Abstract
OBJECTIVE—African-American (AA) children are hyperinsulinemic and insulin resistant compared with American white (AW) children. Previously, we demonstrated that insulin secretion relative to insulin sensitivity was ∼75% higher in AA compared with AW children, suggesting that hyperinsulinemia in AA children is not merely a compensatory response to lower insulin sensitivity. The aim of the present investigation was to assess whether glucose-stimulated insulin response is higher in AA versus AW adolescents who have comparable in vivo insulin sensitivity.
RESEARCH DESIGN AND METHODS—The hyperinsulinemic-euglycemic and hyperglycemic clamp techniques were utilized to assess first- and second-phase insulin secretion. Insulin secretion relative to insulin sensitivity was calculated as the glucose disposition index.
RESULTS—AA adolescents compared with their AW peers with comparable insulin sensitivity and body composition had higher first-phase insulin concentrations.
CONCLUSIONS—The quantitative relationship between insulin sensitivity and first-phase insulin appears to differ among AA and AW adolescents.
African-American (AA) adolescents are hyperinsulinemic and at increased risk for type 2 diabetes when compared with American white (AW) adolescents; however, the mechanisms underlying the increased risk in AA adolescents are unclear. The objective of this study was to assess whether first- and second-phase insulin concentrations are higher in AA versus AW youth if insulin sensitivity is not different between the groups.
RESEARCH DESIGN AND METHODS—
The study was approved by the University of Pittsburgh Institutional Review Board. Recruitment was done using posters, flyers, and newspaper advertisements. A total of 25 healthy AA adolescents (15 male and 10 female, aged 10.0–14.3 years) and 23 healthy AW adolescents (12 male and 11 female, aged 9.9–14.3 years), Tanner stages II–IV, participated. Findings from some of the participants have been reported (1). All subjects who participated in this study are included in this report. Exclusion criteria included ethnicity other than AA or AW, diabetes or other chronic diseases, and use of medications affecting glucose metabolism. Ethnicity was determined by self-report in three generations of the participants’ families.
Metabolic studies
Participants underwent a 3-h 40 mU/m2 per min hyperinsulinemic-euglycemic clamp to assess insulin sensitivity and a 2-h hyperglycemic (12.5 mmol/l) clamp to assess insulin secretion, as described previously (2,3). Fasting hepatic glucose production was evaluated before the hyperinsulinemic-euglycemic clamp as described previously (2). Hepatic glucose production (4), insulin-stimulated glucose disposal (3), insulin sensitivity (3), insulin clearance (3), first- and second-phase insulin concentrations (2), and glucose disposition index (2) were calculated as previously reported. Fasting blood samples were obtained for lipid profile, insulin-like growth factor (IGF)-1, dehydroepiandrosterone sulfate (DHEA-S), estradiol in females, and testosterone in males.
Body composition analysis
Body composition was measured by dual-energy X-ray absorptiometry. Abdominal adiposity was assessed with a 10-mm single axial computed tomography scan at the level of L4–5 vertebrae (4).
Biochemical measurements
Plasma glucose and insulin were measured as previously described (2). IGF-1 levels were measured at Esoterix. Fasting lipid profile was measured using standards of the Centers of Disease Control and Prevention. Plasma free fatty acid levels and deuterium enrichment of glucose in the plasma were determined as previously reported (4). Stored plasma samples from the hyperglycemic clamp for C-peptide measurement were destroyed due to freezer malfunction. Fasting plasma samples for C-peptide from the euglycemic clamp were by immunochemiluminescent assay (Esoterix).
Statistical analysis
Comparisons between AA and AW adolescents were made using two-tailed Student's t test for continuous normally distributed variables. Appropriate nonparametric tests were otherwise used. Data are presented as means ± SEM.
RESULTS—
Physical, hormonal, and metabolic characteristics
AA and AW adolescents had comparable body composition (BMI 21.1 ± 0.7 vs. 20.3 ± 0.8 kg/m2, P = 0.45; percent body fat 21.0 ± 2.3 vs. 22.4 ± 2.2, P = 0.66; visceral adipose tissue 19.5 ± 2.9 vs. 24.9 ± 4.2 cm2, P = 0.48; fat mass 11.2 ± 1.7 vs. 11.2 ± 1.6 kg, P = 0.96), hormonal profiles (estradiol 159 ± 47 vs. 126 ± 20 pmol/l, P = 0.53; testosterone 11.8 ± 2.0 vs. 12.4 ± 1.7 nmol/l, P = 0.83; DHEA-S 3,198 ± 465 vs. 2,962 ± 375 nmol/l, P = 0.85; IGF-1 55 ± 5 vs. 49 ± 3 nmol/l, P = 0.42), and fasting lipid profiles (cholesterol 4.09 ± 0.13 vs. 4.09 ± 0.18 mmol/l, P = 0.97; HDL cholesterol 1.30 ± 0.03 vs. 1.32 ± 0.05 mmol/l, P = 0.60; LDL cholesterol 2.41 ± 0.13 vs. 2.31 ± 0.13 mmol/l, P = 0.61; triglycerides 0.89 ± 0.01 vs. 1.01 ± 0.09 mmol/l, P = 0.30).
Basal metabolic data
Fasting glucose and insulin levels from the hyperinsulinemic-euglycemic and hyperglycemic clamps were averaged. Fasting insulin was not different (122 ± 11 vs. 120 ± 10 pmol/l, P = 0.89) and fasting glucose was lower (5.25 ± 0.05 vs. 5.44 ± 0.06 mmol/l, P = 0.02) in AAs. Fasting C-peptide (0.58 ± 0.05 vs. 0.58 ± 0.06 nmol/l, P = 0.93), free fatty acids (0.35 ± 0.04 vs. 0.34 ± 0.02 mEq/l, P = 0.81), and hepatic glucose production (3.1 ± 0.4 vs. 3.0 ± 0.1 mg · kg−1 · min−1, P = 0.86) were not different.
Insulin sensitivity and clearance
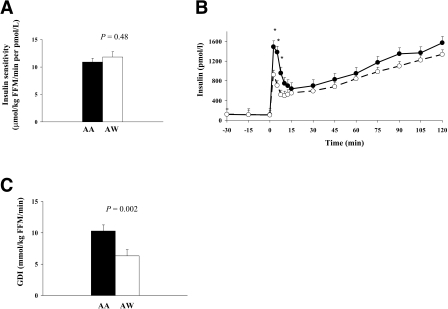
During the hyperinsulinemic-euglycemic clamp, steady-state plasma glucose (AA 5.61 ± 0.03, AW 5.60 ± 0.02 mmol/l, P = 0.67), insulin (AA 690 ± 18, AW 636 ± 36 pmol/l, P = 0.21), insulin-stimulated glucose disposal (AA 74.9 ± 4.4, AW 70.5 ± 4.4 μmol · kg fat-free mass [FFM]−1 · min−1; P = 0.47), and insulin sensitivity (AA 10.9 ± 0.7, AW 11.8 ± 1.0 μmol · kg FFM−1 · min−1 per pmol/l; P = 0.48) were not different (Fig. 1). Insulin clearance was lower in AAs (17.9 ± 0.8 vs. 20.7 ± 1.1 μmol · kg FFM−1 · min−1; P = 0.05).
Figure 1—
In vivo insulin sensitivity measured during a 40 mU/m2 per min hyperinsulinemic-euglycemic clamp (A), insulin concentrations measured during a 2-h hyperglycemic (225 mg/dl) clamp (B), and glucose disposition index (insulin sensitivity × first-phase insulin) (C) in AA and AW adolescents. •, AA; ○, AW.
Insulin secretion and glucose disposition index
During the hyperglycemic clamp, first- and second-phase glucose concentrations were not different (first-phase 11.9 ± 0.2 mmol/l in AA vs. 12.2 ± 0.1 mmol/l in AW, P = 0.25; second-phase 12.3 ± 0.1 mmol/l in AA vs. 12.4 ± 0.1 mmol/l in AW, P = 0.19). First-phase insulin concentration was higher in AAs (1,038 ± 126 vs. 636 ± 108 pmol/l; P = 0.002) (Fig. 1). Second-phase insulin concentration was not different (AA 1,068 ± 120 vs. AW 918 ± 174 pmol/l; P = 0.46). After controlling for insulin clearance, there remained a race difference in first-phase insulin (1,003 ± 121 vs. 647 ± 123 pmol/l, P = 0.05). The glucose disposition index was higher in AAs (10.3 ± 1.0 vs. 6.3 ± 0.7 μmol · kg FFM−1 · min−1, P = 0.002).
CONCLUSIONS—
Several studies have demonstrated that AA children have higher fasting and stimulated insulin levels than AW children (2,5–7); however, insulin sensitivity was lower in AA than in AW youth. Thus, the observed hyperinsulinemia in AAs was explained as a compensatory response to insulin resistance. In the current study, the observation of higher insulin secretion was made despite similar insulin sensitivity. Differences in insulin clearance could partly explain the differences in first-phase insulin concentrations; however, after adjusting for clearance, there remained a race difference in first-phase insulin concentration. Insulin clearance in AA adolescents was ∼14% lower than in AWs, while first-phase insulin was ∼63% higher.
Potential causes of insulin hypersecretion in AAs include dietary/lifestyle factors, genetic differences, and socioeconomic differences. Previously, we reported dietary differences including higher fat-to-carbohydrate ratio in AA children (2). Increased fat-to-carbohydrate ratio correlated negatively with insulin sensitivity and insulin clearance and positively with first-phase insulin levels across racial groups (2). Gower et al. (8) reported that genetic admixture was independently related to insulin sensitivity, fasting insulin, and acute insulin response to glucose, indicating that hyperinsulinemia in AAs has a genetic basis.
The present study demonstrates that AA adolescents have 1) ∼63% higher first-phase insulin and 2) ∼63% higher glucose disposition index even when they have insulin sensitivity comparable with that of their AW peers. There appears to be an upregulated β-cell function in AA adolescents, the mechanism(s) of which should be investigated.
Acknowledgments
This work was supported by U.S. Public Health Service Grants R01-HD27503, K24-HD01357, and K23-RR17250 and the Pediatric Clinical and Translational Research Center at Children's Hospital of Pittsburgh (NIH/NCRR/CTSA grant UL1-RR024153; NIH/NCRR/GCRC grant M01-RR00084).
Published ahead of print at http://care.diabetesjournals.org on 16 April 2008.
The costs of publication of this article were defrayed in part by the payment of page charges. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C Section 1734 solely to indicate this fact.
References
- 1.Lee S, Gungor N, Bacha F, Arslanian S: Insulin resistance: link to the components of the metabolic syndrome and biomarkers of endothelial dysfunction in youth. Diabetes Care 30:2091–2097, 2007 [DOI] [PubMed] [Google Scholar]
- 2.Arslanian SA, Saad R, Lewy V, Danadian K, Janosky J: Hyperinsulinemia in African-American children: decreased insulin clearance and increased insulin secretion and its relationship to insulin sensitivity. Diabetes 51:3014–3019, 2002 [DOI] [PubMed] [Google Scholar]
- 3.DeFronzo RA, Tobin JD, Andres R: Glucose clamp technique: a method for quantifying insulin secretion and resistance. Am J Physiol 237:E214–E223, 1979 [DOI] [PubMed] [Google Scholar]
- 4.Danadian K, Balasekaran G, Lewy V, Meza MP, Robertson R, Arslanian SA: Insulin sensitivity in African-American children with and without family history of type 2 diabetes. Diabetes Care 22:1325–1329, 1999 [DOI] [PubMed] [Google Scholar]
- 5.Gower BA, Nagy TR, Goran MI: Visceral fat, insulin sensitivity, and lipids in prepubertal children. Diabetes 48:1515–1521, 1999 [DOI] [PubMed] [Google Scholar]
- 6.Svec F, Nastasi K, Hilton C, Bao W, Srinivasan SR, Berenson GS: Black-white contrasts in insulin levels during pubertal development: the Bogalusa Heart Study. Diabetes 41:313–317, 1992 [DOI] [PubMed] [Google Scholar]
- 7.Chiu KC, Chuang LM, Yoon C: Comparison of measured and estimated indices of insulin sensitivity and beta cell function: impact of ethnicity on insulin sensitivity and beta cell function in glucose-tolerant and normotensive subjects. J Clin Endocrinol Metab 86:1620–1625, 2001 [DOI] [PubMed] [Google Scholar]
- 8.Gower BA, Fernandez JR, Beasley TM, Shriver MD, Goran MI: Using genetic admixture to explain racial differences in insulin-related phenotypes. Diabetes 52:1047–1051, 2003 [DOI] [PubMed] [Google Scholar]