Abstract
OBJECTIVE—Coinhibitory signals mediated via programmed death 1 (PD-1) receptor play a critical role in downregulating immune responses and in maintaining peripheral tolerance. Programmed death 1 ligand 1 (PD-L1), the interacting ligand for PD-1, widely expressed in many cell types, acts as a tissue-specific negative regulator of pathogenic T-cell responses. We investigated the protective potential of PD-L1 on autoimmune diabetes by transgenically overexpressing PD-L1 in pancreatic β-cells in nonobese diabetic (NOD) mice.
RESEARCH DESIGN AND METHODS—We established an insulin promoter–driven murine PD-L1 transgenic NOD mouse model to directly evaluate the protective effect of an organ-specific PD-L1 transgene against autoimmune diabetes. Transgene expression, insulitis, and diabetic incidence were characterized in these transgenic NOD mice. Lymphocyte development, Th1 cells, and regulatory T-cells were analyzed in these transgenic mice; and T-cell proliferation, adoptive transfer, and islet transplantation were performed to evaluate the PD-L1 transgene–mediated immune-protective mechanisms.
RESULTS—The severity of insulitis in these transgenic mice is significantly decreased, disease onset is delayed, and the incidence of diabetes is markedly decreased compared with littermate controls. NOD/SCID mice that received lymphocytes from transgenic mice became diabetic at a slower rate than mice receiving control lymphocytes. Moreover, lymphocytes collected from recipients transferred by lymphocytes from transgenic mice revealed less proliferative potential than lymphocytes obtained from control recipients. Transgenic islets transplanted in diabetic recipients survived moderately longer than control islets.
CONCLUSIONS—Our results demonstrate the protective potential of transgenic PD-L1 in autoimmune diabetes and illustrate its role in downregulating diabetogenic T-cells in NOD mice.
Programmed death 1 (PD-1) is an immunoreceptor of the CD28/CTLA-4 family whose expression is induced in activated T- and B-cells and in macrophages (1,2). PD-1 has two cytoplasmic tyrosine motifs: one an immunoreceptor tyrosine-based inhibition motif and the other an immunoreceptor tyrosine-based switch motif (ITSM). On interaction of PD-1 with its ligands PD-L1 (B7-H1) or PD-L2 (B7-DC), the tyrosine-phosphorylated ITSM of PD-1 recruits a src homology 2 domain–containing tyrosine phosphatase 2, which mediates the dephosphorylation signaling and reduces lymphocyte activation (3). PD-1−/− mice on different genetic backgrounds develop distinct autoimmune phenotypes, such as lupus-like glomerulonephritis/arthritis in C57Bl/6 (B6) mice or anticardiac troponin I–mediated dilated cardiomyopathy in Balb/c mice (4,5). These observations indicate that PD-1 is a critical negative regulator of lymphocyte activation and that the phenotype of PD-1 deficiency–induced autoimmunity is highly influenced by other genetic factors.
Murine PD-L1 is expressed on many cell types, including stromal cells within many organs, but PD-L2 expression is much more restricted, occurring mainly in dendritic cells, activated monocytes, and macrophages (6,7). Although accumulating data indicate that both PD-L1/PD-1 and PD-L2/PD-1 signals can suppress T-cell proliferation and effector function by blocking cell cycle progression and cytokine production, signaling through PD-L1 interaction is more potent than that through PD-L2 (8). This is consistent with the observations that cytokine production, cytotoxic activity, and clonal expansion were significantly enhanced in T-cells and antigen-presenting cells from PD-L1−/− mice, compared with cells from wild-type or PD-L2−/− mice (9,10). Moreover, the PD-L1−/− mice revealed an increased susceptibility to the induction of autoimmune diseases, such as experimental allergic encephalomyelitis (9), strongly suggesting a protective role of tissue PD-L1 in the maintenance of immune tolerance. Furthermore, treatment of nonobese diabetic (NOD) mice with a combination of agonistic PD-L1.Ig fusion protein and monoclonal antibodies (mAbs) to CD154 induced long-term islet allograft survival, whereas the inhibition of PD-L1–mediated signals by blocking antibody exacerbated autoimmune diabetes (11). Based on these findings, we hypothesize that transgenic expression of PD-L1 in an islet-specific manner may help in preventing T-cell–mediated islet destruction in NOD mice.
To investigate the preventive and/or therapeutic potential of PD-L1 in autoimmune diabetes, we generated transgenic NOD mice overexpressing PD-L1 under control of an insulin promoter. Although a recent report demonstrated that local expression of transgenic PD-L1 on β-cells of B6 mice unexpectedly promotes organ-specific autoimmunity and transplant rejection (12), we hypothesized that overexpression of PD-L1 on islet cells in NOD mice would enhance inhibitory signaling through the PD-1–PD-L1 interaction and protect islet cells from lymphocyte attack. Our results demonstrate that transgenic PD-L1 on islet cells significantly ameliorates the severity of insulitis and incidence of diabetes in NOD mice. Interestingly, our results also indicate that local transgene expression not only protects islets in situ but also mediates a peripheral tolerance. Moreover, transgenic islets transplanted in diabetic recipients survived moderately longer than control islets. Overall, we demonstrate for the first time the preventive potential of transgenic PD-L1 in autoimmune diabetes and provide a theoretical basis for organ-specific genetic manipulation for disease prevention.
RESEARCH DESIGN AND METHODS
NOD and NOD/SCID mice were purchased from The Jackson Laboratories (Bar Harbor, ME), and all mice were subsequently bred at the animal center of the National Defense Medical Center (Taipei, Taiwan, Republic of China) under specific pathogen-free conditions. The spontaneous incidence of diabetes in the colony was 80–90% in females and 20–30% in males by 25 weeks of age.
Generation of PD-L1 transgenic NOD mice.
The PD-L1 gene was cloned from a cDNA library of mouse heart prepared with TRIzol and Superscript II reverse transcriptase (Invitrogen, Carlsbad, CA). It was amplified by PCR with the forward primer 5′-TAGTTCCCAAAACATGAGGATA-3′ and reverse primer 5′-AGAGGGTTCAACACTGCTTAC-3′. The PCR products were cloned into the pCR3.1 vector (Invitrogen) and subsequently sequenced. The pCR3.1-PD-L1 was digested with EcoRI and subcloned into pINS plasmid for the generation of transgenic mice (13). A modified human insulin promoter (pINS) was provided by Dr. Miyazaki (Osaka University, Osaka, Japan). The murine PD-L1 cDNA was inserted into the pINS plasmid to generate a pINS-mPD-L1 construct. The linearized DNA fragment with the human insulin promoter and PD-L1 cDNA was purified and microinjected into the pronuclei of NOD embryos at the one-cell stage and then implanted into pseudopregnant (BALB/c × FVB) F1 females. Southern blotting was performed to confirm the presence of the pINS-PD-L1 transgene. All transgenic mice used in our study were heterozygous for the PD-L1 transgene.
Immunohistochemical analysis.
The pancreas was removed from each mouse, embedded into Tissue-Tek O.C.T. compound, and fixed by acetone. Frozen sections of each sample were incubated with rat anti-mouse PD-L1 mAb (eBioscience) followed by goat anti-rat–horseradish peroxidase (HRP), and then stained with aminoethyl-carbazole reagent (DAKO, Carpinteria, CA) for 10 min. A fluorescent image was collected directly after treatment with phycoerythrin-conjugated anti-mouse PD-L1 mAb, and DAPI was used for background staining.
Assessment of insulitis and diabetes.
The severity of insulitis was scored on hematoxylin-eosin–stained sections of pancreas from mice 14–16 weeks of age by investigators blinded to the identity of the sections. At least 50 islets from each pancreas were randomly chosen for microscopic examination. The severity of insulitis was classified as described previously (14). Urine glucose concentration (glycosuria) was measured weekly or every other day using Chemstrips (Boehringer Mannheim, Indianapolis, IN). Animals scoring positive (>250 mg/dl glucose) on two consecutive tests were classified as diabetic.
T-cell proliferation and division assay.
Splenocytes from 8-week-old mice were treated with Tris-buffered ammonium chloride to eliminate the erythrocytes, washed, and resuspended at a concentration of 5 × 106 cells/ml in RPMI 1640. Cells were stimulated with immobilized anti-CD3 antibody (BD Pharmingen), mouse PD-L1.Ig, or control human IgG1.Ig (R&D Systems). The incorporation of [3H]methyl thymidine was detected at 72 h with a TopCount liquid scintillation counter (Packard). For assessment of cell division, splenocytes were labeled with 5 μmol/l carboxyfluorescein succinimidyl ester (CFSE) solution at room temperature for 5 min and washed three times in PBS containing 5% FBS by centrifugation at room temperature.
Adoptive transfer.
Splenocytes of female transgenic or nontransgenic NOD donors were treated with Tris-buffered ammonium chloride for erythrocyte depletion, and 2 × 107 cells were injected into female NOD/SCID mice via the retro-orbital plexus. Diabetes was assessed as described above. In another experiment using the procedure described above, 6-week-old female transgenic NOD mice or their nontransgenic control littermates were selected as recipients and injected with 2 × 107 cells from 12-week-old female NOD donors.
Isolation of islet-infiltrated lymphocytes.
Pancreata were collected from 16- to 20-week-old transgenic or control NOD mice. Islets were isolated by collagenase digestion and Histopaque gradient purification. Marginal cells were collected and incubated with Cell Dissociation Buffer (Gibco). After passing over a cell strainer, suspended single cells were counted and stained with anti-mouse CD3 antibody for flow cytometric analysis. The percentage of infiltrated CD3+ cells in islets was obtained by gating on lymphocytes.
Flow cytometry.
Lymphocytes from spleen or pancreatic lymph node (PLN) were stained with anti-mouse CD4, CD8α, CD19, CD25, Foxp3 (BD Pharmingen), anti-mouse PD-1, PD-L1 (eBioscience), anti-human CD90 (hThy1, clone 5E10), and anti-mouse CD90.1 (mThy1.1, clone OX-7) (BD Pharmingen). CFSE-labeled lymphocytes were analyzed by triple staining with anti-mouse CD4 and CD8α antibodies. Flow cytometric analysis was performed with a FACSCalibur (BD Biosciences, San Diego, CA).
Islet transplantation.
Regular NOD and PD-L1 transgenic mice at 6–8 weeks of age were used as donors for islet transplants. Newly diabetic female NOD mice with blood glucose concentrations between 300 and 500 mg/dl were collected as recipients. Islets were isolated by collagenase digestion and Histopaque gradient purification. Marginal islets with diameters between 20 and 75 μm were handpicked, and a total of 700 islets were implanted to the left renal capsule of recipient mice. Blood glucose concentrations of recipients were monitored daily. Loss of graft function was defined as a blood glucose result of >300 mg/dl in two consecutive evaluations.
RESULTS
Expression of PD-1 and the inhibitory effect of PD-L1 on NOD T-cells.
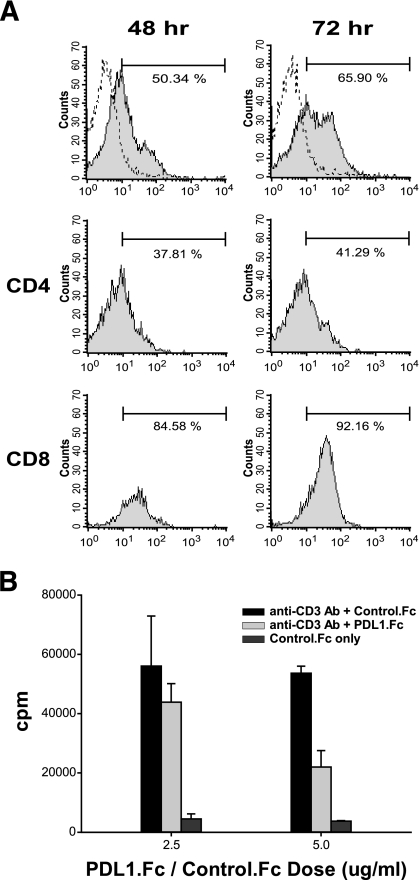
To investigate the interactive role of PD-1 and PD-L1 in the regulation of autoimmune diabetes in NOD mice, we first determined the expression of PD-1 on NOD lymphocytes and evaluated the inhibitory potential of PD-L1. Our results indicated that <1% of splenocytes freshly isolated from 8-week-old female NOD or BALB/c mice expressed PD-1 (data not shown), suggesting a very low PD-1 expression on resting splenocytes in both strains. However, 48 h after anti-CD3 stimulation, the percentage of PD-1+ splenocytes increased to 50.34% (Fig. 1A, left), consistent with previous reports that PD-1 is upregulated after T-cell receptor (TCR) stimulation (1,15), and 72 h after stimulation, the percentage of PD-1+ cells reached 65.90% (Fig. 1A, right), suggesting an increase of PD-1 dependence on activation status. Interestingly, the increase in numbers of PD-1+ CD4+ T-cells (37.81 and 41.29% at 48 and 72 h, respectively) after stimulation is much less significant than that of CD8+ T-cells (84.58 and 92.16% at 48 and 72 h, respectively), suggesting a relative inability of CD4+ T-cells to induce PD-1 on activation.
FIG. 1.
Expression of PD-1 and the inhibitory effect of PD-L1 on NOD T-cells. A: Splenocytes from 8-week-old female NOD mice were cultured in plates coated with 2 μg/ml anti-CD3 antibody for 48 (left) or 72 (right) hours. The stimulated cells were then stained with anti–PD-1 antibody (filled region), and the negative control of unstained cells is indicated as a dotted line. Percentages of PD-1+ cells in CD4 and CD8 populations (middle and bottom panels, respectively) are shown in the top right corner of the histograms. B: Splenocytes were cultured in plates coated with 2 μg/ml anti-CD3 antibody and 2.5 or 5 μg/ml PD-L1.Fc or control IgG1.Fc. Cells were pulsed with 5 μCi/ml [3H]thymidine at 60 h, and the incorporation was measured at 72 h by scintillation counting.
To investigate whether ligand binding to PD-1 can downregulate the activation status of NOD lymphocytes, we coated recombinant PD-L1.Ig on culture plates and measured the proliferation level of anti-CD3–stimulated lymphocytes. Our results clearly indicate a dose-dependent inhibition of proliferation of NOD lymphocytes by PD-L1.Ig (Fig. 1B), and they support the hypothesis that additional PD-1 signaling provided by coated PD-L1 can effectively downregulate NOD lymphocyte activation and proliferation. Therefore, we generated PD-L1 transgenic NOD mice and investigated whether β-cell–specific transgene expression could mediate protection in autoimmune diabetes.
Generation of PD-L1 transgenic NOD mice.
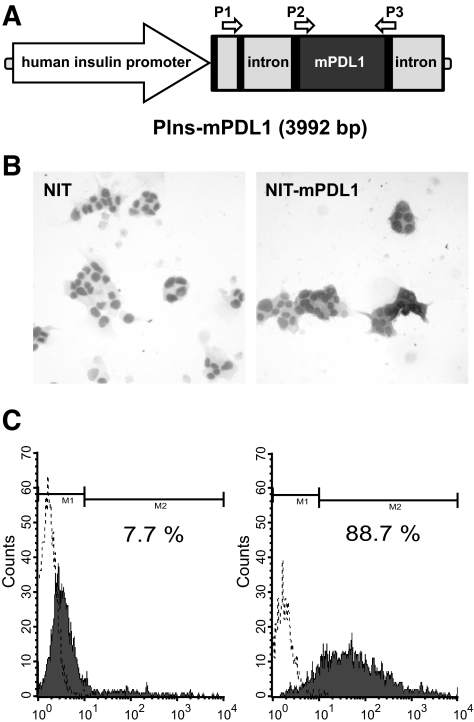
To directly evaluate the protective effect of an organ-specific PD-L1 transgene against autoimmune diabetes, we established an insulin promoter–driven murine PD-L1 (pINS-mPD-L1) transgenic NOD mouse model. The pINS-mPD-L1 construct contains the insulin promoter, the first and part of the second noncoding exons of insulin, an intron from rabbit β-globin, mouse PD-L1 cDNA, and a poly-A signal from rabbit β-globin (Fig. 2A). The insulin promoter is highly specific to β-cells and leads to significant transgene production (13,16). To specifically determine the level of transcription of the PD-L1 transgene and to distinguish it from endogenous PD-L1, we designed a P1 primer to bind to the second exon of the insulin gene and a P3 primer to bind the 3′ end of the coding region of PD-L1. The total expression of endogenous and transgenic PD-L1 was detected by a P2 primer binding to the 5′ end of the coding region of PD-L1 and P3 (Fig. 2A). To test potential expression of pINS-mPDL1 transgene in NOD β-cells, we transfected this construct into the NIT-1 cell line, an NOD insulinoma, and detected its expression by immunocytochemistry (ICC) (Fig. 2B) and flow cytometry (Fig. 2C). NIT-1 cells transfected with pINS-mPD-L1 revealed a strong PD-L1 signal detected by ICC (Fig. 2B), consistent with the flow cytometric data that 88% of cells are PD-L1+ compared with 7.6% of control cells (Fig. 2C). These results clearly demonstrate the expressional availability and feasibility of pINS-mPDL1 construct in NOD β-cells. We therefore microinjected this construct into fertilized NOD eggs to generate the pINS-mPDL1 transgenic mice.
FIG. 2.
Construction and expression of murine PD-L1. A: Schematic diagram of the whole transgene construct. The black areas represent exons, and the gray areas represent introns. The entire first noncoding exon followed by the first intron and 16 bp of the second exon of human insulin gene, which are not translated into protein, were preserved to ensure the stringency of the insulin promoter. A forward primer in the second exon of the insulin gene (P1) and a backward primer in the coding region of mouse PD-L1 (P3) were designed to specifically evaluate PIns-mPD-L1 transcription. The total PD-L1 transcription can be detected by P2 and P3 primers. NIT-1 cells were transfected with PIns-mPD-L1 (right) or control plasmid (left). Cells were then stained by anti–PD-L1 antibody to detect the PD-L1 expression by ICC (B) and flow cytometry (C). Solid histogram indicates NIT-1 transfectants stained with anti–PD-L1 antibody, and dotted line indicates unstained transfectants.
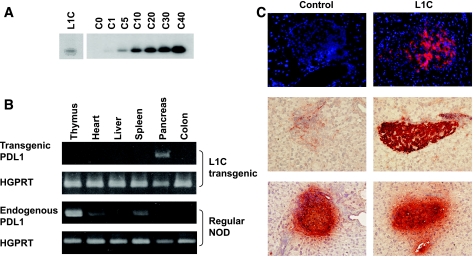
One of the transgenic lines obtained, denoted as L1C, was characterized. Southern blot analysis revealed one to five copies of the transgene in the L1C genome (Fig. 3A). The expression of transgenic PD-L1 was detected by RT-PCR with transgene-specific P1 and P3 primers. Our results demonstrate that transgenic PD-L1 was specifically expressed in pancreas in L1C mice (Fig. 3B), indicating the high stringency of the insulin promoter. Our results also indicate that in normal NOD mice, endogenous PD-L1 detected by P2 and P3 primers is highly expressed in several organs including thymus, heart, and spleen, but that the signal is very low in pancreas (Fig. 3B). High expression of PD-L1 in lymphoid organs suggests a critical role of PD-L1 in the induction and maintenance of immune tolerance, consistent with previous reports of the autoimmune phenotypes developed in PD-L1 knockout mice (9). To evaluate the expression of transgenic or endogenous PD-L1 at the protein level, we performed immunofluorescent (Fig. 3C, top) and immunohistochemical (Fig. 3C, middle) analyses on pancreatic sections from L1C (Fig. 3C, right) or NOD (Fig. 3C, left) mice. The results confirm that, consistent with the RT-PCR results, transgenic PD-L1 is highly expressed on pancreatic islets (Fig. 3C, right side of middle panel) in L1C mice, whereas the pancreatic expression of endogenous PD-L1 is very low (Fig. 3C, left side of middle panel) in control littermates (Fig. 3B). Expression patterns of insulin between L1C and NOD mice are very similar, suggesting that transgenic PD-L1 does not alter the insulin expression in L1C mice (Fig. 3C, bottom). Thus, we successfully generated L1C transgenic NOD mice in which the transgenic PD-L1 is overexpressed in an islet-specific manner.
FIG. 3.
Generation of PD-L1 transgenic NOD mice. A: Southern blot analysis identified the transgenic PD-L1 signal in one of the founders, denoted as L1C, and provided the relative copy number of the PD-L1 transgene in the L1C line. B: Islet-specific expression of the PD-L1 transgene was confirmed by RT-PCR analysis. The spliced transgenic PIns-mPD-L1 and endogenous PD-L1 transcripts from multiple organs of L1C (top) or regular NOD (bottom) mice were assessed. HGPRT transcription served as internal control. C: Frozen pancreatic sections of 6-week-old control NOD (left) or L1C (right) mice were stained with phycoerythrin-conjugated anti–PD-L1 antibody and then observed on a fluorescent microscope. DAPI was used as background staining (top). Immunohistochemical analysis was performed using anti–PD-L1 (middle) or anti-insulin (bottom) and HRP-conjugated secondary antibody (bottom)..
Characterization of the diabetogenic process in L1C mice.
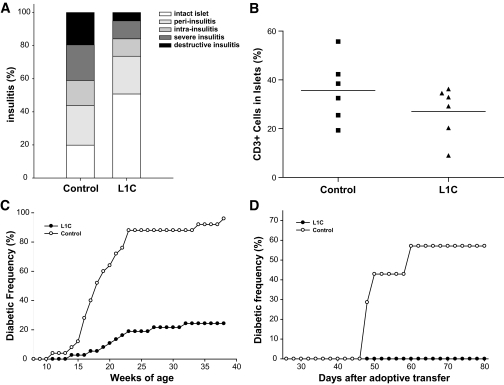
To investigate the protective effects of organ-specific and membrane-bound expression of transgenic PD-L1 in autoimmune diabetes, we analyzed the severity of insulitis and the incidence of spontaneous diabetes in L1C mice. Although expression of the transgene did not completely prevent the development of insulitis, the severity of insulitis in L1C mice was significantly reduced (Fig. 4A). Around 50% of islets from L1C mice were free from lymphocytes compared with 20% in controls (P < 0.05); and only 5% islets from L1C showed destructive insulitis compared with 20% in controls (P < 0.05). In addition to histological analysis of insulitis, we further assessed the lymphocyte composition of the infiltrates within the islet by flow cytometry. Our result indicates that the percentage of CD3 T-cells in L1C islets is significantly decreased compared with control islets (26 vs. 36%, P < 0.05; Fig. 4B). To further investigate the protective efficacy of transgenic PD-L1, we analyzed spontaneous and lymphocyte transfer diabetes in L1C mice. Compared with control littermates, which started to develop diabetes at 11 weeks of age, the first L1C mouse became diabetic after 14 weeks, indicating a delay in onset of disease. At around 20 weeks of age, the diabetic incidence of control mice increased to 64%, but the incidence in L1C mice was only 10.8%. After 25 weeks, >88% of control mice developed diabetes, but the incidence in L1C mice was only 21.6% (Fig. 4C), demonstrating that transgenic PD-L1 significantly inhibits the development of autoimmune diabetes in NOD mice (P < 0.01). Because it is possible that the protection seen in L1C mice is due to modulation of the development of autoreactive T-cells by transgenic expression of PD-L1, we performed adoptive transfer experiments to further investigate whether PD-L1 overexpression can also protect mice from lymphocyte transfer–induced diabetes. Six-week-old female L1C mice were injected intravenously with 2 × 107 splenocytes isolated from 12-week-old female NOD mice. Around 60% of control mice developed diabetes 60 days after transfer, but L1C recipients remained diabetes free until 80 days (Fig. 4D), suggesting that islet-specific expression of PD-L1 not only inhibits the development of spontaneous diabetes but also protects mice from pathogenic lymphocyte transfer–mediated diabetes.
FIG. 4.
Characterization of the diabetogenic process of PD-L1 transgenic mice. A: The insulitis scores were measured in seven mice of each group at 14–16 weeks old. B: The CD3 T-cells infiltrated in the pancreatic islets of L1C (▴) or control littermates (▪) were analyzed by flow cytometry. C: Spontaneous diabetes in female L1C (•, 37 mice) or their control littermates (○, 25 mice) was monitored by weekly measurement of glycosuria. D: The incidence of diabetes in L1C mice or control littermates adoptively transferred with splenocytes from 12-week-old female NOD mice was monitored by testing every other day for glycosuria.
One of the most stringent ways to test whether overexpression of PD-L1 could protect β-cells against immune attack is to examine whether transgenic islet grafts could reverse hyperglycemia in diabetic NOD mice. We isolated islets from L1C mice and transplanted them into kidney capsules of diabetic NOD recipients. Although transgenic islets survived longer than wild-type islets, the result is not statistically significant (7.9 and 6.1 days, respectively, P = 0.06; Table 1), suggesting that expression of transgenic PD-L1 was partially effective in prolonging islet graft survival but did not provide complete protection from diabetes recurrence.
TABLE 1.
Transgenic PD-L1 moderately prolongs the survival period of grafts in isogenic islet transplantation
| Donor islets | Graft survival of each recipient (days) | Mean survival period (days) |
|---|---|---|
| NOD | 5, 5, 5, 6, 6, 6, 7, 9 | 6.1 |
| L1C | 5, 6, 7, 7, 7, 9, 9, 13 | 7.9 |
Pancreatic islets isolated from 6- to 8-week-old mice were transplanted into recipients that were newly diagnosed with diabetes. Blood glucose concentration of recipients was monitored every day. The recurrence of diabetes among the recipients was defined as a recipient blood glucose concentration >16.7 mmol/l in two consecutive evaluations. The day of diabetic recurrence was considered as the end of islet survival.
Lymphocyte development and the antidiabetogenic mechanism in L1C mice.
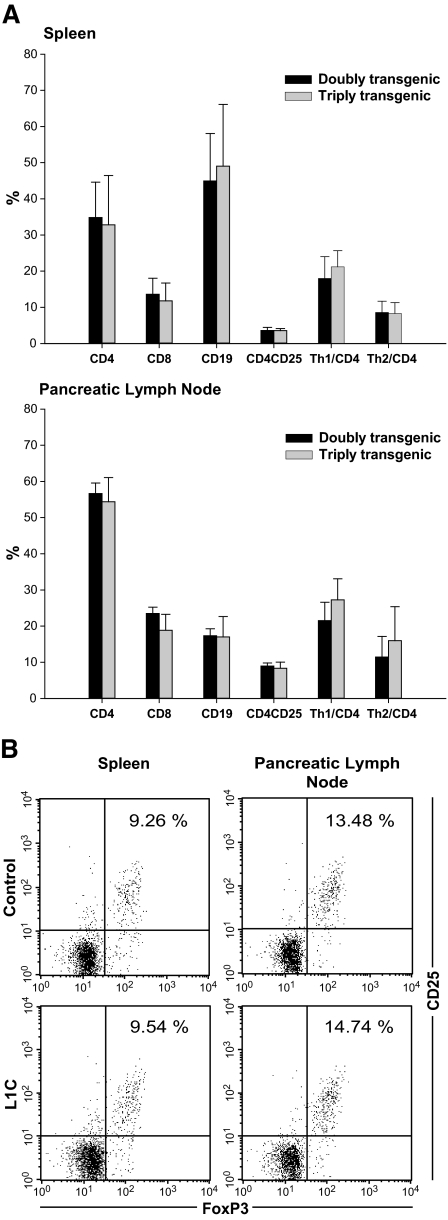
It has been well characterized that an imbalance between Th1 and Th2 responses predisposes NOD mice to developing autoimmune diabetes (17). To investigate whether the protective effect of transgenic PD-L1 works through regulating Th1 and Th2 development in L1C mice, we crossed L1C transgenic mice with T1 and T2 double transgenic NOD mice to generate PD-L1/T1/T2 triple transgenic mice. T1 and T2 double transgenic mice bear two transgenes: human Thy1 (hThy1) under control of the murine interferon-γ (IFN-γ) promoter and murine Thy1.1 (mThy1.1) under control of the murine interleukin-4 (IL-4) promoter (18). Using these PD-L1/T1/T2 triple transgenic mice, we can directly measure the levels of Th1 and Th2 cells by detecting the expression of hThy1 (Th1 marker) and mThy1.1 (Th2 marker), respectively. The absolute cell numbers and distribution of each lymphocyte subpopulation in spleen (Fig. 5A, top) or PLNs (Fig. 5A, bottom) are similar between PD-L1/T1/T2 triple (Fig. 5A, gray bar) and T1/T2 double (Fig. 5A, black bar) transgenic mice, indicating that local expression of transgenic PD-L1 does not alter systemic lymphocyte development in NOD mice. Moreover, the number and percentage of IFN-γ–producing (hThy1+) or IL-4–producing (mThy1.1+) cells in spleen or PLN in PD-L1/T1/T2 triple transgenic mice were not different from those in T1/T2 double transgenic mice, suggesting that transgenic PD-L1 in islets does not suppress systemic IFN-γ–producing cells or induce IL-4–producing cells.
FIG. 5.
Lymphocyte development and analyses of regulatory and helper T-cells in T1/T2 double or L1C/T1/T2 triple transgenic mice. A: The compositions of lymphocytes and the percentages of Th1, Th2, and Tregs in spleen (top) and PLN (bottom) from 8-week-old triple (gray bar) and double transgenic mice (black bar) were analyzed by flow cytometry. B: The population of Tregs of control (top) and L1C (bottom) mice was analyzed by the detection of CD25 and FoxP3 expression as gated on CD4+ lymphocytes in spleen (left) and PLN (right).
It has also been reported that NOD mice have a relative deficiency in CD4+25+ regulatory T-cells (Tregs), leading to an inability to maintain peripheral tolerance (19). To investigate whether transgenic PD-L1 can enhance the development of these Tregs and thus protect L1C mice from autoimmune diabetes, we analyzed the number and percentage of CD4+25+Foxp3 cells in transgenic mice. L1C mice (Fig. 5B, bottom) and control littermates (Fig. 5B, top) exhibited similar numbers and percentages of CD4+25+Foxp3 cells in spleen (Fig. 5B, left) and PLN (Fig. 5B, right), indicating that transgenic PD-L1 is not likely to increase the population of Tregs. These results also suggest that genetic manipulation of immune responses in an organ-specific manner is not likely to induce the systemic development of Tregs.
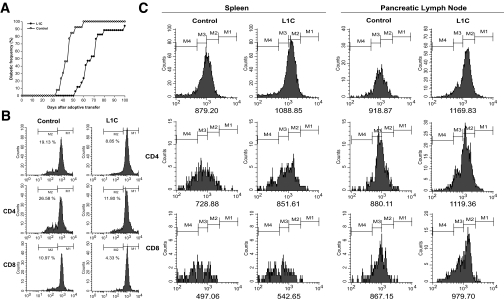
To further investigate the protective mechanism of transgenic PD-L1, we performed adoptive transfer experiments and functionally characterized the pathogenicity of T-cells in L1C mice. NOD/SCID recipients were injected intravenously with L1C or control splenocytes and the diabetic processes in the two groups were compared. In recipients of splenocytes from nontransgenic littermates, diabetes developed at 32 days after injection, whereas recipients of splenocytes from L1C mice developed disease at day 52. At day 60, all recipients of control cells had developed disease, whereas the disease incidence in recipients of L1C splenocytes was only 23.5%, although after this time, recipients of L1C splenocytes rapidly developed diabetes, and the incidence reached 90% at day 100 (Fig. 6A). These results indicate an impairment of L1C splenocytes in disease induction and suggest a potential role of transgenic PD-L1 in regulating autoimmunity in NOD mice. To further characterize the proliferation of L1C lymphocytes, we stimulated those cells with anti-CD3 or ConA. By CFSE-labeling experiments, we demonstrated that anti-CD3–stimulated L1C lymphocytes, both CD4 and CD8, divided much more slowly than control lymphocytes (Fig. 6B). Further analyses on lymphocytes isolated from NOD/SCID recipients also indicate that L1C lymphocytes were less proliferative and divided more slowly than control lymphocytes (Fig. 6C), consistent with the observation that L1C lymphocytes induced disease in recipients with a slower time course (Fig. 6A).
FIG. 6.
Adoptive transfer and lymphocyte division assay. A: The incidence of diabetes in NOD/SCID recipients adoptively transferred with splenocytes from 12- to 14-week-old L1C (•) or control (○) mice was assessed by testing for glycosuria every other day. B: Splenocytes from L1C (right) or control (left) mice were labeled with CFSE, stimulated in plates coated with 4 μg/ml anti-CD3 antibody, and analyzed by flow cytometry 3 days after stimulation. C: CFSE-labeled splenocytes from L1C or control mice were adoptively transferred into NOD/SCID mice to investigate their ability to divide in vivo. The lymphocytes from spleen (left) or PLN (right) of recipients transferred from L1C (right side of each panel) or control mice (left side of each panel) were analyzed by flow cytometry at day 3 after transfer. The mean fluorescence intensity was indicated on the bottom of each plot.
DISCUSSION
PD-1 knockout mice in different genetic backgrounds develop various autoimmune diseases, not only indicating that negative costimulation via PD-1 is essential to downregulate inappropriate immune responses, such as autoimmunity and sustained inflammation, but also suggesting that PD-1 deficiency may exaggerate the genetic predisposition of autoimmune diseases, such as type 1 diabetes, rheumatoid arthritis, or lupus. PD-L1 acts as a tissue-specific negative regulator of pathogenic T-cell responses. Our result that PD-L1 is highly expressed in lymphoid organs, such as thymus, spleen, and lymph nodes, supports a critical role of PD-L1 in the induction and maintenance of immune tolerance, also consistent with previous reports of the autoimmune phenotypes developed in PD-L1 knockout mice (9). Moreover, the observation in our study that heart expresses high levels of PD-L1 may be linked to the abnormal phenotype in mice that develop fatal autoimmune cardiomyopathy due to a lack of PD-1 signaling (5).
In contrast to our results in L1C mice, it has recently been reported that expression of a rat insulin promoter-driven PD-L1 transgene in mice on a B6 background unexpectedly enhanced T-cell priming and effector function and promoted autoimmunity (12). Based on the discrepancy between these two models, we propose that inhibitory regulation by PD-1/PD-L1 interactions may be highly dependent on the genetic background of mouse strains. This is supported by development of distinct autoimmune diseases in PD-1–deficient mice of different genetic backgrounds. NOD mice, unlike B6 mice, are well known for their potential for systemic autoimmunity and progressive pancreatic β-cell–specific destruction. The genetic components involved in the regulation of PD-1 signaling and the eventual outcome of transgenic PD-L1 expression in β-cells may differ between NOD and B6 mice. Although polymorphisms in the PD-1 gene may explain part of these complicated phenomena, the detailed mechanism for these strain-dependent variations of phenotype are not yet clear (20,21).
PD-L1, in addition to binding to PD-1, has been reported to interact with a non–PD-1 receptor and to transmit both costimulatory and apoptotic signals (22). The PD-L1 binding sites for PD-1 and the unknown receptor are distinct. It has also been demonstrated that PD-L1 mutants with impaired PD-1 binding have significantly increased costimulatory potential (23). However, the regulation of the balance for PD-L1 transmitting a negative signal through PD-1 or a positive signal through the unknown receptor is not yet clear. The interactions of PD-L1 with PD-1 or the unknown receptor are distinct, and it is possible that they compete with each other to influence the TCR activation threshold (23). Therefore, it is possible that different threshold levels of regulation through the PD-1/PD-L1 or unknown receptor/PD-L1 pathways could lead to the divergent results in NOD and B6 mice. Finally, the differential levels of expression of transgenic and/or endogenous PD-L1 in β-cells may also contribute to the discrepancy between these two models. It is possible that in L1C NOD mice, overexpressed transgenic PD-L1 binds PD-1 and transduces an inhibitory signal to autoreactive T-cells, whereas in B6 transgenic mice, overexpressed PD-L1 has a dominant-negative effect binding to PD-1 but, for some unknown reason, not transducing a signal.
In our transplantation model, the failure of long-term graft survival may imply two possibilities. One possible explanation may be that insulin promoter–driving expression of PD-L1 was disturbed by the primary nonfunction of transplanted islets. Another possible explanation is that transplantation processes trigger strong oxidative stress- or cytokine-induced apoptosis of transplanted islets, which cannot be prevented by PD-L1.
It has been well characterized that an imbalance between Th1 and Th2 responses predisposes NOD mice to developing autoimmune diabetes (17). Based on our triple transgenic model in this study, we demonstrate that the number and percentage of IFN-γ–producing (hThy1+) or IL-4–producing (mThy1.1+) cells in spleen or PLN in PD-L1/T1/T2 triple transgenic mice were not different from those in T1/T2 double transgenic mice (Fig. 5A), suggesting that transgenic PD-L1 in islets does not suppress systemic IFN-γ–producing cells or induce IL-4–producing cells. This result does not support our original idea that the protective mechanism in L1C mice may be mediated by “rebalancing” Th1 and Th2 development. However, this result is consistent with the primary observation from another group that the numbers of IFN-γ–or IL-4–producing cells in spleen and PLN are similar between PD1−/− and control mice (24). However, further analysis of T-cells infiltrating the pancreas revealed that the percentage of IFN-γ–producing cells in PD-1−/− mice is much greater than in normal NOD mice (8,24). This considerable discrepancy in Th1 cell development between L1C and PD-1−/− mice suggests two possibilities. One possibility is that both PD-L1–and PD-L2–mediated signaling pathways are blocked in PD-1−/− NOD mice and this causes the net effect on Th1 polarization. Alternatively, whereas loss of function in PD-1 from a very early developmental stage substantially promotes the development of Th1 cells, a gain of function and local expression of transgenic PD-L1 insufficiently inhibits Th1 polarization and maintains the systemic Th1/Th2 balance.
Our result also revealed that L1C mice and control littermates exhibited similar numbers and percentages of CD4+25+ cells in spleen and PLN (Fig. 5A), suggesting that transgenic PD-L1 is not likely to increase the population of Tregs. This is supported by the data from L1C and from other transgenic lines generated in our laboratory in which the insulin promoter drives different immunomodulatory genes, including decoy receptor 3 (13), PD-L2, single-chain anti–CTLA-4 Fv, or heme oxygenase-1 (S. Shieh, S. Huang, H.-K.S., unpublished data), where our results suggest that “local” transgene-mediated protection is not through the induction of Tregs. These results also suggest that genetic manipulation of immune responses in an organ-specific manner is not likely to induce the systemic development of Tregs.
The significant protection mediated by islet-specific PD-L1 expression in L1C mice is consistent with a recent report that PD-L1−/− NOD mice displayed an aggressive autoimmunity with a rapid onset of disease and complete diabetic penetrance (8). Consistent with these results, a current report also demonstrates that both the insulin-induced remission in new-onset autoimmune diabetes and the long-term maintenance of immune tolerance in NOD mice are highly dependent on the PD-1-PD-L1 pathway (25). Interestingly, PD-L2−/− mice developed a similar autoimmune phenotype to NOD littermates (8), consistent with our observation that transgenic expression of PD-L2 in islets mediates a weaker immunoprotection (C.-J.W., H.-K.S., unpublished data) than that observed in L1C mice. Both of these results support the uniqueness and importance of the role of PD-L1 in preventing autoimmune diabetes. A very recent report demonstrates that PD-L1 specifically interacting with the B7-1 costimulatory molecule suppresses T-cell responses and inhibits cytokine production (26). These bidirectional inhibitory interactions of PD-L1 to PD-1 and B7-1 provide an additional dimension to immune network of PD family and further support the preferential role of PD-L1 in mediating immunoprotection on autoimmune diseases.
It has been reported that engagement of PD-1 and its ligand results in G0/G1 arrest and inhibits T-cell responses (7,27), and our study demonstrated that L1C lymphocytes were less responsive to TCR stimulation. This lymphocyte arrest may be caused by the interaction of PD-1 and transgenic PD-L1. PD-1/PD-L1–mediated inhibition can be overridden by various factors, such as CD28, IL-2, LPS, CpG, and IL-4 (28,29). The threshold of T-cell activation is decreased in PD-1−/− lymphocytes (30), consistent with the increased autoimmune reactivity in PD-1−/− mice (4,5). The results in our study suggest that local expression of transgenic PD-L1 in the pancreas of L1C mice, where autoantigens are highly expressed, inhibits the priming and clonal expansion of pathogenic lymphocytes at an early stage, suppressing the production of inflammatory factors and thus providing protection.
Promoting an inhibitory signal through the PD-1/L1 pathway can also be applied as a therapeutic strategy in allograft transplantation. The ability of the PD-1/L1 pathway to negatively regulate alloimmunity and promote allograft survival has been demonstrated in various transplantation systems, including cardiac (29), skin (31), and islet transplantation (11). However, enhancing the PD-1 pathway alone is not enough to completely ensure graft survival, and combining treatment with cyclosporine A or CD154 blockade can more efficiently prevent graft rejection (11). Our results with L1C transgenic mice are the first demonstration of the protective potential of transgenic PD-L1 in autoimmune diabetes and provide a theoretical basis for genetic manipulation of islets for future transplantation.
Acknowledgments
H.-K.S. has received grants NSC96-2628-B-016-002-MY3 and NSC96-3112-B-016-001 from the National Science Council (Taiwan, Republic of China). This work was supported in part by the C.Y. Foundation for Advancement of Education, Sciences, and Medicine.
Published ahead of print at http://diabetes.diabetesjournals.org on 16 April 2008.
The costs of publication of this article were defrayed in part by the payment of page charges. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
REFERENCES
- 1.Ishida Y, Agata Y, Shibahara K, Honjo T: Induced expression of PD-1, a novel member of the immunoglobulin gene superfamily, upon programmed cell death. EMBO J 11 :3887 –3895,1992 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Greenwald RJ, Freeman GJ, Sharpe AH: The B7 family revisited. Annu Rev Immunol 23 :515 –548,2005 [DOI] [PubMed] [Google Scholar]
- 3.Chemnitz JM, Parry RV, Nichols KE, June CH, Riley JL: SHP-1 and SHP-2 associate with immunoreceptor tyrosine-based switch motif of programmed death 1 upon primary human T cell stimulation, but only receptor ligation prevents T cell activation. J Immunol 173 :945 –954,2004 [DOI] [PubMed] [Google Scholar]
- 4.Nishimura H, Nose M, Hiai H, Minato N, Honjo T: Development of lupus-like autoimmune diseases by disruption of the PD-1 gene encoding an ITIM motif-carrying immunoreceptor. Immunity 11 :141 –151,1999 [DOI] [PubMed] [Google Scholar]
- 5.Nishimura H, Okazaki T, Tanaka Y, Nakatani K, Hara M, Matsumori A, Sasayama S, Mizoguchi A, Hiai H, Minato N, Honjo T: Autoimmune dilated cardiomyopathy in PD-1 receptor-deficient mice. Science 291 :319 –322,2001 [DOI] [PubMed] [Google Scholar]
- 6.Dong H, Zhu G, Tamada K, Chen L: B7–H1, a third member of the B7 family, co-stimulates T-cell proliferation and interleukin-10 secretion. Nat Med 5 :1365 –1369,1999 [DOI] [PubMed] [Google Scholar]
- 7.Latchman Y, Wood CR, Chernova T, Chaudhary D, Borde M, Chernova I, Iwai Y, Long AJ, Brown JA, Nunes R, Greenfield EA, Bourque K, Boussiotis VA, Carter LL, Carreno BM, Malenkovich N, Nishimura H, Okazaki T, Honjo T, Sharpe AH, Freeman GJ: PD-L2 is a second ligand for PD-1 and inhibits T cell activation. Nat Immunol 2 :261 –268,2001 [DOI] [PubMed] [Google Scholar]
- 8.Keir ME, Liang SC, Guleria I, Latchman YE, Qipo A, Albacker LA, Koulmanda M, Freeman GJ, Sayegh MH, Sharpe AH: Tissue expression of PD-L1 mediates peripheral T cell tolerance. J Exp Med 203 :883 –895,2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Latchman YE, Liang SC, Wu Y, Chernova T, Sobel RA, Klemm M, Kuchroo VK, Freeman GJ, Sharpe AH: PD-L1-deficient mice show that PD-L1 on T cells, antigen-presenting cells, and host tissues negatively regulates T cells. Proc Natl Acad Sci U S A 101 :10691 –10696,2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Shin T, Yoshimura K, Crafton EB, Tsuchiya H, Housseau F, Koseki H, Schulick RD, Chen L, Pardoll DM: In vivo costimulatory role of B7-DC in tuning T helper cell 1 and cytotoxic T lymphocyte responses. J Exp Med 201 :1531 –1541,2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gao W, Demirci G, Strom TB, Li XC: Stimulating PD-1-negative signals concurrent with blocking CD154 co-stimulation induces long-term islet allograft survival. Transplantation 76 :994 –999,2003 [DOI] [PubMed] [Google Scholar]
- 12.Subudhi SK, Zhou P, Yerian LM, Chin RK, Lo JC, Anders RA, Sun Y, Chen L, Wang Y, Alegre ML, Fu YX: Local expression of B7-H1 promotes organ-specific autoimmunity and transplant rejection. J Clin Invest 113 :694 –700,2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sung HH, Juang JH, Lin YC, Kuo CH, Hung JT, Chen A, Chang DM, Chang SY, Hsieh SL, Sytwu HK: Transgenic expression of decoy receptor 3 protects islets from spontaneous and chemical-induced autoimmune destruction in nonobese diabetic mice. J Exp Med 199 :1143 –1151,2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Verdaguer J, Schmidt D, Amrani A, Anderson B, Averill N, Santamaria P: Spontaneous autoimmune diabetes in monoclonal T cell nonobese diabetic mice. J Exp Med 186 :1663 –1676,1997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bennett F, Luxenberg D, Ling V, Wang IM, Marquette K, Lowe D, Khan N, Veldman G, Jacobs KA, Valge-Archer VE, Collins M, Carreno BM: Program death-1 engagement upon TCR activation has distinct effects on costimulation and cytokine-driven proliferation: attenuation of ICOS, IL-4, and IL-21, but not CD28, IL-7, and IL-15 responses. J Immunol 170 :711 –718,2003 [DOI] [PubMed] [Google Scholar]
- 16.Hotta M, Tashiro F, Ikegami H, Niwa H, Ogihara T, Yodoi J, Miyazaki J: Pancreatic beta cell-specific expression of thioredoxin, an antioxidative and antiapoptotic protein, prevents autoimmune and streptozotocin-induced diabetes. J Exp Med 188 :1445 –1451,1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Liblau RS, Singer SM, McDevitt HO: Th1 and Th2 CD4+ T cells in the pathogenesis of organ-specific autoimmune diseases. Immunol Today 16 :34 –38,1995 [DOI] [PubMed] [Google Scholar]
- 18.Hung JT, Liao JH, Lin YC, Chang HY, Wu SF, Chang TH, Kung JT, Hsieh SL, McDevitt H, Sytwu HK: Immunopathogenic role of TH1 cells in autoimmune diabetes: evidence from a T1 and T2 doubly transgenic non-obese diabetic mouse model. J Autoimmun 25 :181 –192,2005 [DOI] [PubMed] [Google Scholar]
- 19.Kukreja A, Cost G, Marker J, Zhang C, Sun Z, Lin-Su K, Ten S, Sanz M, Exley M, Wilson B, Porcelli S, Maclaren N: Multiple immuno-regulatory defects in type-1 diabetes. J Clin Invest 109 :131 –140,2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Prokunina L, Castillejo-Lopez C, Oberg F, Gunnarsson I, Berg L, Magnusson V, Brookes AJ, Tentler D, Kristjansdottir H, Grondal G, Bolstad AI, Svenungsson E, Lundberg I, Sturfelt G, Jonssen A, Truedsson L, Lima G, Alcocer-Varela J, Jonsson R, Gyllensten UB, Harley JB, Alarcon-Segovia D, Steinsson K, Alarcon-Riquelme ME: A regulatory polymorphism in PDCD1 is associated with susceptibility to systemic lupus erythematosus in humans. Nat Genet 32 :666 –669,2002 [DOI] [PubMed] [Google Scholar]
- 21.Ferreiros-Vidal I, Gomez-Reino JJ, Barros F, Carracedo A, Carreira P, Gonzalez-Escribano F, Liz M, Martin J, Ordi J, Vicario JL, Gonzalez A: Association of PDCD1 with susceptibility to systemic lupus erythematosus: evidence of population-specific effects. Arthritis Rheum 50 :2590 –2597,2004 [DOI] [PubMed] [Google Scholar]
- 22.Dong H, Strome SE, Salomao DR, Tamura H, Hirano F, Flies DB, Roche PC, Lu J, Zhu G, Tamada K, Lennon VA, Celis E, Chen L: Tumor-associated B7–H1 promotes T-cell apoptosis: a potential mechanism of immune evasion. Nat Med 8 :793 –800,2002 [DOI] [PubMed] [Google Scholar]
- 23.Wang S, Bajorath J, Flies DB, Dong H, Honjo T, Chen L: Molecular modeling and functional mapping of B7-H1 and B7-DC uncouple costimulatory function from PD-1 interaction. J Exp Med 197 :1083 –1091,2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wang J, Yoshida T, Nakaki F, Hiai H, Okazaki T, Honjo T: Establishment of NOD-Pdcd1−/− mice as an efficient animal model of type I diabetes. Proc Natl Acad Sci U S A 102 :11823 –11828,2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Fife BT, Guleria I, Gubbels Bupp M, Eagar TN, Tang Q, Bour-Jordan H, Yagita H, Azuma M, Sayegh MH, Bluestone JA: Insulin-induced remission in new-onset NOD mice is maintained by the PD-1-PD-L1 pathway. J Exp Med 203 :2737 –2747,2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Butte MJ, Keir ME, Phamduy TB, Sharpe AH, Freeman GJ: Programmed death-1 ligand 1 interacts specifically with the B7-1 costimulatory molecule to inhibit T cell responses. Immunity 27 :111 –122,2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Selenko-Gebauer N, Majdic O, Szekeres A, Hofler G, Guthann E, Korthauer U, Zlabinger G, Steinberger P, Pickl WF, Stockinger H, Knapp W, Stockl J: B7-h1 (programmed death-1 ligand) on dendritic cells is involved in the induction and maintenance of T cell anergy. J Immunol 170 :3637 –3644,2003 [DOI] [PubMed] [Google Scholar]
- 28.Zhong X, Bai C, Gao W, Strom TB, Rothstein TL: Suppression of expression and function of negative immune regulator PD-1 by certain pattern recognition and cytokine receptor signals associated with immune system danger. Int Immunol 16 :1181 –1188,2004 [DOI] [PubMed] [Google Scholar]
- 29.Ito T, Ueno T, Clarkson MR, Yuan X, Jurewicz MM, Yagita H, Azuma M, Sharpe AH, Auchincloss H Jr, Sayegh MH, Najafian N: Analysis of the role of negative T cell costimulatory pathways in CD4 and CD8 T cell-mediated alloimmune responses in vivo. J Immunol 174 :6648 –6656,2005 [DOI] [PubMed] [Google Scholar]
- 30.Freeman GJ, Long AJ, Iwai Y, Bourque K, Chernova T, Nishimura H, Fitz LJ, Malenkovich N, Okazaki T, Byrne MC, Horton HF, Fouser L, Carter L, Ling V, Bowman MR, Carreno BM, Collins M, Wood CR, Honjo T: Engagement of the PD-1 immunoinhibitory receptor by a novel B7 family member leads to negative regulation of lymphocyte activation. J Exp Med 192 :1027 –1034,2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sandner SE, Clarkson MR, Salama AD, Sanchez-Fueyo A, Domenig C, Habicht A, Najafian N, Yagita H, Azuma M, Turka LA, Sayegh MH: Role of the programmed death-1 pathway in regulation of alloimmune responses in vivo. J Immunol 174 :3408 –3415,2005 [DOI] [PubMed] [Google Scholar]