Abstract
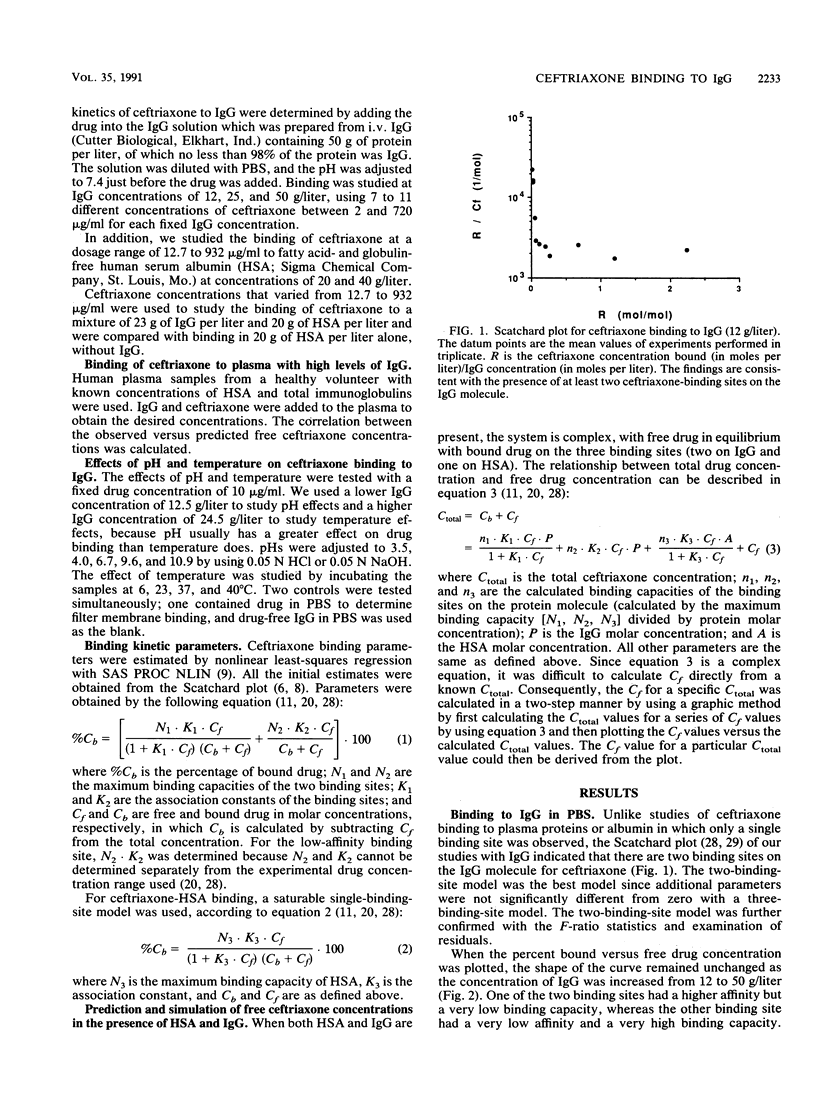
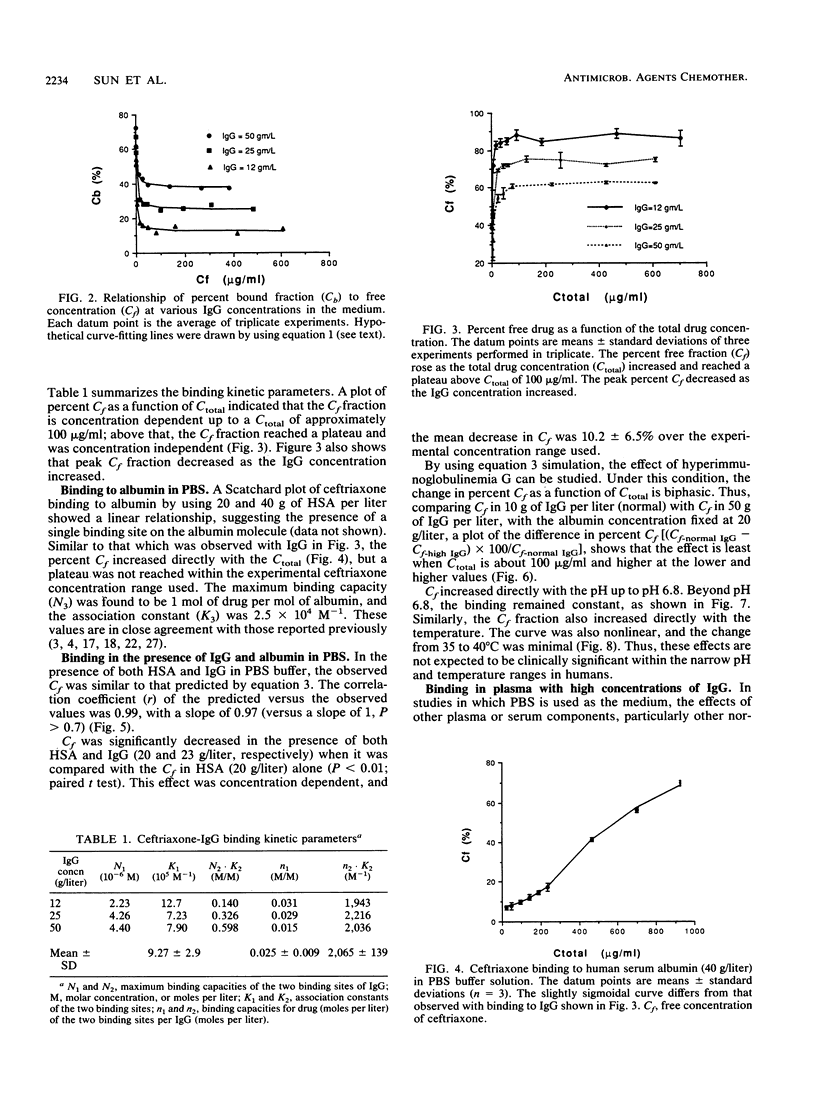
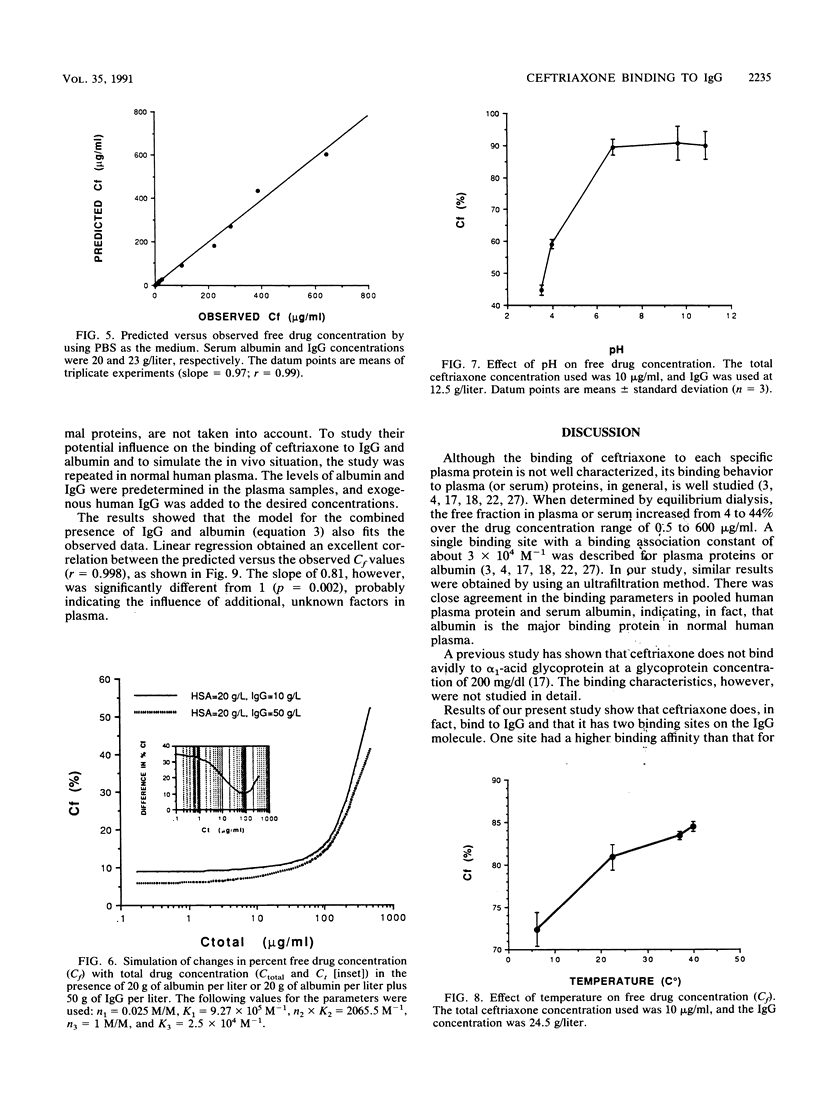
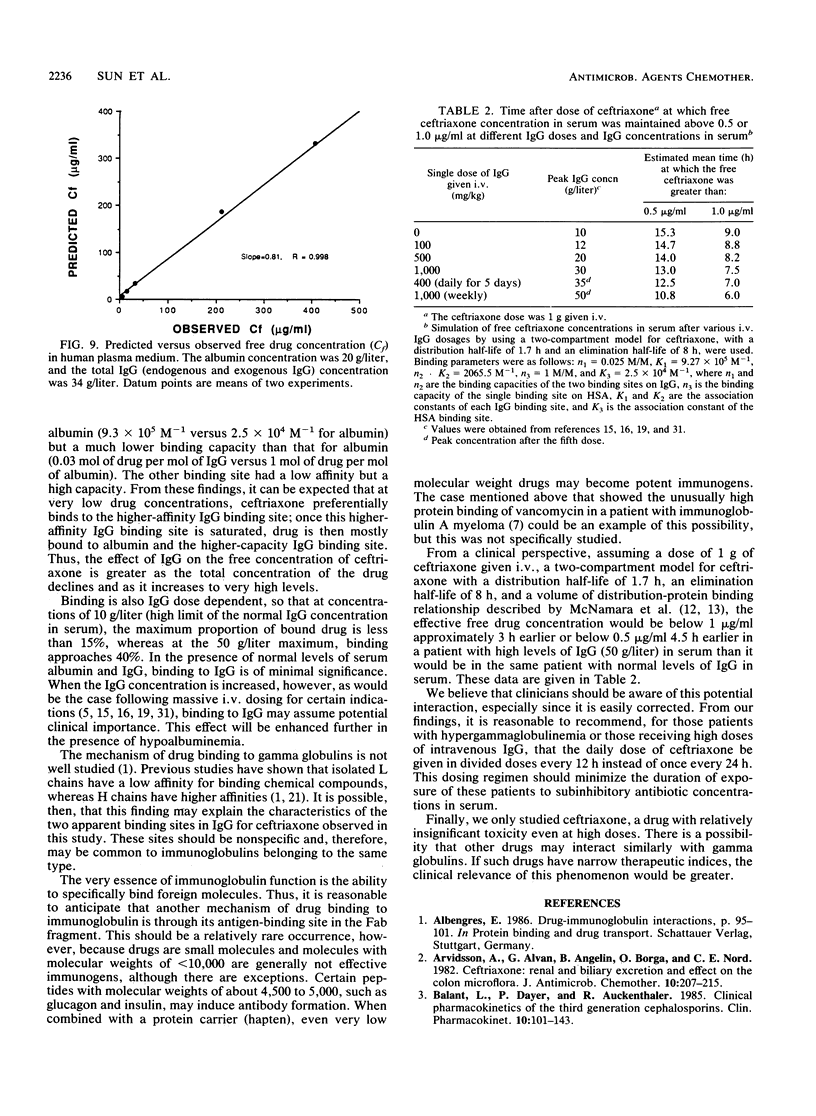
The interaction between immunoglobulin G (IgG) and ceftriaxone was studied. Using an ultrafiltration method, we performed dose ranging studies at a ceftriaxone concentration range of 1 to 720 micrograms/ml in the presence of various concentrations of human IgG, human serum albumin (HSA), and combinations of IgG and HSA at pH 7.4 and 37 degrees C. The results showed that ceftriaxone binding to IgG was nonlinear and was consistent with the presence of two binding sites that possess different binding capacities and affinities. Except for increased peak percent binding as the IgG concentration increased, the binding characteristics did not change with IgG concentration. Binding to HSA was consistent, with the presence of only one high-affinity binding site. A mathematical model based on the observed data was constructed; this model was used to predict protein binding at various concentrations of drug, IgG, HSA, or combinations of IgG and HSA in buffer and in plasma medium. Correlations between the observed versus the predicted values were excellent in both media. Simulations with the model indicated that patients with hypergammaglobulinemia have an increased potential of being exposed to prolonged subinhibitory concentrations of ceftriaxone if the drug is given once every 24 h.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Arvidsson A., Alván G., Angelin B., Borgå O., Nord C. E. Ceftriaxone: renal and biliary excretion and effect on the colon microflora. J Antimicrob Chemother. 1982 Sep;10(3):207–215. doi: 10.1093/jac/10.3.207. [DOI] [PubMed] [Google Scholar]
- Balant L., Dayer P., Auckenthaler R. Clinical pharmacokinetics of the third generation cephalosporins. Clin Pharmacokinet. 1985 Mar-Apr;10(2):101–143. doi: 10.2165/00003088-198510020-00001. [DOI] [PubMed] [Google Scholar]
- Balant L., Fabre J. Clinical relevance of different electrophoretic methods for the analysis of urinary proteins. Curr Probl Clin Biochem. 1979;(9):216–234. [PubMed] [Google Scholar]
- Berkman S. A., Lee M. L., Gale R. P. Clinical uses of intravenous immunoglobulins. Ann Intern Med. 1990 Feb 15;112(4):278–292. doi: 10.7326/0003-4819-112-4-278. [DOI] [PubMed] [Google Scholar]
- Cantú T. G., Dick J. D., Elliott D. E., Humphrey R. L., Kornhauser D. M. Protein binding of vancomycin in a patient with immunoglobulin A myeloma. Antimicrob Agents Chemother. 1990 Jul;34(7):1459–1461. doi: 10.1128/aac.34.7.1459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jungbluth G. L., Pasko M. T., Jusko W. J. Factors affecting ceftriaxone plasma protein binding during open heart surgery. J Pharm Sci. 1989 Oct;78(10):807–811. doi: 10.1002/jps.2600781005. [DOI] [PubMed] [Google Scholar]
- Kim K. S. High-dose intravenous immune globulin impairs antibacterial activity of antibiotics. J Allergy Clin Immunol. 1989 Oct;84(4 Pt 2):579–588. doi: 10.1016/0091-6749(89)90194-2. [DOI] [PubMed] [Google Scholar]
- Luecke R. H., Wosilait W. D. Estimation of drug binding parameters. J Pharmacokinet Biopharm. 1986 Feb;14(1):65–78. doi: 10.1007/BF01059284. [DOI] [PubMed] [Google Scholar]
- McNamara P. J., Gibaldi M., Stoeckel K. Volume of distribution terms for a drug (ceftriaxone) exhibiting concentration-dependent protein binding. I. Theoretical considerations. Eur J Clin Pharmacol. 1983;25(3):399–405. doi: 10.1007/BF01037955. [DOI] [PubMed] [Google Scholar]
- McNamara P. J., Gibaldi M., Stoeckel K. Volume of distribution terms for a drug (ceftriaxone) exhibiting concentration-dependent protein binding. II. Physiological significance. Eur J Clin Pharmacol. 1983;25(3):407–412. doi: 10.1007/BF01037956. [DOI] [PubMed] [Google Scholar]
- McNamara P. J., Stoeckel K., Ziegler W. H. Pharmacokinetics of ceftriaxone following intravenous administration of a 3 g dose. Eur J Clin Pharmacol. 1982;22(1):71–75. doi: 10.1007/BF00606428. [DOI] [PubMed] [Google Scholar]
- Ochs H. D., Fischer S. H., Wedgwood R. J., Wara D. W., Cowan M. J., Ammann A. J., Saxon A., Budinger M. D., Allred R. U., Rousell R. H. Comparison of high-dose and low-dose intravenous immunoglobulin therapy in patients with primary immunodeficiency diseases. Am J Med. 1984 Mar 30;76(3A):78–82. doi: 10.1016/0002-9343(84)90324-3. [DOI] [PubMed] [Google Scholar]
- Patel I. H., Chen S., Parsonnet M., Hackman M. R., Brooks M. A., Konikoff J., Kaplan S. A. Pharmacokinetics of ceftriaxone in humans. Antimicrob Agents Chemother. 1981 Nov;20(5):634–641. doi: 10.1128/aac.20.5.634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pennock B. E. A calculator for finding binding parameters from a Scatchard plot. Anal Biochem. 1973 Nov;56(1):306–309. doi: 10.1016/0003-2697(73)90195-4. [DOI] [PubMed] [Google Scholar]
- Pirofsky B. Intravenous immune globulin therapy in hypogammaglobulinemia. A review. Am J Med. 1984 Mar 30;76(3A):53–60. doi: 10.1016/0002-9343(84)90320-6. [DOI] [PubMed] [Google Scholar]
- Priore R. L., Rosenthal H. E. A statistical method for the estimation of binding parameters in a complex system. Anal Biochem. 1976 Jan;70(1):231–240. doi: 10.1016/s0003-2697(76)80063-2. [DOI] [PubMed] [Google Scholar]
- Rosenthal H. E. A graphic method for the determination and presentation of binding parameters in a complex system. Anal Biochem. 1967 Sep;20(3):525–532. doi: 10.1016/0003-2697(67)90297-7. [DOI] [PubMed] [Google Scholar]
- Schaad U. B., Hayton W. L., Stoeckel K. Single-dose ceftriaxone kinetics in the newborn. Clin Pharmacol Ther. 1985 May;37(5):522–528. doi: 10.1038/clpt.1985.82. [DOI] [PubMed] [Google Scholar]
- Sophianopoulos J. A., Durham S. J., Sophianopoulos A. J., Ragsdale H. L., Cropper W. P., Jr Ultrafiltration is theoretically equivalent to equilibrium dialysis but much simpler to carry out. Arch Biochem Biophys. 1978 Apr 15;187(1):132–137. doi: 10.1016/0003-9861(78)90015-2. [DOI] [PubMed] [Google Scholar]
- Stoeckel K., McNamara P. J., Brandt R., Plozza-Nottebrock H., Ziegler W. H. Effects of concentration-dependent plasma protein binding on ceftriaxone kinetics. Clin Pharmacol Ther. 1981 May;29(5):650–657. doi: 10.1038/clpt.1981.90. [DOI] [PubMed] [Google Scholar]
- Stoeckel K. Pharmacokinetics of Rocephin, a highly active new cephalosporin with an exceptionally long biological half-life. Chemotherapy. 1981;27 (Suppl 1):42–46. doi: 10.1159/000238028. [DOI] [PubMed] [Google Scholar]
- Stoeckel K., Tuerk H., Trueb V., McNamara P. J. Single-dose ceftriaxone kinetics in liver insufficiency. Clin Pharmacol Ther. 1984 Oct;36(4):500–509. doi: 10.1038/clpt.1984.210. [DOI] [PubMed] [Google Scholar]
- Walter C. Graphical procedures for the detection of deviations from the classical model of enzyme kinetics. J Biol Chem. 1974 Feb 10;249(3):699–703. [PubMed] [Google Scholar]
- Whitlam J. B., Brown K. F. Ultrafiltration in serum protein binding determinations. J Pharm Sci. 1981 Feb;70(2):146–150. doi: 10.1002/jps.2600700208. [DOI] [PubMed] [Google Scholar]
- Winston D. J., Ho W. G., Lin C. H., Bartoni K., Budinger M. D., Gale R. P., Champlin R. E. Intravenous immune globulin for prevention of cytomegalovirus infection and interstitial pneumonia after bone marrow transplantation. Ann Intern Med. 1987 Jan;106(1):12–18. doi: 10.7326/0003-4819-106-1-12. [DOI] [PubMed] [Google Scholar]


