Abstract
OBJECTIVE—Despite experimental data suggesting a protective effect of peroxisome proliferator–activated receptor-γ agonists with respect to malignancies, results of available epidemiological studies on the incidence of cancer in rosiglitazone-treated patients are not univocal. The aim of this meta-analysis of randomized clinical trials is to assess the effect of rosiglitazone on the incidence of cancer.
RESEARCH DESIGN AND METHODS—Randomized clinical trials of rosiglitazone with duration of >24 weeks were retrieved through Medline and from the GlaxoSmithKline Web site, which reports main results of all trials sponsored by GlaxoSmithKline; incident malignancies were retrieved from the summary of serious adverse events. Proportions of outcome measures across treatment groups were compared by odds ratios (ORs) and 95% CI. Considering differences in the duration of follow-up among treatment arms in some of the trials, we also calculated the incidence of cancer in rosiglitazone and control groups.
RESULTS—Eighty trials, enrolling 16,332 and 12,522 patients in the rosiglitazone and comparator groups, respectively, were retrieved. Rosiglitazone was not associated with a significant modification of the risk of cancer (OR 0.91 [95% CI 0.71–1.16], P = 0.44). The incidence of malignancies was significantly lower in rosiglitazone-treated patients than in control groups (0.23 [0.19–0.26] vs. 0.44 [0.34–0.58] cases/100 patient-years; P < 0.05).
CONCLUSIONS—The use of rosiglitazone appears to be safe in terms of incidence of cancer, whereas its possible protective effect needs to be further investigated.
Two epidemiological surveys provided discordant results on the effects of rosiglitazone on the incidence of malignancies. One study reported a specific reduction in the incidence of lung cancer (1), whereas another survey suggested an increased risk of malignancies, without providing information on types of cancer (2).
A hypothetical anticancer effect of thiazolidinediones has been suggested on the basis of their pharmacological profile of action. The antimitotic and prodifferentiating effects of peroxisome proliferator–activated receptor (PPAR)-γ agonists, which have been described in vitro and in animal models (3–5), suggested the possible use of these drugs as anticancer therapy, although the results of preliminary trials were contradictory (6–10). On the other hand, the mechanisms underlying a possible mitogenic effect of PPAR-γ activators have not been identified so far. The aim of the present meta-analysis is to assess the risk of cancer associated with rosiglitazone treatment, compared either with placebo or active hypoglycemic drugs.
RESEARCH DESIGN AND METHODS—
Trials were identified through a search of a Web site of GlaxoSmithKline (GSK) (11), manufacturer of rosiglitazone, which contains results of all completed trials sponsored by GSK, with a description of all serious adverse events (including those considered not related to study drug), such as incident malignancies. Published trials sponsored by other companies or by academic institutions were retrieved through a Medline search for all randomized controlled trials with rosiglitazone performed in humans with results published in English up to 5 February 2008. For each trial, all fatal and nonfatal serious adverse events in each treatment arm are listed with a brief description. All studies comparing rosiglitazone with placebo or other active drugs, with a duration >24 weeks, were included in the analysis. Studies of shorter duration were excluded, considering that a brief exposure to a drug is unlikely to have any impact on the incidence of cancer. Occurrences of fatal or nonfatal cancer were extracted from serious adverse events.
After the exclusion of trials with zero events, odds ratios (ORs) and 95% CI, with the Mantel-Haenszel (MH)-OR weighting procedure, were calculated using a random effect model. This procedure was chosen to overcome the limitations of the Peto method (12–14), which had been used in a previous meta-analysis on cardiovascular effects of rosiglitazone (15). In fact, the Peto method overestimates differences between treatments when a large number of small trials, with few events, are included in a meta-analysis (12–14). Separate analyses were performed, whenever possible, for trials with different comparators and for those performed in type 2 diabetic or nondiabetic patients, as well as for trials with duration ≥52 weeks. Separate analyses were also performed for the most common individual types of cancer.
Considering that in the largest trial included in the analysis (16) the duration of follow-up in the rosiglitazone arm is longer than in comparators (17), we also calculated the actual incidence density of cancer in different treatment groups using a random effect model, assuming that rates of loss at follow-up, mortality, and incidence of malignancies were constant throughout the duration of each trial; this analysis also included trials with zero events. Furthermore, after determination of effect sizes for individual trials, ratios between incidence densities were calculated for each trial and combined to obtain a pooled rate ratio. All of the analyses were performed using Comprehensive Meta Analysis (version 2.2.046; Englewood, NJ).
RESULTS—
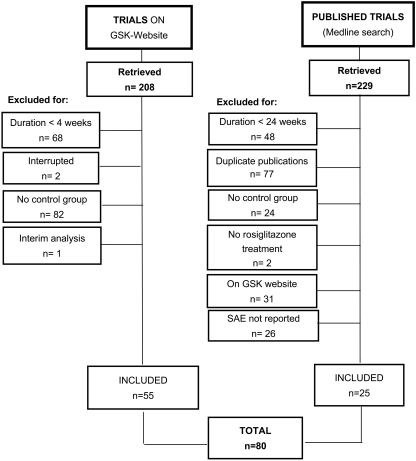
The trial flow is summarized in Fig. 1. The 80 trials included in the analysis enrolled 16,332 and 12,522 patients for rosiglitazone and comparators (15,700 and 18,050 patient-years), respectively, with a weighted mean age of 55.3 years. Of the retrieved trials, 63 and 17 were performed on type 2 diabetic patients (mean A1C 8.1%) or on subjects with different conditions, respectively (Table 1). Most (25 of 51) of the published trials not present on the GSK Web site (11) did not report a detailed description of serious adverse events, including malignancies; only two malignancies had been observed in those trials for which this information was available. A complete list of published trials not on GSK Web site, either included or not included in the meta-analysis, is reported in the online appendix (available at http://dx.doi.org/10.2337/dc07-2308).
Figure 1—
Flow diagram of the trials evaluated for inclusion in the meta-analysis. SAE, serious adverse events.
Table 1—
Main characteristics of clinical trials included in the meta-analysis
| Study (11)* | Characteristics | Comparator | Duration (weeks) | Number R/C | Mean age (years) | Mean A1C (%) | Incident cases of cancer R/C |
|---|---|---|---|---|---|---|---|
| Nondiabetic subjects | |||||||
| Trials on GSK Web site | |||||||
| 100684 | Metabolic syndrome | Placebo | 52 | 43/47 | 45 | — | 0/0 |
| 49653/330 | Plaque psoriasis | Placebo | 52 | 1,181/382 | 44 | — | 3/1 |
| 49653/331 | Plaque psoriasis | Placebo | 52 | 706/325 | 45 | — | 0/1 |
| 49653/334 | Insulin resistant | Placebo | 52 | 178/177 | 68 | — | 4/3 |
| 49653/392 | Insulin resistant | Metformin | 52 | 16/15 | 56 | — | 0/0 |
| 49653/131 | Insulin resistant | Placebo | 26 | 39,427 | 48 | — | 0/0 |
| 49653/452 | Multiple sclerosis | Placebo | 26 | 26/25 | 42 | — | 0/1 |
| ARA102198 | Rheumatoid arthritis | Placebo | 26 | 49/49 | 56 | — | 0/0 |
| AVA100193 | Alzheimer's disease | Placebo | 24 | 394/124 | 71 | — | 0/0 |
| Other published trials | |||||||
| Carr | HIV infection | Placebo | 48 | 53/55 | 45 | — | 1/0 |
| Sidhu | Coronary artery disease | Placebo | 48 | 46/46 | 62 | — | 0/0 |
| Silic | HIV infection | Metformin | 48 | 30/30 | 42 | — | 0/0 |
| van Wijk | HIV infection | Metformin | 26 | 19/20 | 47 | — | 0/0 |
| Coll | HIV infection | Metformin | 26 | 15/16 | 48 | — | 0/0 |
| Cavalcanti | HIV infection | Placebo | 24 | 48/48 | 47 | — | 0/0 |
| Baillargeon | PCOS | Placebo | 24 | 42/30 | 27 | — | 0/0 |
| Lemay | PCOS | None | 24 | 15/13 | 24 | — | 0/0 |
| Type 2 diabetic patients | |||||||
| Trials on GSK Web site | |||||||
| 49653/048 (ADOPT)† | Monotherapy | Glyburide | 208 | 1,456/1,441 | 56 | 7.3 | 63/71 |
| 49653/048 (ADOPT)† | Monotherapy | Metformin | 208 | 1,456/1,454 | 57 | 7.3 | 63/67 |
| 49653/080 | Monotherapy | Glyburide | 156 | 104/99 | 56 | 9.1 | 1/3 |
| 49653/097 | Monotherapy | Glyburide | 148 | 122/120 | 56 | 8.9 | 1/4 |
| 49653/135 | Combined therapy | Placebo | 104 | 116/111 | 68 | 7.4 | 4/7 |
| 49653/211 | NYHA-II, mono-combined | Placebo | 52 | 110/114 | 64 | NR | 2/3 |
| 49653/020 | Monotherapy | Glyburide | 52 | 384/203 | 60 | 8.2 | 3/0 |
| AVM100264 | Combined therapy | Sulfonylureas | 52 | 294/302 | 59 | 8.0 | 2/1 |
| 712753/008 | Combined therapy | None | 48 | 284/135 | 55 | NR | 3/0 |
| 49653/137 | Combined therapy | Glyburide | 32 | 204/185 | 59 | 8.4 | 2/4 |
| BRL49653/185 | Mono-combined | None | 32 | 563/142 | 59 | 7.4 | 4/2 |
| SB-712753/003 | Combined therapy | Placebo | 32 | 254/272 | 59 | 7.2 | 0/1 |
| SB-712753/007† | Monotherapy, OL | Metformin | 32 | 159/154 | 59 | 7.2 | 0/0 |
| SB-712753/007† | Combined therapy, OL | None | 32 | 155/154 | 59 | 7.2 | 0/0 |
| 49653/128 | Combined therapy | Placebo | 28 | 39/38 | 58 | 9.6 | 0/0 |
| 49653/134 | Combined therapy | Placebo | 28 | 561/276 | 55 | 8.7 | 0/2 |
| SB-797620/004 | Monotherapy | Glimepiride | 28 | 232/225 | 53 | 9.0 | 1/0 |
| 49653/024 | Monotherapy | Placebo | 26 | 774/185 | 57 | 8.9 | 5/1 |
| 49653/044 | Combined therapy | Placebo | 26 | 71/34 | 54 | 9.6 | 0/0 |
| 49653/079 | Monotherapy | Glyburide | 26 | 104/106 | 58 | 9.2 | 1/0 |
| 49653/079 | Combined therapy | Placebo | 26 | 99/106 | 58 | 9.2 | 2/0 |
| 49653/082 | Combined therapy | Placebo | 26 | 212/107 | 56 | 9.1 | 0/0 |
| 49653/085 | Combined therapy | Placebo | 26 | 138/139 | 61 | NR | 1/0 |
| 49653/093† | Monotherapy | Metformin | 26 | 107/109 | 59 | 8.7 | 0/0 |
| 49653/093† | Combined therapy | Placebo | 26 | 106/109 | 59 | 8.7 | 0/0 |
| 49653/094 | Combined therapy | Placebo | 26 | 232/116 | 58 | 8.8 | 0/0 |
| 49653/095 | Combined therapy | Placebo | 26 | 196/96 | 58 | 9.0 | 1/0 |
| 49653/096 | Combined therapy | Placebo | 26 | 232/115 | 60 | 9.1 | 2/0 |
| 49653/109 | Monotherapy | Glipizide | 26 | 52/25 | 53 | 8.0 | 0/0 |
| 49653/125 | Combined therapy, OL | None | 26 | 175/173 | 56 | 8.9 | 0/0 |
| 49653/127 | Combined therapy | Placebo | 26 | 56/58 | 60 | 9.0 | 0/2 |
| 49653/136 | Combined therapy | Placebo | 26 | 148/143 | 65 | 8.2 | 2/0 |
| 49653/145 | Combined therapy | None | 26 | 231/242 | 61 | 8.6 | 1/0 |
| 49653/147 | Combined therapy | Placebo | 26 | 89/88 | 54 | 9.1 | 0/0 |
| 49653/162 | Combined therapy | Placebo | 26 | 168/172 | 60 | 8.0 | 2/0 |
| 49653/234 | Combined therapy | Placebo | 26 | 116/61 | 63 | 8.1 | 1/0 |
| 49653/390 | Combined therapy | None | 26 | 33/30 | NR | NR | 1/0 |
| 49653/369 | Monotherapy | Glyburide | 26 | 25/24 | 52 | 6.8 | 0/0 |
| 49653/132 | Combined therapy | Placebo | 24 | 442/112 | 59 | 9.8 | 1/1 |
| 49653/347 | Combined therapy | Placebo | 24 | 418/212 | 53 | 9.0 | 0/1 |
| 49653/015 | Combined therapy | Placebo | 24 | 395/198 | 61 | 9.2 | 4/0 |
| 49653/284 | Combined therapy | Placebo | 24 | 382/384 | 55 | 8.0 | 1/0 |
| SB-712753/002 | Combined therapy | Placebo | 24 | 288/280 | 58 | 7.5 | 1/0 |
| 49653/090 | Monotherapy | Placebo | 24 | 228/75 | 59 | 8.8 | 1/0 |
| 49653/325 | Combined therapy | Placebo | 24 | 196/195 | 53 | 8.0 | 0/1 |
| SB-712753/009 | Combined therapy | Placebo | 24 | 162/160 | 57 | 8.7 | 2/0 |
| AVD102209 | Combined therapy | Placebo | 24 | 132/131 | 56 | 9.6 | 0/0 |
| 49653/143 | Combined therapy | Placebo | 24 | 121/124 | 52 | 9.2 | 1/0 |
| 49653/207 | Children monotherapy | Metformin | 24 | 99/101 | 14 | 8.0 | 0/0 |
| 49653/282 | Combined therapy | Glyburide | 24 | 69/72 | 60 | 7.6 | 0/0 |
| Other published trials | |||||||
| Ko | Combined therapy | Insulin | 52 | 56/56 | 58 | 9.6 | 0/0 |
| Derosa (a) | Combined therapy | Glimepiride | 52 | 49/50 | 53 | 8.0 | 0/0 |
| Derosa (b) | Combined therapy | Pioglitazone | 52 | 48/48 | 55 | 9.0 | 0/0 |
| Derosa (c) | Monotherapy | Pioglitazone | 52 | 45/42 | 54 | 8.1 | 0/0 |
| Rahman | Monotherapy | Placebo | 52 | 11/11 | 47 | 7.5 | 0/0 |
| Kelly | Combined therapy | Glyburide | 26 | 20/16 | 60 | 7.6 | 0/0 |
| Reynolds | Monotherapy | Placebo | 26 | 8/10 | 49 | 9.2 | 0/0 |
| Osman | Monotherapy, PTCA | Placebo | 26 | 8/8 | 55 | 9.6 | 0/0 |
| Zhou | Combined therapy | Placebo | 24 | 442/112 | 56 | 9.8 | 0/0 |
| Goldberg | Monotherapy | Pioglitazone | 24 | 369/366 | 56 | 7.5 | 0/0 |
| Weissman | Combined therapy | Placebo | 24 | 358/351 | 55 | 8.0 | 0/0 |
| Agrawal | Combined therapy | None | 24 | 288/280 | 58 | 7.5 | 0/0 |
| Dailey | Combined therapy | Placebo | 24 | 181/184 | 57 | 8.1 | 0/1 |
| Garber | Combined therapy | Glyburide | 24 | 158/160 | 56 | 8.5 | 0/0 |
| Wang | Mono-combined | None | 24 | 35/35 | 61 | 7.3 | 0/0 |
| Wong | Combined therapy | None | 24 | 26/26 | 62 | 7.2 | 0/0 |
| Jung | Combined therapy | Metformin | 24 | 15/15 | 57 | 9.1 | 0/0 |
| Total | — | — | 39.2 | 16,332/12,522 | 55.3 | 8.1 | 124/178 |
See Appendix for references.
Trials with multiple comparators. ADOPT, A Diabetes Outcome Progression Trial; mono-combined, monotherapy or combined therapy; NR, not reported; NYHA-II, New York Heart Association, Class II; OL, open label; PCOS, polycystic ovary syndrome; PTCA, percutaneous transluminal coronary angioplasty; R/C, rosiglitazone versus comparator.
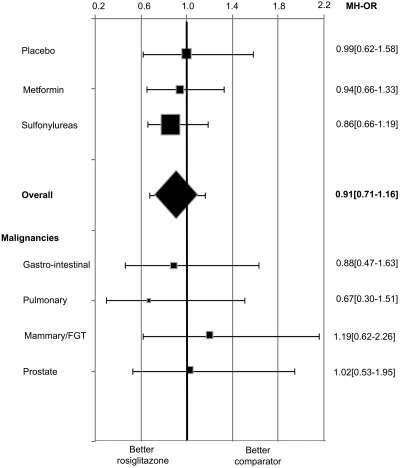
The number of incident malignancies reported in each trial is summarized in Table 1. Of the 302 cases of cancer, 42 (13.9%) were gastrointestinal, 13 (4.3%) pancreatic, 26 (8.6%) pulmonary, 35 (11.6%) of mammary gland/female genital tract, 36 (11.9%) of male urogenital tract, and 105 (34.8%) of other known origin; the type of cancer was not specified in 45 (14.9%) cases. No difference was observed in the proportion of cases between patients allocated to rosiglitazone and comparators. The overall MH-OR [95% CI] for rosiglitazone compared with control groups was 0.91 ([0.71–1.16]; P = 0.44). More than one-half of all malignancies were observed in one large trial, A Diabetes Outcome Progression Trial (ADOPT); the MH-OR after the exclusion of this study was 0.92 (0.61–1.39). When trials with a duration ≥52 weeks were analyzed separately, the MH-OR for rosiglitazone was 0.86 (0.66–1.14). Similar results were obtained for nondiabetic and type 2 diabetic patients (0.93 [0.33–2.65] and 0.91 [0.71–1.17], respectively), for different comparators, and for the most common types of cancer, when analyzed separately (Fig. 2). Separate analyses on individual malignancies in trials with different comparators were not performed because of the insufficient number of events recorded in each group.
Figure 2—
Effects of rosiglitazone on incident malignancies. The size of the data markers represents the relative weight of the trial according to the number of incident cancers. FGT, female genital tract.
The cumulative incidence density of cancer in the rosiglitazone group was significantly (P < 0.05) lower than that in comparators (0.23 [95% CI 0.19–0.26] vs. 0.44 [0.34–0.58] cases/100 patient-years; P < 0.05). The pooled rate ratio for rosiglitazone (versus comparators) was 1.02 (95% CI 0.67–1.57).
CONCLUSIONS—
Available data from randomized clinical trials, summarized in the present meta-analysis, do not support the recent hypothesis of an increased risk of cancer associated with rosiglitazone (2). On the contrary, the incidence of malignancies in patients receiving rosiglitazone is not higher than that observed with comparators, although a possible protective effect of the drug, as suggested by previous observations, was not confirmed by the present data (1). In consideration of the fact that metformin treatment is associated with reduced risk of cancer in epidemiological studies (18), the hypothesis of a protective effect attributable to enhancement of insulin sensitivity and/or reduction of circulating insulin levels should be considered, along with other more direct, PPAR-γ–dependent or –independent effects of the drug (4,5,19). However, the pooled rate ratio did not highlight any effect of rosiglitazone on the risk of cancer. This discrepancy could be due to the fact that the proportion of subjects receiving rosiglitazone and control treatments varies across trials enrolling patients with different characteristics, which may affect the incidence of cancer. It should also be considered that incidence densities and rate ratios reported in the present analysis were obtained on the basis of several problematic assumptions (i.e., that rates of loss at follow-up, mortality, and incidence of malignancies were constant throughout the duration of each trial); these results should therefore be considered with caution. A meta-analysis of rosiglitazone trials based on patient-level data should be performed to gather more reliable information on this issue.
The gold standard for the assessment of the effects of drug treatments on major outcomes is represented by specifically designed and appropriately sized randomized clinical trials. Unfortunately, in the case of hypoglycemic drugs, such trials are often unavailable. Therefore, meta-analyses of events occurring in randomized trials designed with different end points have been used as a surrogate source of information (15). The limitations of this procedure should be clearly recognized; in particular, the classification of outcomes reported as adverse events and not as predefined end points can be problematic. Notably, most published trials do not report any information on incident malignancies; cases could be easily identified only in trials reported on the GSK Web site (11), which contains a detailed description of all serious adverse events. Furthermore, the inclusion in a meta-analysis of many small trials with a very low number of events poses challenging problems in statistical analysis (12–14). It should also be considered that clinical trials usually enroll relatively young patients with low comorbidity and high compliance, who can be considered to have a low risk for cancer.
On the other hand, a trial assessing the effect of a hypoglycemic drug on the incidence of malignancies would be hypothetically very difficult to realize because of the required sample size and duration of follow-up. For this reason, information on this end point can be obtained only through epidemiological studies or meta-analyses of trials designed for other purposes. The epidemiological approach provides the advantage of the possibility of collecting large samples with a long duration of follow-up; however, in observational studies multiple adjustments for confounders can never fully eliminate the prescription bias (i.e., the effect of differences in characteristics of patients on different therapeutic choices). Such a bias could be responsible for the discrepancy between our results and those of a recent cross-sectional survey (2).
The number of events included in the present meta-analysis does not allow a reliable analysis on specific types of cancer. However, our data are consistent with the possibility of specific protection from lung cancer, which has been reported previously in a epidemiological study (1). Considering that the pathogenesis of different forms of cancer is very heterogeneous, the drug could have divergent effects on different malignancies. Interestingly, no reduction of risk for cancer of the female genital tract was detected in rosiglitazone-treated patients, although the drug is used in the treatment of polycystic ovary syndrome (20), which is a known risk factor for these malignancies (21). Larger databases are needed to elucidate the risk profile for individual forms of cancer in rosiglitazone-treated patients.
In summary, the use of rosiglitazone appears to be safe with respect to risk of incident malignancies, whereas further studies are needed to confirm a possible protective effect. The incidence of cancer, which can probably be modified by hypoglycemic drugs, deserves to be considered among the relevant outcomes for the choice of treatment for type 2 diabetes.
Supplementary Material
Acknowledgments
The study was partially supported by funds from Regione Toscana, TRESOR Project. The authors did not receive any compensation for this work, apart from their usual salaries paid with public funds.
This research was performed as a part of the institutional activity of the unit.
N.M. and E.M. have received fees for speaking from GlaxoSmithKline.
The costs of publication of this article were defrayed in part by the payment of page charges. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C Section 1734 solely to indicate this fact.
References
- 1.Govindarajan R, Ratnasinghe L, Simmons DL, Siegel ER, Midathada MV, Kim L, Kim PJ, Owens RJ, Lang NP: Thiazolidinediones and the risk of lung, prostate, and colon cancer in patients with diabetes. J Clin Oncol 25:1476–1481, 2007 [DOI] [PubMed] [Google Scholar]
- 2.Ramos-Nino ME, MacLean CD, Littenberg B: Association between cancer prevalence and use of thiazolidinediones: results from the Vermont Diabetes Information System. BMC Med 5:17, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Nemenoff RA: Peroxisome proliferator-activated receptor-gamma in lung cancer: defining specific versus “off-target” effectors. J Thorac Oncol 2:989–992, 2007 [DOI] [PubMed] [Google Scholar]
- 4.Galli A, Ceni E, Crabb DW, Mello T, Salzano R, Grappone C, Milani S, Surrenti E, Surrenti C, Casini A: Antidiabetic thiazolidinediones inhibit invasiveness of pancreatic cancer cells via PPARγ independent mechanisms. Gut 53:1688–1697, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Yki-Jarvinen H: Thiazolidinediones. N Engl J Med 351:1106–1118, 2004 [DOI] [PubMed] [Google Scholar]
- 6.Kulke MH, Demetri GD, Sharpless NE, Ryan DP, Shivdasani R, Clark JS, Spiegelman BM, Kim H, Mayer RJ, Fuchs CS: A phase II study of troglitazone, an activator of the PPARγ receptor, in patients with chemotherapy-resistant metastatic colorectal cancer. Cancer J 8:395–399, 2002 [DOI] [PubMed] [Google Scholar]
- 7.Mueller E, Smith M, Sarraf P, Kroll T, Aiyer A, Kaufman DS, Oh W, Demetri G, Figg WD, Zhou XP, Eng C, Spiegelman BM, Kantoff PW: Effects of ligand activation of peroxisome proliferator-activated receptor γ in human prostate cancer. Proc Natl Acad Sci USA 97:10990–10995, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Demetri GD, Fletcher CD, Mueller E, Sarraf P, Naujoks R, Campbell N, Spiegelman BM, Singer S: Induction of solid tumor differentiation by the peroxisome proliferator-activated receptor-γ ligand troglitazone in patients with liposarcoma. Proc Natl Acad Sci USA 96:3951–3956, 1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Debrock G, Vanhentenrijk V, Sciot R, Debiec-Rychter M, Oyen R, Van OA: A phase II trial with rosiglitazone in liposarcoma patients. Br J Cancer 89:1409–1412, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Jiang WG, Davies G, Kynaston H, Mason MD, Fodstad O: Does the PGC-1/PPARγ pathway play a role in Com-1/p8 mediated cell growth inhibition in prostate cancer? Int J Mol Med 18:1169–1175, 2006 [PubMed] [Google Scholar]
- 11.GlaxoSmithKline: Clinical trial register [article online], 2007. Available from http://ctr.gsk.co.uk/welcome.asp. Accessed 15 October 2007
- 12.Diamond GA, Kaul S: Rosiglitazone and cardiovascular risk. N Engl J Med 357:938–939, 2007 [PubMed] [Google Scholar]
- 13.Shuster JJ, Jones LS, Salmon DA: Fixed vs random effects meta-analysis in rare event studies: the rosiglitazone link with myocardial infarction and cardiac death. Stat Med 26:4375–4385, 2007 [DOI] [PubMed] [Google Scholar]
- 14.Diamond GA, Bax L, Kaul S: Uncertain effects of rosiglitazone on the risk for myocardial infarction and cardiovascular death. Ann Intern Med 147:578–581, 2007 [DOI] [PubMed] [Google Scholar]
- 15.Nissen SE, Wolski K: Effect of rosiglitazone on the risk of myocardial infarction and death from cardiovascular causes. N Engl J Med 356:2457–2471, 2007 [DOI] [PubMed] [Google Scholar]
- 16.Kahn SE, Haffner SM, Heise MA, Herman WH, Holman RR, Jones NP, Kravitz BG, Lachin JM, O'Neill MC, Zinman B, Viberti G: Glycemic durability of rosiglitazone, metformin, or glyburide monotherapy. N Engl J Med 355:2427–2443, 2006 [DOI] [PubMed] [Google Scholar]
- 17.Mannucci E, Monami M, Marchionni N: Rosiglitazone and cardiovascular risk. N Engl J Med 357:938–940, 2007 [PubMed] [Google Scholar]
- 18.Evans JM, Donnelly LA, Emslie-Smith AM, Alessi DR, Morris AD: Metformin and reduced risk of cancer in diabetic patients. BMJ 330:1304–1305, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Han S, Roman J: Rosiglitazone suppresses human lung carcinoma cell growth through PPARγ-dependent and PPARγ-independent signal pathways. Mol Cancer Ther 5:430–437, 2006 [DOI] [PubMed] [Google Scholar]
- 20.Pillai A, Bang H, Green C: Metformin and glitazones: do they really help PCOS patients? J Fam Pract 56:444–453, 2007 [PubMed] [Google Scholar]
- 21.Navaratnarajah R, Pillay OC, Hardiman P: Polycystic ovary syndrome and endometrial cancer. Semin Reprod Med 26:62–71, 2008 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.