Abstract
OBJECTIVE—Insufficient development of a new intra-islet capillary network after transplantation may be one contributing factor to the failure of islet grafts in clinical transplantation. The present study tested the hypothesis that the angiostatic factor thrombospondin-1 (TSP-1), which is normally present in islets, restricts intra-islet vascular expansion posttransplantation.
RESEARCH DESIGN AND METHODS—Pancreatic islets of TSP-1–deficient (TSP-1−/−) mice or wild-type islets transfected with siRNA for TSP-1 were transplanted beneath the renal capsule of syngeneic or immunocompromised recipient mice.
RESULTS—Both genetically TSP-1−/− islets and TSP-1 siRNA-transfected islet cells demonstrated an increased vascular density when compared with control islets 1 month after transplantation. This was also reflected in a markedly increased blood perfusion and oxygenation of the grafts. The functional importance of the improved vascular engraftment was analyzed by comparing glucose-stimulated insulin release from islet cells transfected with either TSP-1 siRNA or scramble siRNA before implantation. These experiments showed that the increased revascularization of grafts composed of TSP-1 siRNA-transfected islet cells correlated to increments in both their first and second phase of glucose-stimulated insulin secretion.
CONCLUSIONS—Our findings demonstrate that inhibition of TSP-1 in islets intended for transplantation may be a feasible strategy to improve islet graft revascularization and function.
Despite improvements in immunosuppression protocols over the last years, pancreatic islets from at least two donor pancreata are still needed to reverse type 1 diabetes in clinical islet transplantation (1,2). This is far more than the alleged 10–20% of the total islet volume suggested to be enough to maintain normoglycemia in humans. Moreover, in contrast to the results for whole-organ transplantation, there seems to be a continuous decline in islet graft function, and very few patients remain insulin-independent at 5 years posttransplantation (2,3). Because the histocompatibility barrier, the underlying autoimmune disease, and the immunosuppressive agents used are the same for both transplantation procedures, it is likely that issues related to the adaptation of the implanted islets to their new microenvironment play a role for the differences in results.
Pancreatic islets become disconnected from their vascular supply during collagenase digestion before transplantation. Revascularization of transplanted islets has been shown to be concluded within 7–14 days (4). However, the resulting vascular density remains lower than in endogenous islets (5–7) and is associated with an impaired oxygenation (6,8) and endocrine function (7,9,10).
We have recently observed that freshly isolated rodent islets become better revascularized and function better than islets cultured for several days before transplantation (11), although the islet vascular system, also when using freshly isolated islets for transplantation, is far from fully restored. One possible explanation for the improved vascular engraftment in such islets is that not only host blood vessels but also remnant donor islet endothelial cells may participate in the formation of a new islet vascular network (12–14). However, despite the presence of several mitogens for endothelial cells within the islets, such as vascular endothelial growth factor (VEGF), fibroblast growth factor, and matrix metalloproteinases (15–17), intra-islet endothelial cells normally have a very low proliferation rate (18,19). This endothelial quiescence is presumably due to the fact that pro-angiogenic factors normally are counteracted by anti-angiogenic factors present in the islets (20), including the islet endothelial cells themselves (21,22). A possible key factor in this context is thrombospondin-1 (TSP-1), because it is not downregulated by hypoxia (20), which occurs posttransplantation. Moreover, animals deficient of this glycoprotein are characterized by hypervascular islets (23). The present study tested the hypothesis that use of genetically TSP-1−/− islets or transfection of islets in vitro with siRNA for TSP-1 would create a microenvironment permissive for blood vessel growth within islets and improve vascular engraftment and function after transplantation.
RESEARCH DESIGN AND METHODS
Pancreatic islets from wild-type (TSP-1+/+), heterozygous TSP-1+/−, and TSP-1−/− C57BL/6 mice of the F2-F3 generations were used for transplantation. The TSP-1−/− mice were generated by homologous recombination in 129/Sv-derived ES cells implanted in C57BL/6 blastocysts (24). A breeding program of such mice was established at Uppsala University, and male mice 10–12 weeks of age were allocated to the studies. Age-matched wild-type male C57BL/6 mice were used as controls. Recipient C57BL/6 (nu/nu) mice weighing ∼30 g were purchased from M&B Research and Breeding Center (Ry, Denmark). For experiments with siRNA, adult, inbred C57BL/6 mice (M&B) were used both as islet donors and recipients. All animals had free access to water and food throughout the course of the study. The experiments were approved by the animal ethics committee for Uppsala University.
Islet isolation and culture.
Islets from wild-type, TSP-1+/−, and TSP-1−/− C57BL/6 mice were prepared by collagenase digestion (25) and cultured at 37°C free-floating in 5 ml culture medium composed of RPMI 1640 (Sigma-Aldrich, Irvine, U.K.), to which we added 11 mmol/l glucose, 10% (vol/vol) FCS (Sigma-Aldrich), 0.17 mmol/l sodium benzylpenicillate, and 0.17 mmol/l streptomycin.
TSP-1 siRNA transfection of islet cells.
siRNA transfection was performed as previously described (26). Freshly isolated islets were dispersed at 37°C into single cells by addition of 5 mg/ml trypsin (Sigma-Aldrich) for <5 min. The trypsination was terminated with Ca2+-containing culture medium (RPMI 1640), followed by DNase I treatment (33 mU/μl; Amersham Life Sciences, Piscataway, NJ) for 1–2 min. The dispersed cells were washed in RPMI 1640 (Sigma-Aldrich) and divided into two equal fractions. Nonadhesive culture dishes were pretreated with FCS for 30 min at room temperature and then washed with RPMI 1640. Each fraction was placed into a nonadhesive culture dish followed by transfection with 100 nmol/l scramble or an equal mixture of four different TSP-1 siRNA sequences (Table 1) using 10 μg/600 μl Lipofectamine (Invitrogen, Lidingö, Sweden) in 600 μl RPMI 1640. Using this Lipofectamine protocol, we have previously observed uptake of siRNA in >90% of all islet cells (26). After 3 h, the transfection medium was replaced with culture medium. The dispersed islets were re-aggregated with a shaker overnight at standard culture conditions before transplantation.
TABLE 1.
PCR primers and siRNA sequences
| Gene/siRNA | GenBank accession number | Primer/siRNA sequence (5′–3′) |
|---|---|---|
| β-Actin* | NM_007393 | F GCTCTGGCTCCTAGCACC |
| R GAGGAAGCAGTGGCGATACA | ||
| TSP-1* | NM_011580 | F GGAACGGAAAGACAACACTG |
| R AGTTGAGCCCGGTCCTCTTG | ||
| Scramble siRNA† | CAGUCGCGUUUGCGACUGG | |
| TSP-1 siRNA 1‡ | GAUGACUACGCUGGCUUUGUU | |
| TSP-1 siRNA 2‡ | GCCUGCAAGAAAGACGCCUdTT | |
| TSP-1 siRNA 3‡ | CAAAGGCUGCUCCAGCUCUdTT | |
| TSP-1 siRNA 4‡ | CAAUCUGGACAACUGUCCCdTT |
Primers ordered from MWG Biotech (Ebersberg, Germany).
Scramble siRNA sequence ordered from Dharmacon Research (Chicago).
TSP-1 siRNA sequences ordered from Qiagen (Hilden, Germany). F, forward; R, reverse.
TSP-1 mRNA expression analysis.
Dispersed islet cells transfected with scramble or TSP-1 siRNA, or freshly isolated islets, were washed with PBS followed by total RNA isolation using Ultraspec (Biotecx Laboratories, Houston, TX). cDNA synthesis was performed with random nonamers (Sigma-Aldrich) and reverse transcriptase M-MuLV (Moloney murine leukemia virus) H− (Finnzymes, Espoo, Finland). Amplification was obtained with a Lightcycler system (Roche-Diagnostic, Lewes, U.K.) using DyNAmo Capillary SYBR Green qPCR kit (Finnzymes). β-Actin was used as housekeeping gene. For primer sequences used, see Table 1.
TSP-1 protein expression analysis.
Whole islets, kidney and spleen homogenates, and dispersed islet cells transfected with scramble, or TSP-1 siRNA and incubated 1–7 days were washed in cold PBS after lyses in SDS sample buffer (2% SDS, 0.15 mol/l Tris, pH 8.8, 10% glycerol, 5% β-mercaptoethanol, bromphenol blue, and 2 mmol/l phenylmethylsulfonyl fluoride), boiled for 2 min, and separated on a 6% SDS-PAGE gel. Proteins were transferred to Hybond-P membrane (GE Healthcare, Uppsala, Sweden). The membranes were blocked in 2.5% BSA at 4°C overnight followed by incubation with TSP-1 (Ab-2; Ab-4 Labvision, Fremont, CA) (TX-17.10; Santa Cruz Biotechnology, Santa Cruz, CA) and HSP60 antibodies (Stressgen, Ann Arbor, MI) and probed with horseradish peroxidase antibody. The bound antibodies were visualized with Kodak image station 4000MM (Kodak, New Haven, CT) using ECL+ (GE Healthcare). The band intensities were calculated using Kodak Molecular imaging software 4.5.1 SE.
Glucose-stimulated insulin release.
Dispersed islet cells transfected with scramble or TSP-1 siRNA were re-aggregated overnight, cultured for 72 h, and investigated for glucose-stimulated insulin release and insulin content. Groups of islet cells from 10 islets were transferred in triplicate to glass vials containing 250 μl Krebs-Ringer bicarbonate HEPES (KRBH) buffer supplemented with 10 mmol/l HEPES (Sigma-Aldrich) and 2 mg/ml BSA (ICN Biomedicals, Aurora, OH); the buffer is hereafter referred to as KRBH buffer. The KRBH buffer contained 1.67 mmol/l d-glucose during the 1st h of incubation at 37°C (O2:CO2 = 95:5). The medium was removed and replaced by 250 μl KRBH buffer supplemented with 16.7 mmol/l glucose and incubated for a 2nd h. The medium was again removed, and the islet cells were harvested and homogenized in 200 μl redistilled water. The aqueous homogenate was then used for DNA measurements by fluorophotometry (PicoGreen dsDNA Quantitation kit; Molecular Probes, Eugene, OR). A fraction of the homogenate was mixed with acid-ethanol (0.18 mol/l HCl in 95% [vol/vol] ethanol), from which insulin was extracted overnight at 4°C. Insulin contents in incubation medium and homogenates were determined by ELISA (Mercodia, Uppsala, Sweden).
Islet transplantation.
Groups of 250 wild-type, TSP-1+/−, or TSP-1−/− C57BL/6 islets cultured for 3–4 days or re-aggregated scramble or TSP-1 siRNA transfected C57BL/6 islet cells were packed in a braking pipette and syngeneically implanted beneath the capsule of the left kidney in nondiabetic C57BL/6 nu/nu mice anesthetized with avertin (2.5% [vol/vol] solution of 10 g 97% [vol/vol] 2,2,2-tribromoethanol [Sigma-Aldrich] in 10 ml 2-methyl-2-butanol) (Kemila, Stockholm, Sweden). For the transfected material, all re-aggregated islet cells, irrespective of the size of the organoids, were transplanted.
Measurements of blood flow and oxygen tension in transplanted islets.
One month posttransplantation, the animals were anesthetized with avertin (compare with above), placed on a heated operating table (38°C), and tracheotomized. Polyethylene catheters were inserted into the right carotid artery and left jugular vein. The former catheter was connected to a Statham P23dB pressure transducer (Statham Laboratories, Los Angeles, CA) to monitor arterial blood pressure, whereas the latter catheter was used for continuous infusion of Ringer solution (5 ml · kg−1 · h−1) to substitute for loss of body fluid.
After a left subcostal flank incision, the graft-bearing left kidney was immobilized in a plastic cup. The kidney was embedded in cotton wool soaked in Ringer solution and covered with mineral oil (Apoteket, Gothenburg, Sweden) to prevent evaporation and keep the tissue moist at body temperature. The blood perfusion of the islet graft and the adjacent renal cortex was measured by laser-Doppler flowmetry (PF 4001-2; Perimed, Stockholm, Sweden). The laser-Doppler probe (outer tip diameter 0.45 mm) was positioned perpendicular to the immobilized tissue surface by the use of a micromanipulator, and care was taken not to cause any compression of the tissue. At least three blood flow measurements were performed in the transplanted islets and renal cortex in each animal. The mean of these measurements from each animal was calculated and considered to be one experiment. Because it is difficult to calibrate the instrument in physical units of blood flow, all blood flow values are given as arbitrary tissue perfusion units (TPU).
Oxygen tension was measured in the islet graft and the adjacent renal parenchyma with Clark microelectrodes (Unisense, Arhus, Denmark), as described in detail previously (27,28). The electrodes (outer tip diameter 2–6 μm) were inserted into the tissues by the use of a micromanipulator under a stereomicroscope. At least 10 measurements were performed in both the islet graft and the renal cortex. The mean of all measurements, in each tissue and animal, was calculated and considered to be one experiment. During the blood flow and oxygen tension measurements, blood pressure, body temperature, and tissue temperature were continuously monitored with a MacLab Instrument (AD Instruments, Hastings, U.K.). A mean arterial blood pressure <75 mmHg was used as a preset exclusion criteria from the study.
Measurements of blood parameters.
Blood glucose concentrations were determined with test reagent strips (Medisense; Baxter Travenol, Deerfield, IL) from samples obtained from the cut tip of the tail. At the end of blood flow and oxygen tension measurements, a blood sample was collected for analysis of hematocrit and blood gases. Animals with pH <7.30, pO2 <10 kPa, pCO2 >6.8 kPa, or hematocrit <40 were excluded from the study.
Light microscopic evaluation.
The graft-bearing kidneys were removed after the oxygen tension and blood flow measurements, fixed in 10% (vol/vol) formaldehyde, and embedded in paraffin. Consecutive sections (5 μm thick) of the islet grafts were prepared and stained with the lectin Bandeiraea simplicifolia (BS-1) or a monoclonal guinea pig anti-insulin antibody (ICN Biomedicals), as previously described (5), and counterstained with hematoxylin. In each case, ≥10 tissue sections stained with BS-1 or for insulin from all parts of the islet transplants were randomly chosen and evaluated. The blood vessel density and β-cell percentage in pancreatic islets was determined by a direct point-counting method (29). For this purpose, a grid with 121 intersections was placed onto each tissue section under a light microscope (BS-1, ×400; insulin, ×1,000) (19). The number of intersections overlapping endothelial cells (stained with BS-1) or insulin-positive cells was then counted by an examiner unaware of the origin of the samples.
Evaluation of islet graft function.
Grafts from some of the animals transplanted with scramble or TSP-1 siRNA-transfected wild-type islet cells were investigated 1 month posttransplantation for glucose-stimulated insulin secretion as previously described (30). Briefly, the graft-bearing left kidney was removed together with a part of the aorta and inferior vena cava. The ureter and the renal vein were cut, while the aorta was cannulated and infused with a continuously gassed (O2:CO2 = 95:5) Krebs-Ringer bicarbonate buffer supplemented with 2.0% (wt/vol) each of BSA (fraction V; Miles Laboratories, Slough, U.K.) and dextran T70 (Pharmacia, Uppsala, Sweden). At different times during the perfusion, the medium contained either 2.8 or 16.7 mmol/l d-glucose. The medium was administered at a rate of 1 ml/min without recycling for 60 min with a perfusion pressure of ∼40 mmHg. The perfusion experiments started with a 15-min period using medium containing 2.8 mmol/l glucose, which was followed by 30 min using 16.7 mmol/l glucose. The perfusions were concluded by a 15-min perfusion with medium containing 2.8 mmol/l glucose. A 1.0-ml sample was collected at 14, 15, 16, 17, 18, 19, 20, 22, 25, 30, 35, 40, 45, 50, 55, and 60 min. The insulin concentrations of the effluent samples were measured by ELISA (Mercodia). The rate of insulin secretion was calculated by multiplying the insulin concentration in the sample by the flow rate, giving values of insulin expressed as nanograms per minute. The area under the curve (AUC) was then determined from these values.
Statistical analysis.
Values are expressed as the means ± SE. For comparisons of nonparametric data, Wilcoxon's signed rank test was used for comparisons between two groups, and Kruskal-Wallis was used for multiple comparisons. When two groups of parametric data were compared, Student's paired or unpaired two-tailed t test was used. For all comparisons, P values <0.05 were considered statistically significant.
RESULTS
Animal characteristics.
All recipient mice allocated to the study weighed ∼30 g at transplantation and had increased 5–10% in body weight when investigated 1 month later. Both donors of islets, including the TSP-1−/− mice, and recipients were normoglycemic with nonfasting blood glucose concentrations of ∼7 mmol/l. All animals allocated to blood flow and oxygen tension measurements had a mean arterial blood pressure of 90–100 mmHg. No animals had to be excluded based on the preset exclusion criteria for blood gases and hematocrit. However, two animals were excluded from studies of islet graft blood flow and oxygen tension because of hypotension (mean arterial blood pressure <75 mmHg) following perioperative bleeding.
Expression of TSP-1 in different organs.
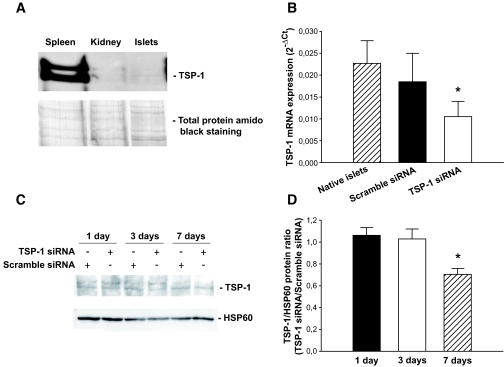
TSP-1 immunoreactivity was observed as a double band. When compared with spleen, pancreatic islets and kidneys contained low amounts of the protein TSP-1 (Fig. 1A).
FIG. 1.
Dispersed islet cells transfected with scramble or TSP-1 siRNA. A: TSP-1 protein expression in C57BL/6 mice tissues. The top panel shows TSP-1 protein expression in spleen, kidney, and freshly isolated islets. The bottom panel shows the same filter stained with amido black for total protein loading. The figure is representative for four separate experiments. B: TSP-1 mRNA expression 24 h posttransfection. Values are means ± SE for 6–11 experiments in each group. Each experiment represents a paired comparison of scramble siRNA and TSP-1 siRNA transfection with islet cells isolated and pooled from two mice. Separate experiments with native expression of TSP-1 in freshly isolated whole islets are included as reference. *P = 0.03 for TSP-1 siRNA when compared with scramble siRNA transfection. C: Islet cells were lysed and separated by SDS-PAGE 1, 3, and 7 days posttransfection. The figure shows TSP-1 and HSP60 (used as loading control) immunoreactivity on the same filter. D: The TSP-1–to–HSP60 protein ratio from filters shown in C was analyzed on days 1, 3, and 7 after the transfection and is expressed as the TSP-1–to–scramble siRNA ratio. *P = 0.02 when compared with scramble siRNA transfection from four separate experiments.
Expression of TSP-1 mRNA and protein in islet cells after siRNA transfection.
Dispersed islet cells transfected with TSP-1 siRNA showed an ∼40% reduction in TSP-1 mRNA expression compared with dispersed islet cells transfected with scramble siRNA when evaluated 24 h posttransfection (Fig. 1B). At the protein expression level, the effects of TSP-1 siRNA transfection caused a delayed effect, with a ∼40% decrease first after 1 week (Fig. 1C and D).
Effects of TSP-1 siRNA transfection on islet function.
There were no differences in insulin release at low glucose concentrations (50.2 ± 5.5 vs. 47.2 ± 6.7 ng · μg DNA−1 · h−1), in insulin release at high glucose concentrations (203.0 ± 18.8 vs. 187.2 ± 19.3 ng · μg DNA−1 · h−1), or in islet insulin content (8.2 ± 0.1 vs. 7.5 ± 0.3 ng/ng DNA) between islet cells transfected with scramble siRNA or TSP-1 siRNA 72 h before measurements.
Blood flow.
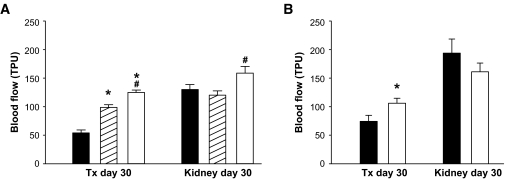
One month posttransplantation, the blood perfusion of grafts composed of wild-type control islets or scramble siRNA-transfected islet cells were similar and 40–50% of that in the adjacent renal cortex (Fig. 2A and B, respectively). In comparison, grafts composed of islet tissue either genetically decreased or deficient in TSP-1 (Fig. 2A) or transfected with siRNA for TSP-1 (Fig. 2B) had a markedly higher blood perfusion when compared with their corresponding control.
FIG. 2.
Blood flow in renal subcapsular islet grafts and adjacent renal cortex 1 month posttransplantation. A: The islet grafts were composed of 250 wild-type (▪), TSP-1+/− (▒), or TSP-1−/− (□) islets. *P ≤ 0.003 when compared with wild-type islets. #P = 0.03 when TSP-1−/− was compared with TSP-1+/−. B: The islet grafts were composed of 250 scramble (▪) or TSP-1 siRNA-transfected (□) islets. *P = 0.04 when compared with scramble siRNA transfected islets. Blood flow values are expressed as TPU. Values are means ± SE for six experiments in each group.
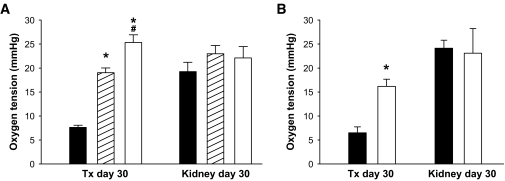
Oxygen tension.
The pO2 levels in islet grafts composed of wild-type control islets (Fig. 3A) or in islet cells transfected with scramble siRNA before transplantation (Fig. 3B) were 7–8 mmHg 1 month posttransplantation. Improved oxygenization compared with this was observed in islet grafts composed of TSP-1+/− islets, TSP-1−/− islets, or islet cells transfected with TSP-1 siRNA before transplantation (Fig. 3A and B, respectively).
FIG. 3.
Oxygen tension in renal subcapsular islet grafts and adjacent renal cortex 1 month posttransplantation. A: The islet grafts were composed of 250 wild-type (▪), TSP-1+/− (▒), or TSP-1−/− (□) islets. B: The islet grafts were composed of 250 scramble (▪) or TSP-1 siRNA-transfected (□) islets. Values are means ± SE for six experiments in each group. *P ≤ 0.001 when compared with control islets (▪) in both A and B. #P = 0.003 when TSP−/− was compared with TSP-1+/−.
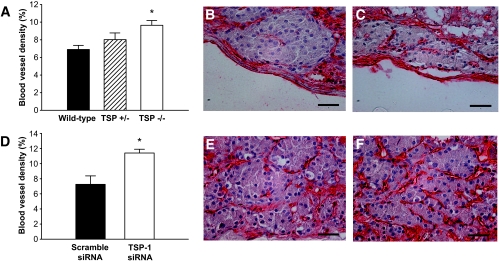
Vascular density.
Both wild-type control islets (Fig. 4A and B) and scramble siRNA-transfected islets tissue (Fig. 4D and E) had a blood vessel density of ∼7% 1 month posttransplantation. By contrast, both TSP-1−/− islets (Fig. 4A and C) and islet tissue transfected with siRNA for TSP-1 (Fig. 4D and F) had an ∼40% increase in vascular density. Islet grafts composed of TSP-1+/− islets had a vascular density in-between wild-type islet grafts and TSP-1−/− islet grafts (Fig. 4A). The increase in blood vessels numbers seemed to occur both in the center and peripheral regions of the TSP-1−/− islets and TSP-1 siRNA-transfected islet tissue.
FIG. 4.
Vascular density in renal subcapsular islet grafts 1 month posttransplantation. A: Percentage of islet area constituted by blood vessels in islet grafts composed of wild-type (▪), TSP-1+/− (▒), or TSP-1−/− (□) islets. *P = 0.046 when compared with wild-type islets. B and C: Micrographs of islet grafts composed of wild-type (B) or TSP-1−/− (C) islets stained with the endothelial marker BS-1 (red). D: Percentage of islet area constituted by blood vessels in islet grafts composed of scramble (▪) or TSP-1 siRNA-transfected (□) islets. *P = 0.016 when compared with scramble siRNA-transfected islets. E and F: Micrographs of islet grafts composed of scramble (E) or TSP-1 (F) siRNA-transfected islets. Values in A and D are means ± SE for five to eight (A) and four experiments in each group, respectively. Scale bars in micrographs are 50 μm.
Islet transplant cell composition.
There were similar percentages of β-cells in grafts composed of wild-type islets or TSP-1−/− islets (84.7 ± 1.3% [n = 4] vs. 86.2 ± 0.9% [n = 5], respectively). Likewise, there was no difference in β-cell percentage in the islet tissue composed of scramble siRNA-transfected and TSP-1 siRNA-transfected islet cells at 1 month follow-up (85.1 ± 0.5 vs. 86.9 ± 1.3%, respectively).
Islet graft function.
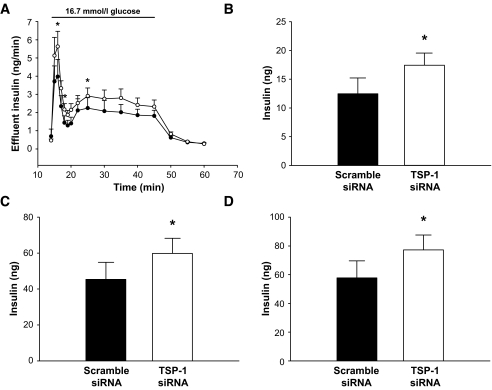
Perfusions of islet graft–bearing kidneys 1 month posttransplantation showed that islet tissue transfected with siRNA for TSP-1 had an improved first and second peak of insulin release when compared with scramble siRNA-transfected islet cells (Fig. 5A–C). Total AUC for glucose-stimulated insulin release in the TSP-1 siRNA-transfected islet tissue was thus increased by ∼40% (Fig. 5D).
FIG. 5.
Functional studies with perfusions of renal subcapsular islet grafts 1 month posttransplantation. A: Insulin release in response to 16.7 mmol/l glucose (bar) from grafts composed of 250 scramble (•) or TSP-1 siRNA-transfected islets (○). B-–D: AUC for the first phase (B) of glucose-stimulated insulin release (min 14–18; *P = 0.006 when compared with control), the second phase (C) of glucose-stimulated insulin release (min 19–50; *P = 0.041 when compared with control), and both the first and second phase (D) of insulin release (min 14–50; *P = 0.025 when compared with control). Values are means ± SE for six experiments with all groups.
DISCUSSION
Adult pancreatic islets have a dense glomerular-like angioarchitecture that requires VEGF-signaling from the β-cells for its formation and maintenance (31–34). However, despite chronic exposure to VEGF, vascular expansion does not normally occur in islets after the 1st postnatal week (35). Likewise, although increased amounts of not only VEGF but also other pro-angiogenic factors such as hepatocyte growth factor are secreted from newly transplanted islets (16,36), new blood vessel formation within the islets also during these conditions is sparse (5–7). Instead, a rich vascular network is formed in the stroma around each single transplanted islet (5). This suggests the presence of matrix- or tissue-bound angiostatic factors within the implanted islets that prevent intra-islet vascular formation. Marked overexpression of VEGF in the donor tissue has previously been shown in experimental studies to overcome this and to improve islet graft revascularization and function (9,10). However, silencing some of the angiostatic factors may be an even simpler strategy.
In this study, we tested the hypothesis that unopposing the action of already-present pro-angiogenic factors through silencing TSP-1 in islets would improve islet graft revascularization and function. The glycoprotein TSP-1 binds to the extracellular matrix (37,38) and exerts its main effects through inducing apoptosis selectively in activated endothelial cells, i.e., those that are forming new blood vessels, but not quiescent endothelium (39). It also inhibits angiogenesis by blocking the mobilization of pro-angiogenic factors, such as matrix metalloproteinase-9 and VEGF, and by inhibiting their access to co-receptors on the endothelial cell surface (40).
We investigated the influence of TSP-1 on islet graft revascularization using two different models: by transplanting genetically TSP-1 deficient islets and by transplanting islet cells transfected with siRNA for TSP-1 in vitro before transplantation. The first model thereby provides a persistent partial (TSP-1+/−) or complete loss (TSP-1−/−) of TSP-1 expression in the islets, whereas the latter causes only a transient decrease in TSP-1 levels. Using both approaches, we observed a markedly improved vascular engraftment of the transplanted islets with improved intra-islet vascular density at 1 month follow-up. In both models, the newly formed blood vessels also seemed to be functional, because the improved vascular density was reflected in an increased blood perfusion and pO2 in the islet grafts. Notably, the pO2 increase was especially prominent in the TSP-1−/− mice, where TSP-1 expression is chronically deficient and of a greater magnitude than the increase in blood vessel numbers. It is possible that this reflects higher blood perfusion in individual blood vessels and thereby better oxygen transport capacities of islet blood vessels in the absence of TSP-1, e.g., due to loss of the previously described counteracting effect of TSP-1 on nitric oxide–mediated vasodilation (41). Nitric oxide is the main mediator of high blood perfusion in endogenous and transplanted islets (42,43). It should be noted that an intra-islet vasodilation is not necessarily reflected in increased total graft blood flow, which is measured by laser-Doppler flowmetry, because total graft blood flow represents not only the nutritive blood flow to the endocrine cells but also blood flow in the vast number of stroma capillaries. In contrast, it could be expected that the blood flow in capillaries in the endocrine parts mainly contributes to the delivery of oxygen to the endocrine cells due to limitations of oxygen diffusion (44).
In our characterization of TSP-1−/− mice, we observed that these mice had fasting and nonfasting blood glucose levels that were normal and did not differ from those of wild-type animals. However, despite that TSP-1−/− mice have been described to have an increased islet mass (23), closer investigations revealed that these animals were slightly glucose intolerant and had a decreased islet function (45), which probably reflects the role of TSP-1 in transforming growth factor-β1 activation (23). Nevertheless, we hypothesized that a transient decrease in islet TSP-1 levels during the acute revascularization phase of transplanted islets may still be beneficial for long-term islet function, considering that such a decrease also can be seen physiologically during the islet vascular expansion of pregnant rats. In our protocol for TSP-1 siRNA transfection of islet cells using Lipofectamine as vector, there was a transient decrease in islet TSP-1 levels at the mRNA and protein levels of a similar magnitude as seen in islets during pregnancy, i.e., ∼40%. In rapidly dividing cells, the silencing effect of siRNA usually lasts for <1 week, but it lasts for up to 3–4 weeks in nondividing cells both in vitro and in vivo (46). Probably because of the low proliferation rate of adult endocrine cells, including β-cells, and turnover rate of the extracellular matrix binding of the TSP-1 protein, silencing of TSP-1 in these cells was not manifested until after 1 week at the protein level. A partial effect of TSP-1 siRNA may also be on the remnant donor islet endothelial cells, because these cells also contain TSP-1 (22). However, in this case, the effect is probably much shorter because of cell division as part of angiogenesis.
When graft function was evaluated by perfusion of graft-bearing kidneys 1 month posttransplantation, islet cells exposed to siRNA for TSP-1 before transplantation performed better than control islet cells exposed to scramble siRNA. The chosen model to evaluate graft function also enabled us to separately evaluate the effects of improved vascular engraftment on the insulin release kinetics from transplanted islets. It is noteworthy that the peak of the first phase of glucose-stimulated insulin secretion was substantially higher in the better revascularized islet grafts, which is similar to what we have previously observed when improving blood perfusion, but not revascularization, by angiotensin II receptor inhibition (47). However, in contrast to when only the blood perfusion was improved, we observed in the present study also an augmented second phase of glucose-stimulated insulin secretion. This suggests that the present findings do not merely reflect a “wash-out” effect of insulin retained in the islet grafts due to low blood perfusion. The general improvement of glucose-stimulated insulin release may instead reflect an improved survival, a better oxygenation, and/or a better endothelial paracrine support of the islet β-cells. The normally low oxygenation of transplanted islets may affect islet survival and function, because it means that their metabolism tends to be much more anaerobic than that of endogenous islets and results in lactate formation and tissue acidosis (48). Several recent reports also indicate that endothelial cell signaling may be important for β-cell function and growth (19,49,50).
We conclude that transient inhibition of TSP-1 early posttransplantation may be used to improve islet graft revascularization and function. Such a strategy, e.g., by development of pharmacological inhibitors, may be more feasible in the clinical setting than trying to increase the expression of pro-angiogenic factors in the transplanted tissue even further.
Acknowledgments
This work was supported by grants from The Swedish Research Council (55X-15043), The Juvenile Diabetes Research Foundation, the European Foundation for the Study of Diabetes/Juvenile Diabetes Research Foundation/Novo Nordisk Research Programme 2006, The Swedish Diabetes Association, The Swedish Juvenile Diabetes Fund, The Anér Foundation, and The Family Ernfors Fund.
The skilled technical assistance of Astrid Nordin, Birgitta Bodin, and Eva Törnelius is gratefully acknowledged.
Published ahead of print at http://diabetes.diabetesjournals.org on 16 April 2008.
The costs of publication of this article were defrayed in part by the payment of page charges. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
REFERENCES
- 1.Shapiro AM, Lakey JR, Ryan EA, Korbutt GS, Toth E, Warnock GL, Kneteman NM, Rajotte RV: Islet transplantation in seven patients with type 1 diabetes mellitus using a glucocorticoid-free immunosuppressive regimen. N Engl J Med 343 :230 –238,2000 [DOI] [PubMed] [Google Scholar]
- 2.Ryan EA, Paty BW, Senior PA, Bigam D, Alfadhli E, Kneteman NM, Lakey JR, Shapiro AM: Five-year follow-up after clinical islet transplantation. Diabetes 54 :2060 –2069,2005 [DOI] [PubMed] [Google Scholar]
- 3.Humar A, Kandaswamy R, Granger D, Gruessner RW, Gruessner AC, Sutherland DE: Decreased surgical risks of pancreas transplantation in the modern era. Ann Surg 231 :269 –275,2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Menger MD, Jaeger S, Walter P, Feifel G, Hammersen F, Messmer K: Angiogenesis and hemodynamics of microvasculature of transplanted islets of Langerhans. Diabetes 38(Suppl. 1) :199 –201,1989 [DOI] [PubMed] [Google Scholar]
- 5.Mattsson G, Jansson L, Carlsson PO: Decreased vascular density in mouse pancreatic islets after transplantation. Diabetes 51 :1362 –1366,2002 [DOI] [PubMed] [Google Scholar]
- 6.Carlsson PO, Palm F, Mattsson G: Low revascularization of experimentally transplanted human pancreatic islets. J Clin Endocrinol Metab 87 :5418 –5423,2002 [DOI] [PubMed] [Google Scholar]
- 7.Contreras JL, Smyth CA, Eckstein C, Bilbao G, Thompson JA, Young CJ, Eckhoff DE: Peripheral mobilization of recipient bone marrow-derived endothelial progenitor cells enhances pancreatic islet revascularization and engraftment after intraportal transplantation. Surgery 134 :390 –398,2003 [DOI] [PubMed] [Google Scholar]
- 8.Carlsson PO, Palm F, Andersson A, Liss P: Markedly decreased oxygen tension in transplanted rat pancreatic islets irrespective of the implantation site. Diabetes 50 :489 –495,2001 [DOI] [PubMed] [Google Scholar]
- 9.Zhang N, Richter A, Suriawinata J, Harbaran S, Altomonte J, Cong L, Zhang H, Song K, Meseck M, Bromberg J, Dong H: Elevated vascular endothelial growth factor production in islets improves islet graft vascularization. Diabetes 53 :963 –970,2004 [DOI] [PubMed] [Google Scholar]
- 10.Lai Y, Schneider D, Kidszun A, Hauck-Schmalenberger I, Breier G, Brandhorst D, Brandhorst H, Iken M, Brendel MD, Bretzel RG, Linn T: Vascular endothelial growth factor increases functional beta-cell mass by improvement of angiogenesis of isolated human and murine pancreatic islets. Transplantation 79 :1530 –1536,2005 [DOI] [PubMed] [Google Scholar]
- 11.Olsson R, Carlsson PO: Better vascular engraftment and function in pancreatic islets transplanted without prior culture. Diabetologia 48 :469 –476,2005 [DOI] [PubMed] [Google Scholar]
- 12.Linn T, Schneider K, Hammes HP, Preissner KT, Brandhorst H, Morgenstern E, Kiefer F, Bretzel RG: Angiogenic capacity of endothelial cells in islets of Langerhans. FASEB J 17 :881 –883,2003 [DOI] [PubMed] [Google Scholar]
- 13.Brissova M, Fowler M, Wiebe P, Shostak A, Shiota M, Radhika A, Lin PC, Gannon M, Powers AC: Intraislet endothelial cells contribute to revascularization of transplanted pancreatic islets. Diabetes 53 :1318 –1325,2004 [DOI] [PubMed] [Google Scholar]
- 14.Nyqvist D, Kohler M, Wahlstedt H, Berggren PO: Donor islet endothelial cells participate in formation of functional vessels within pancreatic islet grafts. Diabetes 54 :2287 –2293,2005 [DOI] [PubMed] [Google Scholar]
- 15.Kuroda M, Oka T, Oka Y, Yamochi T, Ohtsubo K, Mori S, Watanabe T, Machinami R, Ohnishi S: Colocalization of vascular endothelial growth factor (vascular permeability factor) and insulin in pancreatic islet cells. J Clin Endocrinol Metab 80 :3196 –3200,1995 [DOI] [PubMed] [Google Scholar]
- 16.Vasir B, Reitz P, Xu G, Sharma A, Bonner-Weir S, Weir GC: Effects of diabetes and hypoxia on gene markers of angiogenesis (HGF, cMET, uPA and uPAR, TGF-alpha, TGF-beta, bFGF and Vimentin) in cultured and transplanted rat islets. Diabetologia 43 :763 –772,2000 [DOI] [PubMed] [Google Scholar]
- 17.Barro C, Zaoui P, Morel F, Benhamou PY: Matrix metalloproteinase expression in rat pancreatic islets. Pancreas 17 :378 –382,1998 [DOI] [PubMed] [Google Scholar]
- 18.Lou J, Triponez F, Oberholzer J, Wang H, Yu D, Buhler L, Cretin N, Mentha G, Wollheim CB, Morel P: Expression of α-1 proteinase inhibitor in human islet microvascular endothelial cells. Diabetes 48 :1773 –1778,1999 [DOI] [PubMed] [Google Scholar]
- 19.Johansson M, Mattsson G, Andersson A, Jansson L, Carlsson PO: Islet endothelial cells and pancreatic beta-cell proliferation: studies in vitro and during pregnancy in adult rats. Endocrinology 147 :2315 –2324,2006 [DOI] [PubMed] [Google Scholar]
- 20.Tillmar L, Welsh N: In vitro cultured rat islets express genes that both prevent and promote angiogenesis. JOP 5 :81 –91,2004 [PubMed] [Google Scholar]
- 21.Mattsson G, Danielsson A, Kriz V, Carlsson PO, Jansson L: Endothelial cells in endogenous and transplanted pancreatic islets: differences in the expression of angiogenic peptides and receptors. Pancreatology 6 :86 –95,2006 [DOI] [PubMed] [Google Scholar]
- 22.Cantaluppi V, Biancone L, Romanazzi GM, Figliolini F, Beltramo S, Ninniri MS, Galimi F, Romagnoli R, Franchello A, Salizzoni M, Perin PC, Ricordi C, Segoloni GP, Camussi G: Antiangiogenic and immunomodulatory effects of rapamycin on islet endothelium: relevance for islet transplantation. Am J Transplant 6 :2601 –2611,2006 [DOI] [PubMed] [Google Scholar]
- 23.Crawford SE, Stellmach V, Murphy-Ullrich JE, Ribeiro SM, Lawler J, Hynes RO, Boivin GP, Bouck N: Thrombospondin-1 is a major activator of TGF-beta1 in vivo. Cell 93 :1159 –1170,1998 [DOI] [PubMed] [Google Scholar]
- 24.Lawler J, Sunday M, Thibert V, Duquette M, George EL, Rayburn H, Hynes RO: Thrombospondin-1 is required for normal murine pulmonary homeostasis and its absence causes pneumonia. J Clin Invest 101 :982 –992,1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Andersson A: Isolated mouse pancreatic islets in culture: effects of serum and different culture media on the insulin production of the islets. Diabetologia 14 :397 –404,1978 [DOI] [PubMed] [Google Scholar]
- 26.Hagerkvist R, Mokhtari D, Myers JW, Tengholm A, Welsh N: siRNA produced by recombinant dicer mediates efficient gene silencing in islet cells. Ann N Y Acad Sci 1040 :114 –122,2005 [DOI] [PubMed] [Google Scholar]
- 27.Carlsson PO, Liss P, Andersson A, Jansson L: Measurements of oxygen tension in native and transplanted rat pancreatic islets. Diabetes 47 :1027 –1032,1998 [DOI] [PubMed] [Google Scholar]
- 28.Revsbech PN: An oxygen microsensor with a guard cathode. Limnol Oceanogr 34 :474 –478,1989 [Google Scholar]
- 29.Weibel E: Practical methods for biological morphometry. In Stereological Methods. Weibel E, Ed. London, Academic Press,1979. , p.243 –263
- 30.Korsgren O, Jansson L, Andersson A: Effects of hyperglycemia on function of isolated mouse pancreatic islets transplanted under kidney capsule. Diabetes 38 :510 –515,1989 [DOI] [PubMed] [Google Scholar]
- 31.Inoue M, Hager JH, Ferrara N, Gerber HP, Hanahan D: VEGF-A has a critical, nonredundant role in angiogenic switching and pancreatic beta cell carcinogenesis. Cancer Cell 1 :193 –202,2002 [DOI] [PubMed] [Google Scholar]
- 32.Brissova M, Shostak A, Shiota M, Wiebe PO, Poffenberger G, Kantz J, Chen Z, Carr C, Jerome WG, Chen J, Baldwin HS, Nicholson W, Bader DM, Jetton T, Gannon M, Powers AC: Pancreatic islet production of vascular endothelial growth factor-a is essential for islet vascularization, revascularization, and function. Diabetes 55 :2974 –2985,2006 [DOI] [PubMed] [Google Scholar]
- 33.Lammert E, Gu G, McLaughlin M, Brown D, Brekken R, Murtaugh LC, Gerber HP, Ferrara N, Melton DA: Role of VEGF-A in vascularization of pancreatic islets. Curr Biol 13 :1070 –1074,2003 [DOI] [PubMed] [Google Scholar]
- 34.Kamba T, Tam BY, Hashizume H, Haskell A, Sennino B, Mancuso MR, Norberg SM, O'Brien SM, Davis RB, Gowen LC, Anderson KD, Thurston G, Joho S, Springer ML, Kuo CJ, McDonald DM: VEGF-dependent plasticity of fenestrated capillaries in the normal adult microvasculature. Am J Physiol Heart Circ Physiol 290 :H560 –H576,2006 [DOI] [PubMed] [Google Scholar]
- 35.Johansson M, Andersson A, Carlsson PO, Jansson L: Perinatal development of the pancreatic islet microvasculature in rats. J Anat 208 :191 –196,2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Vasir B, Jonas JC, Steil GM, Hollister-Lock J, Hasenkamp W, Sharma A, Bonner-Weir S, Weir GC: Gene expression of VEGF and its receptors Flk-1/KDR and Flt-1 in cultured and transplanted rat islets. Transplantation 71 :924 –935,2001 [DOI] [PubMed] [Google Scholar]
- 37.Lawler J: Thrombospondin-1 as an endogenous inhibitor of angiogenesis and tumor growth. J Cell Mol Med 6 :1 –12,2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Armstrong LC, Bornstein P: Thrombospondins 1 and 2 function as inhibitors of angiogenesis. Matrix Biol 22 :63 –71,2003 [DOI] [PubMed] [Google Scholar]
- 39.Jimenez B, Volpert OV, Crawford SE, Febbraio M, Silverstein RL, Bouck N: Signals leading to apoptosis-dependent inhibition of neovascularization by thrombospondin-1. Nat Med 6 :41 –48,2000 [DOI] [PubMed] [Google Scholar]
- 40.Rodriguez-Manzaneque JC, Lane TF, Ortega MA, Hynes RO, Lawler J, Iruela-Arispe ML: Thrombospondin-1 suppresses spontaneous tumor growth and inhibits activation of matrix metalloproteinase-9 and mobilization of vascular endothelial growth factor. Proc Natl Acad Sci U S A 98 :12485 –12490,2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Isenberg JS, Wink DA, Roberts DD: Thrombospondin-1 antagonizes nitric oxide-stimulated vascular smooth muscle cell responses. Cardiovasc Res 71 :785 –793,2006 [DOI] [PubMed] [Google Scholar]
- 42.Svensson AM, Ostenson CG, Sandler S, Efendic S, Jansson L: Inhibition of nitric oxide synthase by NG-nitro-L-arginine causes a preferential decrease in pancreatic islet blood flow in normal rats and spontaneously diabetic GK rats. Endocrinology 135 :849 –853,1994 [DOI] [PubMed] [Google Scholar]
- 43.Olsson R, Jansson L, Andersson A, Carlsson PO: Local blood flow regulation in transplanted rat pancreatic islets: influence of adenosine, angiotensin II, and nitric oxide inhibition. Transplantation 70 :280 –287,2000 [DOI] [PubMed] [Google Scholar]
- 44.Dionne KE, Colton CK, Yarmush ML: Effect of oxygen on isolated pancreatic tissue. ASAIO Trans 35 :739 –741,1989 [DOI] [PubMed] [Google Scholar]
- 45.Olerud J, Johansson M, Johansson Å, Welsh N, Carlsson P-O: Thrombospondin-1: produced by islet endothelial cells and crucial for beta-cell function. Diabet Med 23(Suppl. 1) :418A ,2006 [Google Scholar]
- 46.Derek W, Bartlett, Davis ME: Insights into the kinetics of siRNA-mediated gene silencing from live-cell and live-animal bioluminescent imaging. Nucleic Acids Res 34 :12 ,2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kampf C, Lau T, Olsson R, Leung PS, Carlsson PO: Angiotensin II type 1 receptor inhibition markedly improves the blood perfusion, oxygen tension and first phase of glucose-stimulated insulin secretion in revascularised syngeneic mouse islet grafts. Diabetologia 48 :1159 –1167,2005 [DOI] [PubMed] [Google Scholar]
- 48.Carlsson PO, Nordin A, Palm F: pH is decreased in transplanted rat pancreatic islets. Am J Physiol Endocrinol Metab 284 :E499 –E504,2003 [DOI] [PubMed] [Google Scholar]
- 49.Nikolova G, Jabs N, Konstantinova I, Domogatskaya A, Tryggvason K, Sorokin L, Fassler R, Gu G, Gerber HP, Ferrara N, Melton DA, Lammert E: The vascular basement membrane: a niche for insulin gene expression and beta cell proliferation. Dev Cell 10 :397 –405,2006 [DOI] [PubMed] [Google Scholar]
- 50.Johansson Å, Johansson M, Carlsson P-O: Islet endothelial cell signaling augment beta cell function. Diabetologia 49(Suppl. 1) :317A –318A,2006 [Google Scholar]