Abstract
OBJECTIVE—Carbohydrate counting is an effective approach to mealtime insulin adjustment in type 1 diabetes but has not been rigorously assessed in type 2 diabetes. We sought to compare an insulin-to-carbohydrate ratio with a simple algorithm for adjusting the dose of prandial insulin glusiline.
RESEARCH AND DESIGN METHODS—This 24-week, multicenter, randomized, controlled study compared two algorithms for adjusting mealtime (glulisine) insulin along with a standard algorithm for adjusting background (glargine) insulin in 273 intent-to-treat patients with type 2 diabetes. Glulisine and glargine were adjusted weekly in both groups based on self-monitored blood glucose (SMBG) results from the previous week. The simple algorithm group was provided set doses of glulisine to take before each meal. The carbohydrate counting (carb count) group was provided an insulin-to-carbohydrate ratio to use for each meal and adjusted their glulisine dose based on the amount of carbohydrate consumed.
RESULTS—A1C levels at week 24 were 6.70% (simple algorithm) and 6.54% (carb count). The respective mean A1C changes from baseline to 24 weeks were −1.46 and −1.59% (P = 0.24). A1C <7.0% was achieved by 73.2% (simple algorithm) and 69.2% (carb count) (P = 0.70) of subjects; respective values for A1C <6.5% were 44.3 and 49.5% (P = 0.28). The total daily dose of insulin was lower, and there was a trend toward less weight gain in carb count group patients. Severe hypoglycemia rates were low and equal in the two groups.
CONCLUSIONS—Weekly basal-bolus insulin adjustments based on premeal and bedtime glucose patterns resulted in significant reductions in A1C. Having two effective approaches to delivering and adjusting rapid-acting mealtime insulin may increase physicians' and patients' willingness to advance therapy to a basal-bolus insulin regimen.
Elevated A1C and postprandial glucose levels have been related to risk for long-term complications in diabetes (1–3). Insulin therapy is often needed to achieve target A1C and postprandial glucose levels in type 2 diabetes (4). Although many insulin regimens are available, in this study we examined the use of a physiological regimen that includes a long-acting insulin to provide a basal insulin level for the entire day and a rapid-acting insulin for bolus administration at mealtimes (5,6).
Establishing the optimal mealtime insulin dose in basal-bolus therapy may be difficult because it often involves calculations that consider multiple factors such as current blood glucose, target blood glucose, insulin-to-carbohydrate ratios, total carbohydrate content of meals, and activity levels (7). Insulin delivery based on carbohydrate counting is the gold standard for improving glycemic control in type 1 diabetes (8,9) but is difficult for some patients (10,11). Neither large, randomized studies of intensive basal-bolus analog insulin therapy in patients with type 2 diabetes nor studies evaluating the efficacy of carbohydrate counting in type 2 diabetes have been conducted. In this 24-week study we compared an insulin-to-carbohydrate ratio with a simple pattern control–based algorithm for adjusting the dose of prandial insulin glulisine.
RESEARCH DESIGN AND METHODS—
This multicenter, controlled, open, randomized, parallel-group study included 2 weeks of screening followed by 24 weeks of treatment. Randomization (1:1) was balanced by metformin administration, baseline number of insulin injections, injection with pen versus syringe and vial, and study center. The study complied with the International Council on Harmonization E6 guideline of May 1996 and with the ethical principles of the Declaration of Helsinki. Institutional review boards approved the protocol and study documents. All patients provided informed written consent.
Participants were aged 18–70 years, had type 2 diabetes for ≥6 months, had A1C of 7–10% at screening, and had taken ≥2 insulin injections/day (36% taking 2 injections and 64% taking >2 injections) with or without metformin (one-third were taking metformin) for ≥3 months before study entry. Upon entry into the study, 37% were using glargine and at least one injection of a rapid-acting insulin analog, 36% were using a premixed insulin, and the remainder were using a mix of various other regimens. Reasons for exclusion were treatment with oral antidiabetic drugs (except metformin) within 3 months before study entry; pregnancy planning, pregnancy, or lactation; serum creatinine ≥1.5 mg/dl in men (≥1.4 mg/dl in women) taking metformin and >3.0 mg/dl for any subject; clinically significant renal disease (other than proteinuria); hepatic disease; New York Heart Association class III–IV heart failure; or any disease or condition that might interfere with study completion.
Treatments
Targets were fasting blood glucose <95 mg/dl, preprandial (before lunch and dinner) blood glucose <100 mg/dl, and bedtime blood glucose <130 mg/dl.
Insulin glargine dosing.
The initial insulin glargine dose was calculated as 50% of the prerandomization total daily insulin dose. Subsequently, dosing was titrated weekly according to the mean of the last 3 days of fasting self-monitored blood glucose (SMBG) (Table 1). A dose increase could be split into ≥2 increments over the week.
Table 1—
Insulin glargine and insulin glulisine dose adjustment based on pattern of mealtime blood glucose values for the past week
| Insulin glargine adjustments: both groups
| |
|---|---|
| Mean of last 3-day fasting SMBG mg/dl | Adjustment |
| >180 mg/dl | Increase 8 units |
| 140–180 mg/dl | Increase 6 units |
| 120–139 mg/dl | Increase 4 units |
| 95–119 mg/dl | Increase 2units |
| 70–94 mg/dl | No change |
| <70 mg/dl | Decrease by the same number of units as insulin glulisine increase that titration week or up to 10% of total insulin glargine dose |
| Insulin glulisine adjustments: simple algorithm group
| ||
|---|---|---|
| Mealtime dose | Pattern of mealtime blood glucose values below target* | Pattern of mealtime blood glucose values above target† |
| ≤10 units | Decrease by 1 unit | Increase by 1 unit |
| >11–19 units | Decrease by 2 units | Increase by 2 units |
| ≥20 units | Decrease by 3 units | Increase by 3 units |
| Insulin glulisine adjustments: carbohydrate counting (insulin-to-carbohydrate ratio) group‡
| ||
|---|---|---|
| Mealtime dose | Pattern of mealtime blood glucose values below target* | Pattern of mealtime blood glucose values above target† |
| 1 unit/20 g | Decrease to 1 unit/25 g | Increase to 1 unit/15 g |
| 1 unit/15 g | Decrease to 1 unit/20 g | Increase to 1 unit/10 g |
| 1 unit/10 g | Decrease to 1 unit/15 g | Increase to 2 units/15 g |
| 2 units/15 g | Decrease to 1 unit/10 g | Increase to 3 units/15 g |
| 3 units/15 g§ | Decrease to 2 units/15 g | Increase to 4 units/15 g |
If more than one-half of the mealtime blood glucose values for the week were below target.
If more than one-half of the mealtime blood glucose values for the week were above target.
Each patient in the carb count group was also given a schedule for a mealtime insulin glulisine correction dose to add a few units if high or subtract a few units if low. §Increase mealtime insulin as needed following this pattern.
Insulin glulisine dosing.
The remaining 50% of the total daily insulin dose was used for mealtime insulin glulisine, which was split to cover three meals: 50% for the largest (most carbohydrate) meal, 33% for the middle-sized meal, and 17% for the smallest meal. Glulisine dose adjustment for both groups was based on prelunch/dinner and bedtime (these three time points are referred to as mealtime) blood glucose patterns from the previous week as shown in Table 1. Simple algorithm patients' premeal doses were set weekly, based on the algorithm in Table 1. Study staff taught the carbohydrate counting (carb count) group about carbohydrate counting and how to use an insulin-to-carbohydrate ratio. The insulin-to-carbohydrate ratios for each meal were determined based on the algorithm in Table 1. An insulin-to-carbohydrate ratio allows patients to adjust their insulin based on the amount of carbohydrate they choose to eat at a meal.
Additional antidiabetic medications.
Patients taking metformin at randomization continued using it at the same dose. No other insulin or oral antidiabetic agents were permitted.
Dietary and lifestyle recommendations
Educational materials were specially designed for this study based on the International Diabetes Center Type 2 Diabetes BASICS client book (12). The materials for the simple algorithm group omitted all references to carbohydrate intake except in the context of treating hypoglycemia and having carbohydrate if alcohol was being consumed.
Patient diaries and study visits
Diaries.
All patients recorded SMBG before meals and at bedtime, insulin doses, food and estimated carbohydrate intake per meal (carb count group), information related to hypoglycemia, activity level, and a 7-point blood glucose profile at weeks 0, 12, 18, and 24.
Visits.
Study visits occurred at screening, at baseline, and at weeks 2, 6, 12, 18, and 24 (end point). Evaluations included physical examinations, vital signs, electrocardiogram, A1C, hematology and chemistry laboratory tests, and diary review. Adverse events, hypoglycemic episodes, and concomitant medications were recorded. There was weekly contact either by a study visit or a phone call to review diaries and adjust insulin doses.
Efficacy and safety variables
Efficacy.
The primary end point was the change in A1C from baseline to week 24. Secondary variables were change in A1C from baseline to individual study time points; changes from baseline to week 24 in fasting plasma glucose (FPG); preprandial and postprandial blood glucose; 7-point blood glucose profile; average basal, bolus, and total insulin doses; lipids; percentages of patients achieving A1C <7.0 and <6.5% at week 24; and weight gain.
Safety.
All adverse events were recorded. Clinical chemistry and hematology values and physical examination results, including weight and vital signs, were documented.
Hypoglycemia
Severe hypoglycemia was defined as requiring assistance and involved either SMBG <36 mg/dl or treatment with oral carbohydrates, intravenous glucose, or glucagon, with a prompt response to that therapy. Symptomatic hypoglycemia was also documented.
Data analysis
Populations.
The safety population included patients who took ≥1 dose of study medication and had any follow-up information. The intent-to-treat (ITT) population included patients evaluated for safety who had baseline and an on-therapy observation for ≥1 efficacy variable but excluded patients who never received treatment or who were treated but had no postbaseline efficacy assessments.
Sample size.
The noninferiority hypothesis was to be tested; a study with 86 evaluable subjects per treatment arm would have 90% power to detect treatment differences of 0.5%. The SD of σ = 1.0 used in the power computations corresponds to the 95% upper confidence limit of the SD of the A1C change from a previous insulin glulisine trial.
Statistical methodology.
A mixed-model repeated-measures analysis including covariates baseline A1C, number of daily injections before the study (2 or >2), metformin use at randomization, injection method (pen or vial), and study site provided adjusted estimates and changes from baseline by visit for weeks 2, 6, 12, 18, and 24 for A1C, FPG, 7-point blood glucose profile, basal and bolus insulin doses, lipids, weight, and BMI. Percentages of patients achieving A1C <7.0 and <6.5% were analyzed by logistic regression that included treatment arm, baseline A1C, and other randomization factors. A Poisson regression model, incorporating overdispersion, was used to analyze the rate of hypoglycemia, and a logistic regression model was used to analyze incidence of hypoglycemia.
RESULTS—
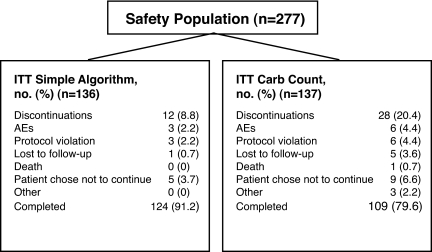
Of 281 patients randomized, 273 comprised the ITT population (Table 2) (136 simple algorithm and 137 carb count) (Fig. 1). Forty ITT patients (12 simple algorithm and 28 carb count) discontinued treatment.
Table 2—
Demographic and clinical characteristics and prior insulin treatment in the ITT population
| Characteristic | Simple algorithm | Carb count | P value |
|---|---|---|---|
| n | 136 | 137 | |
| Age (years) | 55.1 ± 8.8 (29–70) | 55.0 ± 9.5 (28–71) | 0.8026 |
| Sex | |||
| Male | 53 (39.0) | 67 (48.9) | 0.2468 |
| Female | 83 (61.0) | 70 (51.1) | |
| Race | |||
| White | 111 (81.6) | 109 (79.6) | 0.2776 |
| Black | 15 (11.0) | 15 (10.9) | |
| Asian/Oriental | 2 (1.5) | 0 (0.0) | |
| Multiracial | 0 (0.0) | 1 (0.7) | |
| Other | 8 (5.9) | 12 (8.8) | |
| Height (cm) | 169 ± 10.6 (146–198) | 170 ± 9.8 (150–193) | 0.4560 |
| Weight (kg) | 107 ± 24.2 (61–187) | 103 ± 21.7 (52–171) | 0.1217 |
| BMI (kg/cm2) | 37.7 ± 8.1 (21–63) | 35.6 ± 7.2 (17–60) | 0.0416 |
| A1C (%) | 8.1 ± 0.9 (7–10) | 8.3 ± 0.9 (6–11) | 0.0825 |
| FPG (mg/dl) | 162 ± 58.2 (49–306) | 163 ± 54.2 (52–341) | 0.8112 |
| Age at onset (year) | 42.8 ± 10.6 (13–66) | 42.4 ± 9.6 (14–63) | 0.8594 |
| Diabetes duration (years) | 12.9 ± 7.7 (0–40) | 13.0 ± 7.8 (0–36) | 0.9055 |
| Has subject used a pen for insulin administration? | |||
| No | 66 (48.5) | 62 (45.3) | 0.7788 |
| Yes | 69 (50.7) | 75 (54.7) | |
| Number of injections at randomization | |||
| 2 per day | 43 (31.6) | 57 (41.6) | 0.0211 |
| >2 per day | 93 (68.4) | 80 (58.4) | |
| Metformin used at randomization | |||
| No | 90 (66.2) | 89 (65.0) | 0.5793 |
| Yes | 46 (33.8) | 48 (35.0) |
Data are means ± SD (range) or n (%).
Figure 1—
Disposition of patients. AEs, adverse events.
Primary efficacy analysis
For the primary efficacy analysis, ANCOVA was used to compare change from baseline in A1C at week 24 after adjustment for baseline A1C. Noninferiority of the simple algorithm compared with carb count was established because the mean A1C improved in both treatment arms to a similar degree (simple algorithm 1.46% decrease; carb count 1.59% decrease), and the 95% confidence bounds on the mean difference were well within the noninferiority margin of 0.5% specified in the study protocol (week 24: carb count − simple algorithm = −0.13%) with 95% confidence bounds (−0.35 to 0.09%).
Secondary efficacy analyses
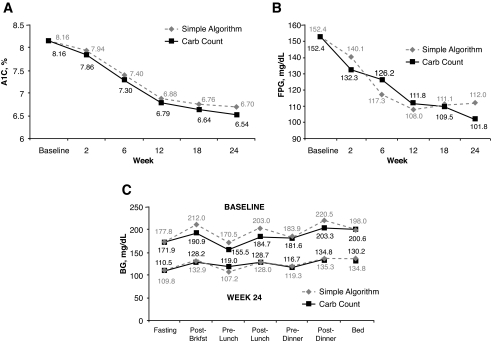
A1C levels at week 24 were 6.70% (simple algorithm) and 6.54% (carb count) (Fig. 2A); at each time point, changes from baseline were statistically significant with both treatments (P < 0.0001). By 12 weeks both groups had achieved an average A1C of <7.0%. At the end point, 73.0% (simple algorithm) and 69.2% (carb count) of patients had A1C <7.0% (P = 0.70); respective values for A1C <6.5% were 44.3 and 49.5% (P = 0.28).
Figure 2—
A: A1C: change from baseline in simple algorithm and carb count groups at weeks 2, 6, 12, 18, and 24 (ITT population). B: FPG: change from baseline in simple algorithm and carb count groups at weeks 2, 6, 12, 18, and 24 (ITT population). C: Glucose profiles from 7-point SMBG testing at baseline and week 24 in simple algorithm and carb count groups (ITT population).
By week 24, both arms had significantly improved FPG adjusted means from baseline (simple algorithm 112.0 mg/dl; carb count 101.8 mg/dl; P < 0.0001 for both). The change from baseline was −40.4 mg/dl in the simple algorithm group patients and −50.6 mg/dl in the carb count group patients (P = 0.059) (Fig. 2B). By 12 weeks the average FPG was 108 mg/dl (simple algorithm) and 112 mg/dl (carb count). Blood glucose values at each visit declined in both arms, and the within-group change from baseline was statistically significant over all daily time points and study visits (Fig. 2C).
Changes in insulin doses
At baseline, the mean insulin glulisine, insulin glargine, and total insulin doses were 53.9, 53.9, and 107.8 units (simple algorithm) and 50.5, 50.5, and 100.9 units (carb count), respectively. At week 24, the adjusted mean insulin glulisine (P = 0.0011), insulin glargine (P < 0.0001), and total insulin doses (P = 0.0002) were significantly higher in simple algorithm than in carb count group patients (108.7, 102.5, and 207.4 units vs. 88.9, 86.4, and 175.5 units, respectively). At 24 weeks, the total insulin dose was 1.9 units/kg (simple algorithm group) and 1.7 units/kg (carb count group) (see Table 3 of the online appendix available at http://dx.doi.org/10.2337/dc07-2137).
Lipids
Adjusted mean total cholesterol decreased slightly in both groups from baseline to week 24. A significant decrease was observed only for week 12 among the carb count group patients: from 175.0 to 168.5 mg/dl (−8.35 mg/dl; P < 0.01). Neither HDL nor LDL cholesterol changed significantly from baseline to week 24 in either group; no between-group differences were observed at week 12 or 24. Triglycerides decreased significantly from baseline to week 12 in both the carb count (144.0 to 128.5 mg/dl) and simple algorithm (164.7 to 148.3 mg/dl) groups (−18.27 mg/dl, P < 0.0001 and −15.14 mg/dl, P < 0.004, respectively). There was also a significant reduction in triglycerides from baseline to week 24 in the carb count (144.0 to 133.0 mg/dl) group but not in the simple algorithm (164.7 to 153.4 mg/dl) group (−13.19 mg/dl, P = 0.008 and −8.19 mg/dl P = 0.170, respectively).
Weight and BMI
Both groups gained weight at 24 weeks: simple algorithm 3.6 kg (3.4%) and carb count 2.4 kg (2.3%) (P = 0.06 for the between-group difference at week 24). Both groups showed a small but significant increase in BMI at 24 weeks: simple algorithm 1.28 kg/m2 and carb count 0.83 kg/m2 (both P < 0.0001 vs. baseline; P = 0.037 between groups).
Safety
Adverse events.
The population used to determine adverse events and hypoglycemia came from the safety population. Overall, 102 (73.9%) simple algorithm and 98 (70.5%) carb count group patients reported ≥1 treatment-emergent adverse events. In both groups, the most common adverse events were upper respiratory tract infection (simple algorithm 17.4%; carb count 10.8%), nasopharyngitis (8.7% vs. 5.8%, respectively), sinusitis (8.0% vs. 6.5%), and influenza (5.1% vs. 6.5%). Of 42 serious adverse events, 41 were nonfatal: 22 (15.9%) in the simple algorithm group and 19 (13.7%) in the carb count group. One death due to myocardial infarction occurred in the carb count group. Adverse events caused six (4.3%) patients in the carb count group and three (2.2%) patients in the simple algorithm group to discontinue treatment.
Hypoglycemia.
The simple algorithm group had 53 episodes of severe hypoglycemia in 19 patients, and the carb count group had 37 episodes in 19 patients, leading to estimates of 0.89 and 0.67 events/patient-year for the two groups (P = 0.58). SMBG <70 mg/dl with symptoms was not statistically significant between the two groups (P = 0.08). However, SMBG <50 mg/dl with symptoms was slightly but statistically significantly more common in the carb count group than in the simple algorithm group (8.0 vs. 4.9 events/patient-year, P = 0.02).
Clinical and laboratory examinations.
Changes from baseline were minor and not clinically significant.
CONCLUSIONS—
This is one of the few randomized controlled trials to evaluate basal-bolus analog insulin therapy in obese patients with type 2 diabetes and one of the first studies to evaluate the use of carbohydrate counting in patients with type 2 diabetes. Using a simple algorithm to adjust mealtime insulin glulisine each week based on SMBG patterns was as effective as adjusting mealtime insulin using insulin-to-carbohydrate ratios. Both approaches yielded a reduction of about 1.5% in A1C with no significant differences in mean A1C change from baseline or in the percentage of patients achieving A1C goals of <6.5% (13) or <7.0% (14). Both regimens were well tolerated. The risk for severe hypoglycemia was low and not significantly different between groups.
Other studies (15,16) have shown that patients whose diabetes is well-controlled with insulin often have a basal:bolus insulin ratio close to 50%:50%. At week 24, after multiple weekly titrations of insulin based on SMBG patterns, both the simple algorithm and the carb count groups had basal-bolus insulin ratios of ∼49%:51%. In addition, at the end of the study, the bolus insulin dose was split between breakfast, lunch, and dinner: ∼27, 35, and 38% in the simple algorithm group and 25, 34, and 41% in the carb count group, respectively. At week 24, both groups required large total daily insulin doses (simple algorithm 1.9 units/kg and carb count 1.7 units/kg). The lower total insulin doses for the carb count group patients may reflect more matching of insulin doses to carbohydrate intake at each meal as opposed to the set meal doses of those in the simple algorithm group.
In a recent study comparing three approaches to starting a single type of analog insulin in patients with type 2 diabetes (basal, biphasic premixed, and prandial), Holman et al. (17) concluded that although each regimen improved glucose control, most patients were likely to need more than one type of insulin to achieve target glucose levels. The percentages of patients who achieved the target A1C of the study (<6.5%) were 8.1% basal, 17.0% biphasic, and 23.9% prandial. Although one cannot directly compare insulin regimens from different study populations, it seems that diabetes in our study population would be more difficult to control because patients had a longer duration of diabetes and were more obese than subjects in the trial of Holman et al. In the study reported here, however, patients with type 2 diabetes using basal:bolus therapy achieved an A1C <6.5% almost half of the time (simple algorithm 44.3% and carb count 49.5%).
Although basal-bolus insulin therapy can improve glycemic control in type 1 (6,18) and type 2 (19,20) diabetes, the most effective use of SMBG for adjusting the basal-bolus insulin doses has not been clearly established (21–24). Some feel that postprandial monitoring is critical to establishing good glycemic control, whereas others are not convinced that postprandial testing is essential (25). In the present study, we showed that patients with type 2 diabetes using fasting and mealtime SMBG testing alone to monitor and adjust their rapid-acting and long-acting insulin analogs achieved excellent glucose control as measured by A1C, with minimal severe hypoglycemia.
The key elements of successful insulin therapy are optimizing glycemic control while minimizing hypoglycemia. Patients can learn to adjust mealtime insulin by either using a simple algorithm or learning to use insulin-to-carbohydrate ratios. Insulin-to-carbohydrate ratios allow flexibility in food choices and enable relatively precise matching of mealtime insulin needs but can seem complex and may be difficult for some patients to implement (10,11). We have shown that a standard mealtime insulin glulisine dose adjusted weekly on the basis of preprandial blood glucose patterns can achieve the same goals as a regimen that adjusts mealtime insulin based on insulin-to-carbohydrate ratios. It may be that our simple algorithm group patients either consumed fairly consistent amounts of carbohydrates, thus minimizing needed changes in insulin dosing, or learned to modify their carbohydrate intake based on SMBG measurements.
In summary, having two effective approaches to deliver and adjust rapid-acting mealtime insulin (a simple algorithm and insulin-to-carbohydrate ratio) may increase patients' and clinicians' willingness to undertake basal:bolus insulin therapy, a step that is often needed to achieve optimal glucose control in type 2 diabetes.
Acknowledgments
Editorial support was provided through the sanofi-aventis US Group.
The authors acknowledge David M. Kendall, MD, and Poul Strange, MD, PhD, for their contributions to the protocol design; Michael Miller, PhD, for statistical analysis; Elizabeth Lee, MD, for overall study management; all of the study site investigators; Kelli Bradbury, ARNP-C; Gary Graf, ARNP-C; all the study coordinators; and Mary Ann Carr for her expert manuscript preparation assistance.
Published ahead of print at http://care.diabetesjournals.org on 25 March 2008.
Clinical trial reg. no. NCT00135057, clinicaltrials.gov.
R.M.B. receives grant funds from, serves on an advisory panel for, and is a consultant for sanofi-aventis. All financial compensation goes to Park Nicollet for research and education. P.H. receives grant funds from and serves on the speakers bureau and advisory panel for sanofi-aventis.
The costs of publication of this article were defrayed in part by the payment of page charges. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C Section 1734 solely to indicate this fact.
See accompanying editorial, p. 1467.
References
- 1.Home P: Contributions of basal and post-prandial hyperglycaemia to micro- and macrovascular complications in people with type 2 diabetes. Curr Med Res Opin 21:989–998, 2005 [DOI] [PubMed] [Google Scholar]
- 2.Heine RJ, Balkau B, Ceriello A, Del Prato S, Horton ES, Taskinen M-R: What does postprandial hyperglycaemia mean? Diabet Med 21:208–213, 2004 [DOI] [PubMed] [Google Scholar]
- 3.Ceriello A: Postprandial hyperglycemia and diabetes complications: is it time to treat? Diabetes 54:1–7, 2005 [DOI] [PubMed] [Google Scholar]
- 4.Wright A, Burden AC, Paisey RB, Cull CA, Holman RR, UK Prospective Diabetes Study Group: Sulfonylurea inadequacy: efficacy of addition of insulin over 6 years in patients with type 2 diabetes in the UK Prospective Diabetes Study (UKPDS 57). Diabetes Care 25:330–336, 2002 [DOI] [PubMed] [Google Scholar]
- 5.Gerich JE: Novel insulins: expanding options in diabetes management. Am J Med 113:308–316, 2002 [DOI] [PubMed] [Google Scholar]
- 6.Dreyer M, Prager R, Robinson A, Busch K, Ellis G, Souhami E, Van Leendert R: Efficacy and safety of insulin glulisine in patients with type 1 diabetes. Horm Metab Res 37:702–707, 2005 [DOI] [PubMed] [Google Scholar]
- 7.Gross TM, Kayne D, King A, Rother C, Juth S: A bolus calculator is an effective means of controlling postprandial glycemia in patients on insulin pump therapy. Diabetes Technol Ther 5:365–369, 2003 [DOI] [PubMed] [Google Scholar]
- 8.Kalergis M, Pacaud D, Strychar I, Meltzer S, Jones PJ, Yale JF: Optimizing insulin delivery: assessment of three strategies in intensive diabetes management. Diabetes Obes Metab 2:299–305, 2000 [DOI] [PubMed] [Google Scholar]
- 9.DAFNE Study Group: Training in flexible, intensive insulin management to enable dietary freedom in people with type 1 diabetes: dose adjustment for normal eating (DAFNE) randomised controlled trial. BMJ 325:746–751, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Peragallo-Dittko V (Ed.): A Core Curriculum for Diabetes Education. 4th ed. Chicago, American Association of Diabetes Educators, 2001
- 11.Kaufman FR, Halvorson M, Carpenter S: Use of a plastic insulin dosage guide to correct blood glucose levels out of the target range and for carbohydrate counting in subjects with type 1 diabetes. Diabetes Care 22:1252–1257, 1999 [DOI] [PubMed] [Google Scholar]
- 12.Rickheim P, Flader J, Carstensen KM: Type 2 Diabetes BASICS. 2nd ed. Minneapolis, International Diabetes Center, 2004
- 13.American Association of Clinical Endocrinologists/American College of Endocrinology: The American Association of Clinical Endocrinologists medical guidelines for the management of diabetes mellitus: the AACE system of intensive diabetes self-management—2002 update. Endocr Pract 8:41–82, 2002 [PubMed] [Google Scholar]
- 14.American Diabetes Association: Standards of medical care in diabetes. Diabetes Care 29(Suppl. 1):S4–S42, 2006 [PubMed] [Google Scholar]
- 15.Raskin P, Klaff L, Bergenstal R, Halle JP, Donley D, Mecca T: A 16-week comparison of the novel insulin analog insulin glargine (HOE 901) and NPH human insulin used with insulin lispro in patients with type 1 diabetes. Diabetes Care 23:1666–1671, 2000 [DOI] [PubMed] [Google Scholar]
- 16.De Leeuw I, Vague P, Selam JL, Skeie S, Lang H, Draeer E, Elte JWF: Insulin detemir used in basal-bolus therapy in people with type 1 diabetes is associated with a lower risk of nocturnal hypoglycaemia and less weight gain over 12 months in comparison to NPH insulin. Diabetes Obes Metab 7:73–82, 2005 [DOI] [PubMed] [Google Scholar]
- 17.Holman RR, Thorne KI, Farmer AJ, Davies MJ, Keenan JF, Paul S, Levy JC, 4-T Study Group: Addition of biphasic, prandial, or basal insulin to oral therapy in type 2 diabetes. N Engl J Med 1716–1730, 2007 [DOI] [PubMed]
- 18.Garg SK, Rosenstock J, Ways K: Optimized basal-bolus insulin regimens in type 1 diabetes: insulin glulisine versus regular human insulin in combination with basal insulin glargine. Endocr Pract 11:11–17, 2005 [DOI] [PubMed] [Google Scholar]
- 19.Dailey G, Rosenstock J, Moses RG, Ways K: Insulin glulisine provides improved glycemic control in patients with type 2 diabetes. Diabetes Care 27:2363–2368, 2004 [DOI] [PubMed] [Google Scholar]
- 20.Rosenstock J, Ahmann AJ, Colon G, Scism-Bacon J, Jiang H, Martin S: Advancing insulin therapy in type 2 diabetes previously treated with glargine plus oral agents. Diabetes Care 31:20–25, 2008 [DOI] [PubMed] [Google Scholar]
- 21.Reichard P, Nilsson BY, Rosenqvist U: The effect of long-term intensified insulin treatment on the development of microvascular complications of diabetes mellitus. N Engl J Med 329:304–309, 1993 [DOI] [PubMed] [Google Scholar]
- 22.Diabetes Control and Complications Trial Research Group: The effect of intensive treatment of diabetes on the development and progression of the long-term complications of insulin-dependent diabetes mellitus. N Engl J Med 329:977–986, 1993 [DOI] [PubMed] [Google Scholar]
- 23.Ohkubo Y, Kishikawa H, Araki E, Isami S, Motoyoshi S, Kojima Y, Furuyoshi N, Shichiri M: Intensive insulin therapy prevents the progression of diabetic microvascular complications in Japanese patients with non-insulin-dependent diabetes mellitus: a randomized prospective 6-year study. Diabetes Res Clin Pract 28:103–117, 1995 [DOI] [PubMed] [Google Scholar]
- 24.UK Prospective Diabetes Study Group: Intensive blood-glucose control with sulphonylureas or insulin compared with conventional treatment and risk of complications in patients with type 2 diabetes (UKPDS 33). Lancet 352:837–853, 1998 [PubMed] [Google Scholar]
- 25.Buse JB: Should postprandial glucose be routinely measured and treated to a particular target? No! Diabetes Care 26:1615–1618, 2003 [DOI] [PubMed] [Google Scholar]