Abstract
OBJECTIVE—Increased intramuscular triacylglycerol (IMTG) storage is a characteristic of the obese insulin-resistant state. We aimed to investigate whether a blunted fasting or β-adrenergically mediated lipolysis contributes to this increased IMTG storage in obesity.
RESEARCH DESIGN AND METHODS—Forearm skeletal muscle lipolysis was investigated in 13 lean and 10 obese men using [2H5]glycerol combined with the measurement of arteriovenous differences before and during β-adrenergic stimulation using the nonselective β-agonist isoprenaline (ISO). Muscle biopsies were taken from the vastus lateralis muscle before and during ISO to investigate hormone-sensitive lipase (HSL) protein expression and serine phosphorylation.
RESULTS—Baseline total glycerol release across the forearm was significantly blunted in obese compared with lean subjects (P = 0.045). This was accompanied by lower HSL protein expression (P = 0.004), HSL phosphorylation on PKA sites Ser563 (P = 0.041) and Ser659 (P = 0.09), and HSL phosphorylation on the AMPK site Ser565 (P = 0.007), suggesting a blunted skeletal muscle lipolysis in obesity. Total forearm glycerol uptake during baseline did not differ significantly between groups, whereas higher net fatty acid uptake across the forearm was observed in the obese (P = 0.064). ISO induced an increase in total glycerol release from skeletal muscle, which was not significantly different between groups. Interestingly, this was accompanied by an increase in HSL Ser659 phosphorylation in obese subjects during ISO compared with baseline (P = 0.008).
CONCLUSIONS—Obesity is accompanied by impaired fasting glycerol release, lower HSL protein expression, and serine phosphorylation. It remains to be determined whether this is a primary factor or an adaptation to the obese insulin-resistant state.
The obese insulin-resistant state is characterized by increased triacylglycerol (TAG) storage in adipose and nonadipose tissues (ectopic fat), such as skeletal muscle (1). A strong link between increased intramuscular TAG (IMTG) stores and skeletal muscle insulin resistance has been shown in lean and obese subjects (2,3). Recent studies have, however, indicated that intramuscular accumulation of lipid intermediates rather than TAG per se may be the direct cause of skeletal muscle insulin resistance, through interference with insulin signaling (4). Impaired uptake and a reduced oxidation of fatty acids has been reported in skeletal muscle under postabsorptive conditions, during β-adrenergic stimulation and moderate-intensity exercise in obese subjects with type 2 diabetes (5–7). Besides impaired fatty acid handling, disturbances in the regulation of skeletal muscle lipolysis may contribute to the increased storage of IMTG and lipid metabolites. So far, little is known on the in vivo regulation of skeletal muscle lipolysis in obesity. Data from our laboratory indicate that the catecholamine-induced increase in interstitial glycerol concentration and local blood flow are blunted in obese subjects (8), a factor that may contribute to an increase in content of muscle TAG and diacylglycerol.
Although the molecular mechanisms that underlie muscle lipolysis are not known in detail, it has been shown that hormone-sensitive lipase (HSL) is expressed in skeletal muscle of rodents (9,10) and humans (11). HSL activity appears to be regulated by site-specific phosphorylation on several serine residues. It has been demonstrated that skeletal muscle HSL can be phosphorylated on at least five serine residues (Ser563, Ser565, Ser600, Ser659, and Ser660) (11–13). Catecholamines increase intracellular cyclic AMP concentration, resulting in the activation of protein kinase A (PKA). HSL Ser563, Ser659, and Ser660 are major PKA phosphorylation sites, although Ser563 may not affect HSL activity directly (14). It is still unclear which of the PKA phosphorylation sites on HSL are important in mediating the effect of catecholamines on in vivo muscle HSL activity. Ser659 appears to be a likely candidate because HSL Ser659 phosphorylation and HSL activity show a similar response to exercise with concomitant increase in circulating epinephrine (13). In vitro studies on purified bovine adipocyte HSL have shown that AMP-activated protein kinase (AMPK) phosphorylates HSL on Ser565, thereby abolishing PKA-induced HSL activation (15). In human skeletal muscle, changes in AMPK activity during exercise were also associated with an increased HSL Ser565 phosphorylation, but this was not accompanied by an increased HSL activity, suggesting that AMPK can phosphorylate HSL on Ser565 but that AMPK is of minor importance as a regulator of HSL activity in human skeletal muscle during exercise (11).
So far, limited data are available on the differences in in vivo regulation of skeletal muscle lipolysis between lean and obese subjects. The aim of the present study was to investigate whether in vivo baseline and/or catecholamine-induced lipolysis is impaired in skeletal muscle of obese compared with lean subjects. For this reason, [2H5]glycerol tracer methodology was used to investigate in vivo whole-body and regional forearm skeletal muscle lipolysis in lean and obese subjects after an overnight fast and during β-adrenergic stimulation, using the nonselective β-adrenergic agonist isoprenaline (ISO). To obtain more information on the underlying mechanism at the molecular level, we measured skeletal muscle HSL protein expression and serine phosphorylation on Ser563, Ser565, and Ser659.
RESEARCH DESIGN AND METHODS
Three healthy lean (two women and one man; age 20 ± 1 years; BMI 22.3 ± 1.1 kg/m2) subjects participated in a pilot experiment during which [2H5]glycerol enrichment was investigated during 6-h infusion to determine the time required to achieve an isotopic steady state. Thirteen lean and 10 obese nonsmoking men participated in the actual muscle lipolysis experiment during which [2H5]glycerol was infused for 3 h. Clinical characteristics of the subjects included in the experiment are summarized in Table 1. Body weight and body density (by hydrostatic weighing), used for calculations of percent body fat, fat mass, and fat-free mass (FFM), were determined after an overnight fast, as previously described (16). All subjects were in good health as assessed by medical history, were free of any medication, and spent ≤3 h/week in organized sports activities. The Medical Ethical Committee of Maastricht University approved the study protocol, and all subjects gave their written informed consent before participating in the study.
TABLE 1.
Subject characteristics
| Lean | Obese | |
|---|---|---|
| n | 13 | 10 |
| Age (years) | 49 ± 9 | 54 ± 8 |
| Weight (kg) | 75 ± 6 | 102 ± 10* |
| Height (m) | 1.81 ± 0.07 | 1.79 ± 0.07 |
| BMI (kg/m2) | 23.0 ± 1.8 | 31.9 ± 1.9* |
| Body fat percentage (kg) | 20.2 ± 3.5 | 31.7 ± 1.5* |
| FFM (kg) | 60.1 ± 5.4 | 69.7 ± 6.7* |
| Waist-to-hip ratio | 0.91 ± 0.04 | 1.01 ± 0.03* |
| Systolic blood pressure (mmHg) | 126 ± 11 | 137 ± 13* |
| Diastolic blood pressure (mmHg) | 77 ± 7 | 85 ± 9* |
| HOMAIR | 1.8 ± 0.7 | 3.4 ± 0.9* |
Data are means ± SD. All subjects are men.
P < 0.05 obese vs. lean.
Experimental protocol.
In a pilot study in three subjects, the time course of [2H5]glycerol enrichment was determined to investigate when steady-state concentrations were achieved. Glycerol enrichment was measured in arterialized blood and venous blood draining the forearm during primed (3 μmol · kg−1) constant infusion of [2H5]glycerol (0.20 μmol · kg−1 · min−1) for 6 h. Blood samples were taken simultaneously from the two sites at baseline for background enrichment (t0) and at 10 time points during [2H5]glycerol infusion (t60, t90, t120, t150, t180, t210, t240, t330, t345, and t360).
During the actual muscle lipolysis experiment, glycerol enrichment and exchange across the forearm were investigated during primed (3 μmol · kg−1) constant infusion of [2H5]glycerol (0.20 μmol · kg−1 · min−1) for 3 h. After a 120-min baseline period, ISO was infused at a rate of 20 ng · kg−1 FFM · min−1 for 60 min. At this infusion rate, plasma ISO concentrations are comparable in lean and obese subjects (17). At the beginning of the experiment, an arterialized blood sample was taken for measurement of background enrichment. Furthermore, arterialized and deep venous blood samples were taken simultaneously at three baseline time points (t90, t105, and t120) and at three time points during the last 30 min of ISO infusion (t150, t165, and t180). In both the pilot and muscle lipolysis experiment, forearm blood flow (FBF) was measured just before blood sampling to calculate substrate fluxes across the forearm (see fbf). Skeletal muscle biopsies were taken from the vastus lateralis muscle under local anesthesia of skin and fascia (Xylocaine; AstraZeneca, Zoetermeer, the Netherlands) immediately before the baseline period (t0) and just before the end of ISO infusion (t180). Muscle biopsies were immediately frozen in liquid nitrogen and stored at −80°C until further analysis. During the experiment, heart rate was recorded continuously by means of a three-lead electrocardiogram (ECG). When heart rate increased >30 beats/min or in case of ECG irregularities, ISO infusion was stopped (n = 2, 1 lean/1 obese).
Clinical methods.
All subjects were asked to refrain from drinking alcohol and to perform no strenuous exercise for a period of 24 h before the experiment. Subjects came to the laboratory by car or bus at 8:00 a.m. after an overnight fast. Before initiating the experiment, a catheter was inserted retrogradely into a superficial dorsal hand vein to obtain arterialized venous blood. The hand was warmed in a hotbox, which was maintained at 60°C to achieve adequate arterialization (18). In the same arm, a second catheter was inserted in a forearm antecubital vein for the infusion of [2H5]glycerol tracer and ISO. In the contralateral arm, a third catheter was introduced retrogradely in an antecubital vein of the forearm for sampling of deep venous blood draining forearm skeletal muscle. The subjects rested in a supine position for the entire duration of the study.
FBF.
FBF was measured by venous occlusion plethysmography (EC5R plethysmograph; Hokanson, Bellevue, WA) using mercury-in-silastic strain gauges applied to the widest part of the forearm (19). During measurement periods, the hand circulation was occluded by rapid inflation of a sphygmomanometer cuff (E20 rapid cuff inflator; Hokanson) placed around the wrist to a pressure of 200 mmHg. In this way, FBF can be assessed without interference of the hand circulation. A second cuff, placed just above the anticubital fossa, was inflated to 45 mmHg (which was lower than the diastolic blood pressure, which was >70 mmHg in all subjects) to achieve venous occlusion and obtain plethysmographic recordings. During venous occlusion, the plethysmographic recordings reflect the rate of arterial inflow, indicating FBF.
Muscle lysates.
Muscle tissue was freeze-dried; dissected free of all visible adipose tissue, connective tissue, and blood under a microscope; and subsequently homogenized (1:80 [wt/vol]) in a buffer containing 50 mmol/l HEPES (pH 7.5), 150 mmol/l NaCl, 20 mmol/l sodium pyrophosphate, 20 mmol/l l-glycerophosphate, 10 mmol/l NaF, 2 mmol/l sodium orthovanadate, 2 mmol/l EDTA, 1% Nonidet P-40, 10% glycerol, 2 mmol/l phenylmethylsulfonyl fluoride, 1 mmol/l MgCl2, 1 mmol/l CaCl2, 10 μg/ml leupeptin, 10 μg/ml aprotinin, and 3 mmol/l benzamidine. Homogenates were rotated end over end for 1 h at 4°C and then cleared by centrifugation for 1 h at 17,500g, 4°C. Protein content in the supernatant was measured by the bicinchoninic acid protein assay (Pierce, Rockford, IL).
Western blotting.
Expression of HSL protein and phosphorylation of HSL Ser563, Ser565, and Ser659 was detected by Western blotting on the muscle lysates. The lysates were boiled in Laemmli buffer before being subjected to SDS-PAGE and immunoblotting. Primary antibodies were rabbit anti-HSL (donated by Prof. Cecilia Holm [Department of Cell and Molecular Biology, Lund University, Sweden]) and sheep anti–phospho-HSL Ser563, sheep anti–phospho-HSL Ser565 (11), and sheep anti–phospho-HSL Ser659 (13). Secondary antibodies were horseradish peroxidase–conjugated anti-rabbit (catalog no. P0448; DAKO, Glostrup, Denmark) and anti-sheep (catalog no. 81-8620; Zymed, San Francisco, CA). Antigen/antibody complexes were visualized using enhanced chemiluminescence (ECL+; Amersham Biosciences, Buckinghamshire, U.K.) and quantified by a Kodak Image Station E440CF (Kodak, Glostrup, Denmark).
Analytical methods.
A small portion of blood was used for the measurement of oxygen saturation to ensure adequate arterialization (ABL510; Radiometer, Copenhagen). Blood was collected in tubes containing EDTA and centrifuged for 10 min at 1,000g, 4°C. The supernatant (plasma) was used for the enzymatic colorimetric quantification of fatty acids (NEFA C kit; Wako Chemicals, Neuss, Germany), free glycerol (Boehringer, Mannheim, Germany), and TAG (Sigma, St. Louis, MO) on a COBAS FARA centrifugal spectrophotometer (Roche Diagnostica, Basel, Switzerland). Plasma glucose concentration (ABX Diagnostics, Montpellier, France) and lactate (ABX Diagnostics) were measured enzymatically on a COBAS MIRA automated spectrophotometer (Roche Diagnostica). Plasma insulin was measured with a double antibody radioimmunoassay (Linco Research, St. Charles, MO). Insulin sensitivity was assessed by the homeostasis model assessment index for insulin resistance (HOMAIR), calculated from baseline glucose and insulin (20). Hematocrit was measured using a microcapillary system (Hirschmann Laborgeräte, Eberstadt, Germany).
Isotope enrichment.
To determine isotopic enrichment of glycerol, samples were first derivatized. One milliliter acetone was added to 150 μl plasma, and each tube was vortexed for 2 min and centrifuged for 20 min at 17,500g, 4°C. The supernatant was transferred to a clean tube, dried under nitrogen at 37°C, and derivatized using 80 μl ethyl acetate (catalog no. 45765; Sigma-Aldrich, Seelze, Germany) and 80 μl heptafluorobutyric acid anhydride (catalog no. 63164; Pierce). The tubes were vortexed for 2 min and incubated for 1 h at 70°C. Samples were then rotated end over end for 5 min at 25°C and evaporated under nitrogen at room temperature. Seventy microliters ethyl acetate was added before injection into the gas chromatograph–mass spectrometer (MAT 252; Finnigan, Bremen, Germany) for measurement of glycerol enrichment by selectively monitoring the mass-to-charge ratio of molecular ions 253 and 257 for glycerol (21).
Calculations.
The exchange of metabolites across the forearm was calculated by multiplying the arteriovenous plasma concentration difference of metabolites by forearm plasma flow. Plasma flow was calculated as FBF × (1 − hematocrit), with hematocrit expressed as a fraction. A positive net exchange indicates net uptake, whereas a negative net exchange indicates net release.
The expected deep venous glycerol enrichment, in case of no glycerol uptake, was calculated as arterialized enrichment multiplied by arterialized glycerol concentration and subsequently divided by deep venous glycerol concentration.
The rate of appearance (Ra) of glycerol was calculated according to the following steady-state equation:
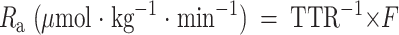 |
where TTR is tracer-to-tracee ratio and F is the isotope infusion rate (μmol · kg−1 · min−1).
The fractional extraction (fract) of glycerol across the forearm was calculated by dividing the arteriovenous concentration difference of [2H5]glycerol by the arterialized [2H5]glycerol concentration. Total glycerol uptake across the forearm was then calculated as follows:
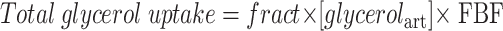 |
where the unit is nmol · 100 ml−1 tissue · min−1; [glycerolart] is arterialized glycerol concentration (μmol/l); and FBF is forearm skeletal muscle blood flow (ml · 100 ml−1 tissue · min−1). Forearm total glycerol release was calculated from the formula:
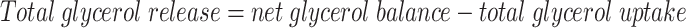 |
Net glycerol balance (exchange) was calculated as explained above.
Statistical analysis.
Differences within groups (i.e., between baseline and ISO) were tested using the paired Student's t test (two-sided). Comparisons between groups (at baseline and during ISO infusion and changes from baseline to ISO, respectively) were made using Student's two-sample t test (equal variance assumed). Statistical calculations were performed using SPSS for Macintosh (version 11.0; SPSS, Chicago). Data are presented as mean ± SE if not otherwise stated. P < 0.05 was considered statistically significant.
RESULTS
Pilot experiment.
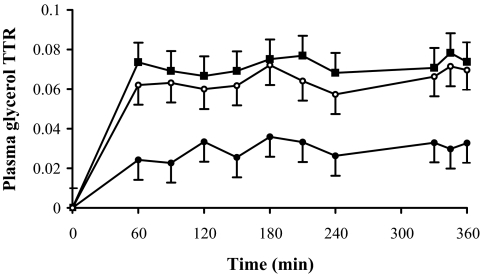
In Fig. 1, we show that arterialized and deep venous TTR, obtained with a 6-h [2H5]glycerol infusion, reached a steady state after 1 h of infusion. The measured deep venous enrichment was consistently lower than the expected enrichment, implying uptake of glycerol across the forearm (Fig. 1). In the actual muscle lipolysis experiment (3-h [2H5]glycerol infusion), TTR also reached a steady state after 1 h and remained stable during ISO infusion (data not shown). Thus, our data support the use of a relatively short infusion time (≥1 h) to accurately study glycerol metabolism.
FIG. 1.
Plasma glycerol TTR during 6-h primed constant infusion of [2H5]glycerol (n = 3) in arterialized blood (▪), forearm venous blood (•), and expected forearm venous enrichment (○). The expected deep venous glycerol enrichment was calculated as arterialized enrichment multiplied by arterialized glycerol concentration divided by deep venous glycerol concentration. The measured venous enrichment was consistently lower than the expected deep venous enrichment (P < 0.05), implying uptake of glycerol across the forearm. Values are means ± SE.
Circulating metabolites.
Baseline plasma-arterialized TAG concentration was twice as high for the obese than the lean subjects (P < 0.001; Table 2). β-Adrenergic stimulation increased plasma-arterialized TAG concentrations in obese (P = 0.047; Table 2) subjects, whereas in lean subjects, TAG concentrations decreased during ISO (P = 0.08; Table 2). Thus, the change in plasma-arterialized TAG concentrations from baseline to ISO was different between lean and obese subjects (P = 0.004; Table 2). Because of an irregular ECG or failure of the cannulation, there are three individuals less in each group in the ISO experiment. Therefore, Δ values for the remaining 10 lean and 7 obese subjects are included in Table 2. Baseline values for this subgroup did not differ from those of the whole group.
TABLE 2.
Circulating arterialized metabolite concentrations during baseline and isoprenaline infusion in lean and obese subjects
| Lean |
Obese |
|||||
|---|---|---|---|---|---|---|
| Baseline | ISO | Δ | Baseline | ISO | Δ | |
| n | 13 | 10 | 10 | 10 | 7 | 7 |
| TAG (μmol/l) | 701 ± 66 | 648 ± 64* | −46 ± 23 | 1,464 ± 190† | 1,667 ± 217*† | 112 ± 44‡ |
| FFA (μmol/l) | 661 ± 41 | 942 ± 53* | 271 ± 46 | 638 ± 42 | 1,124 ± 82* | 469 ± 82‡ |
| Glycerol (μmol/l) | 102 ± 5 | 118 ± 7* | 15 ± 5 | 106 ± 4 | 147 ± 10*† | 44 ± 9‡ |
| Glycerol Ra (μmol/min) | 199 ± 12 | 311 ± 28* | 109 ± 13 | 220 ± 15 | 391 ± 30* | 172 ± 19 |
| Glycerol Ra/FFM (μmol · kg−1 FFM · min−1) | 3.4 ± 0.2 | 5.5 ± 0.5* | 2.0 ± 0.5 | 3.3 ± 0.3 | 5.8 ± 0.6* | 2.5 ± 0.5 |
| Glucose (mmol/l) | 5.3 ± 0.1 | 5.4 ± 0.1 | 0.14 ± 0.06 | 5.5 ± 0.2 | 5.4 ± 0.1 | −0.12 ± 0.10 |
| Insulin (mU/l) | 7.2 ± 0.6 | 10.7 ± 0.9* | 3.4 ± 0.6 | 13.6 ± 1.0† | 24.0 ± 2.3*† | 10.6 ± 1.5‡ |
| Lactate (mmol/l) | 0.61 ± 0.04 | 0.72 ± 0.03 | 0.09 ± 0.04 | 0.98 ± 0.11† | 0.99 ± 0.06† | 0.05 ± 0.07 |
Data are means ± SE.
P < 0.05 ISO vs. baseline.
P < 0.05 obese vs. lean.
P < 0.05 change (Δ) from baseline obese vs. lean.
Baseline plasma-arterialized FFA and glycerol concentrations did not differ significantly between lean and obese subjects. β-Adrenergic stimulation increased FFA and glycerol in lean (P < 0.001 and P = 0.015, respectively) and obese (P = 0.001 and P < 0.001, respectively) subjects (Table 2). Moreover, the β-adrenergic–mediated increase in arterialized FFA and glycerol was more pronounced in obese subjects (P = 0.037 and 0.008; Table 2), suggesting a higher whole-body lipolytic response in the obese. Likewise, β-adrenergic stimulation increased whole-body glycerol Ra in lean and obese subjects (P < 0.001; Table 2), and this increase tended to be higher in the obese (P = 0.067; Table 2). Expressed per unit FFM, baseline glycerol Ra was not significantly different between groups. β-Adrenergic stimulation increased the glycerol Ra per unit FFM in lean and obese subjects (P < 0.001; Table 2), but this increase in glycerol Ra per unit FFM was not significantly different between groups.
Plasma-arterialized insulin and lactate concentrations were higher in obese than in lean subjects during baseline and ISO (P = 0.002; Table 2), whereas glucose did not differ significantly between lean and obese subjects in both conditions. β-Adrenergic stimulation increased circulating insulin concentrations in lean and obese (P < 0.001; Table 2). This increase in circulating insulin concentrations was significantly higher in obese than in lean subjects (P < 0.001; Table 2). Circulating glucose and lactate concentrations were unchanged during β-adrenergic stimulation.
Regional forearm metabolism.
Baseline FBF was not different between lean and obese subjects (P = 0.15; Table 3). FBF was significantly elevated during β-adrenergic stimulation in both lean and obese subjects (P < 0.001), but the increase in FBF during β-adrenergic stimulation was not significantly different between groups.
TABLE 3.
Regional forearm blood flow and net metabolite flux during baseline and isoprenaline infusion in lean and obese subjects
| Lean |
Obese |
|||
|---|---|---|---|---|
| Baseline | ISO | Baseline | ISO | |
| n | 13 | 10 | 10 | 7 |
| FBF (ml · 100 ml−1 tissue · min−1) | 2.9 ± 0.2 | 4.6 ± 0.4* | 2.5 ± 0.3 | 3.5 ± 0.3* |
| Forearm net flux (nmol · 100 ml−1 tissue · min−1) | ||||
| TAG | 17 ± 5 | 24 ± 19† | −2 ± 9† | 46 ± 31† |
| FFA | 6 ± 59† | −53 ± 143† | 156 ± 42 | 230 ± 88 |
| Glycerol | −21 ± 11† | −39 ± 33† | 11 ± 5†‡ | 6 ± 21† |
| Glucose | 142 ± 51 | 213 ± 117† | 226 ± 81 | 289 ± 223† |
| Lactate | −90 ± 27 | −171 ± 73 | 8 ± 45 | −327 ± 78 |
Data are means ± SE. Positive flux = net uptake; negative flux = net release.
P < 0.05 ISO vs. baseline.
Exchange not different from zero.
P < 0.05 obese vs. lean.
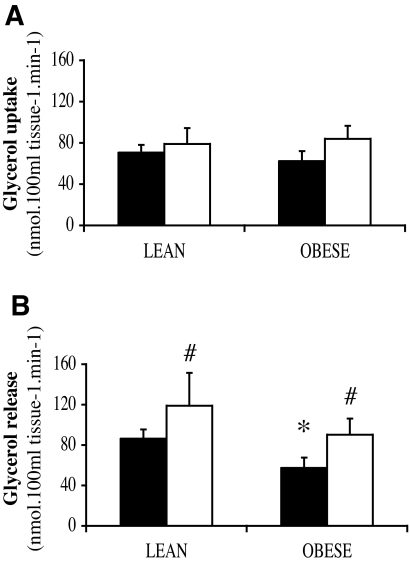
Fractional extraction of [2H5]glycerol from the circulation (lean vs. obese, 40.2 ± 3.4 vs. 40.5 ± 6.1%) was not significantly different between groups. Significant glycerol uptake across the forearm was observed in both obese and lean subjects (P < 0.001 compared with 0; Fig. 2A). The increase in total glycerol uptake during β-adrenergic stimulation was not significantly different between groups.
FIG. 2.
Total glycerol uptake (A) and release (B) across the forearm during baseline (▪) and ISO infusion (□) using a [2H5]glycerol tracer in lean and obese subjects. *P < 0.05 obese vs. lean; #P < 0.05 ISO vs. baseline. Values are means ± SE.
Baseline net glycerol efflux across the forearm was significantly lower in the obese than in the lean subjects (P = 0.025; Table 3). Accordingly, obese subjects showed significantly less total glycerol release across the forearm at baseline compared with lean subjects (P = 0.045; Fig. 2B). These data indicate a blunted glycerol release during baseline in obese subjects. Total glycerol uptake expressed relative to total glycerol release at baseline was not significantly different between lean and obese subjects (lean vs. obese, 92.7 ± 13.5 vs. 91.7 ± 23.9% of total release). Furthermore, obese subjects had higher net fatty acid uptake across the forearm at baseline (P = 0.064; Table 3). β-Adrenergic stimulation increased total glycerol release in lean and obese subjects (P = 0.037 and 0.042; Fig. 2B), but this increase was not significantly different between groups. Finally, the increase in net lactate efflux during β-adrenergic stimulation tended to be higher in obese than in lean subjects (P = 0.06; Table 3).
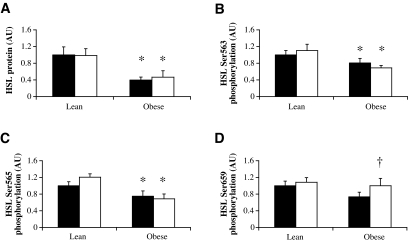
Total HSL protein expression and serine phosphorylation in the vastus lateralis muscle.
Muscle HSL protein expression was significantly lower in obese compared with lean subjects (P = 0.004) and did not change during β-adrenergic stimulation (Fig. 3A). Baseline HSL phosphorylation on Ser563 (P = 0.041), Ser565 (P = 0.007), and Ser659 (P = 0.09) was reduced in obese compared with lean subjects (Fig. 3B–D). When corrected for total HSL protein, HSL Ser563, Ser565, and Ser659 phosphorylation was comparable between lean and obese subjects (data not shown). No effect of β-adrenergic stimulation was observed on HSL Ser563 or HSL Ser565 phosphorylation in both lean and obese subjects (Fig. 3B and C). Obese subjects showed an increased HSL Ser659 phosphorylation (P = 0.008; Fig. 3D), whereas in lean subjects, HSL Ser659 phosphorylation was unchanged after ISO infusion.
FIG. 3.
HSL protein expression (A) and Ser563 (B), Ser565 (C), and Ser659 (D) phosphorylation during baseline (▪) and ISO infusion (□) in lean and obese subjects. Data are expressed as arbitrary units (AU). *P < 0.05 obese vs. lean; †P < 0.01 obese vs. lean in change between baseline and ISO. Values are means ± SE.
DISCUSSION
The present study revealed a blunted fasting muscle glycerol release in obese compared with lean men (Fig. 2B). This blunted skeletal muscle glycerol release was accompanied by a lower total HSL protein expression and phosphorylation of HSL Ser563, Ser565, and Ser659 (Fig. 3), suggesting a blunted fasting muscle lipolysis in obesity. The β-adrenergic–mediated muscle lipolytic response was not significantly different between lean and obese subjects. In contrast to lean subjects, an increased HSL Ser659 phosphorylation was observed in skeletal muscle of obese subjects during β-adrenergic stimulation compared with baseline.
Whole-body lipolysis.
There seemed to be a directionally opposite effect of β-adrenergic stimulation on circulating TAG. Circulating TAG concentrations during β-adrenergic stimulation increased in obese subjects, whereas lean subjects showed slightly decreased circulating TAG concentration during β-adrenergic stimulation. This could indicate a greater TAG clearance in lean than in obese subjects after β-adrenergic stimulation. In the present study, skeletal muscle TAG clearance was not significantly different between groups. Adipose tissue lipoprotein lipase (LPL)-mediated TAG hydrolysis might be the cause of an increased net TAG extraction during β-adrenergic stimulation. An increased rate of action of LPL has been shown during epinephrine infusion in lean subjects (22). Furthermore, it has been shown that obese subjects have a diminished adipose tissue LPL activity during postprandial conditions (23). However, it remains to be elucidated whether an impaired adipose tissue LPL activity during β-adrenergic stimulation in obese compared with lean subjects can explain the difference in circulating TAG concentration.
Baseline muscle glycerol uptake.
The present observation of significant uptake of glycerol across the forearm (Fig. 2A) is in agreement with previous reports (24). The first indications for significant metabolism of glycerol in muscle came from Elia et al. (25) showing 50% loss of enriched glycerol across the forearm. More recently, studies confirmed the finding of significant uptake of glycerol by forearm muscle (26) and vastus lateralis muscle (27). The enzymatic machinery for utilization of glycerol seems to be present in skeletal muscle. Glycerol dehydrogenase, the enzyme that could initiate glycerol oxidation by skeletal muscle, has been demonstrated in humans (28), and oxidation of glycerol by skeletal muscle has been shown to occur in humans (28). Furthermore, glycerol kinase expression has been demonstrated in human muscle cells (29). Thus, in humans, glycerol taken up from the circulation might be oxidized or incorporated into IMTG, as shown in rats (30).
Baseline muscle glycerol release.
Our data show a blunted baseline total glycerol release per unit muscle mass in obese subjects (Fig. 2B). This blunted baseline total glycerol release was accompanied by a lower total HSL protein expression in skeletal muscle of obese subjects (Fig. 3A), suggesting a blunted baseline muscle lipolysis. However, it can be argued that glycerol tracer release does not only reflect lipolysis. Thus, a blunted glycerol release might reflect an increased intramuscular glycerol use (i.e., oxidation or TAG synthesis). To our knowledge, our data provide the first indication of a reduced muscle HSL protein expression in obese compared with lean subjects. It is well known that expression of HSL is markedly decreased in subcutaneous adipocytes and differentiated adipocytes from obese subjects. This suggests that, at least in adipose tissue, a decreased HSL expression is a primary defect in obesity (31,32). However, we cannot exclude that the blunted muscle lipolysis in obese subjects was a secondary phenomenon caused by a higher degree of hyperinsulinemia. Still, it has been suggested that muscle lipolysis is primarily regulated by substrate supply and to a lesser degree is under hormonal control (33). This seems to be supported by studies showing no apparent suppression of in vivo skeletal muscle lipolysis by insulin (34,35). Furthermore, our data suggest that phosphorylation of HSL on the PKA target sites Ser563 and Ser659, and on the AMPK target site Ser565, was lower in obese than in lean subjects. It should be recognized, however, that when corrected for total HSL protein, HSL Ser563, Ser565, and Ser659 phosphorylation was comparable between lean and obese subjects, suggesting that a similar percentage of HSL molecules was phosphorylated on these three serine sites in lean and obese subjects. Nevertheless, the reduced absolute number of HSL molecules phosphorylated on Ser659 may at least partly explain the blunted baseline glycerol release in obese compared with lean subjects. On the other hand, HSL Ser563 and Ser565 phosphorylation have been suggested not to be major regulators of HSL activity in human skeletal muscle (11,13). Thus, the reduced phosphorylation of HSL on these two sites may not have been important in determining the blunted baseline lipolysis in obese subjects. For practical reasons, the arteriovenous differences were measured across the forearm muscle, and biopsies were taken from the vastus lateralis muscle. Because there may be heterogeneity in lipolysis between different muscle groups (36), the combination of forearm substrate fluxes with lipolytic enzymes in muscle biopsies from the thigh has to be interpreted with caution. Finally, it should be mentioned that other lipases might also contribute to the blunted baseline muscle glycerol release observed in obese subjects. Recently, we identified adipose triglyceride lipase (ATGL) expression in human skeletal muscle (37). More research is needed to elucidate the potential role of ATGL in human skeletal muscle lipolysis.
Baseline net muscle fatty acid uptake.
An increased basal net fatty acid uptake was observed across the forearm of obese subjects. An impaired fatty acid uptake and oxidation by both the leg (5) and the arm (21,38) have been observed previously in obese type 2 diabetic subjects compared with healthy individuals. Disturbances in fatty acid handling and an impaired muscle lipolysis may contribute to the increased IMTG storage in obese subjects. However, because this also depends on lipid turnover, both TAG synthesis and breakdown have to be known to draw final conclusions with respect to the mechanisms underlying increased IMTG content in obesity. For practical reasons and because our primary objective was to study glycerol metabolism, in the present study, no carbon-labeled long-chain fatty acid tracer was used to measure fluxes and oxidation rates across the forearm.
Muscle glycerol release during β-adrenergic stimulation.
The present results showed equal forearm glycerol release during systemic infusion of the nonselective β-adrenergic agonist ISO in lean and obese subjects, suggesting a comparable lipolytic response. Previously, in situ microdialysis using a β-2 agonist, salbutamol, showed a blunted lipolysis in the gastrocnemius muscle of obese insulin-resistant subjects compared with lean subjects (8). Differences in systemic versus local infusion of β-adrenergic agonists might partly explain this discrepancy. Also, in microdialysis studies, interstitial glycerol is used as a measure of lipolysis. As mentioned previously, glycerol is taken up by skeletal muscle, suggesting that interstitial glycerol may not reflect the overall rate of lipolysis but may instead be the net result of TAG and glycerol metabolism in muscle, thus reflecting net glycerol turnover (39). Finally, there may be marked heterogeneity in lipolysis between different muscle groups, possibly correlated to composition of fiber types (36). Accordingly, in rats, it was shown that muscles with a majority of type 1 fibers had greater HSL activity compared with muscles with a majority of type 2 fiber (10). The higher content of type 1 fibers in the gastrocnemius muscle compared with forearm muscle may not only cause a generally higher lipolytic sensitivity to β-adrenergic stimulation but may also influence the difference in β-adrenergically stimulated lipolysis between lean and obese subjects.
It is known from studies with purified bovine adipocyte HSL (15) and in different cell lines transfected with wild-type and mutant forms of HSL (14) that β-adrenergic stimulation increases HSL activity through phosphorylation on several serine residues. In the present study, HSL Ser659 phosphorylation significantly increased during β-adrenergic stimulation in skeletal muscle of obese subjects, whereas no effect was seen in lean subjects. A previous study in men and women during exercise has shown that muscle Ser659 phosphorylation and muscle HSL activity show a very similar pattern with respect to exercise response and dependency on sex, indicating that Ser659 serves an important role in the regulation of HSL activity in human skeletal muscle (13), as has been demonstrated in adipocytes (14). It can be speculated that obese subjects increase HSL Ser659 phosphorylation during β-adrenergic stimulation to deal with a reduced total HSL protein expression, increasing muscle HSL activity to a level comparable with lean subjects. In addition, HSL Ser660 appears to be a major PKA target site and HSL activity–controlling site (14). In the present study, HSL Ser660 phosphorylation was not measured. Finally, phosphorylation of the PKA target site Ser563 on HSL did not increase significantly during β-adrenergic stimulation. This is in accordance with previous studies, in which HSL Ser563 phosphorylation was not increased during exercise despite an increase in circulating epinephrine (11,13). Maybe HSL Ser563 is already maximally phosphorylated in the basal, resting state. Moreover, it has been argued that HSL Ser563 may not be an important regulator of HSL activity in human skeletal muscle (11).
Muscle lactate release during β-adrenergic stimulation.
Net lactate release across the forearm increased during β-adrenergic stimulation. This increase was higher in obese compared with lean subjects, suggesting that the glycolytic flux was stimulated to a greater extent by ISO in obese than in lean subjects. This seems in line with previous findings showing an increased lactate release during β-adrenergic stimulation in obese subjects (17) that persisted after weight reduction (40), indicating that this disturbance might be an early factor in the etiology of obesity.
Limitations of the study.
Considering the increased discomfort and risk associated with arterial catheters, we used arterialized hand vein blood as substitute for arterial blood. Arterialized blood has been shown to be an acceptable alternative to arterial sampling (41,42). Arterialization was achieved by heating the hand in a warm air box at 60°C for at least 30 min, which has been previously validated as the appropriate procedure for obtaining arterialized blood (43). In the present study, mean oxygen saturation was 94.5% in both lean and obese subjects, indicating comparable and successful arterialization in both groups.
In conclusion, the obese insulin-resistant state is characterized by a reduced muscle glycerol release during baseline fasting conditions, which was accompanied by a lower HSL protein expression and phosphorylation on the PKA target sites Ser563 and Ser659 and on the AMPK target site Ser565. This suggests a blunted fasting skeletal muscle lipolysis in obesity, which may be an important factor contributing to the increased lipid storage in skeletal muscle of obese insulin-resistant subjects. Further studies are necessary to address in more detail whether these disturbances are primary factors or adaptation responses to the obese insulin-resistant state.
Acknowledgments
C.R. has received support from the Commission of the European Communities (contract no. LSHM-CT-2004-005272 EXGENESIS), the Copenhagen Muscle Research Centre, and the Ministry of Food, Agriculture and Fisheries. B.K. has received support from the Commission of the European Communities (contract no. LSHM-CT-2004-005272 EXGENESIS), the Copenhagen Muscle Research Centre, and the Ministry of Food, Agriculture and Fisheries. E.E.B. has received a grant from the Netherlands Organization for Health Research and Development (NWO/ZonMw contract no. 015.01.095).
We greatly appreciate the technical support of Jos Stegen and Anneke van Hees and the willingness of the volunteers to participate in this study. We thank Prof. D. Grahame Hardie (Division of Molecular Physiology, School of Life Sciences, University of Dundee, Dundee, Scotland, U.K.) for providing the anti–phospho-HSL Ser antibodies.
Published ahead of print at http://diabetes.diabetesjournals.org on 8 April 2008.
The costs of publication of this article were defrayed in part by the payment of page charges. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
See accompanying commentary, p. 1784.
REFERENCES
- 1.Sinha R, Dufour S, Petersen KF, LeBon V, Enoksson S, Ma YZ, Savoye M, Rothman DL, Shulman GI, Caprio S: Assessment of skeletal muscle triglyceride content by 1H nuclear magnetic resonance spectroscopy in lean and obese adolescents: relationships to insulin sensitivity, total body fat, and central adiposity. Diabetes 51 :1022 –1027,2002 [DOI] [PubMed] [Google Scholar]
- 2.Krssak M, Falk Petersen K, Dresner A, DiPietro L, Vogel SM, Rothman DL, Roden M, Shulman GI: Intramyocellular lipid concentrations are correlated with insulin sensitivity in humans: a 1H NMR spectroscopy study. Diabetologia 42 :113 –116,1999 [DOI] [PubMed] [Google Scholar]
- 3.Perseghin G, Scifo P, De Cobelli F, Pagliato E, Battezzati A, Arcelloni C, Vanzulli A, Testolin G, Pozza G, Del Maschio A, Luzi L: Intramyocellular triglyceride content is a determinant of in vivo insulin resistance in humans: a 1H-13C nuclear magnetic resonance spectroscopy assessment in offspring of type 2 diabetic parents. Diabetes 48 :1600 –1606,1999 [DOI] [PubMed] [Google Scholar]
- 4.Petersen KF, Shulman GI: Etiology of insulin resistance. Am J Med 119 :S10 –S16,2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kelley DE, Simoneau JA: Impaired free fatty acid utilization by skeletal muscle in non-insulin-dependent diabetes mellitus. J Clin Invest 94 :2349 –2356,1994 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Blaak EE: Fatty acid metabolism in obesity and type 2 diabetes mellitus. Proc Nutr Soc 62 :753 –760,2003 [DOI] [PubMed] [Google Scholar]
- 7.Blaak EE: Basic disturbances in skeletal muscle fatty acid metabolism in obesity and type 2 diabetes mellitus. Proc Nutr Soc 63 :323 –330,2004 [DOI] [PubMed] [Google Scholar]
- 8.Blaak EE, Schiffelers SL, Saris WH, Mensink M, Kooi ME: Impaired beta-adrenergically mediated lipolysis in skeletal muscle of obese subjects. Diabetologia 47 :1462 –1468,2004 [DOI] [PubMed] [Google Scholar]
- 9.Langfort J, Ploug T, Ihlemann J, Enevoldsen LH, Stallknecht B, Saldo M, Kjaer M, Holm C, Galbo H: Hormone-sensitive lipase (HSL) expression and regulation in skeletal muscle. Adv Exp Med Biol 441 :219 –228,1998 [DOI] [PubMed] [Google Scholar]
- 10.Langfort J, Ploug T, Ihlemann J, Saldo M, Holm C, Galbo H: Expression of hormone-sensitive lipase and its regulation by adrenaline in skeletal muscle. Biochem J 340 :459 –465,1999 [PMC free article] [PubMed] [Google Scholar]
- 11.Roepstorff C, Vistisen B, Donsmark M, Nielsen JN, Galbo H, Green KA, Hardie DG, Wojtaszewski JF, Richter EA, Kiens B: Regulation of hormone-sensitive lipase activity and Ser563 and Ser565 phosphorylation in human skeletal muscle during exercise. J Physiol 560 :551 –562,2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Watt MJ, Holmes AG, Pinnamaneni SK, Garnham AP, Steinberg GR, Kemp BE, Febbraio MA: Regulation of HSL serine phosphorylation in skeletal muscle and adipose tissue. Am J Physiol Endocrinol Metab 290 :E500 –E508,2006 [DOI] [PubMed] [Google Scholar]
- 13.Roepstorff C, Donsmark M, Thiele M, Vistisen B, Stewart G, Vissing K, Schjerling P, Hardie DG, Galbo H, Kiens B: Sex differences in hormone-sensitive lipase expression, activity, and phosphorylation in skeletal muscle at rest and during exercise. Am J Physiol Endocrinol Metab 291 :E1106 –E1114,2006 [DOI] [PubMed] [Google Scholar]
- 14.Anthonsen MW, Ronnstrand L, Wernstedt C, Degerman E, Holm C: Identification of novel phosphorylation sites in hormone-sensitive lipase that are phosphorylated in response to isoproterenol and govern activation properties in vitro. J Biol Chem 273 :215 –221,1998 [DOI] [PubMed] [Google Scholar]
- 15.Garton AJ, Campbell DG, Carling D, Hardie DG, Colbran RJ, Yeaman SJ: Phosphorylation of bovine hormone-sensitive lipase by the AMP-activated protein kinase: a possible antilipolytic mechanism. Eur J Biochem 179 :249 –254,1989 [DOI] [PubMed] [Google Scholar]
- 16.Goossens GH, Blaak EE, Saris WH, van Baak MA: Angiotensin II-induced effects on adipose and skeletal muscle tissue blood flow and lipolysis in normal-weight and obese subjects. J Clin Endocrinol Metab 89 :2690 –2696,2004 [DOI] [PubMed] [Google Scholar]
- 17.Blaak EE, Van Baak MA, Kemerink GJ, Pakbiers MT, Heidendal GA, Saris WH: Beta-adrenergic stimulation of energy expenditure and forearm skeletal muscle metabolism in lean and obese men. Am J Physiol 267 :E306 –E315,1994 [DOI] [PubMed] [Google Scholar]
- 18.Abumrad NN, Rabin D, Diamond MP, Lacy WW: Use of a heated superficial hand vein as an alternative site for the measurement of amino acid concentrations and for the study of glucose and alanine kinetics in man. Metabolism 30 :936 –940,1981 [DOI] [PubMed] [Google Scholar]
- 19.Webb DJ: The pharmacology of human blood vessels in vivo. J Vasc Res 32 :2 –15,1995 [DOI] [PubMed] [Google Scholar]
- 20.Matthews DR, Hosker JP, Rudenski AS, Naylor BA, Treacher DF, Turner RC: Homeostasis model assessment: insulin resistance and beta-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia 28 :412 –419,1985 [DOI] [PubMed] [Google Scholar]
- 21.Blaak EE, Wagenmakers AJ, Glatz JF, Wolffenbuttel BH, Kemerink GJ, Langenberg CJ, Heidendal GA, Saris WH: Plasma FFA utilization and fatty acid-binding protein content are diminished in type 2 diabetic muscle. Am J Physiol Endocrinol Metab 279 :E146 –E154,2000 [DOI] [PubMed] [Google Scholar]
- 22.Samra JS, Simpson EJ, Clark ML, Forster CD, Humphreys SM, Macdonald IA, Frayn KN: Effects of epinephrine infusion on adipose tissue: interactions between blood flow and lipid metabolism. Am J Physiol 271 :E834 –E839,1996 [DOI] [PubMed] [Google Scholar]
- 23.Coppack SW, Evans RD, Fisher RM, Frayn KN, Gibbons GF, Humphreys SM, Kirk ML, Potts JL, Hockaday TD: Adipose tissue metabolism in obesity: lipase action in vivo before and after a mixed meal. Metabolism 41 :264 –272,1992 [DOI] [PubMed] [Google Scholar]
- 24.Frayn KN, Coppack SW, Humphreys SM: Glycerol and lactate uptake in human forearm. Metabolism 40 :1317 –1319,1991 [DOI] [PubMed] [Google Scholar]
- 25.Elia M, Khan K, Calder G, Kurpad A: Glycerol exchange across the human forearm assessed by a combination of tracer and arteriovenous exchange techniques. Clin Sci (Lond) 84 :99 –104,1993 [DOI] [PubMed] [Google Scholar]
- 26.Coppack SW, Persson M, Judd RL, Miles JM: Glycerol and nonesterified fatty acid metabolism in human muscle and adipose tissue in vivo. Am J Physiol 276 :E233 –E240,1999 [DOI] [PubMed] [Google Scholar]
- 27.van Hall G, Sacchetti M, Radegran G, Saltin B: Human skeletal muscle fatty acid and glycerol metabolism during rest, exercise and recovery. J Physiol 543 :1047 –1058,2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hagenfeldt L, Wahren J: Human forearm muscle metabolism during exercise: II. Uptake, release and oxidation of individual FFA and glycerol. Scand J Clin Lab Invest 21 :263 –276,1968 [DOI] [PubMed] [Google Scholar]
- 29.Montell E, Lerin C, Newgard CB, Gomez-Foix AM: Effects of modulation of glycerol kinase expression on lipid and carbohydrate metabolism in human muscle cells. J Biol Chem 277 :2682 –2686,2002 [DOI] [PubMed] [Google Scholar]
- 30.Guo Z, Jensen MD: Blood glycerol is an important precursor for intramuscular triacylglycerol synthesis. J Biol Chem 274 :23702 –23706,1999 [DOI] [PubMed] [Google Scholar]
- 31.Large V, Reynisdottir S, Langin D, Fredby K, Klannemark M, Holm C, Arner P: Decreased expression and function of adipocyte hormone-sensitive lipase in subcutaneous fat cells of obese subjects. J Lipid Res 40 :2059 –2066,1999 [PubMed] [Google Scholar]
- 32.Lofgren P, Hoffstedt J, Ryden M, Thorne A, Holm C, Wahrenberg H, Arner P: Major gender differences in the lipolytic capacity of abdominal subcutaneous fat cells in obesity observed before and after long-term weight reduction. J Clin Endocrinol Metab 87 :764 –771,2002 [DOI] [PubMed] [Google Scholar]
- 33.Wicklmayr M, Dietze G, Rett K, Mehnert H: Evidence for a substrate regulation of triglyceride lipolysis in human skeletal muscle. Horm Metab Res 17 :471 –475,1985 [DOI] [PubMed] [Google Scholar]
- 34.Moberg E, Sjoberg S, Hagstrom-Toft E, Bolinder J: No apparent suppression by insulin of in vivo skeletal muscle lipolysis in nonobese women. Am J Physiol Endocrinol Metab 283 :E295 –E301,2002 [DOI] [PubMed] [Google Scholar]
- 35.Qvisth V, Hagstrom-Toft E, Enoksson S, Sherwin RS, Sjoberg S, Bolinder J: Combined hyperinsulinemia and hyperglycemia, but not hyperinsulinemia alone, suppress human skeletal muscle lipolytic activity in vivo. J Clin Endocrinol Metab 89 :4693 –4700,2004 [DOI] [PubMed] [Google Scholar]
- 36.Hagstrom-Toft E, Qvisth V, Nennesmo I, Ryden M, Bolinder H, Enoksson S, Bolinder J, Arner P: Marked heterogeneity of human skeletal muscle lipolysis at rest. Diabetes 51 :3376 –3383,2002 [DOI] [PubMed] [Google Scholar]
- 37.Jocken JW, Smit E, Goossens GH, Essers YP, van Baak MA, Mensink M, Saris WH, Blaak EE: Adipose triglyceride lipase (ATGL) expression in human skeletal muscle is type I (oxidative) fiber specific. Histochem Cell Biol 129 :535 –538,2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Blaak EE, Wagenmakers AJ: The fate of [U-13C]palmitate extracted by skeletal muscle in subjects with type 2 diabetes and control subjects. Diabetes 51 :784 –789,2002 [DOI] [PubMed] [Google Scholar]
- 39.Sjostrand M, Gudbjornsdottir S, Holmang A, Strindberg L, Ekberg K, Lonnroth P: Measurements of interstitial muscle glycerol in normal and insulin-resistant subjects. J Clin Endocrinol Metab 87 :2206 –2211,2002 [DOI] [PubMed] [Google Scholar]
- 40.Blaak EE, Van Baak MA, Kemerink GJ, Pakbiers MT, Heidendal GA, Saris WH: Beta-adrenergic stimulation of skeletal muscle metabolism in relation to weight reduction in obese men. Am J Physiol 267 :E316 –E322,1994 [DOI] [PubMed] [Google Scholar]
- 41.Frayn KN, Macdonald IA: Methodological considerations in arterialization of venous blood. Clin Chem 38 :316 –317,1992 [PubMed] [Google Scholar]
- 42.Jensen MD, Heiling VJ: Heated hand vein blood is satisfactory for measurements during free fatty acid kinetic studies. Metabolism 40 :406 –409,1991 [DOI] [PubMed] [Google Scholar]
- 43.Blaak EE, Van Baak MA, Kempen KP, Saris WH: Effect of hand heating by a warm air box on O2 consumption of the contralateral arm. J Appl Physiol 72 :2364 –2368,1992 [DOI] [PubMed] [Google Scholar]