Crystal structure of glycogen synthase kinase 3 β: structural basis for phosphate-primed substrate specificity and autoinhibition. Cell 105:721–732 [DOI] [PubMed] [Google Scholar]
- Bach S, Knockaert M, Reinhardt J, Lozach O, Schmitt S, Baratte B, Koken M, Coburn SP, Tang L, Jiang T, Liang DC, Galons H, Dierick JF, Pinna LA, Meggio F, Totzke F, Schachtele C, Lerman AS, Carnero A, Wan Y, Gray N, Meijer L 2005 Roscovitine targets, protein kinases and pyridoxal kinase. J Biol Chem 280:31208–31219 [DOI] [PubMed] [Google Scholar]
- Rajagopalan-Gupta RM, Lamm ML, Mukherjee S, Rasenick MM, Hunzicker-Dunn M 1998 Luteinizing hormone/choriogonadotropin receptor-mediated activation of heterotrimeric guanine nucleotide binding proteins in ovarian follicular membranes. Endocrinology 139:4547–4555 [DOI] [PubMed] [Google Scholar]
- Conti M, Hsieh M, Park JY, Su YQ 2006 Role of the epidermal growth factor network in ovarian follicles. Mol Endocrinol 20:715–723 [DOI] [PubMed] [Google Scholar]
- Hunzicker-Dunn M, Mayo K 2006 Gonadotropin signaling in the ovary. In: The physiology of reproduction. 3rd ed. San Diego, CA: Elsevier; 569–614 [Google Scholar]
- Park JY, Su YQ, Ariga M, Law E, Jin SL, Conti M 2004 EGF-like growth factors as mediators of LH action in the ovulatory follicle. Science 303:682–684 [DOI] [PubMed] [Google Scholar]
- Hsieh M, Lee D, Panigone S, Horner K, Chen R, Theologis A, Lee DC, Threadgill DW, Conti M 2007 Luteinizing hormone-dependent activation of the epidermal growth factor network is essential for ovulation. Mol Cell Biol 27:1914–1924 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morris JK, Richards JS 1995 Luteinizing hormone induces prostaglandin endoperoxide synthase-2 and luteinization in vitro by A-kinase and C-kinase pathways. Endocrinology 136:1549–1558 [DOI] [PubMed] [Google Scholar]
- Doble BW, Woodgett JR 2003 GSK-3: tricks of the trade for a multi-tasking kinase. J Cell Sci 116:1175–1186 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chatfield K, Eastman A 2004 Inhibitors of protein phosphatases 1 and 2A differentially prevent intrinsic and extrinsic apoptosis pathways. Biochem Biophys Res Commun 323:1313–1320 [DOI] [PubMed] [Google Scholar]
- De Baere I, Derua R, Janssens V, Van HC, Waelkens E, Merlevede W, Goris J 1999 Purification of porcine brain protein phosphatase 2A leucine carboxyl methyltransferase and cloning of the human homologue. Biochemistry 38:16539–16547 [DOI] [PubMed] [Google Scholar]
- Ogris E, Du X, Nelson KC, Mak EK, Yu XX, Lane WS, Pallas DC 1999 A protein phosphatase methylesterase (PME-1) is one of several novel proteins stably associating with two inactive mutants of protein phosphatase 2A. J Biol Chem 274:14382–14391 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brautigan DL 1995 Flicking the switches: phosphorylation of serine/threonine protein phosphatases. Semin Cancer Biol 6:211–217 [DOI] [PubMed] [Google Scholar]
- Begum N, Ragolia L 1996 cAMP counter-regulates insulin-mediated protein phosphatase-2A inactivation in rat skeletal muscle cells. J Biol Chem 271:31166–31171 [DOI] [PubMed] [Google Scholar]
- Usui H, Inoue R, Tanabe O, Nishito Y, Shimizu M, Hayashi H, Kagamiyama H, Takeda M 1998 Activation of protein phosphatase 2A by cAMP-dependent protein kinase-catalyzed phosphorylation of the 74-kDa B″ (δ) regulatory subunit in vitro and identification of the phosphorylation sites. FEBS Lett 430:312–316 [DOI] [PubMed] [Google Scholar]
- Salvador LM, Park Y, Cottom J, Maizels ET, Jones JCR, Schillace RV, Carr DW, Cheung P, Allis CD, Jameson JL, Hunzicker-Dunn M 2001 Follicle stimulating hormone stimulates protein kinase A mediated histone H3 phosphorylation and acetylation leading to select gene activation in ovarian granulosa cells. J Biol Chem 276:40146–40155 [DOI] [PubMed] [Google Scholar]
- Hunzicker-Dunn M, Cutler Jr RE, Maizels ET, DeManno DA, Lamm MLG, Erlichman J, Sanwal BD, LaBarbera AR 1991 Isozymes of cAMP-dependent protein kinase present in the rat corpus luteum. J Biol Chem 266:7166–7175 [PubMed] [Google Scholar]
- Bender FE, Douglass LW, Kramer A 1982 Statistical methods for food and agriculture. Westport, CT.: AVI Publishing Co., Inc.; 87–107 [Google Scholar]