Abstract
OBJECTIVE—The G319S HNF1A variant is associated with an increased risk of type 2 diabetes in the Canadian Oji-Cree population. We hypothesized that the variant site at the 3′ end of exon 4 might influence splicing and characterized mRNA transcripts to investigate the mutational mechanism underlying this susceptibility to diabetes.
RESEARCH DESIGN AND METHODS—We established lymphoblastoid cell lines from a G319S homozygote and controls. HNF1A transcripts were characterized in the cell lines and pancreatic tissue by sequence analysis of RT-PCR products and quantification using real-time PCR. Susceptibility to mRNA surveillance was investigated using cycloheximide.
RESULTS—Full-length G319S mRNA accounted for 24% of mRNA transcripts in the homozygous G319S cell line. A novel isoform lacking the terminal 12 bases of exon 4 was upregulated (55% of mRNA transcripts) compared with control cell lines (33%) and human pancreatic tissue (17%). Two abnormal transcripts present only in the G319S cell line included premature termination codons as a result of the inclusion of seven nucleotides from intron 4 or the deletion of exon 8. Cycloheximide treatment increased the levels of both transcripts.
CONCLUSIONS—The G319S variant results in the production of two abnormal transcripts and an alteration in the relative balance of normal splicing products. This is predicted to lead to a reduction in total HNF1A transcript levels, but residual hepatocyte nuclear factor-1α protein activity in G319S homozygotes may still reach up to 66% of normal levels. A combination of abnormal splicing and reduced activity of the G319S protein may explain the diabetes susceptibility.
The Oji-Cree are an isolated population from North Central Canada who are among the most diabetes-prone subpopulations in the world; almost 40% of adults (1) and a high proportion of adolescents (2) have diabetes. Clinical heterogeneity in the disease phenotype suggests that diabetes in the Oji-Cree may have both genetic and environmental components (2–4). The most common genetic susceptibility determinant is a G>A base substitution at nucleotide 955 (c.955G>A) in the hepatocyte nuclear factor (HNF)1 homeobox A (HNF1A) gene, rare mutations that cause maturity-onset diabetes of the young (MODY) (5). This variant is found only in the Oji-Cree and is predicted to result in a glycine to serine substitution at codon 319 (p.Gly319Ser; G319S). Almost 40% of diabetic subjects carry the G319S variant, with an odds ratio of 1.97 for heterozygotes and 4.00 for G319S homozygotes compared with subjects without the variant (6).
Oji-Cree subjects without the variant show the characteristic features of type 2 diabetes with high levels of obesity, high plasma insulin, and insulin resistance (2). The diabetic phenotype of G319S carriers is more consistent with a defect in insulin secretion, with an earlier onset of diabetes, less obesity, and lower insulin levels than in diabetic noncarriers (2). Although some of the insulin-resistance features that have been reported in Oji-Cree diabetic subjects may arise from their unique genetic background, it is more likely that most of these features arise from the concurrent obesity in this population (2).
The G319S variant is located within the proline-rich domain II of the transactivation domain (7) but outside the regions important for nuclear localization or maximum transactivation capacity (8). In vitro transient transactivation studies demonstrated reduced transactivation activity of the HNF-1α protein by 54% compared with the wild-type protein. No dominant-negative effects or influences on protein stability or DNA binding were observed (4). The G319S missense variant results from a base substitution at the most 3′ nucleotide of exon 4. This base is located within the conserved intron 4 splice donor site, and we hypothesized that the c.955G>A variant might affect splicing. In this study, we determined the sequence and levels of HNF1A mRNA transcripts expressed in Epstein Barr virus (EBV)-transformed lymphoblastoid cells derived from a G319S homozygote and two controls. We demonstrate that the G319S variant leads to the production of two abnormal transcripts and disrupts the normal balance of HNF1A expression.
RESEARCH DESIGN AND METHODS
The G319S homozygous subject was diagnosed with diabetes at 13 years of age. He was asymptomatic at presentation, with no history of polyuria, polydypsia, or weight loss. He had no acanthosis nigricans, dyslipidemia, hypertension, or evidence of nonalcoholic fatty liver disease. There was a family history of diabetes affecting two maternal aunts, the maternal grandmother, the father, and two paternal uncles. Informed consent was obtained from all participants. The study was approved by the Health Research Ethics Board, University of Manitoba, and was carried out in accordance with the Declaration of Helsinki. Commercially available human pancreatic RNA was obtained from a pool of five donors aged 24–77 years (Clontech, Oxford, U.K.).
EBV transformation.
Cell lines were established from the G319S homozygous subject and two unrelated control subjects by EBV transformation of peripheral blood lymphocytes (ECACC, Porton Down, Salisbury, U.K.). Cell lines were maintained in 1× RPMI-1640 (Gibco Life Technologies, Paisley, U.K.), supplemented with 10% fetal calf serum (Gibco Life Technologies).
RNA extraction and reverse transcription.
Total RNA was extracted from ∼1 × 106 lymphoblastoid cells using the Perfect RNA Mini RNA kit (Eppendorf, Hamburg, Germany). Complementary DNA (cDNA) was synthesized from 4.5 μg total RNA using the Thermoscript RT-PCR system (Gibco Life Technologies), with 50°C as the incubation temperature.
Characterization of HNF1A transcripts.
HNF1A transcripts were amplified in triplicate from G319S and control mRNAs as previously described (9). Individual splice products were then isolated by bandstab PCR and sequenced (10). To investigate the occurrence of variants in cis/trans, long-range PCR was used with primers EX4FP, EX4FS, EX9RP, and EX9RS (Table 1).
TABLE 1.
Nested and real-time PCR probe and primer sequences
| Probe/primer name | Sequence | Labels |
|---|---|---|
| EX4FP | 5’ TGCGTGTCTACAACTGGTTTG 3’ | None |
| EX9RP | 5’ AGGGTGGTGGCCTGAGAT 3’ | None |
| EX4FS | 5’ TGGCCATGGACACGTACAG 3’ | None |
| EX9RS | 5’ GTGAAGCCCGGACTCACTG 3’ | None |
| HNF1A-WT-F | 5’ CCCTCTCCCCCAGTAAGGT 3’ | None |
| HNF1A-WT-R | 5’ AGGGTACTTCTGCAGTCTCACT 3’ | None |
| HNF1A-WT-P | 5’ CACGGTGTGCGCTATG 3’ | 5’ 6-FAM, 3’-MGB |
| G319S-FL-F | 5’ TGCCCTCTCCCCCAGTAAG 3’ | None |
| G319S-FL-R | 5’ AGGGTACTTCTGCAGTCTCACT 3’ | None |
| G319S-FL-P | 5’ TCCACAGTGTGCGCTATG 3’ | 5’ 6-FAM, 3’-MGB |
| Ex412bpdel-F | 5’ CCTCCACCTGCCCTCTC 3’ | None |
| Ex412bpdel-R | 5’ AGGGTACTTCTGCAGTCTCACT 3’ | None |
| Ex412bpdel-P | 5’ CCCCAGTGTGCGCTATG 3’ | 5’ 6-FAM, 3’-MGB |
| IVS4ins7-F | 5’ CTCCACCTGCCCTCTCC 3’ | None |
| IVS4ins7-R | 5’ TCGCAGGCTGTCCATAGC 3’ | None |
| IVS4ins7-P | 5’ ACCACTTACTGTGGACCTTA 3’ | 5’ 6-FAM, 3’-MGB |
| Ex8del-F | 5’ CCCCTTCATGGCCACCAT 3’ | None |
| Ex8del-R | 5’ GTGTGAAGCCCGGACTCA 3’ | None |
| Ex8del-P | 5’ CCCCACGGTCTTCACC 3’ | 5’ 6-FAM, 3’-MGB |
The sequences of nested PCR primers and oligonucleotides used in the real-time quantification are given. The label 6-FAM refers to 6-fluorosceine and MGB refers to the minor groove binding non-fluorescent quencher. Forward primers are given by F suffix, reverse primers by R suffix, and probes by P suffix.
Real-time PCR quantification of G319S and normal transcripts.
Ectopic mRNA transcripts were amplified from lymphoblastoid cells using a single-tube TaqMan approach on the ABI Prism 7900 platform (Applied Biosystems, Warrington, U.K.). Primer and probe sequences are given in Table 1. Triplicate single-round reactions were carried out for each cell line using 2 μl cDNA. PCR products were detected by the use of mutation-specific probes that were identical except for the site of the mutation (Table 1), as previously described (9,10). Final profiles were pooled from at least two separate reverse transcriptions to ensure accuracy.
Relative quantification of normal and mutant transcripts.
Relative quantification of the two transcripts was carried out using the equation 2−ΔΔCt described by Applied Biosystems (Foster City, CA) (11). The ubiquitously expressed β-2 microglobulin (β2M) gene was selected as an endogenous control. An assay for this gene (Assay Hs00187842) was purchased by Assays-on-Demand from Applied Biosystems (Foster City, CA).
Inhibition of nonsense-mediated mRNA decay.
To determine whether any observed reductions in the amount of mutant transcripts were due to nonsense-mediated mRNA decay (NMD), cell cultures were split and treated for 4 h with either solvent alone (1% DMSO) or cycloheximide (100 μg/ml in DMSO) (Sigma, Poole, U.K.). Cells were then washed once with PBS (Gibco Life Technologies) and harvested by centrifugation.
In silico prediction of splicing patterns and mRNA folding.
The software package used for splice site prediction is available from www.fruitfly.org, and the secondary structure of mRNAs was predicted using MFOLD software (http://www.bioinfo.rpi.edu/applications/mfold) (12,13).
RESULTS
Bioinformatic tools predicted a high score (0.99) for the native splice donor site of intron 4, which was reduced to 0.94 by the G319S variant. An alternative cryptic splice site 12 bp 5′ of the native splice donor site was also indicated with a score of 0.94 in both normal and G319S sequences.
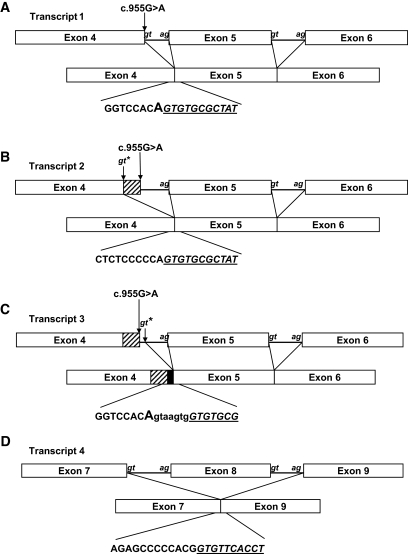
Analysis of cDNA from the homozygous G319S subject revealed four different sequences (Fig. 1A–D). Transcript 1 corresponds with the full-length HNF1A product including the G319S substitution (Fig. 1A). Transcript 2 corresponds with use of the alternative splice donor site and the generation of an mRNA transcript lacking 12 base pairs at the 3′ end of exon 4, which includes nucleotide 955 (the site of the G319S substitution) (Fig. 1B). These mRNAs will result in the translation of an in-frame protein of either 631 or 627 amino acids, respectively. Transcript 3 shows a small insertion of 7 bp of intron 4 immediately following the G319S variant (Fig. 1C). This results from the use of a further intron 4 splice donor site 3′ of the native site. The 7 bp insertion disrupts the reading frame, and a premature termination codon is introduced at codon 332 in exon 5. The fourth transcript shows complete skipping of exon 8 in addition to the 7 bp insertion from intron 4 predicted to result in premature termination at codon 332 (Fig. 1D). Long-range PCR analysis confirmed that the IVS4nt + 7 insertion and the exon 8 deletion are present within the same transcript (in cis).
FIG. 1.
Schematic of G319S variant transcripts. The mRNA sequences of transcript 1 (A), transcript 2 (B), transcript 3 (C), and transcript 4 (D) are given below. Lowercase letters in italics refer to the conserved splice sites present in the introns. The position of G319S at nt955 is marked by an arrow. Cryptic splice sites are marked with stars. The junction sequences of the abnormal transcripts are provided. The sequence deriving from the 5′ exon are in normal type, and the sequence deriving from the 3′ spliced exon are underlined and given in italics. Sequences derived from introns are in lowercase. The position of the 12-bp deletion is given by a hatched box, and the position of the 7-bp insertion is given by a black box. The position of the G319S variant on the sequence is marked in capitals where appropriate.
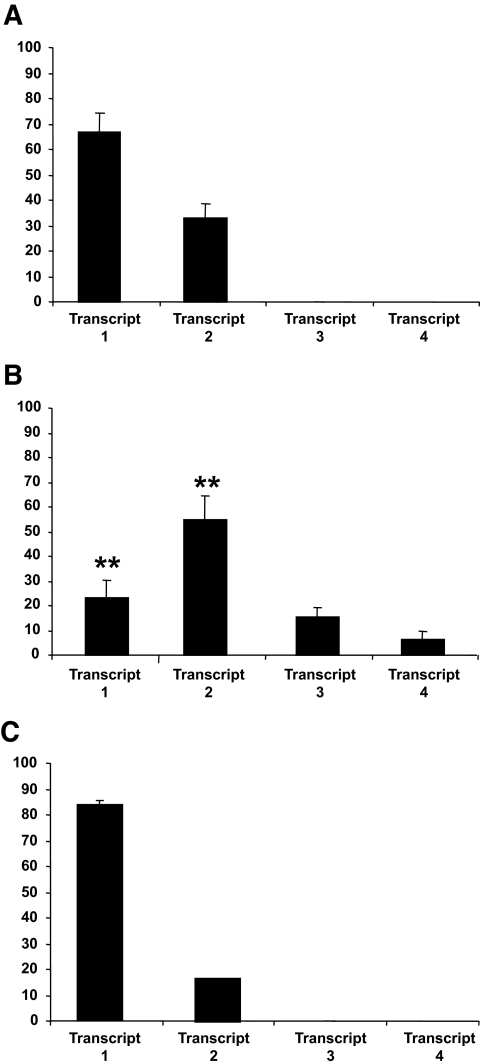
The four mRNA transcripts identified in the G319S homozygous cell line were quantified by real-time PCR (Fig. 2A). The most abundant transcript was transcript 2, which comprised 55% of HNF1A mRNA transcripts. This transcript results from the use of a cryptic spice site 12 bp proximal to the native splice donor site of intron 4. The full-length G319S transcript (transcript 1) represented 24% of total HNF1A expression, with transcripts 3 and 4 at ∼15 and 6%, respectively. The levels of transcripts 3 and 4 were increased 1.6- and 1.7-fold, respectively, by the addition of the translation blocker cycloheximide, which abolishes the nonsense-mediated decay mRNA surveillance pathway (14). Transcript 2 was also present in both normal cell lines and in human pancreatic tissue (28, 38, and 17% of total HNF1A expression, respectively), which suggests that it is a normal splice variant and may not compromise HNF-1α activity (Fig. 2B and C).
FIG. 2.
Real-time PCR quantification of G319S and wild-type transcripts in lymphoblastoid cells and normal human pancreas. Real-time quantitative data for G319S and normal transcripts are given for normal (A) homozygous G319S cells (B) and normal human pancreas (C). The percentages given are calculated relative to β-2 microglobulin (B2M) expression. The X-axis refers to the transcript identity, and the Y-axis refers to the percentage of total HNF1A expression represented by each transcript. Error bars represent the upper and lower limits of quantification based on three independent measurements. Differences in expression profile were analyzed statistically by comparison of the ΔCt values (i.e., the crossing point of the test transcript relative to the endogenous control) for full-length and 12-bp deletion-bearing transcripts in lymphoblastoid cells by Mann-Whitney U test analysis.
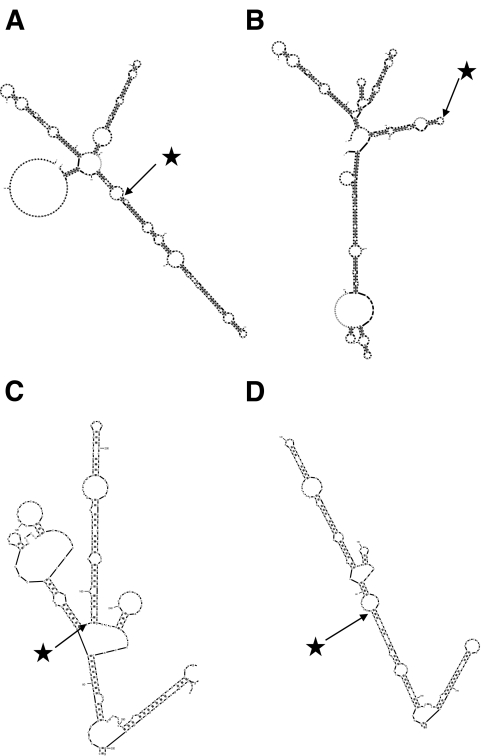
The c.955G>A base substitution is predicted to alter the secondary structure of the HNF1A pre-RNA (Fig. 3A and B). When guanine is present at nucleotide 955, the sequences flanking the splice donor site of exon 4 are held in a relatively open conformation, but when adenine is substituted, they are incorporated into a double-stranded region (Figs. 3C and D). These changes in mRNA secondary structure predict a global change in conformation, which may obscure binding sites for splicing factors.
FIG. 3.
Predicted secondary structure of G319S hnRNAs and mRNAs. The predicted secondary structures of G319 prespliced hnRNA (A), S319 prespliced hnRNAs (B), G319S full-length processed mRNA (C), and G319S IVS4ins7 processed mRNA transcripts (D) are given. The positions of residue 319 within the transcript are marked by stars. Note the alteration of secondary structure apparent in C and D.
DISCUSSION
The HNF1A G319S variant may moderate diabetes susceptibility through the disruption of normal mRNA splicing. We detected two abnormal transcripts (transcripts 3 and 4) in the G319S cell line that result in premature termination codons. We also identified a novel isoform, transcript 2, that arises from the use of a cryptic splice donor site 12 bases upstream of the native intron 4 splice donor site. This is the major HNF1A isoform (55%) in G319S lymphoblastoid cells but was also present in normal control cell lines and human pancreas at lower levels (∼33 and 17%, respectively). The G319S variant does not completely abolish the use of the native splice donor site, since full-length G319S transcripts were also present (24% of transcripts).
The identification of an mRNA transcript lacking exon 8 was unexpected since this exon is located 4.6 kb downstream from the c.955G>A substitution. Mutations located close to one splice site causing deletion of remote exons have been reported before (15), but they are rare. One possible explanation may be that alterations in the conformation of the nascent hnRNA (pre-mRNA) transcripts may influence both splice site choice and the stability of the resultant mRNA (16–18). The inclusion of sequences from intron 4 may therefore disrupt the secondary structure of the transcript and prevent binding of proteins to additional splicing factors such as exon splicing enhancers. The predicted changes in RNA secondary structure resulting from the intron 4 insertion (Fig. 3B) are consistent with this hypothesis.
This study has identified a novel HNF1A isoform (transcript 2) expressed in lymphoblastoid cell lines and adult human pancreas. This isoform lacks the 3′ terminal four amino acid residues of exon 4 that are not located within any of the regions required for maximal transactivation capacity or nuclear localization (8). It seems unlikely that this isoform would have reduced functional activity. Previous in vitro functional studies of full-length G319S protein showed a reduction in HNF-1α activity by ∼54% (4). This transcript represented 24% of total mRNA and is estimated to account for ∼11% of active HNF-1α protein in G319S homozygotes. This, together with the predicted 55% activity attributable to transcript 2, suggests that G319S homozygotes might retain up to 66% of HNF-1α activity. The levels of active HNF-1α protein in a G319S heterozygote are predicted to be intermediate between these and normal levels.
The Oji-Cree diabetic phenotype associated with the G319S variant is consistent with reduced insulin secretion and is likely to be a consequence of reduced HNF-1α activity. Our results suggest that the residual level of transactivation activity is greater than that observed in HNF1A-MODY, where the mutational mechanism of haploinsufficiency was confirmed by the identification of heterozygous gene deletions (20–22). The reduction in HNF-1α activity may result from a combination of NMD-mediated reduction in mRNA levels due to the premature termination codon–containing transcripts and the reduced function of the full-length G319S protein. The aberrant splicing resulting from the base substitution at the 3′ end of exon 4 moderates the diabetic phenotype through the upregulation of a novel isoform that is predicted to have normal transactivation activity.
We considered the possibility that other coding polymorphisms associated with type 2 diabetes might also affect splicing, but the HNF1A coding single nucleotide polymorphisms A98V and I27L are not located close to conserved splice sites or exonic splice enhancers. Our study demonstrates that susceptibility to type 2 diabetes in the Oji-Cree may be mediated in part by aberrant splicing, highlighting the potential utility of mRNA analysis in the investigation of disease susceptibility alleles.
Acknowledgments
We are grateful to the Research and Development Directorate at the Royal Devon and Exeter NHS Foundation Trust for funding this study.
We are grateful to Dr. Heather Dean for clinical collaboration and constructive comments on the manuscript. We thank Andrew Parrish for assistance in preparing the figures. We also acknowledge the European Collection of Cell Cultures (ECACC) for providing the transformed cell lines. A.T.H. is a Wellcome Trust Research Leave Fellow, and L.W.H. is a Research Councils UK fellow.
Published ahead of print at http://diabetes.diabetesjournals.org on 14 April 2008.
L.W.H. and M.J.S. contributed equally to this work.
The costs of publication of this article were defrayed in part by the payment of page charges. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
REFERENCES
- 1.Hegele RA, Cao H, Harris SB, Hanley AJ, Zinman B: Hepatocyte nuclear factor 1α G319S: a private mutation in Oji-Cree associated with diabetes. Diabetes Care 22 :524 ,1999 [DOI] [PubMed] [Google Scholar]
- 2.Sellers EAC, Triggs-Raine BL, Rockman-Greenberg C, Dean HJ: The prevalence of the HNF-1α G319S mutation in Canadian aboriginal youth with type 2 diabetes. Diabetes Care 25 :2202 –2206,2002 [DOI] [PubMed] [Google Scholar]
- 3.Hegele RA, Cao H, Harris SB, Zinman B, Hanley AJ, Anderson CM: Gender, obesity, hepatic nuclear factor-1alpha G319S and the age-of-onset of type 2 diabetes in Canadian Oji-Cree. Int J Obesity 24 :1062 –1064,2000 [DOI] [PubMed] [Google Scholar]
- 4.Triggs-Raine BL, Kirkpatrick RD, Kelly SL, Norquay LD, Cattini PA, Yamagata K, Hanley AJ, Zinman B, Harris SB, Barrett PH, Hegele RA: HNF-1alpha G319S, a transactivation-deficient mutant, is associated with altered dynamics of diabetes onset in an Oji-Cree community. Proc Natl Acad Sci U S A 99 :4614 –4619,2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Owen K, Hattersley A: Maturity-onset diabetes of the young: from clinical description to molecular genetic characterization. Best Pract Res Clin Endocrinol Metab 15 :309 –323,2001 [DOI] [PubMed] [Google Scholar]
- 6.Hegele RA, Cao H, Harris SB, Hanley AJ, Zinman B: The hepatic nuclear factor-1alpha G319S variant is associated with early-onset type 2 diabetes in Canadian Oji-Cree. J Clin Endocrinol Metab 84 :1077 –1082,1999 [DOI] [PubMed] [Google Scholar]
- 7.Vaxillaire M, Abderrahmani A, Boutin P, Bailleul B, Froguel P, Yaniv M, Pontoglio M: Anatomy of a homeoprotein revealed by the analysis of human MODY3 mutations. J Biol Chem 274 :35639 –35646,1999 [DOI] [PubMed] [Google Scholar]
- 8.Bjorkhaug L, Bratland A, Njolstad PR, Molven A: Functional dissection of the HNF-1alpha transcription factor: a study on nuclear localization and transcriptional activation. DNA Cell Biol 24 :661 –669,2005 [DOI] [PubMed] [Google Scholar]
- 9.Bulman MP, Harries LW, Hansen T, Shepherd M, Kelly WF, Hattersley AT, Ellard S: Abnormal splicing of hepatocyte nuclear factor 1 alpha in maturity-onset diabetes of the young. Diabetologia 45 :1463 –1467,2002 [DOI] [PubMed] [Google Scholar]
- 10.Harries LW, Ellard S, Jones RW, Hattersley AT, Bingham C: Abnormal splicing of hepatocyte nuclear factor-1 beta in the renal cysts and diabetes syndrome. Diabetologia 47 :937 –942,2004 [DOI] [PubMed] [Google Scholar]
- 11.Applied-Biosystems: Relative Quantitation of Gene Expression. Applied Biosystems, User Bulletin #2, p.11 –15,2001
- 12.Zuker M: Mfold web server for nucleic acid folding and hybridization prediction. Nucleic Acid Res 31 :3406 –3415,2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Matthews DH, Sabina J, Zuker M, Turner DH: Expanded sequence dependence of thermodynamic parameters improves prediction of RNA secondary structure. J Mol Biol 288 :911 –940,1999 [DOI] [PubMed] [Google Scholar]
- 14.Frischmeyer PA, Dietz HC: Nonsense-mediated mRNA decay in health and disease. Hum Mol Genet 8 :1893 –1900,1999 [DOI] [PubMed] [Google Scholar]
- 15.Lo Ten Foe JR, Kryut F, Zweekhorst M, Pals G, Gibson R, Mathew CG, Joenje H, Arwert F: Exon 6 skipping in the Fanconi anemia C gene associated with a nonsense/missense mutation (775C–>T) in exon 5: the first example of a nonsense mutation in one exon causing skipping of another downstream. Hum Mutat (Suppl. 1):S25 –S27,1998 [DOI] [PubMed]
- 16.Chamary JV, Hurst L: Evidence for selection on synonymous mutations affecting stability of mRNA secondary structure in mammals. Genome Biology 6 :R75 ,2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chamary JV, Parmley JL, Hurst L: Hearing silence: non-neutral evolution at synonymous sites in mammals. Nat Rev Genet 7 :98 –108,2006 [DOI] [PubMed] [Google Scholar]
- 18.Shabalina S, Oqurtsov A, Spiridonov N: A periodic pattern of mRNA secondary structure created by the genetic code. Nucleic Acid Res 34 :2428 –2437,2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Winckler W, Burtt NP, Holmkvist J, Cervin C, de Bakker PI, Sun M, Almgren P, Tuomi T, Gaudet D, Hudson TJ, Ardlie KG, Daly MJ, Hirschhorn JN, Altshuler D, Groop L: Association of common variation in the HNF1α gene region with risk of type 2 diabetes. Diabetes 54 :2336 –2342,2005 [DOI] [PubMed] [Google Scholar]
- 20.Thomas H, Badenberg B, Bulman M, Lemm I, Lausen J, Kind S, Roosen S, Ellard S, Hattersley AT, Ryffel G: Evidence for haploinsufficiency of the HNF1[alpha] gene revealed by functional characterisation of MODY3-associated mutations. Biol Chem 383 :1691 –1700,2002 [DOI] [PubMed] [Google Scholar]
- 21.Harries LW, Hattersley AT, Ellard S: Messenger RNA transcripts of the hepatocyte nuclear factor-1α gene containing premature termination codons are subject to nonsense-mediated decay. Diabetes 53 :500 –504,2004 [DOI] [PubMed] [Google Scholar]
- 22.Ellard S, Thomas K, Edghill EL, Owens M, Ambye L, Cropper J, Little J, Strachan M, Stride A, Ersoy B, Eiberg H, Pedersen O, Shepherd MH, Hansen T, Harries LW, Hattersley AT: Partial and whole gene deletion mutations of the GCK and HNF1A genes in maturity-onset diabetes of the young. Diabetologia 50 :2313 –2317,2007 [DOI] [PubMed] [Google Scholar]