Abstract
OBJECTIVE—The hypothesis of carotenoid having a preventive role in diabetes is suggested by their antioxidant properties. In this report, we investigated the relationship between baseline total plasma carotenoid levels and 9-year onset of dysglycemia (impaired fasting glucose or type 2 diabetes) in a healthy elderly population.
RESEARCH DESIGN AND METHODS—The Epidemiology of Vascular Ageing Study is a 9-year longitudinal study including 1,389 volunteers aged 59–71 years. Fasting plasma glucose was measured at baseline and at 2, 4, and 9 years after inclusion. The relationship between plasma carotenoid at baseline and incidence of dysglycemia was determined by Cox proportional hazards regression analysis adjusting for potential confounders.
RESULTS—At 9 years, 127 incident cases of dysglycemia had occurred. Risk of dysglycemia was significantly lower in participants with plasma carotenoid in the highest quartile (Q4) compared with participants in the lowest quartile (Q1) (Q4 vs. Q1: relative risk 0.26 [95% CI 0.14–0.49], P < 10−4; Q3 vs. Q1: 0.55 [0.34–0.89], P = 0.01; and Q2 vs. Q1: 0.82 [0.51–1.31], P = 0.40). After controlling for sociodemographic variables, lifestyle habits, cardiovascular disease, blood pressure, BMI, and lipid profile, risk of dysglycemia remained significantly lower in participants in the highest quartile of total plasma carotenoid compared with participants in the lowest quartile (Q4 vs. Q1: 0.42 [0.22–0.82], P = 0.01; Q3 vs. Q1: 0.69 [0.41–1.15], P = 0.16; and Q2 vs. Q1: 0.80 [0.48–1.32], P = 0.38).
CONCLUSIONS—This study prospectively confirms that plasma carotenoid levels have an independent relationship to onset of dysglycemia.
Carotenoids are natural pigments synthesized by plants and microorganisms but not by animals or by humans. These pigments are found in food, especially in fruits and vegetables. It is highly suggested that they play a protective role in chronic diseases (1) or cancers. Even if the biological mechanisms for such a protection are currently unclear (2), their protective effects could come from their antioxidant properties (3).
Several cross-sectional (4–6) and case-control (7–9) studies have shown an inverse relationship between serum carotenes and type 2 diabetes status. A longitudinal study (10) on dietary intake of antioxidants found a significant relationship between β-cryptoxanthin intake and reduced risk of type 2 diabetes; however, the association between serum carotenoid and diabetes was called into question by the results of two other longitudinal studies (11,12) and one randomized double-blind trial (13).
To investigate if carotenoids could have a role in diabetes incidence in the elderly, possibly through their antioxidant capacity, we explored the relationships between total plasma carotenoid levels at baseline and 9-year occurrence of type 2 diabetes or impaired fasting glucose (IFG) in a healthy elderly population.
RESEARCH DESIGN AND METHODS—
The Epidemiology of Vascular Ageing (EVA) study is a 9-year longitudinal study with six waves of follow-up (14). At baseline (EVA0, 1991–1993), 1,389 volunteers (574 men and 815 women) born between 1922 and 1932 (mean age of 65 years) residing in the town of Nantes (western France) were recruited from electoral rolls and, to a lesser extent, via information campaigns. The subsequent follow-up waves with biological measurements were EVA2 (2-year follow-up, n = 1,272), EVA3 (4-year follow-up, n = 1,188), and EVA6 (9-year follow-up, n = 781). The study protocol was approved by the ethical committee of the University Center Hospital of Kremlin-Bicêtre, Paris, France. Signed informed consent was obtained from all participants at enrollment. For the purpose of the present article, analyses were carried out on 1,165 participants who were normoglycemic at inclusion, defined by a fasting blood glucose (FBG) ≤6.1 mmol/l and not being on antidiabetes drugs, and for whom plasma carotenoid measurement was available.
Blood samples were drawn between 8:30 and 9:30 a.m. after a 12-h fast. Biological procedures for the determination of glucose levels have been described elsewhere (15). According to the World Health Organization definition (16), participants with an FBG ≥7.00 mmol/l or who used antidiabetes drugs were defined as having diabetes. Participants with IFG were characterized by 6.1 > FBG < 7 mmol/l (16). Dysglycemia was defined by presence of IFG or diabetes status. During the 9-year follow-up, cumulative incidence of dysglycemia was considered. To account for the reversibility of IFG, we defined persistent IFG as having dysglycemia at least two times on the three follow-up waves.
At baseline, the general questionnaire allowed us to obtain information on sociodemographic factors (sex, age, and education) and lifestyle habits (smoking habits and alcohol intake). In addition, height and weight were measured. Two independent measures of systolic and diastolic blood pressure were taken with a digital electronic tensiometer after a 10-min rest. Health characteristics considered in these analyses were BMI, total-to-HDL cholesterol ratio, use of lipid-lowering drugs, hypertension (systolic or diastolic blood pressure ≥140 or ≥90 mmHg, respectively, or use of hypertensive drugs), and history of vascular diseases (self-reported history of myocardial infarction, angina pectoris, or stroke).
Total plasma carotenoid levels were determined only at baseline by using a spectrophotometric assay. Samples were immediately frozen at −20°C then stored at −80°C, and all the determinations were done at the same time (2 years after the first inclusion). After precipitation of plasma proteins with ethanol, carotenoids were extracted with hexane and measurements of absorbance on the hexane phase at 350, 450, and 550 nm were performed (spectrophotometer Uvikon 860; Kontron, Rotkreuz, Switzerland). Concentrations were calculated on the basis of a molecular extinction factor at 450 nm of 134,000 l · mol−1 · cm−1. Absorbance values at 350 and 550 nm were used to correct the absorbance obtained at 450 nm by applying an adequate equation. Coefficients of intra- and interassay variation were 5.4 and 4.9%, respectively.
Statistical methods
Sex, education (primary school or lower/high school or higher), smoking status (current, ex-smokers, or nonsmokers), alcohol intake (≥20 ml/<20 ml per day), cardiovascular disease antecedents (yes/no), and use of lipid-lowering drugs (yes/no) were considered as categorical variables. Age, BMI, diastolic and systolic blood pressure, and the total-to-HDL cholesterol ratio were considered as continuous variables. The total plasma carotenoid level was considered by quartile. The median and range values for each quartile were 1.42 μmol/l (0.21–1.82) for Q1 (<25th), 2.16 μmol/l (1.83–2.53) for Q2 (≥25th, <50th), 2.90 μmol/l (2.55–3.43) for Q3 (≥50th, <75th), and 4.14 μmol/l (3.44–10.1) for Q4 (≥75th). By using the Student's t test (for continuous variables) and the χ2 test (for categorical variables), baseline characteristics were compared between participants who developed dysglycemia during the follow-up and those who did not and between participants who completed the 9-year follow-up and those who did not. Survival analyses by actuarial methods were used to assess the probability of not developing dysglycemia according to levels of plasma carotenoids. The effects of total plasma carotenoid levels on onset of dysglycemia were determined by Cox proportional hazards regression models, in which age (in years) during the study was used as the time axis, with left truncation at age of study entry. To take into account the reversibility of IFG, additional sensitivity analyses were performed after considering persistent IFG. To explore whether relationships with carotenoids could be confounded by their antioxidant capacity, similar analyses were performed on participants (n = 875) for whom different markers of antioxidant status, such as thiobarbiturate reactive substances, vitamin E, activity of glutathione peroxydases, and superoxyde dismutase, were measured as baseline (method described in Berr et al. [15]). The proportional hazards assumption was verified by adding a time-dependent variable to the model. Results of Cox multivariate regressions were expressed by hazard ratio (95% CI). All interactions between total plasma carotenoids and other variables were tested. Statistical analyses were performed using SAS software version 9.1 (SAS Institute, Cary, NC).
RESULTS—
During the 9-year follow-up, 127 new cases of dysglycemia (including 29 cases of type 2 diabetes) occurred. Sixty-four cases (including nine cases of diabetes) occurred during the first 2 years of follow-up (EVA2, baseline). Forty cases (including 10 cases of diabetes) occurred between EVA2 and EVA3, and 23 cases (including 10 of diabetes) occurred between EVA3 and EVA6. Characteristics of the 635 participants who completed the 9-year follow-up were compared with the 530 who did not (including 101 deaths). Participants who did not complete the whole study were more likely to be current or former smokers (43.4 vs. 37.5%, P = 0.04), to have higher baseline diastolic blood pressure (79.5 ± 11.0 vs. 78. 2 ± 10.7 mmHg, P = 0.03), and have lower baseline total plasma carotenoid levels (2.71 ± 1.23 vs. 2.91 ± 1.32 μmol/l, P = 0.008).
Among the 127 participants who developed a dysglycemia during the 9-year follow-up, the proportion of men, current or former smokers, and regular consumers of alcohol was significantly higher compared with the 1,038 participants who did not develop dysglycemia (Table 1). A significantly higher BMI and diastolic and systolic blood pressure and lower HDL cholesterol concentration at baseline were also observed in the dysglycemic group. No significant difference was observed in baseline total cholesterol, proportion of lipid-lowering drug users, cardiovascular diseases history, and age between the two groups. Bivariate analyses also showed that total baseline plasma carotenoid levels were significantly lower in participants who developed dysglycemia compared with those who did not (Table 1).
Table 1—
Comparison of baseline characteristics between participants who developed dysglycemia during the 9-year follow-up and those who did not: results of bivariate analyses
| Participants
|
P value | ||
|---|---|---|---|
| Did not develop dysglycemia | Developed dysglycemia | ||
| n | 1,038 | 127 | |
| Sociodemographic factors | |||
| Women | 63.9 | 44.7 | <10−4 |
| Age at baseline (years) | 65.0 ± 3.0 | 64.9 ± 3.1 | 0.72 |
| Higher education* | 48.5 | 47.1 | 0.78 |
| Consumption factors | |||
| Current/former smokers | 38.7 | 52.8 | 0.002 |
| Alcohol consumer (>20 ml/day)* | 26.0 | 39.2 | 0.002 |
| Health factors | |||
| BMI (kg/m2)* | 24.7 ± 3.4 | 27.0 ± 3.7 | <10−4 |
| HDL cholesterol (mmol/l)* | 1.69 ± 0.43 | 1.50 ± 0.42 | <10−4 |
| Total cholesterol (mmol/l)* | 6.43 ± 1.01 | 6.30 ± 0.98 | 0.17 |
| Total-to-HDL cholesterol ratio* | 4.03 ± 1.15 | 4.49 ± 1.45 | 0.01 |
| Lipid-lowering drugs user | 22.8 | 27.6 | 0.23 |
| Systolic blood pressure (mmHg)* | 130.0 ± 17.6 | 136.4 ± 16.0 | 0.0001 |
| Diastolic blood pressure (mmHg)* | 78.4 ± 10.8 | 82.0 ± 11.1 | 0.0006 |
| Cardiovascular disease antecedents | 8.6 | 8.9 | 0.91 |
| Total plasma carotenoid (μmol/l) | 2.87 ± 1.29 | 2.37 ± 1.10 | <10−4 |
Data are means ± SD or percent.
Analyses were performed on 1,164 participants for education level, 1,144 for alcohol consumption, 1,164 for blood pressure, 1,162 for BMI, 1,121 for LDL cholesterol, 1,130 for HDL cholesterol, and 1,139 for total cholesterol.
Factors associated with total plasma carotenoids at baseline are described in Table 2. A higher proportion of women were found in higher quartiles of total plasma carotenoids, and a higher percentage of smokers and alcohol consumers were observed in lower quartiles. BMI, diastolic and systolic blood pressure, and cardiovascular disease history decreased with higher levels of plasma carotenoids. High levels of carotenoids were associated with an increase of total cholesterol and a decrease of HDL cholesterol, and there was a higher proportion of lipid-lowering drug users in low quartiles of plasma carotenoid levels.
Table 2—
Factors associated with total plasma carotenoids at baseline
| Plasma carotenoid quartiles
|
P trend | ||||
|---|---|---|---|---|---|
| Quartile 1 (<1.82 μmol/l) | Quartile 2 (1.82–2.55 μmol/l) | Quartile 3 (2.55–3.43 μmol/l) | Quartile 4 (≥3.43 μmol/l) | ||
| Sociodemographic factors | |||||
| Women | 40.1 | 52.5 | 65.8 | 83.2 | <10−4 |
| Age at baseline (years) | 64.7 ± 2.9 | 65.3 ± 2.8 | 65.0 ± 3.1 | 64.8 ± 3.0 | 0.06 |
| Higher education | 43.0 | 49.3 | 50.8 | 49.4 | 0.28 |
| Consumption factors | |||||
| Current/former smokers | 56.2 | 48.2 | 37.9 | 22.5 | <10−4 |
| Alcohol consumer (>20 ml/day) | 41.2 | 33.2 | 24.7 | 14.1 | <10−4 |
| Health factors | |||||
| BMI (kg/ m2) | 26.5 ± 3.7 | 25.4 ± 3.4 | 24.6 ± 3.3 | 23.6 ± 3.0 | <10−4 |
| HDL cholesterol (mmol/l) | 1.55 ± 0.41 | 1.56 ± 0.35 | 1.71 ± 0.42 | 1.82 ± 0.46 | <10−4 |
| Total cholesterol (mmol/l) | 5.98 ± 0.99 | 6.23 ± 0.91 | 6.47 ± 0.91 | 6.86 ± 1.01 | <10−4 |
| Total-to-HDL cholesterol ratio | 4.10 ± 1.13 | 4.22 ± 1.37 | 4.01 ± 1.09 | 4.00 ± 1.17 | 0.09 |
| Lipid-lowering drugs user | 32.5 | 26.9 | 20.4 | 15.9 | <10−4 |
| Diastolic blood pressure (mmHg) | 81.2 ± 11.8 | 79.8 ± 11.0 | 77.9 ± 10.8 | 76.9 ± 9.7 | <10−4 |
| Systolic blood pressure (mmHg) | 133.4 ± 18.1 | 134.2 ± 18.0 | 130.0 ± 18.0 | 126.2 ± 15.1 | <10−4 |
| Cardiovascular disease antecedents | 13.2 | 11.7 | 4.70 | 6.3 | 0.0004 |
Data are means ± SD or percent.
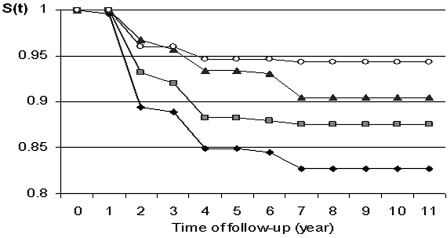
Comparisons of survival distributions between quartiles of plasma carotenoids showed that the lower the quartile the greater the occurrence of dysglycemia (Fig. 1). The analysis showed that risk of dysglycemia decreased significantly in participants with plasma carotenoids in Q4 and Q3 compared with participants in Q1 (Q4 vs. Q1: relative risk 0.26 [95% CI 0.14–0.49], P < 10–4; Q3 vs. Q1: 0.55 [0.34–0.89], P = 0.01; and Q2 vs. Q1: 0.82 [0.51–1.31], P = 0.40). Interactions tested between plasma carotenoids and different characteristics were not statistically significant. Results of multivariate Cox models (Table 3), after controlling for sociodemographic factors, smoking habits, alcohol intake, cardiovascular disease history, blood pressure, BMI, and lipid profile, showed that risk of dysglycemia during the 9-year follow-up remained significantly lower in participants in the highest quartile of total plasma carotenoids compared with participants in the lowest quartile (Q4 vs. Q1: 0.42 [0.22–0.82], P = 0.01). The other adjusted risks did not remain significant (Q3 vs. Q1: 0.69 [0.41–1.15], P = 0.16; and Q2 vs. Q1: 0.80 [0.48–1.32], P = 0.38). To take into account reversibility of IFG, sensitivity analyses with persistent IFG as an end point were performed and showed a similar graded association between quartiles of carotenoids and dysglycemia (data not shown).
Figure 1—
Nonoccurrence of dysglycemia for each total plasma carotenoid quartile group. Q1: <1.82 μmol/l, Q2: 1.82–2.55 μmol/l, Q3: 2.55–3.43 μmol/l, Q4: 3.43 μmol/l. ♦, Q1;  , Q2; ▴, Q3; ○, Q4.
, Q2; ▴, Q3; ○, Q4.
Table 3—
Effects of total plasma carotenoids on onset of dysglycemia during the EVA study follow-up: results of multivariate Cox proportional hazards regression analyses
| Complete model | Multivariate models*
|
|
|---|---|---|
| Hazard ratio (95% CI) | P | |
| Total plasma carotenoids (μmol/l) | ||
| Q4 vs. Q1 | 0.42 (0.22–0.82) | 0.01† |
| Q3 vs. Q1 | 0.69 (0.41–1.15) | 0.16† |
| Q2 vs. Q1 | 0.80 (0.48–1.32) | 0.38† |
| Sex | 0.90 (0.53–1.54) | 0.71 |
| Education (high versus low level) | 0.91 (0.62–1.34) | 0.63 |
| Smoking habits (current/former versus nonsmoker) | 1.08 (0.67–1.75) | 0.74 |
| Alcohol intake (>20 vs. ≤20 ml) | 1.18 (0.75–1.85) | 0.47 |
| Diastolic blood pressure (mmHg) | 0.99 (0.96–1.01) | 0.37 |
| Systolic blood pressure (mmHg) | 1.01 (0.99–1.03) | 0.11 |
| Cardiovascular disease antecedents (yes versus no) | 0.69 (0.34–1.40) | 0.30 |
| Total-to-HDL cholesterol ratio | 1.22 (1.06–1.40) | 0.005 |
| Lipid-lowering drugs user (yes versus no) | 1.30 (0.85–1.98) | 0.22 |
| BMI (kg/m2) | 1.11 (1.05–1.17) | 0.0002 |
Adjustment for all variables listed in the table; model performed on 1,035 participants.
P trend = 0.08.
To test if the relationship was maintained after adjustment for antioxidant markers, similar analyses were performed after adjustment for markers of antioxidant status (thiobarbiturate reactive substances, vitamin E, activity of glutathione peroxydase, and superoxyde dismutase). Relative risks of dysglycemia associated with quartile of total plasma carotenoid levels were similar to those described in Table 3 (Q4 vs. Q1: relative risk 0.38 [95% CI 0.19–0.79], P = 0.009; Q3 vs. Q1: 0.66 [0.40–1.15], P = 0.15; and Q2 vs. Q1: 0.74 [0.43–1.27], P = 0.28). None of the markers of antioxidant stress measured at baseline was significantly associated with 9-year risk of dysglycemia.
CONCLUSIONS—
Our results showed that high levels of plasma carotenoids at baseline were significantly associated with a lower 9-year risk of onset of dysglycemia in a healthy elderly population, independently of factors classically associated with dysglycemia, such as sociodemographic factors, lifestyle habits, cardiovascular disease history, blood pressure, BMI, and dyslipidemia. Furthermore, the relation persisted after adjustment for markers of antioxidant status (thiobarbiturate reactive substances, vitamin E, activity of glutathione peroxydase, and superoxyde dismutase). This suggests that plasma carotenoids could be in a direct relation with dysglycemia. To our knowledge, our study is one of the few that longitudinally explore the relationship between carotenoid and dysglycemia.
Many cross-sectional studies (4–9) have shown this association. In a population at high risk of type 2 diabetes, the Botnia Dietary Study showed that high dietary intake of α-carotene, β-carotene, and lycopene and high plasma β-carotene concentration were beneficially associated with glucose metabolism in participants (6). The third National Health and Nutrition Examination Survey (5) and the Australian Diabetes, Obesity, and Lifestyle Study (4) showed a significant association between low serum β-carotene levels and type 2 diabetes. In a previous publication (17), using baseline data of the EVA study, we also reported a significant correlation between total plasma carotenoids and glycemia. Finally, case-control studies (8–9) also found lower plasma carotenoid levels in diabetic patients than in control subjects, and one of them (8) was carried out on an elderly population. However, in this cross-sectional framework, it is not possible to know if low levels of carotenoids in diabetic participants are a consequence (the results of increased utilization due to the oxidative stress effects of the disease) or a cause: are the low concentrations involved in the pathogenesis of the disease?
Our longitudinal findings confirm results from the prospective study led by Montonen et al. (10) (23 years of follow-up), which showed, in a cohort of 4,303 participants free of diabetes at baseline, that β-cryptoxanthin intake was significantly associated with a reduced risk of type 2 diabetes (relative risk 0.58 [95% CI 0.44–0.78]). Other longitudinal results are more conflicting. In a nested case-control study (11) (106 case and 201 control subjects, mean age of 59.9 years), risk of diabetes was found to be lower in the highest serum β-carotene tertile group compared with the lowest tertile; however, the significant association did not remain after adjustment for cardiovascular risk factors (0.94 [0.38–2.32]). More recently, results of a nested case-control study (470 case and 470 control subjects) conducted on middle-aged and older U.S. women (aged >45 years) by Wang et al. (12) did not confirm the prospective association between different baseline plasma carotenoids compounds (lycopene, α-carotene, β-carotene, β-cryptoxanthin, lutein, and zeaxanthin) and risk of type 2 diabetes. The different methods and the biovariability of carotenoids, which is influenced by several factors such as characteristics of the food sources, interactions with other dietary factors, and various participant characteristics, could explain the differences between studies. Finally, a double-blind randomized controlled trial (13) of 12-year supplementation of β-carotene in a healthy U.S. male cohort (n = 22,071, aged 40–84 years) did not show a significant benefit on risk of type 2 diabetes (396 incident cases in the supplemented group versus 402 cases in the placebo group; relative risk 0.98 [0.85–1.12]). However, results of this trial should be interpreted with caution, as type 2 diabetes was not the primary end point and diagnosis of diabetes was self-reported without screening for glucose tolerance.
Our study has limitations. The EVA study included volunteers with higher educational status and higher incomes than the average elderly French population; however, this should not have any effects on the relationship between plasma carotenoid levels and dysglycemia. For reasons of statistical power (only 29 incident cases of diabetes), we considered IFG in the dysglycemia definition. IFG is a risk situation for diabetes; however, we cannot exclude that some participants have reversed to normal. Thus, we cannot exclude that we overdiagnosed dysglycemia. However, if this is the case, it is likely to have weakened the true association between carotenoids and dysglycemia. Moreover, our sensitivity analyses using the persistent IFG definition showed that the relation was maintained. Another limitation comes from our carotenoid measurement, insofar as only one measurement was made using a spectrophotometry method, while the reversed-phase high-performance liquid chromatography method provides more accurate measures with information on different carotenoids. Unfortunately, in 1991, when the EVA study started, this assay was not available for epidemiological purposes. Furthermore, the blood samples were equally collected throughout the year in the EVA study. Finally, another limitation is the high rate of attrition in this cohort. Participants lost to follow-up had lower baseline total plasma carotenoids. Most likely, this caused an underestimation of the incidence of dysglycemia and consequently decreased the power of the study. Despite these limitations, the relationships we showed were strong, almost in a dose-dependent fashion, and remained significant in the highest quartile after adjusting for various potential confounders.
Currently, the mechanism of this potential relationship is still under debate, and, as it has been described by Paiva et al. (2), several hypotheses can explain this observation. One of them involves antioxidant properties. In our study, analyses were repeated after controlling for various antioxidative markers, and our results remained unchanged, suggesting that the association between total plasma carotenoids and diabetes observed in our cohort is independent of the oxidative stress status of subjects. Other human studies have failed to show a clear benefit of antioxidants (18) and some studies have even suggested that they can be potentially harmful (18,19).
High plasma carotenoid is also a marker of fruit and vegetable consumption (20). A reduced risk of type 2 diabetes with vegetable consumption was suggested in several studies (21,22) but not all (23). This possible protective effect of fruit and vegetable consumption in diabetes described in these studies could result from the combined action of many protective compounds, including antioxidants, and could explain the controversial literature results between studies that were interested in blood measurement levels of carotenoids and those that were interested in carotenoid-rich fruit and vegetable consumption. Finally, we cannot exclude that carotenoids might have been serving as markers for other protective lifestyle habits and health behaviors but are not acting as effective agents themselves.
In conclusion, our results bring support to a possible role of carotenoids in onset of IFG and type 2 diabetes in elderly people. Further studies are necessary to explore the mechanism that could explain the relationship and hopefully design original measures that could help prevent dysglycemia.
Acknowledgments
T.N.A. was supported by a grant from the Société Française de Nutrition.
The EVA study was carried out under an agreement between the Institut National de la Santé et de la Recherche Medicalé and the Merck, Sharp, and Dohme-Chibret Laboratories (WestPoint, PA) and was supported by the Eisai Laboratory, Paris, France.
Published ahead of print at http://care.diabetesjournals.org on 4 April 2008.
The costs of publication of this article were defrayed in part by the payment of page charges. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C Section 1734 solely to indicate this fact.
References
- 1.Hung HC, Joshipura KJ, Jiang R, Hu FB, et al: Fruit and vegetable intake and risk of major chronic disease. J Natl Cancer Inst 96:1577–1584, 2004 [DOI] [PubMed] [Google Scholar]
- 2.Paiva SA, Russell RM: Beta-carotene and other carotenoids as antioxidants. J Am Coll Nutr 18:426–433, 1999 [DOI] [PubMed] [Google Scholar]
- 3.Maritim AC, Sanders RA, Watkins JB 3rd: Diabetes, oxidative stress, and antioxidants: a review. J Biochem Mol Toxicol 17:24–38, 2003 [DOI] [PubMed] [Google Scholar]
- 4.Coyne T, Ibiebele TI, Baade PD, et al: Diabetes mellitus and serum carotenoids: findings of a population-based study in Queensland. Australia Am J Clin Nutr 82:685–693, 2005 [DOI] [PubMed] [Google Scholar]
- 5.Ford ES, Will JC, Bowman BA, et al: Diabetes mellitus and serum carotenoids: findings from the Third National Health and Nutrition Examination Survey. Am J Epidemiol 149:168–176, 1999 [DOI] [PubMed] [Google Scholar]
- 6.Ylonen K, Alfthan G, Groop L, et al: Dietary intakes and plasma concentrations of carotenoids and tocopherols in relation to glucose metabolism in subjects at high risk of type 2 diabetes: the Botnia Dietary Study. Am J Clin Nutr 77:1434–1441, 2003 [DOI] [PubMed] [Google Scholar]
- 7.Suzuki K, Ito Y, Nakamura S, et al: Relationship between serum carotenoids and hyperglycemia: a population-based cross-sectional study. J Epidemiol 12:357–366, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Polidori MC, Mecocci P, Stahl W, et al: Plasma levels of lipophilic antioxidants in very old patients with type 2 diabetes. Diabetes Metab Res Rev 16:15–19, 2000 [DOI] [PubMed] [Google Scholar]
- 9.Abahusain MA, Wright J, Dickerson JW, et al: Retinol, alpha-tocopherol and carotenoids in diabetes. Eur J Clin Nutr 53:630–635, 1999 [DOI] [PubMed] [Google Scholar]
- 10.Montonen J, Knekt P, Jarvinen R, et al: Dietary antioxidant intake and risk of type 2 diabetes. Diabetes Care 27:362–366, 2004 [DOI] [PubMed] [Google Scholar]
- 11.Reunanen A, Knekt P, Aaran RK, et al: Serum antioxidants and risk of non-insulin dependent diabetes mellitus. Eur J Clin Nutr 52:89–93, 1998 [DOI] [PubMed] [Google Scholar]
- 12.Wang L, Liu S, Pradhan AD, Manson JE, et al: Plasma lycopene, other carotenoids, and the risk of type 2 diabetes in women. Am J Epidemiol 164:576–585, 2006 [DOI] [PubMed] [Google Scholar]
- 13.Liu S, Ajani U, Chae C, et al: Long-term beta-carotene supplementation and risk of type 2 diabetes mellitus: a randomized controlled trial. JAMA 282:1073–1075, 1999 [DOI] [PubMed] [Google Scholar]
- 14.Akbaraly NT, Arnaud J, Hininger-Favier I, et al: Selenium and mortality in the elderly: results from the EVA study. Clin Chem 51:2117–2123, 2005 [DOI] [PubMed] [Google Scholar]
- 15.Berr C, Richard MJ, Roussel AM, et al: Systemic oxidative stress and cognitive performance in the population-based EVA study: Etude du Vieillissement Arteriel. Free Radic Biol Med 24:1202–1208, 1998 [DOI] [PubMed] [Google Scholar]
- 16.World Health Organization: Definition, Diagnosis and Classification of Diabetes Mellitus and Its Complications. Part 1: Diagnosis and Classification of Diabetes Mellitus. Geneva, World Health Org., 1999
- 17.Fontbonne A, Berr C, Ducimetiere P, et al: Changes in cognitive abilities over a 4-year period are unfavorably affected in elderly diabetic subjects: results of the Epidemiology of Vascular Aging Study. Diabetes Care 24:366–370, 2001 [DOI] [PubMed] [Google Scholar]
- 18.Yim S, Malhotra A, Veves A: Antioxidants and CVD in diabetes: where do we stand now. Curr Diab Rep 7:8–13, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ward NC, Wu JH, Clarke MW, et al: The effect of vitamin E on blood pressure in individuals with type 2 diabetes: a randomized, double-blind, placebo-controlled trial. J Hypertens 25:227–234, 2007 [DOI] [PubMed] [Google Scholar]
- 20.Al-Delaimy WK, Ferrari P, Slimani N, et al: Plasma carotenoids as biomarkers of intake of fruits and vegetables: individual-level correlations in the European Prospective Investigation into Cancer and Nutrition (EPIC). Eur J Clin Nutr 59:1387–1396, 2005 [DOI] [PubMed] [Google Scholar]
- 21.Feskens EJ, Virtanen SM, Rasanen L, et al: Dietary factors determining diabetes and impaired glucose tolerance: a 20-year follow-up of the Finnish and Dutch cohorts of the Seven Countries Study. Diabetes Care 18:1104–1112, 1995 [DOI] [PubMed] [Google Scholar]
- 22.Snowdon DA, Phillips RL: Does a vegetarian diet reduce the occurrence of diabetes? Am J Public Health 75:507–512, 1985 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hamer M, Chida Y: Intake of fruit, vegetables, and antioxidants and risk of type 2 diabetes: systematic review and meta-analysis. J Hypertens 25:2361–2369, 2007 [DOI] [PubMed] [Google Scholar]