Abstract
OBJECTIVE—Adiponectin is an adipocyte-derived hormone that plays an important role in glucose and lipid metabolism. The main aims of this study are to investigate the effects of adiponectin on VLDL triglyceride (VLDL-TG) metabolism and the underlying mechanism.
RESEARCH DESIGN AND METHODS—Adenoviruses were used to generate a mouse model with elevated circulating adiponectin. HepG2 and C2C12 cells were treated with recombinant human adiponectin.
RESULTS—Three days after Ad-mACRP30 adenovirus injection, plasma adiponectin protein levels were increased 12-fold. All three main multimeric adiponectin molecules were proportionally elevated. Fasting plasma TG levels were significantly decreased (∼40%) in the mice with elevated adiponectin in circulation, as were the plasma levels of large and medium VLDL subclasses. Although apolipoprotein B mRNA levels were robustly suppressed in the livers of adiponectin-overexpressing mice and in cultured HepG2 cells treated with recombinant human adiponectin, hepatic VLDL-TG secretion rates were not altered by elevated plasma adiponectin. However, Ad-mACRP30–treated mice exhibited a significant increase of postheparin plasma lipoprotein lipase (LPL) activity compared with mice that received control viral vector. Skeletal muscle LPL activity and mRNA levels of LPL and VLDL receptor (VLDLr) were also increased in Ad-mACRP30–treated mice. Recombinant human adiponectin treatment increased LPL and VLDLr mRNA levels in differentiated C1C12 myotubes.
CONCLUSIONS—These results suggest that adiponectin decreases plasma TG levels by increasing skeletal muscle LPL and VLDLr expression and consequently VLDL-TG catabolism.
Dyslipidemia is a major component of the metabolic syndrome and a strong risk factor for the development of cardiovascular disease. Adiponectin is an adipocyte-derived hormone that enhances insulin sensitivity and plays an important role in glucose metabolism (1). Paradoxically, adiponectin gene expression is diminished with the development of obesity (1). Thus, hypoadiponectinemia and dyslipidemia are commonly observed in obesity and obesity-associated metabolic syndrome. In addition, studies have shown that plasma adiponectin levels are inversely correlated with VLDL triglyceride (VLDL-TG) and positively correlated with HDL cholesterol (2–4), which suggest that adiponectin may influence in lipid metabolism.
Effects of adiponectin on lipid metabolism have been studied in adiponectin transgenic and gene-deficient mouse models. In adiponectin transgenic mice, although plasma adiponectin is only moderately elevated, plasma TG concentrations are markedly reduced (5–7). In contrast, hypertriglyceridemia has been reported in adiponectin-deficient mice (8). Furthermore, administration of adiponectin normalized high-fat diet–induced hypertriglyceridemia in mice (9). These studies demonstrate that adiponectin plays an important role in TG metabolism. However, the mechanism by which adiponectin regulates TG metabolism is largely unknown.
VLDL is the main carrier of circulating TG during fasting. Plasma VLDL-TG level is determined by the balance of VLDL-TG hepatic secretion and catabolism in peripheral tissues. VLDL-TG is synthesized and secreted by hepatocytes after a series of complex intracellular events involving the synthesis of apolipoprotein B (apoB) and lipid and their assembly into lipoprotein particles. TG is the main lipid component of VLDL. At the luminal surface of capillaries in peripheral tissues, VLDL-TG is hydrolyzed by lipoprotein lipase (LPL) with the release of fatty acids, which are transported into adipocytes for storage or into heart and skeletal muscle for oxidation as fuel (10).
To determine the effect and underlying mechanism of adiponectin on TG metabolism, a mouse model with acute elevation of plasma adiponectin was generated for this study using adenovirus-mediated gene transduction. Recombinant human adiponectin and cultured myotubes were also used. Our study showed that elevation of plasma adiponectin reduced fasting circulating TG in mice. Despite decreased apoB mRNA levels in adiponectin-overexpressing mice, elevated adiponectin did not affect hepatic VLDL-TG or hepatic apoB protein secretion. Increases in plasma adiponectin did not alter mean VLDL particle size. However, the large and medium VLDL subclass particle concentrations were significantly reduced in adiponectin-overexpressed mice. Furthermore, significant increases of postheparin LPL activity and of LPL and VLDL receptor (VLDLr) expression in skeletal muscle were observed in the mice with elevated adiponectin. Therefore, we propose that adiponectin reduces plasma TG by increasing LPL, VLDLr expression, and VLDL-TG catabolism in peripheral tissues.
RESEARCH DESIGN AND METHODS
Recombinant human adiponectin and anti-mouse adiponectin antibody were purchased from R&D Systems (Minneapolis, MN). Poloxamer-407 was generously provided by BASF (Mount Olive, NJ). Fatty acid–free BSA and actinomycin D were purchased from Sigma (St. Louis, MO). The L-type TG-H and HDL cholesterol kits were purchased from Wako Diagnostics (Richmond, VA). Penicillin, streptomycin, Dulbecco's modified Eagle's medium (DMEM), and horse serum were purchased from Invitrogen (Carlsbad, CA).
Male C57BL/6J mice were purchased from The Jackson Laboratories (Bar Harbor, ME). All mice were maintained under standardized conditions with 12-h/12-h light/dark cycle and used between 8 and 10 weeks. The mice were randomly divided into two groups (n = 6). The experiments using mouse models were carried out under the Association for Assessment and Accreditation of Laboratory Animal Care guidelines with approval of the University of Kentucky Animal Care and Use Committee. Purified adenoviruses were diluted in PBS immediately before infusion. The adenovirus was injected through tail vein with a dosage of 1 × 109 plaque-forming units/mouse in 100-μl volume (11). Most experiments were conducted 3 days after injection unless otherwise stated. Adenovirus-encoding green fluorescent protein (GFP) was used as control.
Cell culture.
HepG2 and C2C12 myoblasts were purchased from American Type Culture Collection. The cells were maintained in DMEM supplemented with 10% fetal bovine serum (Gemini Bio-Products, Woodland, CA), 200 mmol/l l-glutamine, 200 units/ml penicillin, and 50 μg/ml streptomycin in an atmosphere of 95% air and 5% CO2. When C2C12 cells reached 90% confluency, myotube differentiation was induced by switching the medium to DMEM supplemented with 2% horse serum. The culture medium was changed daily. Multinucleated myotubes were obtained within 4 days. For adiponectin treatment studies, both HepG2 and C2C12 myotubes were washed twice with PBS. Recombinant human adiponectin (10 μg/ml) was added to serum-free DMEM and cultured overnight or for the indicated times.
mRNA stability measurement.
After treating HepG2 cells with adiponectin overnight, 10 μg/ml actinomycin D was added into medium. The cells were further cultured for the indicated times. Total mRNA was extracted and mRNA levels were quantified by real-time PCR using primers listed in Table 1. 18S ribosomal RNA was used as an internal control.
TABLE 1.
Primer sequences for real-time PCR
| Gene | Sequences (5′ to 3′) |
GenBank | |
|---|---|---|---|
| Forward | Reverse | ||
| m-18S rRNA | CGAAAGCATTTGCCAAGAAT | AGTCGGCATCGTTTATGGTC | X00686 |
| m-LPL | CAGAGTTTGACCGCCTTCC | AATTTGCTTTCGATGTCTGAGAA | NM_008509 |
| m-VLDLr | GAGCCCCTGAAGGAATGCC | CCTATAACTAGGTCTTTGCAGATATGG | NM_013703 |
| m-apoB | GGCACTGTGGGTCTGGAT | TTCTTCTCTGGAGGGGACTG | NM_009693 |
| h-apoB | TGCTCTCATCAAAGGCATGA | TGCTTCCTCTTAGCGTCCAG | NM_000384 |
| m-apoC-III | CGCTAAGTAGCGTGCAGGA | TCTGAAGTGATTGTCCATCCAG | NM_023114 |
| h-apoC-III | GTGCAGGAGTCCCAGGTG | AGTAGTCTTTCAGGGAACTGAAGC | NM_000040 |
| m-apoA-V | CAGTGTTCGCAAGCACTCAG | GAAGCTGCCTTTCAGGTTCTC | NM_080434 |
| h-apoA-V | AGGCACGGAAAGGCTTCT | GCTCAAGGCTGTCTTTCAGG | NM_052968 |
| m-CD36 | TGGCCTTACTTGGGATTGG | CCAGTGTATATGTAGGCTCATCCA | NM_007643 |
Hepatic VLDL-TG secretion and apoB secretion.
Hepatic VLDL-TG secretion and apoB secretion rates were measured using poloxamer-407, which inhibits endogenous LPL with fewer adverse effects on lipoprotein metabolism than Triton WR-1339 (12). Mice were fasted for 4 h before detergent injection. Poloxamer-407 was dissolved in PBS and injected into mouse (1,000 mg/kg) through tail vein (12). Blood samples were drawn in heparin capillary tubes at the indicated times, and serum TG concentrations were measured using a Wako kit. ApoB protein levels were quantified by Western blotting. Serum samples were prepared in nonreducing protein loading buffer (10 mmol/l Tris-HCl, 5% glycerol, and 1% SDS, pH 6.8) or twofold concentrated Laemmli buffer (4% SDS, 20% glycerol, 10% 2-mercaptoethanol, and 0.125 mol/l Tris-HCl, pH 6.8) without boiling. The anti-mouse apoB-48/100 antibody was purchased from Meridian Life Science (Saco, ME). The antibody was specifically designed to recognize both apoB-100 and apoB-48.
The total lipoprotein fraction of plasma (100 μl) was prepared by gel filtration chromatography using a Superose 6 10/300GL column (GE), run at a flow rate of 0.5 ml/min. Fractions were collected at 0.5-min intervals. Total cholesterol concentrations of fractions were measured and plotted to identify the positions of the major lipoprotein classes. Fractions of 25 μl (12–35) were mixed with nonreducing protein loading buffer and separated by SDS-PAGE. ApoB protein was probed by Western blotting.
LPL activity measurement.
Postheparin and tissue LPL activity was measured using a kit and protocol provided by the manufacturer (Research Diagnostics). Fluorescent TG, which is intramolecularly quenched by a trinitrophenyl group, was used as substrate. Hydrolysis by lipase results in pyrene fluorescence. Hepatic lipase activity can be distinguished in the presence of 1 mol/l NaCl, which totally abolishes LPL activity. Plasma samples were collected from mice 5 min after a tail vein injection of 300 units/kg heparin (13). Tissue samples were collected after overnight fasting and homogenized for LPL activity measurement.
Lipoprotein particle size and subclass profile.
Three days after virus injection and overnight fasting, blood samples were collected by heart puncture. Plasma was prepared and stored at 4°C. Assays were carried out within 3 days after collecting plasma samples. Lipoprotein particle size and subclass profile were analyzed using whole-plasma sample and nuclear magnetic resonance (NMR) lipoProfile-II at LipoScience (Raleigh, NC). All lipoprotein particle concentrations are provided in units of moles of particle per liter (14). One mole of particles equals 6.02 × 1023 particles per liter. The measured NMR signal amplitude from each lipoprotein subclass is directly proportional to the particle concentration of that subclass and not the amount of cholesterol or TG in that particle (14).
Plasmid constructs and generation of adenovirus.
Mouse adiponectin cDNA was cloned by RT-PCR and inserted into pENTR vector (Invitrogen). The sequences of the primers are the following: forward primer, 5′-caccaggATGgTACTGTTGCAAGC-3′; and reverse primer, 5′-TCAGTTGGTATCATGGTAGA-3′. Adiponectin-encoding DNA was then transferred to pAd/CMV/V5-Dest vector using the Gateway technique following protocol provided by the manufacturer (Invitrogen). Adenovirus was produced in HEK293 cells and purified by cesium chloride as described previously (15).
Serum adiponectin immunoblotting.
Serum adiponectin protein levels and adiponectin multimers were analyzed by Western blotting. Blood samples were collected through the tail vein after overnight fasting. For total adiponectin measurements, serum (0.4 μl) was separated by SDS-PAGE using Laemmli sample buffer. For multimeric adiponectin measurements, serum samples were prepared by nonreducing loading buffer (1% SDS, 5% glycerol, and 10 mmol/l Tris-HCl, pH 6.8) at room temperature for 10 min and separated by 4–20% gradient SDS-PAGE. Proteins were transferred to polyvinylidine fluoride membrane, and adiponectin was probed with antibody raised against mouse adiponectin. Protein bands were quantified using Bio-Rad Chemidoc system with Quantity 1 software.
Quantitative RT-PCR analysis.
Total RNA was prepared from liver tissues, HepG2 cells, and C2C12 myotubes with TRIzol following the manufacturer's protocol (Invitrogen). cDNA was synthesized using SuperScript III Reverse Transcriptase and oligo(dT)12–18 primer (Invitrogen). Real-time PCR was performed using a Mx3000P Real-Time PCR system (Stratagene) and SYBR Green dye (Molecular Probes, Eugene, OR). The sequences for the primers are listed in Table 1. The levels of PCR product were calculated from standard curves established for each primer pair. Expression data were normalized to the amount of 18S rRNA.
Statistical analysis.
Data are expressed as mean ± SE. Statistical analysis was performed using the Student's t test or ANOVA, followed by contrast test with Tukey's or Dunnett's error protection. Differences were considered significant at P < 0.05.
RESULTS
Adenovirus-mediated hepatic adiponectin transduction increases circulating adiponectin in mice.
Most previous animal studies regarding the modulatory effects of adiponectin on lipid metabolism were conducted in adiponectin transgenic or gene-deficient mouse models, which illustrate a long-term impact of adiponectin on lipid metabolism. A life-time increase or absence of adiponectin may induce changes in multiple systems that may obscure the direct modulatory effects of adiponectin on lipid metabolism in vivo. To investigate the effects of adiponectin on lipid metabolism, we generated a mouse model with an acute elevation of plasma adiponectin using adenovirus-mediated in vivo gene transduction (15). Similar mouse or rat models have been reported in several studies for other purposes (16,17).
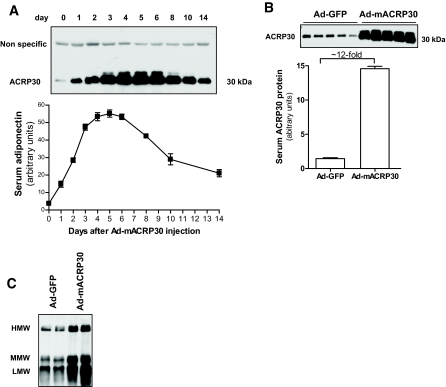
Consistent with our previous study (15), viral vector–mediated adiponectin gene expression was observed almost exclusively in liver tissues (data not shown). Plasma adiponectin protein levels were measured by Western blot. As shown in Fig. 1A, a significant increase of serum adiponectin was detected 24 h after viral vector injection. Serum adiponectin concentration reached maximal levels around days 4–5. Ad-mACRP30–treated mice exhibited a 12-fold increase of circulating adiponectin 72 h after injection (Fig. 1B), without any significant changes of body weight compared with Ad-GFP–treated mice (24.45 ± 1.64 vs. 24.10 ± 1.45 g). To minimize any possible effects of virus-induced inflammation, subsequent experiments were performed 48–72 h after injection. These results demonstrate that adenovirus vector–mediated adiponectin gene expression in liver increases adiponectin levels in plasma.
FIG. 1.
Adenovirus-mediated adiponectin overexpression in mice. Purified adenovirus vectors were injected into mice through the tail vein. Plasma adiponectin protein levels were measured by Western blot. A: Time course of total serum adiponectin protein in Ad-mACRP30 viral vector–treated mice. Top panel is a representative autoradiograph of four mice. B: Serum total adiponectin protein levels were increased (∼12-fold) 3 days after viral vector injection. C: Proportional increase of circulating multimeric forms of adiponectin in Ad-mACRP30 vector–treated mice. The multimeric forms of adiponectin were analyzed by nonreducing PAGE. HMW, high molecular weight; MMW, medium molecular weight; LMW, low molecular weight.
Circulating adiponectin proteins can exist as multimers. It has been suggested that the different multimeric forms of adiponectin may have different biological functions or potency (1,18). Our results showed that adenovirus-mediated adiponectin gene transduction proportionally increased all three main molecular weight adiponectin multimers in circulation (Fig. 1C).
Adiponectin reduces fasting plasma TG and free fatty acid in mice.
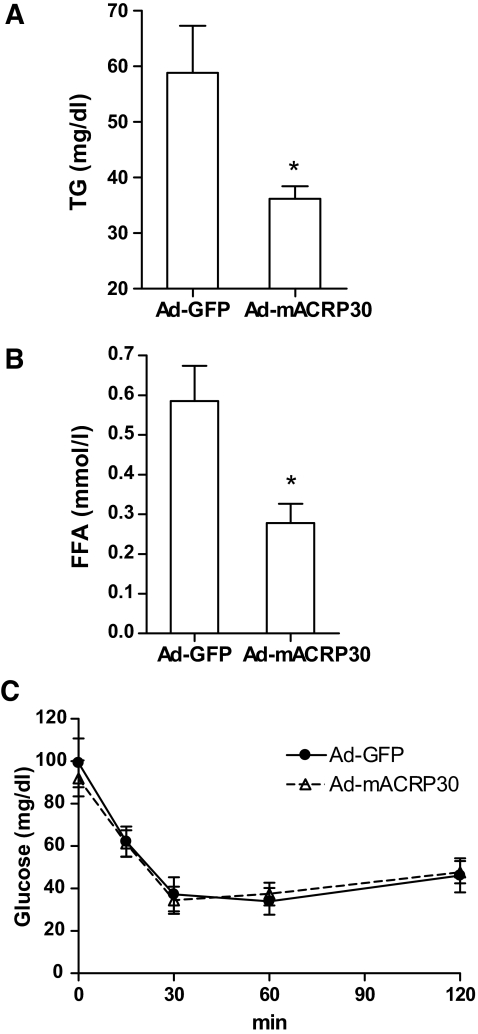
Three days after virus injection, mice were fasted for 6 h, and plasma total TG and free fatty acid (FFA) concentrations were determined. As shown in Fig. 2A, plasma TG concentrations were reduced ∼40% (P < 0.01) in Ad-mACRP30–treated mice. A similar reduction in plasma FFA was observed (Fig. 2B). Fasting plasma HDL cholesterol concentrations were significantly increased in Ad-mACRP30–treated mice compared with the control group (48.08 ± 6.86 vs. 25.59 ± 5.65 mg/dl, P = 0.021). The finding that adiponectin reduces fasting total TG is consistent with the observed reduction in plasma TG in adiponectin transgenic mice (5). However, 4 h after re-feeding, the difference of TG between Ad-mACRP30–and Ad-GFP–treated mice vanished (Supplemental Fig. 1, available in the online appendix at http://dx.doi.org/10.2337/db07-0435), presumably due to high levels of chylomicrons.
FIG. 2.
Reduction of serum TG and FFA in adiponectin-overexpressing mice. Three days after viral vector injection, the mice were fasted for 6 h. Blood samples were collected, and serum TG (A) and FFA (B) concentrations were measured using Wako kit, n = 6. C: Insulin challenge tests were carried out after overnight fasting, n = 5. *P < 0.05 vs. Ad-GFP vector–treated control mice.
An insulin challenge test, conducted 72 h after adenovirus administration, showed that there was no significant difference in insulin-stimulated glucose disposal between Ad-GFP–and Ad-mACRP30–treated mice (Fig. 2C). In addition, there was no difference in fasting plasma glucose concentrations between these two groups of mice (Fig. 2C). Fasting plasma insulin levels in Ad-mACRP30–and Ad-GFP–treated mice were similar (0.3346 ± 0.04 vs. 0.3105 ± 0.01 ng/ml). However, consistent with a previous study (19), significant decreases of PEPCK and glucose-6-phosphatase mRNA were observed in the livers of mice with elevated plasma adiponectin (data not shown). More precise measurements, e.g., hyperinsulinemic-euglycemic clamps, are required to determine the effect of acutely increased adiponectin on insulin sensitivity. Our results imply that adiponectin may modulate lipid metabolism through a mechanism independent of adiponectin-enhanced insulin sensitivity.
Adiponectin reduces apoB mRNA but does not alter hepatic apoB protein secretion.
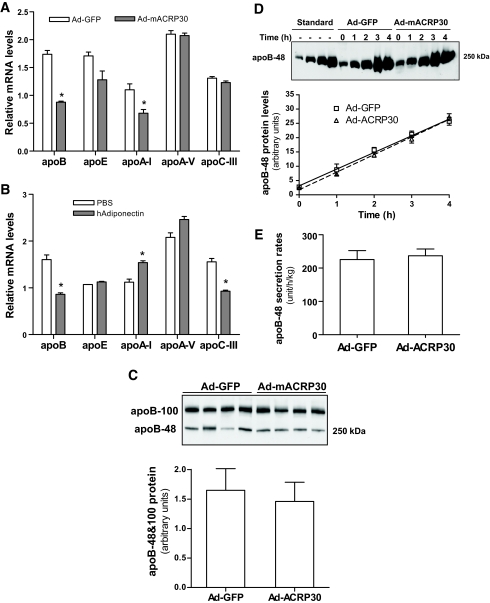
We measured mRNA levels of apoB and several other apolipoproteins in the livers of Ad-mACRP30–treated mice to test whether adiponectin regulates apolipoprotein expression. As shown in Fig. 3A, hepatic apoB mRNA was reduced by nearly 50% in Ad-mACRP30–treated mice compared with control mice. The expression of apoE, apoA-I, apoA-V, and apoC-III was also measured. Studies have reported that adiponectin can regulate peroxisome proliferator–activated receptor α (PPARα) (18) and that PPARα regulates both apoA-V and apoC-III transcription (20,21). However, our results showed no differences in the mRNA levels of either apoA-V or apoC-III between Ad-mACRP30–and Ad-GFP–treated mice (Fig. 3A). However, apoA-I mRNA levels were decreased in Ad-mACRP30–treated mice (Fig. 3A).
FIG. 3.
Adiponectin reduces apoB mRNA but has no effect on hepatic apoB secretion. A: Total mRNA was extracted from livers of mice 3 days after virus injection. mRNA levels were measured by real-time PCR using 18S as internal control, n = 6. B: Confluent HepG2 cells were treated with 10 μg/ml recombinant human adiponectin or BSA control for 24 h, n = 4. Mouse serum samples were collected after 4 h of fasting (C) and at indicated time points after P-407 injection (D). Plasma was separated in SDS-PAGE with nonreducing loading buffer, and apoB protein was probed using anti-mouse apoB-specific antibody. The standards were made by stepwise dilution (1:2 dilution in PBS) using a plasma sample from a wild-type mouse 2 h after P-407 injection. E: Hepatic apoB-48 secretion rates were calculated based on the increase of plasma apoB-48 protein levels after P-407 injection. *P < 0.05 vs. Ad-GFP–or BSA-treated control mice, respectively, for liver and cultured cells.
To further verify these observations, an in vitro study was carried out using HepG2 hepatocytes and recombinant human adiponectin protein. Consistent with the in vivo results, the apoB mRNA level was also markedly suppressed in HepG2 cells after 24 h of 10 μg/ml adiponectin treatment (P < 0.01; Fig. 3B). Adiponectin treatment did not alter apoA-V mRNA; however, it reduced apoC-III mRNA (Fig. 3B). The increase in apoA-I mRNA level after adiponectin treatment was the opposite of the results of the in vivo study (Fig. 3A). These differences in the effects of adiponectin on apoA-I and apoC-III expression in the in vivo and in vitro studies may reflect differences in these two models.
We also measured the effects of adiponectin treatment on apoB mRNA stability in HepG2 cells. The results showed that there was no significant difference of apoB mRNA half-life between adiponectin-treated and control cells (data not shown). Together, these studies indicate that adiponectin suppresses hepatic apoB gene expression without altering apoB mRNA stability, implying that adiponectin inhibits apoB expression at the transcription level.
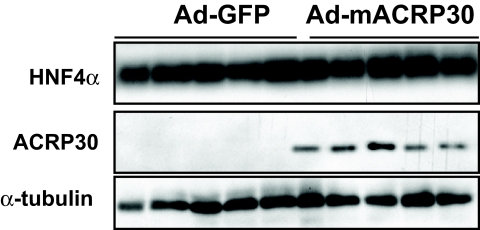
While this manuscript was in preparation, Neumeier et al. (22) reported that adiponectin reduced apoB expression in human hepatocytes by reducing hepatocyte nuclear factor-4α (HNF4α). However, our study did not show any changes in HNF4α at both protein (Fig. 4) and mRNA levels (data not shown). Microsomal TG transfer protein plays a key role in VLDL assembly in liver. Our studies showed that the microsomal triglyceride transfer protein mRNA levels were not significantly altered in the livers of Ad-mACRP30–treated mice or adiponectin-incubated HepG2 cells (data not shown).
FIG. 4.
Elevated adiponectin has no effect on HNF4α protein levels in mouse liver. Three days after viral vector injection, liver tissues were collected in the fasting state after perfusion. Total liver protein was prepared, and HNF4α and adiponectin were analyzed by Western blotting.
To further study the effect of adiponectin on apoB expression in liver, basal serum apoB protein levels and hepatic apoB protein secretion rates were studied using fasting serum and serum samples collected at different time points after LPL inhibition by Poloxamer-407 (see details in research design and methods for measurement of hepatic VLDL secretion rate). Mouse hepatocytes edit apoB mRNA, and both apoB-100 and apoB-48 protein can be produced from mouse liver (23). As shown in Fig. 3C, despite slightly reduced fasting apoB-100 and apoB-48 levels in Ad-mACRP30–treated mice, there was no statistical difference of basal total apoB protein levels between Ad-mACRP30–and Ad-GFP–treated mice (P > 0.05).
Unexpectedly, the fasting plasma apoB-100 band was markedly shifted in Ad-mACRP30–treated mice under nonreducing conditions, whereas the size difference disappeared under reducing conditions (Fig. 3C). These observations suggest that elevated adiponectin may induce a conformational change or modification of plasma apoB-100 in the treated mice. Further studies are being undertaken to identify the mechanism through which adiponectin alters plasma apoB-100 protein mobility and its biological consequences.
Results in Supplemental Fig. 2A show that plasma apoB-100 protein levels are higher than apoB-48 levels in mice in the fasting state. Plasma lipoproteins were fractionated by fast-protein liquid chromatography, and cholesterol concentrations in the fractions were determined. Interestingly, the large bulk of apoB-100 in Ad-mACRP30–treated mice and also control mice was detected in fractions corresponding to the position of LDL (Supplemental Fig. 2B). The study also showed that at 4 h after inhibition of LPL by Poloxamer-407 (12,24), there was an expected large accumulation of apoB-48 and also of apoB-100, although to a lesser extent (Fig. 3D; Supplemental Fig. 2A). However, the increased rate of apoB-100 protein was significantly less than of apoB-48 (Supplemental Fig. 2A), which may be caused by P-407–induced blockage of formation of new LDL and continued catabolism of preexisting LDL (Supplemental Fig. 2B). Therefore, we used apoB-48 protein accumulation to calculate hepatic apoB secretion rate (Fig. 3D). Our study showed no significant difference of hepatic apoB-48 secretion between adiponectin-overexpressing and control mice (Fig. 3E). These results suggest that adiponectin has no effect on hepatic apoB protein secretion despite reducing apoB mRNA in hepatocytes.
Elevated plasma adiponectin has no effect on hepatic VLDL-TG secretion.
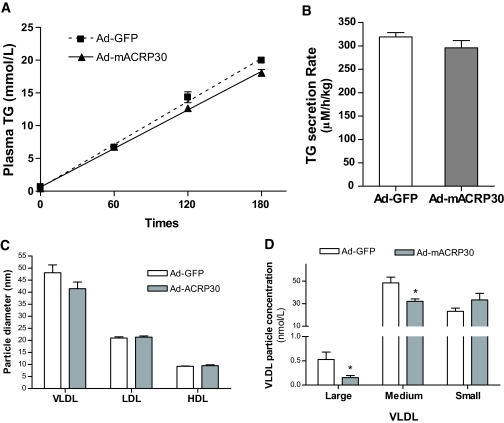
The rate of hepatic VLDL-TG secretion was directly measured by the technique in which Poloxamer-407 is used to inhibit endogenous LPL and VLDL processing (12,24). Serum TG levels increased markedly after Poloxamer-407 addition and at the same rate in the Ad-mACRP30–treated and control mice. Calculated hepatic VLDL-TG secretion rates in the two groups of mice were similar (Fig. 5B). This study indicates that although adiponectin suppresses apoB transcription, hepatic VLDL-TG secretion is not inhibited. This conclusion is supported by previous studies, which suggest that apoB gene expression in liver is constitutive and that apoB synthesis is not usually rate limiting for VLDL assembly and VLDL-TG secretion (25,26).
FIG. 5.
Elevated plasma adiponectin has no effect on hepatic VLDL-TG secretion and lipoprotein particle size. A and B: Hepatic VLDL-TG secretion rates were studied by measuring serum TG accumulation using Poloxamer-407 to inhibit endogenous LPL. The mice were fasted for 4 h, 3 days after viral vector injection. Blood samples were collected at indicated time points, and TG was measured. C and D: Four-hour fasting blood samples were collected 3 days after virus injection. Mean lipoprotein particle size and subclass profile were measured using a proton NMR analyzer, n = 6. Diameter range for VLDL subclass: large, >60 nm; medium, 35–60 nm; small, 27–35 nm. *P < 0.05 vs. Ad-GFP vector–treated control mice.
Decrease of large and medium VLDL particle levels in adiponectin-overexpressing mice.
Studies have demonstrated that VLDL particle size plays a significant role in VLDL catabolism and that multimeric adiponectin is related to lipoprotein subclass profiles (27). Our studies showed that blood TG concentrations were significantly reduced in adiponectin-overexpressing mice without an alteration in the rate of hepatic apoB and VLDL-TG secretion. This raises the question of whether VLDL particle size is altered in adiponectin-overexpressing mice. We measured lipoprotein particle size and the subclass profile using NMR. As shown in Fig. 5C, mean particle sizes of LDL or HDL were comparable between Ad-mACRP30–treated and control mice. VLDL mean particle size was slightly decreased in adiponectin-overexpressing mice but without statistical significance. However, the levels of large and medium VLDL subclasses in Ad-mACRP30–treated mice were significantly lower than in control mice (Fig. 5D). Interestingly, small VLDL levels tended to increase in Ad-mACRP30–treated mice (Fig. 5D). These results are consistent with the human study, which reported that circulating total and high–molecular weight adiponectin are negatively correlated with large VLDL particle levels (27).
Postheparin plasma LPL activity is increased in the mice with elevated plasma adiponectin.
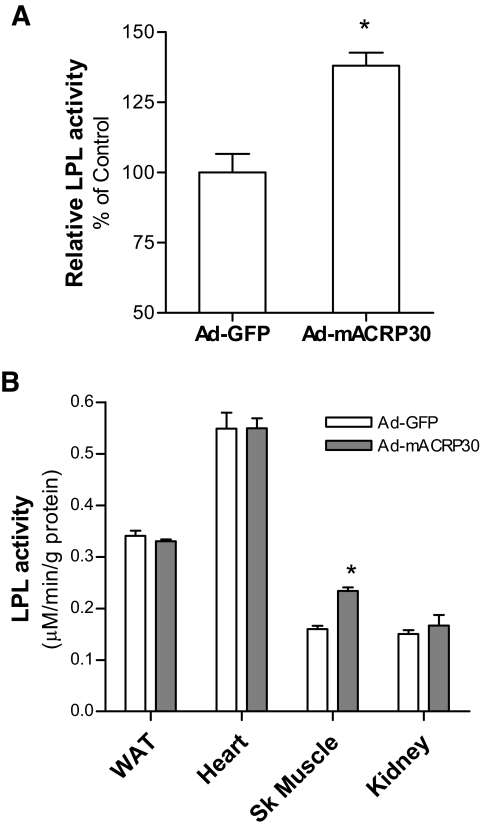
The level of fasting plasma VLDL-TG is determined by the balance of hepatic VLDL-TG secretion and peripheral VLDL-TG catabolism. Our results showed that elevated adiponectin reduces serum TG concentrations and has no effect on hepatic VLDL-TG secretion. Therefore, adiponectin-induced low-serum TG is most likely caused by increasing VLDL-TG catabolism.
Our results showed that postheparin plasma LPL activities were significantly increased in mice that received Ad-mACRP30 treatment (Fig. 6A). Tissue LPL activity was also measured using homogenized epididymal fat, gastrocnemius muscle, or heart. No difference was detected in LPL activities in epididymal fat or heart between Ad-mACRP30–and Ad-GFP virus–treated mice (Fig. 6B). However, skeletal muscle LPL activities were increased by 41% in Ad-mACRP30–treated mice (P < 0.05; Fig. 6B).
FIG. 6.
Adiponectin increases postheparin LPL activity in mice. A: Postheparin plasma was collected from mice after 3 days of viral vector injection, and LPL activity was measured, n = 6. B: Tissue LPL activity was measured using homogenized protein samples, n = 5. *P < 0.05 vs. Ad-GFP–treated mice.
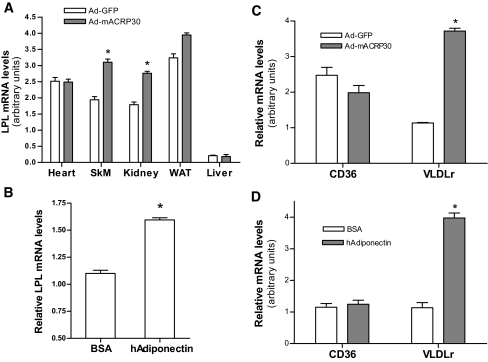
Adiponectin increases LPL and VLDLr expression in skeletal muscle.
Altered LPL gene expression and posttranslational modification are two mechanisms that regulate LPL activity. The mRNA levels of LPL were measured by real-time PCR. Results showed that LPL mRNA was significantly increased in both skeletal muscle and kidney of Ad-mACRP30–treated mice (P < 0.01; Fig. 7A). No difference was observed in heart, epididymal fat, or liver between these two groups of mice.
FIG. 7.
Adiponectin enhances LPL and VLDLr gene expression in skeletal muscle. A: LPL mRNA levels were measured by real-time PCR, using mRNA samples extracted from gastrocnemius muscle, heart, kidney, and epididymal fat from Ad-mACRP30–or Ad-GFP–treated mice, n = 6. B: Differentiated C2C12 myotubes were treated with 10 μg/ml recombinant human adiponectin or BSA control for 24 h, n = 4. The mRNA levels of CD36 and VLDLr in gastrocnemius muscle (C) and recombinant human adiponectin-treated C2C12 myotubes (D) were also analyzed by real-time PCR. *P < 0.05 vs. Ad-GFP–or BSA-treated control mice. SkM, skeletal muscle; WAT, epididymal fat.
To further verify the effects of adiponectin on LPL expression, cultured C2C12 myotubes were treated with 10 μg/ml recombinant human adiponectin. A 24-h incubation of cells with adiponectin significantly increased LPL mRNA levels, consistent with the results from the in vivo studies (P < 0.05; Fig. 7B). These data indicate that adiponectin directly increases LPL gene expression in skeletal muscle.
VLDLr plays an important role in VLDL-TG catabolism. It not only interacts with and enhances LPL activity but also binds and internalizes VLDL remnants into cells (28). We measured VLDLr mRNA levels and found that they were robustly increased (3.6-fold) in skeletal muscle of Ad-mACRP30–treated mice (P < 0.001; Fig. 7C). In contrast, the VLDLr mRNA levels in epididymal fat, heart, and kidney were similar in adiponectin-overexpressing and control mice (data not shown). A robust increase of VLDLr mRNA levels was also observed in adiponectin-treated C2C12 myotubes (P < 0.05; Fig. 7D).
Fatty acid translocase (FAT/CD36) is one of the main proteins that facilitate fatty acid diffusion across plasma membrane. Our study showed that CD36 mRNA levels were not altered in either skeletal muscle of Ad-mACRP30–treated mice or human adiponectin–incubated myotubes (Fig. 7C and D).
DISCUSSION
Although compelling evidence indicates that adiponectin modulates lipid metabolism, the underlying mechanisms are largely unknown. Using adenovirus-mediated adiponectin overexpression in vivo and a cultured cellular model, our current study specifically investigated the modulatory effect of adiponectin on VLDL-TG metabolism. Consistent with previous studies, our study showed that elevated adiponectin reduces plasma TG in mice. Despite the decrease of apoB mRNA levels in the livers of Ad-mACRP30–treated mice and in adiponectin-treated HepG2 hepatocytes, elevated adiponectin did not alter hepatic VLDL-TG or apoB protein secretion in mice. On the other hand, skeletal muscle LPL transcription and postheparin plasma LPL activities, together with VLDLr transcription, were robustly increased in adiponectin-overexpressing mice. Consistent with these findings, recombinant adiponectin increased LPL and VLDLr gene expression in cultured skeletal muscle cells. Therefore, we propose that adiponectin reduces plasma TG by increasing VLDL catabolism in skeletal muscle.
Since the discovery of the insulin-sensitizing property of adiponectin, numerous studies have observed an inverse relationship between plasma TG and adiponectin levels. Studies using adiponectin transgenic or gene-deficient mice further indicated that adiponectin modulates lipid metabolism (5–9). However, transgenic or gene-deficient mouse models represent long-term effects of increased or absent adiponectin on lipid metabolism. Using adenovirus-mediated gene transduction technique, we generated a mouse model with acute elevations of plasma adiponectin. Our study shows that an acute increase of circulating adiponectin reduces serum TG and also increases HDL cholesterol in mice. Together, our study further confirms that adiponectin improves the anti-atherogenic lipoprotein profile in mice. A recent study suggested that the positive link between HDL and adiponectin may be caused by reduced apoA1 degradation (29). Our study found a decrease of apoA1 mRNA in the livers of adiponectin-overexpressing mice, but an opposite effect was observed in HepG2 cells (Fig. 3A and B). Further studies are required to elucidate the underlying mechanism by which adiponectin increases HDL cholesterol.
ApoB is the major apolipoprotein in VLDL, and both apoB-100 and apoB-48 are produced in rodent hepatocytes (23). The results from our in vivo and in vitro studies show that adiponectin suppresses apoB transcription in hepatocyte, which is in agreement with the findings of a most recent report (22). Importantly, our in vivo study demonstrated that adiponectin did not alter basal serum apoB protein levels or the rate of hepatic apoB secretion despite decreasing apoB transcription. These results are in line with previous reports that hepatic apoB mRNA is abundant and that the rate of apoB translation exceeds apoB secretion (25,26). Thus, the majority of newly synthesized apoB protein is degraded within hepatocytes before lipoprotein assembly and secretion (26,30). In addition, our study found that adiponectin overexpression did not alter liver VLDL-TG secretion in mice. These results lead us to conclude that although adiponectin reduces hepatic apoB gene transcription, adiponectin has no significant effect on hepatic apoB and VLDL-TG secretion. Nevertheless, our study clearly showed that fasting plasma TG was decreased in adiponectin-overexpressing mice. Therefore, we postulate that increased VLDL-TG catabolism, rather than decreased synthesis, accounts for the reduced plasma TG levels in response to adiponectin.
LPL has been considered a gatekeeper for VLDL-TG catabolism (10). LPL hydrolyzes VLDL-TG and releases fatty acids for oxidation as fuel for skeletal or cardiac muscle or for storage in adipose tissue (10,31). Recent studies have reported a positive relationship between plasma adiponectin levels and postheparin LPL activities (32,33). Consistent with these correlative human studies, our study demonstrated that there was significant increase of postheparin LPL activity in the mice with elevated adiponectin. These data indicate that adiponectin increases VLDL-TG hydrolysis in peripheral tissues, thus leading to a decrease in circulating TG. Furthermore, our study showed that LPL expression and activity were increased in skeletal muscle from the mice with elevated adiponectin. In fasted animals, adipose tissue LPL expression and activity are suppressed, and plasma LPL is mainly contributed by skeletal muscle and heart (34). Skeletal muscle plays an important role in TG clearance, taking into account its large mass in the body. Therefore, increased postheparin LPL activities in adiponectin-overexpressing mice were likely due to increased skeletal muscle LPL expression. A previous study has reported that adiponectin increased TG clearance by increasing LPL expression in white adipose tissues in female adiponectin transgenic mice (5). However, our study did not show a significant increase of LPL expression and activity in epididymal fat of adiponectin-overexpressing mice. Differences between these animal models may cause the discrepancy. The mouse model we used is acute adiponectin overexpression. In addition, adiponectin was ectopically overexpressed and secreted from liver after adenovirus transduction. Under normal physiological conditions, adiponectin is predominantly synthesized in adipose tissues. Furthermore, our mice were male, unlike the female mice used in the previous study (5). However, in our current study and in the previous report (5), serum FFAs were found to be reduced in adiponectin-overexpressing mice.
VLDLr is a member of LDL receptor family (35). Similar to LPL, VLDLr is highly abundant in heart, skeletal muscle, and adipose tissue, whereas it is barely detectable in liver (35–37). Recent studies demonstrate that VLDLr is involved in VLDL-TG catabolism by interacting with LPL (13,35–38). Our findings show that elevation of plasma adiponectin induced a robust increase of VLDLr mRNA in skeletal muscle and cultured myotubes. Therefore, we postulate that the combined effects of adiponectin on VLDLr and LPL in skeletal muscle contribute to increased VLDL-TG catabolism. Our recent findings have revealed that adiponectin enhances LPL gene transcription via AMP kinase activation in skeletal muscle (L.Q., J.S., unpublished data). The detailed mechanisms are under investigation.
In summary, our study indicates that adiponectin reduces blood TG but has no effect on hepatic VLDL-TG secretion. Increased LPL and VLDLr expression in skeletal muscle and plasma LPL activity were observed in mice with elevated plasma adiponectin. Therefore, we propose that adiponectin reduces plasma TG by increasing VLDL-TG catabolism in skeletal muscle, at least in part by increasing LPL and VLDLr expression.
Supplementary Material
Acknowledgments
J.S. has received American Diabetes Association Grants 1-04-JF-44 and 7-07-CD-23, National Institutes of Health Grant 1R21-DK-077643-01, and American Heart Association Grant 0665289B.
We thank Yingxia Li, Xuebing Wang, and Ira M. Mains for technical support of lipoprotein fraction.
Published ahead of print at http://diabetes.diabetesjournals.org on 28 March 2008.
The costs of publication of this article were defrayed in part by the payment of page charges. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
REFERENCES
- 1.Berg AH, Scherer PE: Adipose tissue, inflammation, and cardiovascular disease. Circ Res 96 :939 –949,2005 [DOI] [PubMed] [Google Scholar]
- 2.Ng TWK, Watts GF, Farvid MS, Chan DC, Barrett PHR: Adipocytokines and VLDL metabolism: independent regulatory effects of adiponectin, insulin resistance, and fat compartments on VLDL apolipoprotein B-100 kinetics? Diabetes 54 :795 –802,2005 [DOI] [PubMed] [Google Scholar]
- 3.Tschritter O, Fritsche A, Thamer C, Haap M, Shirkavand F, Rahe S, Staiger H, Maerker E, Haring H, Stumvoll M: Plasma adiponectin concentrations predict insulin sensitivity of both glucose and lipid metabolism. Diabetes 52 :239 –243,2003 [DOI] [PubMed] [Google Scholar]
- 4.Baratta R, Amato S, Degano C, Farina MG, Patane G, Vigneri R, Frittitta L: Adiponectin relationship with lipid metabolism is independent of body fat mass: evidence from both cross-sectional and intervention studies. J Clin Endocrinol Metab 89 :2665 –2671,2004 [DOI] [PubMed] [Google Scholar]
- 5.Combs TP, Pajvani UB, Berg AH, Lin Y, Jelicks LA, Laplante M, Nawrocki AR, Rajala MW, Parlow AF, Cheeseboro L, Ding Y-Y, Russell RG, Lindemann D, Hartley A, Baker GRC, Obici S, Deshaies Y, Ludgate M, Rossetti L, Scherer PE: A transgenic mouse with a deletion in the collagenous domain of adiponectin displays elevated circulating adiponectin and improved insulin sensitivity. Endocrinology 145 :367 –383,2004 [DOI] [PubMed] [Google Scholar]
- 6.Yamauchi T, Kamon J, Waki H, Imai Y, Shimozawa N, Hioki K, Uchida S, Ito Y, Takakuwa K, Matsui J, Takata M, Eto K, Terauchi Y, Komeda K, Tsunoda M, Murakami K, Ohnishi Y, Naitoh T, Yamamura K, Ueyama Y, Froguel P, Kimura S, Nagai R, Kadowaki T: Globular adiponectin protected ob/ob mice from diabetes and ApoE-deficient mice from atherosclerosis. J Biol Chem 278 :2461 –2468,2003 [DOI] [PubMed] [Google Scholar]
- 7.Bauche IB, El Mkadem SA, Pottier A-M, Senou M, Many M-C, Rezsohazy R, Penicaud L, Maeda N, Funahashi T, Brichard SM: Overexpression of adiponectin targeted to adipose tissue in transgenic mice: impaired adipocyte differentiation. Endocrinology 148 :1539 –1549,2007 [DOI] [PubMed] [Google Scholar]
- 8.Kubota N, Terauchi Y, Yamauchi T, Kubota T, Moroi M, Matsui J, Eto K, Yamashita T, Kamon J, Satoh H, Yano W, Froguel P, Nagai R, Kimura S, Kadowaki T, Noda T: Disruption of adiponectin causes insulin resistance and neointimal formation. J Biol Chem 277 :25863 –25866,2002 [DOI] [PubMed] [Google Scholar]
- 9.Yamauchi T, Kamon J, Waki H, Terauchi Y, Kubota N, Hara K, Mori Y, Ide T, Murakami K, Tsuboyama-Kasaoka N, Ezaki O, Akanuma Y, Gavrilova O, Vinson C, Reitman ML, Kagechika H, Shudo K, Yoda M, Nakano Y, Tobe K, Nagai R, Kimura S, Tomita M, Froguel P, Kadowaki T: The fat-derived hormone adiponectin reverses insulin resistance associated with both lipoatrophy and obesity. Nat Med 7 :941 –946,2001 [DOI] [PubMed] [Google Scholar]
- 10.Merkel M, Eckel RH, Goldberg IJ: Lipoprotein lipase: genetics, lipid uptake, and regulation. J Lipid Res 43 :1997 –2006,2002 [DOI] [PubMed] [Google Scholar]
- 11.Qiao L, MacLean PS, Schaack J, Orlicky DJ, Darimont C, Pagliassotti M, Friedman JE, Shao J: C/EBPα regulates human adiponectin gene transcription through an intronic enhancer. Diabetes 54 :1744 –1754,2005 [DOI] [PubMed] [Google Scholar]
- 12.Millar JS, Cromley DA, McCoy MG, Rader DJ, Billheimer JT: Determining hepatic triglyceride production in mice: comparison of poloxamer 407 with Triton WR-1339. J Lipid Res 46 :2023 –2028,2005 [DOI] [PubMed] [Google Scholar]
- 13.Yagyu H, Lutz EP, Kako Y, Marks S, Hu Y, Choi SY, Bensadoun A, Goldberg IJ: Very low density lipoprotein (VLDL) receptor-deficient mice have reduced lipoprotein lipase activity: possible causes of hypertriglyceridemia and reduced body mass with vldl receptor deficiency. J Biol Chem 277 :10037 –10043,2002 [DOI] [PubMed] [Google Scholar]
- 14.Jeyarajah EJ, Cromwell WC, Otvos JD: Lipoprotein particle analysis by nuclear magnetic resonance spectroscopy. Clin Lab Med 26 :847 –870,2006 [DOI] [PubMed] [Google Scholar]
- 15.Qiao L, MacLean PS, You H, Schaack J, Shao J: Knocking down liver CCAAT/enhancer-binding protein {alpha} by adenovirus-transduced silent interfering ribonucleic acid improves hepatic gluconeogenesis and lipid homeostasis in db/db mice. Endocrinology 147 :3060 –3069,2006 [DOI] [PubMed] [Google Scholar]
- 16.Okamoto Y, Kihara S, Ouchi N, Nishida M, Arita Y, Kumada M, Ohashi K, Sakai N, Shimomura I, Kobayashi H, Terasaka N, Inaba T, Funahashi T, Matsuzawa Y: Adiponectin reduces atherosclerosis in apolipoprotein E-deficient mice. Circulation 106 :2767 –2770,2002 [DOI] [PubMed] [Google Scholar]
- 17.Satoh H, Nguyen MTA, Trujillo M, Imamura T, Usui I, Scherer PE, Olefsky JM: Adenovirus-mediated adiponectin expression augments skeletal muscle insulin sensitivity in male Wistar rats. Diabetes 54 :1304 –1313,2005 [DOI] [PubMed] [Google Scholar]
- 18.Kadowaki T, Yamauchi T: Adiponectin and adiponectin receptors. Endocr Rev 26 :439 –451,2005 [DOI] [PubMed] [Google Scholar]
- 19.Berg AH, Combs TP, Du X, Brownlee M, Scherer PE: The adipocyte-secreted protein Acrp30 enhances hepatic insulin action. Nat Med 7 :947 –953,2001 [DOI] [PubMed] [Google Scholar]
- 20.Qu S, Su D, Altomonte J, Kamagate A, He J, Perdomo G, Tse T, Jiang Y, Dong HH: PPAR{alpha} mediates the hypolipidemic action of fibrates by antagonizing FoxO1. Am J Physiol Endocrinol Metab 292 :E421 –E434,2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Schultze AE, Alborn WE, Newton RK, Konrad RJ: Administration of a PPAR{alpha} agonist increases serum apolipoprotein A-V levels and the apolipoprotein A-V/apolipoprotein C-III ratio. J Lipid Res 46 :1591 –1595,2005 [DOI] [PubMed] [Google Scholar]
- 22.Neumeier M, Sigruener A, Eggenhofer E, Weigert J, Weiss TS, Schaeffler A, Schlitt HJ, Aslanidis C, Piso P, Langmann T, Schmitz G, Scholmerich J, Buechler C: High molecular weight adiponectin reduces apolipoprotein B and E release in human hepatocytes. Biochem Biophys Res Commun 352 :543 –548,2007 [DOI] [PubMed] [Google Scholar]
- 23.Greeve J, Altkemper I, Dieterich JH, Greten H, Windler E: Apolipoprotein B mRNA editing in 12 different mammalian species: hepatic expression is reflected in low concentrations of apoB-containing plasma lipoproteins. J Lipid Res 34 :1367 –1383,1993 [PubMed] [Google Scholar]
- 24.Muurling M, Jong MC, Mensink RP, Hornstra G, Dahlmans VE, Pijl H, Voshol PJ, Havekes LM: A low-fat diet has a higher potential than energy restriction to improve high-fat diet-induced insulin resistance in mice. Metabolism 51 :695 –701,2002 [DOI] [PubMed] [Google Scholar]
- 25.Fisher EA, Ginsberg HN: Complexity in the secretory pathway: the assembly and secretion of apolipoprotein B-containing lipoproteins. J Biol Chem 277 :17377 –17380,2002 [DOI] [PubMed] [Google Scholar]
- 26.Borchardt RA, Davis RA: Intrahepatic assembly of very low density lipoproteins: rate of transport out of the endoplasmic reticulum determines rate of secretion. J Biol Chem 262 :16394 –16402,1987 [PubMed] [Google Scholar]
- 27.Lara-Castro C, Luo N, Wallace P, Klein RL, Garvey WT: Adiponectin multimeric complexes and the metabolic syndrome trait cluster. Diabetes 55 :249 –259,2006 [PubMed] [Google Scholar]
- 28.Argraves KM, Battey FD, MacCalman CD, McCrae KR, Gafvels M, Kozarsky KF, Chappell DA, Strauss JF III, Strickland DK: The very low density lipoprotein receptor mediates the cellular catabolism of lipoprotein lipase and urokinase-plasminogen activator inhibitor type I complexes. J Biol Chem 270 :26550 –26557,1995 [DOI] [PubMed] [Google Scholar]
- 29.Verges B, Petit JM, Duvillard L, Dautin G, Florentin E, Galland F, Gambert P: Adiponectin is an important determinant of ApoA-I catabolism. Arterioscler Thromb Vasc Biol 26 :1364 –1369,2006 [DOI] [PubMed] [Google Scholar]
- 30.Dixon JL, Furukawa S, Ginsberg HN: Oleate stimulates secretion of apolipoprotein B-containing lipoproteins from Hep G2 cells by inhibiting early intracellular degradation of apolipoprotein B. J Biol Chem 266 :5080 –5086,1991 [PubMed] [Google Scholar]
- 31.Levak-Frank S, Hofmann W, Weinstock PH, Radner H, Sattler W, Breslow JL, Zechner R: Induced mutant mouse lines that express lipoprotein lipase in cardiac muscle, but not in skeletal muscle and adipose tissue, have normal plasma triglyceride and high-density lipoprotein-cholesterol levels. Proc Natl Acad Sci U S A 96 :3165 –3170,1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.von Eynatten M, Schneider JG, Humpert PM, Rudofsky G, Schmidt N, Barosch P, Hamann A, Morcos M, Kreuzer J, Bierhaus A, Nawroth PP, Dugi KA: Decreased plasma lipoprotein lipase in hypoadiponectinemia: an association independent of systemic inflammation and insulin resistance. Diabetes Care 27 :2925 –2929,2004 [DOI] [PubMed] [Google Scholar]
- 33.De Vries R, Wolffenbuttel BH, Sluiter WJ, van Tol A, Dullaart RP: Post-heparin plasma lipoprotein lipase, but not hepatic lipase activity, is related to plasma adiponectin in type 2 diabetic patients and healthy subjects. Clin Lab 51 :403 –409,2005 [PubMed] [Google Scholar]
- 34.Zechner R: The tissue-specific expression of lipoprotein lipase: implications for energy and lipoprotein metabolism. Curr Opin Lipidol 8 :77 –88,1997 [DOI] [PubMed] [Google Scholar]
- 35.Takahashi S, Sakai J, Fujino T, Miyamori I, Yamamoto TT: The very low density lipoprotein (VLDL) receptor: a peripheral lipoprotein receptor for remnant lipoproteins into fatty acid active tissues. Mol Cell Biochem 248 :121 –127,2003 [DOI] [PubMed] [Google Scholar]
- 36.Takahashi S, Kawarabayasi Y, Nakai T, Sakai J, Yamamoto T: Rabbit very low density lipoprotein receptor: a low density lipoprotein receptor-like protein with distinct ligand specificity. Proc Natl Acad Sci U S A 89 :9252 –9256,1992 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Tacken PJ, Hofker MH, Havekes LM, van Dijk KW: Living up to a name: the role of the VLDL receptor in lipid metabolism. Curr Opin Lipidol 12 :275 –279,2001 [DOI] [PubMed] [Google Scholar]
- 38.Obunike JC, Lutz EP, Li Z, Paka L, Katopodis T, Strickland DK, Kozarsky KF, Pillarisetti S, Goldberg IJ: Transcytosis of lipoprotein lipase across cultured endothelial cells requires both heparan sulfate proteoglycans and the very low density lipoprotein receptor. J Biol Chem 276 :8934 –8941,2001 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.