Increased lipid availability is strongly associated with both β-cell dysfunction and insulin resistance, two key facets of type 2 diabetes. Isoforms of the protein kinase C (PKC) family have been viewed as candidates for mediating the effects of fat oversupply because they are lipid-dependent kinases with wide-ranging roles in signal transduction, including the positive and negative modulation of insulin action. Until recently, their involvement was based on correlative studies, but now causative roles for distinct PKC isoforms have also been addressed, in both pancreatic β-cells and insulin-sensitive tissues. Our goal here, therefore, is to review the hitherto disparate fields of PKC function in insulin signaling/resistance on the one hand and in regulating β-cell biology on the other hand. By integrating these two areas, we provide a reappraisal of the current paradigm regarding PKC and type 2 diabetes. In particular, we propose that PKCɛ warrants further investigation, not merely as a treatment for insulin resistance as previously supposed, but also as a positive regulator of insulin availability.
DIACYLGLYCEROL-MEDIATED ACTIVATION OF PROTEIN KINASE C
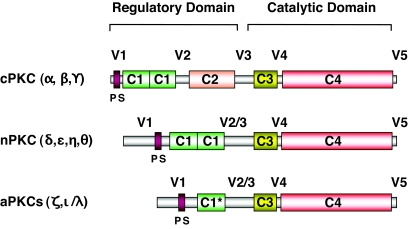
The protein kinase C (PKC) family comprises 10 isoforms that have been subdivided into three groups (Fig. 1) based on sequence homology and mechanisms of activation (rev. in 1). While differentiated by their sensitivity to Ca2+, both the conventional PKCs (cPKCα, -β, and -γ) and novel PKCs (nPKCδ, -ɛ, -η, and -θ) are dependent on diacylglycerol (DAG) for full activation. These isoforms are therefore responsive to the stimulation of G protein–coupled receptors or receptor tyrosine kinases, which activate phospholipase C, inducing the hydrolysis of phosphatidylinositol 4,5-bisphosphate at the plasma membrane and the resultant generation of DAG and Ca2+. Evidence for the acute elevation of DAG in this fashion by insulin was reported in early studies (2), although the identities of the putative phospholipase(s) and phospholipid substrates involved were never clarified. On the other hand, chronic elevation of DAG through de novo synthesis during periods of lipid oversupply, as in the case of obesity, has been widely correlated with cPKC and nPKC activation, although in this case, DAG is first synthesized in the endoplasmic reticulum, perhaps resulting in PKC activation at intracellular sites.
FIG. 1.
The PKC family of lipid-activated protein kinases. PKC isoforms contain constant regions (C1–4) and variable regions (V1–5) and can be divided into three subgroups. cPKCs are activated in the presence of calcium, which binds to the C2 domain, and DAG, which binds to the C1 domains. nPKCs lack C2 domains and are Ca2+-independent but still require DAG for full activation. aPKCs possess only one nonfunctional C1 domain (C1*) and no C2 domain and are both Ca2+- and DAG-independent. The C3 regions (ATP-binding) and C4 regions (protein substrate binding) are highly conserved between isoforms. In each case, the pseudosubstrate (PS) sequences, found in the V1 variable region, interfere with the catalytic domains to inhibit substrate phosphorylation until conformational changes induced by activators allow full activation.
As a consequence of the interaction between PKC and membrane-delimited DAG, cPKC and nPKC isoforms generally translocate from a cytosolic to a membrane-associated compartment. PKC isoform translocation, observed by immunoblotting subcellular fractions, is thus commonly used as an indication of activation, particularly because in vitro kinase assays discriminate poorly between isoforms. Longer-term stimulation leads to PKC downregulation by proteolysis, although susceptibility varies between isoforms and depends on cell type.
PKCs AS INSULIN SIGNAL TRANSDUCERS
The atypical isoforms (aPKCζ and aPKCι/λ) constitute a third group within the PKC family and are independent of both Ca2+ and DAG (Fig. 1). (There is confusion in the literature concerning PKCλ [3], which is not a distinct isoform but in fact the mouse ortholog of human PKCι [4]. In all species, the gene symbol for this isoform is now Prkci.) Instead, these kinases can be activated in response to stimulation of the insulin receptor substrate (IRS)/phosphatidylinositol (PI) 3-kinase pathway, which enables phosphorylation of aPKCs at the “activation loop” near the catalytic site by PI 3-dependent kinase 1 (5).
Atypical PKCs signal in parallel to Akt in muscle and adipose tissue during the stimulation of glucose metabolism, especially via translocation of GLUT4 (6). There appears to be redundancy between aPKCζ and aPKCι in this respect because one can substitute for the other in overexpression studies. Diminished IRS-1/PI 3-kinase–dependent aPKC activation is observed in muscle and adipose tissue during insulin resistance and type 2 diabetes (6) but remains intact in liver. In this instance, activation occurs predominantly through the IRS-2/PI 3-kinase pathway and is more important for the lipogenic action of insulin, so its continued function may play a role in lipid dysregulation upon hyperinsulinemia in insulin-resistant states (6).
Insulin has also been reported to stimulate the activity of cPKC and nPKC isoforms to promote glucose disposal (7). Putative mechanisms of activation include tyrosine phosphorylation of PKCδ and alternative splicing of PKCβ to increase PKCβII levels, but these have not been widely substantiated, and the positive effects of these kinases need to be reconciled with the negative regulation of insulin action detailed below.
PKC AND INSULIN RESISTANCE
An association between PKC activation and insulin resistance in skeletal muscle became apparent from studies linking PKC translocation with defective insulin-stimulated glucose metabolism (8–10). Most often, nPKC isoforms, especially PKCθ and PKCɛ, were implicated together with an elevation of lipid intermediates such as DAG. Thus, skeletal muscle from rats fed a high-fat diet for 3 weeks exhibited an increase in the translocation of PKCθ and PKCɛ in conjunction with elevated lipid content and diminished glucose disposal (11). PKC redistribution was reversed upon treatment with the insulin sensitizer rosiglitazone (12). Similar alterations in nPKC isoforms were also observed in genetic models of obesity and diabetes (13,14). In more acute models of insulin resistance, PKCθ translocation was also observed after 5-h lipid infusion (15), whereas 1- or 4-day infusion of glucose, which increased muscle lipid content, promoted activation of PKCɛ (16).
PKCɛ has been the isoform most often implicated in the generation of insulin resistance in liver. This was initially demonstrated using liver biopsies from obese subjects with type 2 diabetes (17). In addition, short-term (3-day) fat feeding of rats, which induces hepatic steatosis, led to translocation of PKCɛ in concert with a diminished ability of insulin to reduce endogenous glucose production (18). Other isoforms have been implicated to a lesser extent. Alterations in the cellular localization of PKCα and PKCζ, in addition to PKCɛ, were observed in diabetic liver (17). PKCδ translocation has also been observed in muscle of high-fat fed rats (11), as well as after lipid infusion in both liver (19) and muscle where PKCβ was also activated (20). These studies support the hypothesis that activation of one or more PKC isoforms through increased lipid availability, especially PKCθ and PKCɛ, can interfere with insulin-stimulated glucose disposal. It is not clear why nPKC isoforms are more often implicated in these studies when cPKCs are also sensitive to elevations in DAG. This may be related to the additional sensitivity of cPKCs to Ca2+, which may not become elevated upon fat oversupply.
MECHANISMS OF PKC-INDUCED INSULIN RESISTANCE
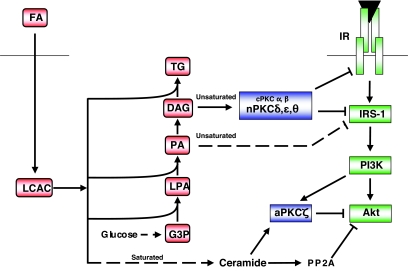
PKC has been reported to inhibit several components of the insulin signaling cascade, as well as downstream metabolic enzymes such as glycogen synthase (rev. in 10). These studies were often based on in vitro phosphorylation or overexpression of PKC and potential substrates in cultured cells and require careful interpretation. In vivo, PKC has also been suggested to act indirectly, such as by upregulation of inflammatory pathways (21). Nevertheless, most studies have addressed the simpler hypothesis that PKC directly phosphorylates serine residues of the insulin receptor substrates, especially IRS-1 (Fig. 2). This results in diminished tyrosine phosphorylation of IRS-1, reduced downstream signaling through the PI 3-kinase/Akt pathway to glucose metabolism, and, ultimately, enhanced IRS-1 degradation (22,23). Several PKC isoforms, especially PKCδ, have been shown to phosphorylate IRS-1 directly, at least in vitro or in intact cells (Table 1). In addition, PKC may act upstream of other ser/thr kinases (24–26). Thus, PKC activation may enhance the ability of kinases, such as Jun NH2-terminal kinase (JNK) and inhibitor of κB kinase (IKK)-β, to phosphorylate IRS-1 at Ser-307, a key regulatory site located close to the domain that interacts with the insulin receptor (27). PKC may also act upstream of p42/44 MAPK to promote Ser-612 phosphorylation, which more specifically modulates PI 3-kinase activation (28,29).
FIG. 2.
Lipid oversupply leads to the generation of distinct intracellular mediators of insulin resistance. Fatty acids entering the cell are activated by the formation of LCAC. Saturated fatty acid favors ceramide accumulation due to the requirement for palmitate during de novo synthesis, which in turn leads to the inhibition of Akt, in part due to aPKCζ action. In contrast, DAG species derived from unsaturated fatty acids favor nPKC activation and inhibition at the level of the insulin receptor (IR), or IRS-1. In addition, another unsaturated fatty acid–derived species, dilinoleoyl-phosphatidic acid (PA), can reduce IRS-1 tyrosine phosphorylation in a PKC-independent manner (49). G3P, glycerol 3-phosphate; LPA, lysophosphatidic acid; TG, triglyceride. See text for further details.
TABLE 1.
PKC-mediated IRS-1 phosphorylation
| Action | Effect | Reference | |
|---|---|---|---|
| PKCα | IRS-1 Ser/Thr phosphorylation | Inhibition of IR kinase activity | 30 |
| IRS-1 Ser24 phosphorylation | Reduced IRS-1 phosphatidylinositol-4,5-bisphosphate binding | 31 | |
| PKCδ | IRS-1 Ser/Thr phosphorylation | Inhibition of IR kinase activity | 30 |
| IRS-1 Ser307,323,574 phosphorylation | Reduced IRS-1 Tyr phosphorylation | 32 | |
| IRS-1 Ser24 phosphorylation | Reduced IRS-1 phosphatidylinositol-4,5-bisphosphate binding | 33 | |
| IRS-1 Ser318 phosphorylation | Early positive but longer-term negative regulation of IRS-1 signaling | 34 | |
| IRS-1 Ser357 phosphorylation | Reduced IRS-1 Tyr phosphorylation | 35 | |
| PKCθ | IRS-1 Ser/Thr phosphorylation | Inhibition of IR kinase activity | 30 |
| IRS-1 Ser1101 phosphorylation | Reduced IRS-1 Tyr phosphorylation | 36 | |
| PKCζ | IRS-1 Ser/Thr phosphorylation | Reduced IRS-1 Tyr phosphorylation | 37,38 |
| IRS-1 Ser318 phosphorylation | Reduced IRS-1 Tyr phosphorylation | 39,40 |
IRS-1 phosphorylation sites are listed as they appear in individual references. Ser302, 307, and 318 in mouse IRS-1 are Ser307, 312, and 323 in human IRS-1. IR, insulin receptor.
The insulin receptor itself, as well as IRS-1, may be a site of negative regulation by PKC (Fig. 2), particularly PKCɛ. While direct phosphorylation by PKC does not appear to modulate tyrosine kinase activity (41), an interaction of PKCɛ with the receptor in liver may affect its ability to phosphorylate its substrates (42). Alternatively, such an association may affect insulin receptor trafficking in hepatocytes (43) and hence both the clearance of insulin by the liver (44) and degradation of the receptor itself (14).
THE RELATIONSHIP BETWEEN DAG ACCUMULATION, PKC ACTIVATION, AND INSULIN RESISTANCE
The studies described above have helped to establish the hypothesis that aberrant PKC activation plays a role in the development of insulin resistance. It is generally assumed that elevations in DAG, secondary to intracellular lipid accumulation, provide the essential link in this scheme. But intracellular DAG could also be increased by insulin (45) or from de novo synthesis from glucose (46). Thus, PKCs may contribute to the secondary insulin resistance caused by the hyperinsulinemia and/or hyperglycemia occurring in the glucose-intolerant state. A further corollary of these and similar studies is that different species of fatty acids induce insulin resistance via different mechanisms (rev. in 8). PKC activation is relatively specific to DAG species composed of unsaturated fatty acid side chains (47). Thus, we have demonstrated that the elevation in DAG observed in muscle cells treated with the saturated fatty acid palmitate does not greatly stimulate PKC translocation, while the relatively minor DAG changes caused by unsaturated fatty acids can do so (48,49). Because both saturated and unsaturated fatty acids cause insulin resistance, this supports the contention that saturated fatty acids do not act principally via the DAG/PKC pathway, but through other mechanisms including ceramide-dependent signaling (50–53). (Ceramide is a potential activator of aPKCζ [Fig. 2], and a cross-talk mechanism involving sequestration of Akt by PKCζ in caveolae has also been proposed to contribute to the disruption of insulin signaling caused by saturated fatty acids [52].)
The integral link between specific fatty acids, DAG, and PKC activation has been confirmed in studies addressing the effects of DAG kinase (DGK) isoforms, which lower intracellular DAG levels and reverse the activation of PKC, but these have also highlighted the complexity of DAG signaling (49,54,55). On the one hand, a reduction in DGKδ activity potentially contributes to hyperglycemia-induced activation of PKCδ and the resultant inhibition of insulin signaling in skeletal muscle from subjects with type 2 diabetes (55). On the other hand, overexpression of DGKɛ in muscle cells, while reducing the activation of several PKCs as expected because of chronic exposure to unsaturated fatty acid, in fact compounds defects in insulin signaling (49). Such discrepancies are perhaps explained by the different substrate specificities, regulation, and cellular localization of the DGK isoforms (56) involved. In addition, and taken together with the failure of dominant-negative nPKC mutants to overcome insulin resistance in cultured myotubes (48), it can be argued that the diminished insulin action arising from lipid oversupply is not always simply explained by the accompanying DAG accumulation and PKC activation. Indeed, other lipid intermediates such as dilinoleoyl-phosphatidic acid (49) have been recently implicated as mediating insulin resistance induced by unsaturated fatty acids via pathways independent of PKC. This makes it essential to define precisely the situations where PKC does play a role and to quantify its contribution using in vivo models.
RECENT FINDINGS FROM PKC TRANSGENIC AND KNOCKOUT MICE
The importance of the correlation between the translocation of various PKC isoforms and insulin resistance has now been addressed in a more causative fashion, mostly using PKC knockout mice. Deletion studies of conventional PKC isoforms have argued against these enzymes acting to significantly reduce insulin action in vivo (57,58), although it now appears that PKCβ can impinge on this indirectly by reducing lipid oxidation in adipose tissue and thereby enhancing fat accumulation (59).
PKCθ has been extensively investigated, because it is relatively highly expressed in skeletal muscle compared with other tissues, suggesting it as a prime candidate for regulating glucose homeostasis. A complex picture has emerged. First, PKCθ knockout mice are protected against the short-term insulin resistance and defective insulin signaling in skeletal muscle generated by acute lipid infusion (60). In contrast, longer-term studies revealed that these animals exhibit an increased susceptibility to obesity and diet-induced insulin resistance (61). Likewise, a dominant-negative approach to block PKCθ by overexpressing a kinase-inactive mutant in skeletal muscle also resulted in obesity, glucose intolerance, and hyperinsulinemia (62). It therefore appears that while PKCθ can acutely inhibit insulin signal transduction, it plays other important roles that protect against obesity. Pertinently, activation of PKCθ has usually been inferred from an increased membrane-to-cytosolic ratio, but this is often the consequence of the reduction in cytosolic protein rather than an increase in membrane association (11,12,63). These features could equally be interpreted as an overall downregulation of PKCθ and diminished functional capacity, consistent with it playing a positive role in insulin sensitivity in the longer term.
Other studies addressing causation have indicated multiple roles for hepatic PKCɛ in the modulation of glucose homeostasis. Antisense oligonucleotides against PKCɛ were able to reverse a defect in insulin receptor kinase activity and the suppression of hepatic glucose production observed in rats fed for 3 days on a high-fat diet based on safflower oil (rich in the unsaturated fatty acid linoleate) (42). This extends the correlation between PKCɛ translocation and liver insulin resistance observed under short-term conditions (18). In contrast, no improvement in hepatic insulin signaling was observed in mice globally deleted in PKCɛ and maintained on a long-term diet rich in saturated fatty acids and sucrose, which induces obesity and profound insulin resistance (44). Surprisingly, however, glucose tolerance was completely restored in the PKCɛ knockout mice. This was explained by a greater availability of insulin, which compensated for, rather than reversed, insulin resistance. Two phenotypes in the PKCɛ null mice contributed to this compensation: an enhancement in glucose-stimulated insulin secretion by pancreatic β-cells (see below) and a reduction in hepatic insulin clearance (44). Importantly, there was no increase in hepatic triglyceride or fatty acid content in PKCɛ knockout mice, indicating that the transiently enhanced insulin levels in response to a glucose challenge did not promote steatosis.
The contrasting interpretations of the role of PKCɛ in hepatic insulin action arising from these studies might be explained by differences in both the type and the duration of the fat diets used (42,44). First, the use of a diet rich in unsaturated fat is more likely to activate PKC (48), while a saturated fat diet may act predominantly through the elevation of ceramide (50,51,53). Second, it is possible that PKCɛ contributes significantly to hepatic insulin resistance in an acute fashion but that other PKCɛ-independent mechanisms become prevalent over time, such that PKCɛ deletion is no longer sufficient to protect insulin signaling. Finally, compensation for PKCɛ deletion by other PKC isoforms, such as PKCδ, might occur in liver and muscle, but not in β-cells. Under these conditions, enhanced insulin secretion (see below) might overcome the ongoing loss of insulin sensitivity because of the high-fat diet.
Overall, it can be concluded that individual PKC isoforms, especially PKCθ and PKCɛ, play multiple roles in the modulation of insulin action, even within one particular tissue such as muscle or liver. In the case of PKCθ, these roles appear to be discordant, making this enzyme less suitable as a therapeutic target. In contrast, although deletion or reduction of PKCɛ has been shown to produce divergent effects, collectively these should all be beneficial for glucose homeostasis. It still remains to be determined, however, whether the key mechanism of action of PKCɛ relates to the generation of insulin resistance, as originally envisioned, or whether the newly recognized role in regulating insulin availability predominates.
PKC IN β-CELLS
Surprisingly, given the longstanding focus on PKCs in insulin resistance, there have been relatively few studies addressing a corresponding role in β-cell dysfunction. Before describing this work, however, it is necessary to outline what is known about the normal physiological functions of PKC in β-cells, particularly its controversial role in nutrient-stimulated insulin secretion and its regulation of β-cell mass. Other aspects less directly relevant to type 2 diabetes, such as the well-established involvement of PKC downstream of muscarinic cholinergic receptors, have been reviewed elsewhere and will not be addressed further here (64,65).
PKC ISOFORMS AND GLUCOSE-STIMULATED INSULIN SECRETION
It has been known for more than 25 years that activation of PKC, using pharmacological stimulators such as phorbol esters, was sufficient to augment insulin secretion, particularly in conjunction with a stimulus that raised intracellular Ca2+ (66). Because glucose promoted phosphoinositide hydrolysis in β-cells, secondary to voltage-gated Ca2+ influx and a Ca2+-dependent stimulation of phospholipase C (67), it was envisioned that the DAG/PKC pathway might contribute to glucose-stimulated insulin secretion (GSIS). However, DAG could also be generated via de novo synthesis, either from glucose directly (68,69) or secondarily to a switch in endogenous fatty acid metabolism away from β-oxidation and toward esterification products. The latter phenomenon has been widely documented and arises via glucose-enhanced anaplerosis leading to increases in malonyl CoA, which subsequently inhibits CPT1, a key control point of β-oxidation (70). The further realization that CoA derivatives of long-chain fatty acids (long-chain acyl-CoA [LCAC]) could themselves activate PKCs has underpinned ongoing support for this proposed signaling function of lipid partitioning. Thus, irrespective of whether PKC activation was triggered by DAG generated from phosphoinositide hydrolysis or by LCACs from endogenous metabolism, the net result would be an amplification of the more proximal Ca2+-dependent signals that initiate GSIS (70,71).
Nevertheless, for technical reasons, it has been difficult to prove or disprove this role of PKC. Rat and mouse islets and (to variable extents) β-cell lines express multiple PKC isoforms, including PKCα, -βII, -δ, -ɛ, -ζ, and -ι (72–75). Moreover, the framework within which PKCs function might differ between rat and mouse β-cells. Rat islets display a second phase of GSIS, which is both greatly exaggerated compared with mouse and much more sensitive to the (nonspecific) PKC inhibitor staurosporine (76). Human islets behave more similarly to mouse than to rat islets, displaying a modest second phase that is resistant to staurosporine (69,77). Another technical limitation is that although measuring PKC translocation by immunoblotting is more specific than activity assays, this may suffer from redistribution artifacts during sample processing. Subcellular localization by microscopy is potentially compounded by cross-reactivity of antisera (for studies of endogenous protein) or overexpression artifacts (when using transfected reporters). Optimal visualization of the latter often also relies on complex correction of background signals and/or somewhat artificial conditions.
Nevertheless, there is a general consensus that glucose promotes a rapid Ca2+-dependent translocation of cPKC isoforms (particularly PKCα) to the plasma membrane (78–80). Glucose also stimulates a redistribution of PKCɛ, in this case independently of Ca2+ (79,81), to sites containing insulin granules at either the plasma membrane (82) or near the nucleus (83). Translocation does not necessarily equate to functional activation, however, and demonstrations of glucose-stimulated phosphorylation of PKC substrates in β-cells have been inconsistent or poorly correlated with secretion (75,84–86). We demonstrated that glucose normally induces only a modest and transient increase in phosphorylation of cPKC substrates in islets, but this is significantly enhanced in the presence of phosphatase inhibitors (75). This implies that a concomitant stimulation of protein phosphatases by glucose (64) might counteract the consequences of PKC activation.
Determining whether PKC activation is necessary for GSIS has also been problematic. Global downregulation of PKC activity by chronic exposure to phorbol esters, or using nonspecific inhibitors, has produced variable results (84–88) possibly explained by species differences. Nevertheless, there is consistent evidence that inhibitors of conventional PKCs completely block insulin secretion in response to phorbol esters, but affect GSIS only modestly at best (75,86,89,90). Overexpression of a kinase-dead PKCα construct in rat islets augmented first-phase, and slightly diminished second-phase, GSIS in batch incubations (91), but this was not observed when assessed directly using islet perifusion (75). Thus, the general conclusion from these studies is that conventional PKCs are not required for a robust first- or second-phase GSIS, although their activation by glucose might exert a minor modulatory influence.
A role for PKCɛ in GSIS has also been proposed (79,82). In one study, in which rat islets were “skinned” to facilitate entry of a PKCɛ inhibitory peptide, a partial requirement for PKCɛ in GSIS was demonstrated (79). Using the same peptide, but in a cell-permeant form, we observed no requirement for PKCɛ in GSIS from mouse islets (44). Another report concluded that overexpression of PKCɛ in β-cells of ob/ob mice was sufficient to enhance secretion, although this was not assayed directly, but extrapolated from changes in membrane capacitance during the initial recording (82). Capacitance changes due to glibenclamide (82) or inositol hexakisphosphate (92) were also blocked by kinase-dead and/or antisense constructs, but this approach was not extended to examine a direct requirement of PKCɛ in GSIS. In contrast, using PKCɛ knockout mice, glucose tolerance and insulin excursions during whole-body glucose tolerance tests were similar to wild-type animals (44). Likewise, no differences in GSIS were observed when control and PKCɛ null islets were compared in batch incubations or perifusion studies ex vivo (44).
PKC AND INSULIN SECRETION DUE TO FATTY ACIDS
There are also indications of PKC involvement in the potentiation of GSIS by fatty acids (71). First, treatment of β-cells with fatty acids has been variously shown to activate PKC (93–96). Second, general PKC inhibitors partially attenuate the enhancement of insulin secretion due to fatty acids (97–99), even in mouse islets where these inhibitors barely affect GSIS (97,98). Isoform specificity is uncertain, since selective inhibitors or overexpression strategies have not been used in this context. Most evidence, however, points to an involvement of non-cPKCs and therefore novel or atypical isoforms (99–101). Early studies emphasized the importance of endogenous lipid metabolism, and especially a switch from β-oxidation to esterification products, in the mechanism of activation (94). This is consistent with demonstrations that the novel PKCs in particular can be directly activated by LCACs (96). Another possibility, yet to be explored fully, is that PKC could be activated downstream of the cell surface GPR40 receptor, which binds a variety of fatty acids and is known to couple to phospholipase C and hence presumably to phosphoinositide hydrolysis (102).
REGULATION OF β-CELL MASS OR DIFFERENTIATION BY PKC
There is strong evidence to suggest that PKCζ positively regulates β-cell proliferation in response to a variety of growth factors, although the signaling partners up- and downstream of PKCζ are yet to be determined (103–105). Specificity was confirmed in one study by demonstrating that proliferation was not altered by knockdown of PKCι expression (105), consistent with observations that islets from PKCι knockout mice are no smaller than those from wild-type animals (106). There is also evidence that PKCζ can positively regulate expression of the key β-cell transcription factor Pdx-1 in response to either IGF-1 (104) or glucose (107). This would seem more relevant to the control of differentiation, as opposed to proliferation, and is consistent with a requirement for PKCζ in the glucose-dependent expression of K+ channel subunits required for GSIS (108). A developmental role for PKCι has also been strongly supported. PKCι knockout mice display defective GSIS in vivo and ex vivo, but this is explained less by a specific role in stimulus secretion coupling than by a general effect on β-cell differentiation exerted upstream of the HNF3β transcription factor (106). This is not dissimilar to the purported roles of PKCζ in the glucose-dependent differentiation program described above (107,108) and so may point to redundant roles for aPKCs in this particular instance.
There is less extensive evidence for involvement of nPKC isoforms in regulating β-cell mass. Fatty acids disrupt signaling downstream of receptors important for β-cell proliferation, and nPKC isoforms have been implicated in this context (96). Islet mass was not altered by PKCɛ deletion in mice maintained on a chow diet (44). On a high-fat diet, however, islet cell mass and proliferation were augmented in wild-type animals in partial compensation for the accompanying insulin resistance. These increases in mass and proliferation were not observed in the PKCɛ knockout mice (44). This may represent an adaptive response, in that compensation would no longer be required because of the improved glucose tolerance observed under these conditions. Alternatively, PKCɛ might play a more active role in β-cell proliferation, which is absent in the knockout mice. Further work is required to resolve these issues.
As in many cell types, PKCδ exerts a pro-apoptotic role in pancreatic β-cells (95,109,110). This was first shown in cytokine-mediated cell destruction and involved an increase in mRNA stabilization for transcripts of inducible NO synthase (109). A more distal role, secondary to generation of a constitutively active PKCδ fragment after its cleavage by caspase 3, was also implicated (110). Inhibition of PKCδ also partially protected against lipoapoptosis (95). In this instance, activation appeared to be downstream of Gq, which potentially implicates a receptor such as GPR40. Further investigation of this role of PKCδ would be of interest. Seemingly at odds with this pro-apoptotic function, other authors have demonstrated a partial requirement in GSIS using islets from PKCδ knockout mice (111). This contrasted with an earlier study using overexpression of kinase-dead PKCδ in isolated rat islets using adenovirus (75). Moreover, translocation of PKCδ has never been observed when tested in response to glucose (80,83,100,111).
PKC IN SECRETORY DYSFUNCTION OF β-CELLS
We have recently established an unexpected role for PKCɛ in the development of β-cell lipotoxicity (44). As discussed above, deletion of PKCɛ resulted in a normalization of glucose tolerance in fat-fed mice because of an enhancement of insulin availability rather than improved insulin sensitivity. This was confirmed by comparing GSIS from wild-type or PKCɛ null islets chronically exposed to elevated fatty acids ex vivo. The secretory defects induced under these conditions were prevented by deletion of PKCɛ, and likewise GSIS was improved in islets of diabetic db/db mice when treated ex vivo with a PKCɛ inhibitory peptide. In all cases, in vivo and ex vivo, the enhancement of insulin secretion was dependent on a diabetic milieu or prior lipid exposure; unimpaired GSIS was not altered by functional inhibition of PKCɛ (44). This suggests that activation of PKCɛ is either intimately involved in the actual process whereby secretory defects are induced by chronically elevated fatty acid or lipid overload or that it acts proximally to that process.
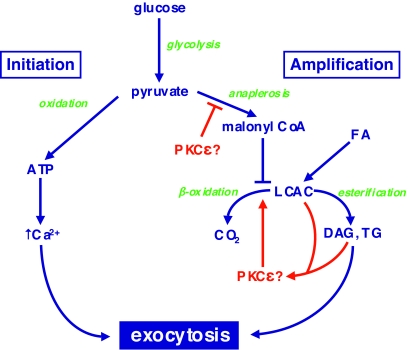
PKCɛ most probably affects secretion via multiple mechanisms. Deletion of this isoform resulted in slight (∼25%) increases in both insulin content and insulin mRNA, suggesting that it might play a minor role in regulating insulin gene expression (44). But the modesty of these increases, and their lack of dependence on prior lipid exposure, suggests they do not make a major contribution to the reversal of defective secretion. Instead, our findings implicated the amplification pathway of GSIS, based on observations of lipid partitioning in β-cells during acute exposure to glucose (44). Normally this is associated with a switch from β-oxidation toward esterification pathways, but chronic pretreatment with fatty acids disrupts this switch by upregulating β-oxidation (70). Deletion of PKCɛ helps to restore the appropriate balance between esterification and oxidation, and this may contribute to the normalization of insulin secretion (Fig. 3). At present, however, the role of lipid partitioning in regulating GSIS remains somewhat controversial. It is unclear whether this phenomenon is itself causal, activating signaling cascades that augment secretion, or whether it is merely a readout of potentially more important events occurring upstream in anaplerotic pathways (Fig. 3). It is possible that further defining the role of PKCɛ might actually help resolve some of these basic unsolved questions in stimulus-secretion coupling in β-cells.
FIG. 3.
Putative site of action of PKCɛ in the amplification pathway of GSIS. Pyruvate, derived from glycolysis, undergoes two metabolic fates in mitochondria that together regulate GSIS. In the initiation pathway, it is subjected to oxidative phosphorylation to generate ATP, which leads to closure of ATP-dependent K+ channels, depolarization, and the gating of Ca2+ influx. In the amplification pathway, pyruvate augments Krebs cycle intermediates (anaplerosis), some of which can be exported to the cytosol to generate malonyl-CoA. This results in an inhibition of the β-oxidation of LCACs derived from exogenous fatty acids or mobilization of endogenous lipid stores. This favors the formation of esterification products di- or triacylglycerol (DAG or TG, respectively). PKCɛ, potentially activated by LCACs or DAG, appears to promote oxidation of lipid fuels at the expense of esterification pathways that are implicated in the amplification pathway. Whether PKCɛ acts directly at this site, or upstream at a step in anaplerosis, remains to be determined. For further explanation, see the text and Ref. 44.
PKC AS A THERAPEUTIC TARGET FOR TYPE 2 DIABETES
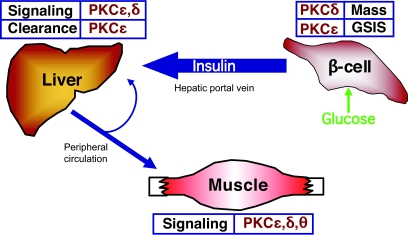
Much of the work described above is based on the premise that inhibition of PKCs could be of benefit in the treatment of type 2 diabetes. Within that framework, we will now attempt to summarize our view of the current state of the field (Fig. 4). Most speculation over the last decade has centered on PKC antagonists as potential insulin sensitizers. This is based on a body of work demonstrating that PKCs possess the capacity to disrupt insulin signaling and that they are activated in muscle and liver during insulin resistance. A causal relationship, however, between PKC activation and insulin resistance has been difficult to substantiate. In particular, the early promise of PKCθ as potential mediator of muscle insulin resistance has been clouded by an additional role in protecting against obesity (61,62). In liver, inhibition of PKCɛ might improve insulin sensitivity in short-term models (42); this was not the case in longer-term dietary regimens that are arguably more representative of obesity-induced insulin resistance (44). Further work is required to resolve these issues, particularly because a compensatory activation of other PKC isoforms may have masked the effects of PKCɛ deletion on insulin sensitivity in the knockout model. A possible candidate in that regard is PKCδ, which has been implicated in studies of IRS phosphorylation (30,32–35), although its role in vivo has not been assessed directly.
FIG. 4.
Potential sites at which individual PKC isoforms might be beneficially targeted for the treatment of type 2 diabetes. Inhibition of PKCɛ is predicted to improve insulin availability by restoring defective GSIS and by diminishing hepatic insulin clearance. This may also improve insulin sensitivity in liver and muscle. Targeting PKCδ may be of benefit in maintaining β-cell mass and in treating insulin resistance in muscle and liver. See text for details.
Regardless of the ultimate involvement or not of PKCs in insulin resistance, the rationale for targeting PKCs in the treatment of type 2 diabetes has been extended by our recent demonstration of an unexpected role for PKCɛ in regulating insulin availability (Fig. 4). Indeed, targeting PKCɛ is likely to have several advantages over existing therapies that enhance insulin secretion. Most importantly, PKCɛ inhibition appears to act very close to the actual cause of impaired secretion, or at least specifically addresses its consequences. To our knowledge, no other strategy for promoting insulin secretion shows this benefit. Existing therapies, such as sulfonylureas or GLP-1 agonists, bypass the secretory defect rather than addressing it directly. Another advantage is that PKCɛ deletion selectively augmented the first phase of GSIS, which is crucial for regulating glucose tolerance and which is lost early in the development of type 2 diabetes. Finally, the effects of inhibiting PKCɛ in β-cells were complemented by a reduction in hepatic insulin clearance (44). Both aspects contributed to the enhanced availability of insulin. Taken together, these features suggest that a single therapy, based on inhibition of PKCɛ, could act at multiple sites and in manners different from, and therefore complimentary to, existing treatments for type 2 diabetes.
Obviously, it is difficult to develop truly selective inhibitors of protein kinases and, indeed, it remains to be seen whether other potential consequences of PKCɛ inhibition might preclude its adoption as a therapy. On the other hand, off-target effects might actually prove to be beneficial if they included, for example, inhibition of PKCδ, which might help preserve β-cell mass and/or overcome insulin resistance (Fig. 4). Regardless of the ultimate clinical utility of PKC inhibitors, however, it seems that there is much useful information to be obtained in the short term by the further study of the roles of PKC in regulating glucose homeostasis. This is a particular need for mechanistic insight into how PKCɛ controls insulin uptake in hepatocytes and restores GSIS in diabetic β-cells.
Acknowledgments
Work from the authors’ laboratories has been supported by grants from the National Health and Medical Research Council, Diabetes Australia Research Trust, Eli Lilly Australia, and the Juvenile Diabetes Research Foundation. The authors apologize to those whose work could not be cited, owing to lack of space.
The costs of publication of this article were defrayed in part by the payment of page charges. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
REFERENCES
- 1.Mellor H, Parker PJ: The extended protein kinase C superfamily. Biochem J 332 :281 –292,1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Farese RV: Phospholipid signaling systems in insulin action. Am J Med 85 :36 –43,1988 [DOI] [PubMed] [Google Scholar]
- 3.Akimoto K, Mizuno K, Osada S, Hirai S, Tanuma S, Suzuki K, Ohno S: A new member of the third class in the protein kinase C family, PKCλ, expressed dominantly in an undifferentiated mouse embryonal carcinoma cell line and also in many tissues and cells. J Biol Chem 269 :12677 –12683,1994 [PubMed] [Google Scholar]
- 4.Selbie LA, Schmitz-Peiffer C, Sheng YH, Biden TJ: Molecular cloning and characterization of PKCι, an atypical isoform of protein kinase-C derived from insulin-secreting cells. J Biol Chem 268 :24296 –24302,1993 [PubMed] [Google Scholar]
- 5.Newton AC: Regulation of the ABC kinases by phosphorylation: protein kinase C as a paradigm. Biochem J 370 :361 –371,2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Farese RV, Sajan MP, Standaert ML: Insulin-sensitive protein kinases (atypical protein kinase C and protein kinase B/Akt): actions and defects in obesity and type II diabetes. Exp Biol Med 230 :593 –605,2005 [DOI] [PubMed] [Google Scholar]
- 7.Sampson SR, Cooper DR: Specific protein kinase C isoforms as transducers and modulators of insulin signaling. Mol Genet Metab 89 :32 –47,2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Schmitz-Peiffer C: Signalling aspects of insulin resistance in skeletal muscle: mechanisms induced by lipid oversupply. Cell Signal 12 :583 –594,2000 [DOI] [PubMed] [Google Scholar]
- 9.Idris I, Gray S, Donnelly R: Protein kinase C activation: isozyme-specific effects on metabolism and cardiovascular complications in diabetes. Diabetologia 44 :659 –673,2001 [DOI] [PubMed] [Google Scholar]
- 10.Schmitz-Peiffer C: Protein kinase C and lipid-induced insulin resistance in skeletal muscle. Ann N Y Acad Sci 967 :146 –157,2002 [DOI] [PubMed] [Google Scholar]
- 11.Schmitz-Peiffer C, Browne CL, Oakes ND, Watkinson A, Chisholm DJ, Kraegen EW, Biden TJ: Alterations in the expression and cellular localization of protein kinase C isozymes ɛ and θ are associated with insulin resistance in skeletal muscle of the high-fat-fed rat. Diabetes 46 :169 –178,1997 [DOI] [PubMed] [Google Scholar]
- 12.Schmitz-Peiffer C, Oakes ND, Browne CL, Kraegen EW, Biden TJ: Reversal of chronic alterations of skeletal muscle protein kinase C from fat-fed rats by BRL-49653. Am J Physiol 273 :E915 –E921,1997 [DOI] [PubMed] [Google Scholar]
- 13.Qu X, Seale JP, Donnelly R: Tissue and isoform-selective activation of protein kinase C in insulin-resistant obese Zucker rats: effects of feeding. J Endocrinol 162 :207 –214,1999 [DOI] [PubMed] [Google Scholar]
- 14.Ikeda Y, Olsen GS, Ziv E, Hansen LL, Busch AK, Hansen BF, Shafrir E, Mosthaf-Seedorf L: Cellular mechanism of nutritionally induced insulin resistance in Psammomys obesus: overexpression of protein kinase Cɛ in skeletal muscle precedes the onset of hyperinsulinemia and hyperglycemia. Diabetes 50 :584 –592,2001 [DOI] [PubMed] [Google Scholar]
- 15.Griffin ME, Marcucci MJ, Cline GW, Bell K, Barucci N, Lee D, Goodyear LJ, Kraegen EW, White MF, Shulman GI: Free fatty acid-induced insulin resistance is associated with activation of protein kinase C θ and alterations in the insulin signaling cascade. Diabetes 48 :1270 –1274,1999 [DOI] [PubMed] [Google Scholar]
- 16.Laybutt DR, Schmitz-Peiffer C, Saha AK, Ruderman NB, Biden TJ, Kraegen EW: Muscle lipid accumulation and protein kinase C activation in the insulin-resistant chronically glucose-infused rat. Am J Physiol 277 :E1070 –E1076,1999 [DOI] [PubMed] [Google Scholar]
- 17.Considine RV, Nyce MR, Allen LE, Morales LM, Triester S, Serrano J, Colberg J, Lanzajacoby S, Caro JF: Protein kinase C is increased in the liver of humans and rats with noninsulin-dependent diabetes mellitus: an alteration not due to hyperglycemia. J Clin Invest 95 :2938 –2944,1995 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Samuel VT, Liu ZX, Qu XQ, Elder BD, Bilz S, Befroy D, Romanelli AJ, Shulman GI: Mechanism of hepatic insulin resistance in non-alcoholic fatty liver disease. J Biol Chem 279 :32345 –32353,2004 [DOI] [PubMed] [Google Scholar]
- 19.Lam TKT, Yoshii H, Haber CA, Bogdanovic E, Lam L, Fantus IG, Giacca A: Free fatty acid-induced hepatic insulin resistance: a potential role for protein kinase Cδ. Am J Physiol 283 :E682 –E691,2002 [DOI] [PubMed] [Google Scholar]
- 20.Itani SI, Ruderman NB, Schmieder F, Boden G: Lipid-induced insulin resistance in human muscle is associated with changes in diacylglycerol, protein kinase C, and IκB-α. Diabetes 51 :2005 –2011,2002 [DOI] [PubMed] [Google Scholar]
- 21.Boden G, She P, Mozzoli M, Cheung P, Gumireddy K, Reddy P, Xiang X, Luo Z, Ruderman N: Free fatty acids produce insulin resistance and activate the proinflammatory nuclear factor-κB pathway in rat liver. Diabetes 54 :3458 –3465,2005 [DOI] [PubMed] [Google Scholar]
- 22.Schmitz-Peiffer C, Whitehead JP: IRS-1 regulation in health and disease. IUBMB Life 55 :367 –374,2003 [DOI] [PubMed] [Google Scholar]
- 23.Zick Y: Ser/Thr phosphorylation of IRS proteins: a molecular basis for insulin resistance. Sci STKE 2005 :pe4 ,2005 [DOI] [PubMed] [Google Scholar]
- 24.Khoshnan A, Bae D, Tindell CA, Nel AE: The physical association of protein kinase C θ with a lipid raft-associated inhibitor of κB factor kinase (IKK) complex plays a role in the activation of the NF-κB cascade by TCR and CD28. J Immunol 165 :6933 –6940,2000 [DOI] [PubMed] [Google Scholar]
- 25.Tojima Y, Fujimoto A, Delhase M, Chen Y, Hatakeyama S, Nakayama K, Kaneko Y, Nimura Y, Motoyama N, Ikeda K, Karin M, Nakanishi M: NAK is an IκB kinase-activating kinase. Nature 404 :778 –782,2000 [DOI] [PubMed] [Google Scholar]
- 26.Jaeschke A, Davis RJ: Metabolic stress signaling mediated by mixed-lineage kinases. Mol Cell Neurosci 27 :498 –508,2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Aguirre V, Werner ED, Giraud J, Lee YH, Shoelson SE, White MF: Phosphorylation of Ser(307) in insulin receptor substrate-1 blocks interactions with the insulin receptor and inhibits insulin action. J Biol Chem 277 :1531 –1537,2002 [DOI] [PubMed] [Google Scholar]
- 28.De Fea K, Roth RA: Protein kinase c modulation of insulin receptor substrate-1 tyrosine phosphorylation requires serine 612. Biochemistry 36 :12939 –12947,1997 [DOI] [PubMed] [Google Scholar]
- 29.De Fea K, Roth RA: Modulation of insulin receptor substrate-1 tyrosine phosphorylation and function by mitogen-activated protein kinase. J Biol Chem 272 :31400 –31406,1997 [DOI] [PubMed] [Google Scholar]
- 30.Kellerer M, Mushack J, Seffer E, Mischak H, Ullrich A, Haring HU: Protein kinase c isoforms α, δ, θ, and require insulin receptor substrate-1 to inhibit the tyrosine kinase activity of the insulin receptor in human kidney embryonic cells (hek 293 cells). Diabetologia 41 :833 –838,1998 [DOI] [PubMed] [Google Scholar]
- 31.Nawaratne R, Gray A, Jorgensen CH, Downes CP, Siddle K, Sethi JK: Regulation of insulin receptor substrate 1 pleckstrin homology domain by protein kinase C: role of serine 24 phosphorylation. Mol Endocrinol 20 :1838 –1852,2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Greene MW, Morrice N, Garofalo RS, Roth RA: Modulation of human insulin receptor substrate-1 tyrosine phosphorylation by protein kinase C δ. Biochem J 378 :105 –116,2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Greene MW, Ruhoff MS, Roth RA, Kim JA, Quon MJ, Krause JA: PKCδ-mediated IRS-1 Ser24 phosphorylation negatively regulates IRS-1 function. Biochem Biophys Res Comm 349 :976 –986,2006 [DOI] [PubMed] [Google Scholar]
- 34.Weigert C, Hennige AM, Lehmann R, Brodbeck K, Baumgartner F, Schauble M, Haring HU, Schleicher ED: Direct cross-talk of interleukin-6 and insulin signal transduction via insulin receptor substrate-1 in skeletal muscle cells. J Biol Chem 281 :7060 –7067,2006 [DOI] [PubMed] [Google Scholar]
- 35.Waraich RS, Weigert C, Kalbacher H, Hennige AM, Lutz S, Haring HU, Schleicher ED, Voelter W, Lehmann R: Phosphorylation of Ser357of rat insulin receptor substrate-1 mediates adverse effects of protein kinase C-δ on insulin action in skeletal muscle cells. J Biol Chem 283 :11226 –11233,2008 [DOI] [PubMed] [Google Scholar]
- 36.Li Y, Soos TJ, Li XH, Wu J, DeGennaro M, Sun XJ, Littman DR, Birnbaum MJ, Polakiewicz RD: Protein kinase C θ inhibits insulin signaling by phosphorylating IRS1 at Ser(1101). J Biol Chem 279 :45304 –45307,2004 [DOI] [PubMed] [Google Scholar]
- 37.Liu YF, Paz K, Herschkovitz A, Alt A, Tennenbaum T, Sampson SR, Ohba M, Kuroki T, LeRoith D, Zick Y: Insulin stimulates PKCζ-mediated phosphorylation of insulin receptor substrate-1 (IRS-1): a self-attenuated mechanism to negatively regulate the function of IRS proteins. J Biol Chem 276 :14459 –14465,2001 [DOI] [PubMed] [Google Scholar]
- 38.Ravichandran LV, Esposito DL, Chen J, Quon MJ: Protein kinase C-ζ phosphorylates insulin receptor substrate-1 and impairs its ability to activate phosphatidylinositol 3-kinase in response to insulin. J Biol Chem 276 :3543 –3549,2001 [DOI] [PubMed] [Google Scholar]
- 39.Moeschel K, Beck A, Weigert C, Lammers R, Kalbacher H, Voelter W, Schleicher ED, Haring HU, Lehmann R: Protein kinase C-ζ-induced phosphorylation of Ser(318) in insulin receptor substrate-1 (IRS-1) attenuates the interaction with the insulin receptor and the tyrosine phosphorylation of IRS-1. J Biol Chem 279 :25157 –25163,2004 [DOI] [PubMed] [Google Scholar]
- 40.Weigert C, Hennige AM, Brischmann T, Beck A, Moeschel K, Schauble M, Brodbeck K, Haring HU, Schleicher ED, Lehmann R: The phosphorylation of Ser(318) of insulin receptor substrate 1 is not per se inhibitory in skeletal muscle cells but is necessary to trigger the attenuation of the insulin-stimulated signal. J Biol Chem 280 :37393 –37399,2005 [DOI] [PubMed] [Google Scholar]
- 41.Coghlan MP, Siddle K: Phorbol esters induce insulin receptor phosphorylation in transfected fibroblasts without affecting tyrosine kinase activity. Biochem Biophys Res Comm 193 :371 –377,1993 [DOI] [PubMed] [Google Scholar]
- 42.Samuel VT, Liu ZX, Wang A, Beddow SA, Geisler JG, Kahn M, Zhang XM, Monia BP, Bhanot S, Shulman GI: Inhibition of protein kinase Cɛ prevents hepatic insulin resistance in nonalcoholic fatty liver disease. J Clin Invest 117 :739 –745,2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Hribal ML, D’Alfonso R, Giovannone B, Lauro D, Liu YY, Borboni P, Federici M, Lauro R, Sesti G: The sulfonylurea glimepiride regulates intracellular routing of the insulin-receptor complexes through their interaction with specific protein kinase C isoforms. Mol Pharmacol 59 :322 –330,2001 [DOI] [PubMed] [Google Scholar]
- 44.Schmitz-Peiffer C, Laybutt DR, Burchfield JG, Gurisik E, Narasimhan S, Mitchell CJ, Pedersen DJ, Braun U, Cooney GJ, Leitges M, Biden TJ: Inhibition of PKCɛ improves glucose-stimulated insulin secretion and reduces insulin clearance. Cell Metab 6 :320 –328,2007 [DOI] [PubMed] [Google Scholar]
- 45.Standaert ML, Bandyopadhyay G, Zhou XP, Galloway L, Farese RV: Insulin stimulates phospholipase D-dependent phosphatidylcholine hydrolysis, Rho translocation, de novo phospholipid synthesis, and diacylglycerol/protein kinase C signaling in L6 myotubes. Endocrinology 137 :3014 –3020,1996 [DOI] [PubMed] [Google Scholar]
- 46.Ishizuka T, Hoffman J, Cooper DR, Watson JE, Pushkin DB, Farese RV: Glucose-induced synthesis of diacylglycerol de novo is associated with translocation (activation) of protein kinase C in rat adipocytes. FEBS Lett 249 :234 –238,1989 [DOI] [PubMed] [Google Scholar]
- 47.Hodgkin MN, Pettitt TR, Martin A, Michell RH, Pemberton AJ, Wakelam MJ: Diacylglycerols and phosphatidates: which molecular species are intracellular messengers? Trends Biochem Sci 23 :200 –204,1998 [DOI] [PubMed] [Google Scholar]
- 48.Cazzolli R, Craig DL, Biden TJ, Schmitz-Peiffer C: Inhibition of glycogen synthesis by fatty acid in C2C12 muscle cells is independent of PKC-α, -ɛ, and -θ. Am J Physiol 282 :E1204 –E1213,2002 [DOI] [PubMed] [Google Scholar]
- 49.Cazzolli R, Mitchell TW, Burchfield JG, Pedersen DJ, Turner N, Biden TJ, Schmitz-Peiffer C: Dilinoleoyl-phosphatidic acid mediates reduced IRS-1 tyrosine phosphorylation in rat skeletal muscle cells and mouse muscle. Diabetologia 50 :1732 –1742,2007 [DOI] [PubMed] [Google Scholar]
- 50.Schmitz-Peiffer C, Craig DL, Biden TJ: Ceramide generation is sufficient to account for the inhibition of the insulin-stimulated PKB pathway in C2C12 skeletal muscle cells pretreated with palmitate. J Biol Chem 274 :24202 –24210,1999 [DOI] [PubMed] [Google Scholar]
- 51.Cazzolli R, Carpenter L, Biden TJ, Schmitz-Peiffer C: A role for protein phosphatase 2A-like activity, but not atypical protein kinase C ζ, in the inhibition of protein kinase B/Akt and glycogen synthesis by palmitate. Diabetes 50 :2210 –2218,2001 [DOI] [PubMed] [Google Scholar]
- 52.Hajduch E, Turban S, Le Liepvre X, Le Lay S, Lipina C, Dimopoulos N, Dugail I, Hundal HS: Targeting of PKCζ and PKB to caveolin-enriched microdomains represents a crucial step underpinning the disruption in PKB-directed signaling by ceramide. Biochem J 410 :369 –379,2008 [DOI] [PubMed] [Google Scholar]
- 53.Holland WL, Brozinick JT, Wang LP, Hawkins ED, Sargent KM, Liu Y, Narra K, Hoehn KL, Knotts TA, Siesky A, Nelson DH, Karathanasis SK, Fontenot GK, Birnbaum MJ, Summers SA: Inhibition of ceramide synthesis ameliorates glucocorticoid-, saturated-fat-, and obesity-induced insulin resistance. Cell Metab 5 :167 –179,2007 [DOI] [PubMed] [Google Scholar]
- 54.Pettitt TR, Wakelam M: Diacylglycerol kinase ɛ, but not ζ selectively removes polyunsaturated diacylglycerol, inducing altered protein kinase C distribution in vivo. J Biol Chem 274 :36181 –36186,1999 [DOI] [PubMed] [Google Scholar]
- 55.Chibalin AV, Leng Y, Vieira E, Krook A, Bjornholm M, Long YC, Kotova O, Zhong Z, Sakane F, Steiler T, Nylen C, Wang J, Laakso M, Topham MK, Gilbert M, Wallberg-Henriksson H, Zierath JR: Downregulation of diacylglycerol kinase δ contributes to hyperglycemia-induced insulin resistance. Cell 132 :375 –386,2008 [DOI] [PubMed] [Google Scholar]
- 56.Topham MK: Signaling roles of diacylglycerol kinases. J Cell Biochem 97 :474 –484,2006 [DOI] [PubMed] [Google Scholar]
- 57.Leitges M, Plomann M, Standaert ML, Bandyopadhyay G, Sajan MP, Kanoh Y, Farese RV: Knockout of PKCα enhances insulin signaling through PI3K. Mol Endocrinol 16 :847 –858,2002 [DOI] [PubMed] [Google Scholar]
- 58.Standaert ML, Bandyopadhyay G, Galloway L, Soto J, Ono Y, Kikkawa U, Farese RV, Leitges M: Effects of knockout of the protein kinase C β gene on glucose transport and glucose homeostasis. Endocrinology 140 :4470 –4477,1999 [DOI] [PubMed] [Google Scholar]
- 59.Bansode R, Huang W, Roy S, Mehta M, Mehta KD: PKCβ deficiency increases fatty acid oxidation and reduces fat storage. J Biol Chem 283 :231 –236,2008 [DOI] [PubMed] [Google Scholar]
- 60.Kim JK, Fillmore JJ, Sunshine MJ, Albrecht B, Higashimori T, Kim D-W, Liu Z-X, Soos TJ, Cline GW, O’Brien WR, Littman DR, Shulman GI: PKC-θ knockout mice are protected from fat-induced insulin resistance. J Clin Invest 114 :823 –827,2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Gao Z, Wang Z, Zhang X, Butler AA, Zuberi A, Gawronska-Kozak B, Lefevre M, York D, Ravussin E, Berthoud HR, McGuinness O, Cefalu WT, Ye J: Inactivation of PKCθ leads to increased susceptibility to obesity and dietary insulin resistance in mice. Am J Physiol 292 :E84 –E91,2007 [DOI] [PubMed] [Google Scholar]
- 62.Serra C, Federici M, Buongiorno A, Senni MI, Morelli S, Segratella E, Pascuccio M, Tiveron C, Mattei E, Tatangelo L, Lauro R, Molinaro M, Giaccari A, Bouche M: Transgenic mice with dominant negative PKC-θ in skeletal muscle: a new model of insulin resistance and obesity. J Cell Physiol 196 :89 –97,2003 [DOI] [PubMed] [Google Scholar]
- 63.Yu C, Chen Y, Zong H, Wang Y, Bergeron R, Kim JK, Cline GW, Cushman SW, Cooney GJ, Atcheson B, White MF, Kraegen EW, Shulman GI: Mechanism by which fatty acids inhibit insulin activation of IRS-1 associated phosphatidylinositol 3-kinase activity in muscle. J Biol Chem 277 :50230 –50236,2002 [DOI] [PubMed] [Google Scholar]
- 64.Jones PM, Persaud SJ: Protein kinases, protein phosphorylation, and the regulation of insulin secretion from pancreatic β-cells. Endocr Rev 19 :429 –461,1998 [DOI] [PubMed] [Google Scholar]
- 65.Gilon P, Henquin JC: Mechanisms and physiological significance of the cholinergic control of pancreatic β-cell function. Endocr Rev 22 :565 –604,2001 [DOI] [PubMed] [Google Scholar]
- 66.Zawalich W, Brown C, Rasmussen H: Insulin secretion: combined effects of phorbol ester and A23187. Biochem Biophys Res Comm 117 :448 –455,1983 [DOI] [PubMed] [Google Scholar]
- 67.Biden TJ, Peter-Riesch B, Schlegel W, Wollheim CB: Ca2+-mediated generation of inositol 1,4,5-triphosphate and inositol 1,3,4,5-tetrakisphosphate in pancreatic islets: studies with K+, glucose, and carbamylcholine. J Biol Chem 262 :3567 –3571,1987 [PubMed] [Google Scholar]
- 68.Peter-Riesch B, Fathi M, Schlegel W, Wollheim CB: Glucose and carbachol generate 1,2-diacylglycerols by different mechanisms in pancreatic islets. J Clin Invest 81 :1154 –1161,1988 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Wolf BA, Easom RA, McDaniel ML, Turk J: Diacylglycerol synthesis de novo from glucose by pancreatic islets isolated from rats and humans. J Clin Invest 85 :482 –490,1990 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Deeney JT, Prentki M, Corkey BE: Metabolic control of β-cell function. Semin Cell Dev Biol 11 :267 –275,2000 [DOI] [PubMed] [Google Scholar]
- 71.Yaney GC, Corkey BE: Fatty acid metabolism and insulin secretion in pancreatic β-cells. Diabetologia 46 :1297 –1312,2003 [DOI] [PubMed] [Google Scholar]
- 72.Knutson KL, Hoenig M: Identification and subcellular characterization of protein kinase-C isoforms in insulinoma β-cells and whole islets. Endocrinology 135 :881 –886,1994 [DOI] [PubMed] [Google Scholar]
- 73.Tian YM, Urquidi V, Ashcroft SJ: Protein kinase C in β-cells: expression of multiple isoforms and involvement in cholinergic stimulation of insulin secretion. Mol Cell Endocrinol 119 :185 –193,1996 [DOI] [PubMed] [Google Scholar]
- 74.Kaneto H, Suzuma K, Sharma A, Bonner-Weir S, King GL, Weir GC: Involvement of protein kinase C β2 in c-myc induction by high glucose in pancreatic β-cells. J Biol Chem 277 :3680 –3685,2002 [DOI] [PubMed] [Google Scholar]
- 75.Carpenter L, Mitchell CJ, Xu ZZ, Poronnik P, Both GW, Biden TJ: PKCα is activated but not required during glucose-induced insulin secretion from rat pancreatic islets. Diabetes 53 :53 –60,2004 [DOI] [PubMed] [Google Scholar]
- 76.Zawalich WS, Zawalich KC: Species differences in the induction of time-dependent potentiation of insulin secretion. Endocrinology 137 :1664 –1669,1996 [DOI] [PubMed] [Google Scholar]
- 77.Henquin JC, Dufrane D, Nenquin M: Nutrient control of insulin secretion in isolated normal human islets. Diabetes 55 :3470 –3477,2006 [DOI] [PubMed] [Google Scholar]
- 78.Ganesan S, Calle R, Zawalich K, Greenawalt K, Zawalich W, Shulman GI, Rasmussen H: Immunocytochemical localization of α-protein kinase-C in rat pancreatic β-cells during glucose-induced insulin secretion. J Cell Biol 119 :313 –324,1992 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Yedovitzky M, Mochlyrosen D, Johnson JA, Gray MO, Ron D, Abramovitch E, Cerasi E, Nesher R: Translocation inhibitors define specificity of protein kinase C isoenzymes in pancreatic β-cells. J Biol Chem 272 :1417 –1420,1997 [DOI] [PubMed] [Google Scholar]
- 80.Pinton P, Tsuboi T, Ainscow EK, Pozzan T, Rizzuto R, Rutter GA: Dynamics of glucose-induced membrane recruitment of protein kinase C β II in living pancreatic islet β-cells. J Biol Chem 277 :37702 –37710,2002 [DOI] [PubMed] [Google Scholar]
- 81.Zaitsev SV, Efendic S, Arkhammar P, Bertorello AM, Berggren PO: Dissociation between changes in cytoplasmic free Ca2+ concentration and insulin secretion as evidenced from measurements in mouse single pancreatic islets. Proc Natl Acad Sci U S A 92 :9712 –9716,1995 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Mendez CF, Leibiger IB, Leibiger B, Hoy M, Gromada J, Berggren PO, Bertorello AM: Rapid association of protein kinase C-ɛ with insulin granules is essential for insulin exocytosis. J Biol Chem 278 :44753 –44757,2003 [DOI] [PubMed] [Google Scholar]
- 83.Warwar N, Efendic S, Ostenson CG, Haber EP, Cerasi E, Nesher R: Dynamics of glucose-induced localization of PKG isoenzyines in pancreatic β-cells: diabetes-related changes in the GK rat. Diabetes 55 :590 –599,2006 [DOI] [PubMed] [Google Scholar]
- 84.Easom RA, Hughes JH, Landt M, Wolf BA, Turk J, McDaniel ML: Comparison of effects of phorbol esters and glucose on protein kinase C activation and insulin secretion in pancreatic islets. Biochem J 264 :27 –33,1989 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Arkhammar P, Nilsson T, Welsh M, Welsh N, Berggren PO: Effects of protein kinase C activation on the regulation of the stimulus-secretion coupling in pancreatic β-cells. Biochem J 264 :207 –215,1989 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Gonelle-Gispert C, Costa M, Takahashi M, Sadoul K, Halban P: Phosphorylation of SNAP-25 on serine-187 is induced by secretagogues in insulin-secreting cells, but is not correlated with insulin secretion. Biochem J 368 :223 –232,2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Hii CS, Jones PM, Persaud SJ, Howell SL: A re-assessment of the role of protein kinase C in glucose-stimulated insulin secretion. Biochem J 246 :489 –493,1987 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Zawalich WS, Zawalich KC, Kelley GG: Regulation of insulin release by phospholipase C activation in mouse islets: differential effects of glucose and neurohumoral stimulation. Endocrinology 136 :4903 –4909,1995 [DOI] [PubMed] [Google Scholar]
- 89.Harris TE, Persaud SJ, Jones PM: Atypical isoforms of PKC and insulin secretion from pancreatic β-cells: evidence using Go 6976 and Ro 31–8220 as PKC inhibitors. Biochem Biophys Res Comm 227 :672 –676,1996 [DOI] [PubMed] [Google Scholar]
- 90.Zawalich WS, Zawalich KC: Effects of protein kinase C inhibitors on insulin secretory responses from rodent pancreatic islets. Mol Cell Endocrinol 177 :95 –105,2001 [DOI] [PubMed] [Google Scholar]
- 91.Zhang H, Nagasawa M, Yamada S, Mogami H, Suzuki Y, Kojima I: Bimodal role of conventional protein kinase C in insulin secretion from rat pancreatic β cells. J Physiol 561 :133 –147,2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Hoy M, Berggren PO, Gromada J: Involvement of protein kinase Cɛ in inositol hexakisphosphate-induced exocytosis in mouse pancreatic β-cells. J Biol Chem 278 :35168 –35171,2003 [DOI] [PubMed] [Google Scholar]
- 93.Knutson KL, Hoenig M: Arachidonic acid-induced down-regulation of protein kinase C δ in β-cells. J Cell Biochem 62 :543 –552,1996 [DOI] [PubMed] [Google Scholar]
- 94.Alcazar O, Qiu-yue Z, Gine E, Tamarit-Rodriguez J: Stimulation of islet protein kinase C translocation by palmitate requires metabolism of the fatty acid. Diabetes 46 :1153 –1158,1997 [DOI] [PubMed] [Google Scholar]
- 95.Eitel K, Staiger H, Rieger J, Mischak H, Brandhorst H, Brendel MD, Bretzel RG, Haring HU, Kellerer M: Protein kinase C δ activation and translocation to the nucleus are required for fatty acid-induced apoptosis of insulin-secreting cells. Diabetes 52 :991 –997,2003 [DOI] [PubMed] [Google Scholar]
- 96.Wrede CE, Dickson LM, Lingohr MK, Briaud I, Rhodes CJ: Fatty acid and phorbol ester-mediated interference of mitogenic signaling via novel protein kinase C isoforms in pancreatic β-cells (INS-1). J Mol Endocrinol 30 :271 –286,2003 [DOI] [PubMed] [Google Scholar]
- 97.Warnotte C, Gilon P, Nenquin M, Henquin JC: Mechanisms of the stimulation of insulin release by saturated fatty acids: a study of palmitate effects in mouse β-cells. Diabetes 43 :703 –711,1994 [DOI] [PubMed] [Google Scholar]
- 98.Thams P, Capito K: Differential mechanisms of glucose and palmitate in augmentation of insulin secretion in mouse pancreatic islets. Diabetologia 44 :738 –746,2001 [DOI] [PubMed] [Google Scholar]
- 99.Littman ED, Pitchumoni S, Garfinkel MR, Opara EC: Role of protein kinase C isoenzymes in fatty acid stimulation of insulin secretion. Pancreas 20 :256 –263,2000 [DOI] [PubMed] [Google Scholar]
- 100.Knutson KL, Hoenig M: Regulation of distinct pools of protein kinase C δ in β cells. J Cell Biochem 60 :130 –138,1996 [DOI] [PubMed] [Google Scholar]
- 101.Yaney GC, Korchak HM, Corkey BE: Long-chain acyl CoA regulation of protein kinase C and fatty acid potentiation of glucose-stimulated insulin secretion in clonal β-cells. Endocrinology 141 :1989 –1998,2000 [DOI] [PubMed] [Google Scholar]
- 102.Fujiwara K, Maekawa F, Yada T: Oleic acid interacts with GPR40 to induce Ca2+ signaling in rat islet β-cells: mediation by PLC and L-type Ca2+ channel and link to insulin release. Am J Physiol 289 :E670 –E677,2005 [DOI] [PubMed] [Google Scholar]
- 103.Buteau J, Foisy S, Rhodes CJ, Carpenter L, Biden TJ, Prentki M: Protein kinase C ζ activation mediates glucagon-like peptide-1-induced pancreatic β-cell proliferation. Diabetes 50 :2237 –2243,2001 [DOI] [PubMed] [Google Scholar]
- 104.Hennige AM, Fritsche A, Strack V, Weigert C, Mischak H, Borboni P, Renn W, Haring HU, Kellerer M: PKCζ enhances insulin-like growth factor 1-dependent mitogenic activity in the rat clonal β cell line RIN 1046–38. Biochem Biophys Res Comm 290 :85 –90,2002 [DOI] [PubMed] [Google Scholar]
- 105.Vasavada RC, Wang L, Fujinaka Y, Takane KK, Rosa TC, Mellado-Gil JM, Friedman PA, Garcia-Ocana A: Protein kinase C-ζ activation markedly enhances β-cell proliferation: an essential role in growth factor-mediated β-cell mitogenesis. Diabetes 56 :2732 –2743,2007 [DOI] [PubMed] [Google Scholar]
- 106.Hashimoto N, Kido Y, Uchida T, Matsuda T, Suzuki K, Inoue H, Matsumoto M, Ogawa W, Maeda S, Fujihara H, Ueta Y, Uchiyama Y, Akimoto K, Ohno S, Noda T, Kasuga M: PKCλ regulates glucose-induced insulin secretion through modulation of gene expression in pancreatic β cells. J Clin Invest 115 :138 –145,2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Furukawa N, Shirotani T, Araki E, Kaneko K, Todaka M, Matsumoto K, Tsuruzoe K, Motoshima H, Yoshizato K, Kishikawa H, Shichiri M: Possible involvement of atypical protein kinase C (PKC) in glucose-sensitive expression of human insulin gene: DNA-binding activity and transcriptional activity of pancreatic and duodenal homeobox gene-1 (PDX-1) are enhanced via calphostin C-sensitive but phorbol 12-myristate 13-acetate (PMA) and Go 6976-insensitive pathway. Endocr J 46 :43 –58,1999 [DOI] [PubMed] [Google Scholar]
- 108.Miele C, Raciti GA, Cassese A, Romano C, Giacco F, Oriente F, Paturzo F, Andreozzi F, Zabatta A, Troncone G, Bosch F, Pujol A, Chneiweiss H, Formisano P, Beguinot F: PED/PEA-15 regulates glucose-induced insulin secretion by restraining potassium channel expression in pancreatic β-cells. Diabetes 56 :622 –633,2007 [DOI] [PubMed] [Google Scholar]
- 109.Carpenter L, Cordery D, Biden TJ: Protein kinase C δ activation by interleukin-1β stabilizes inducible nitric-oxide synthase mRNA in pancreatic β-cells. J Biol Chem 276 :5368 –5374,2001 [DOI] [PubMed] [Google Scholar]
- 110.Carpenter L, Cordery D, Biden TJ: Inhibition of protein kinase C δ protects rat INS-1 cells against interleukin-1β and streptozotocin-induced apoptosis. Diabetes 51 :317 –324,2002 [DOI] [PubMed] [Google Scholar]
- 111.Uchida T, Iwashita N, Ohara-Imaizumi M, Ogihara T, Nagai S, Choi JB, Tamura Y, Tada N, Kawamori R, Nakayama KI, Nagamatsu S, Watada H: Protein kinase C δ plays a non-redundant role in insulin secretion in pancreatic β cells. J Biol Chem 282 :2707 –2716,2007 [DOI] [PubMed] [Google Scholar]