Abstract
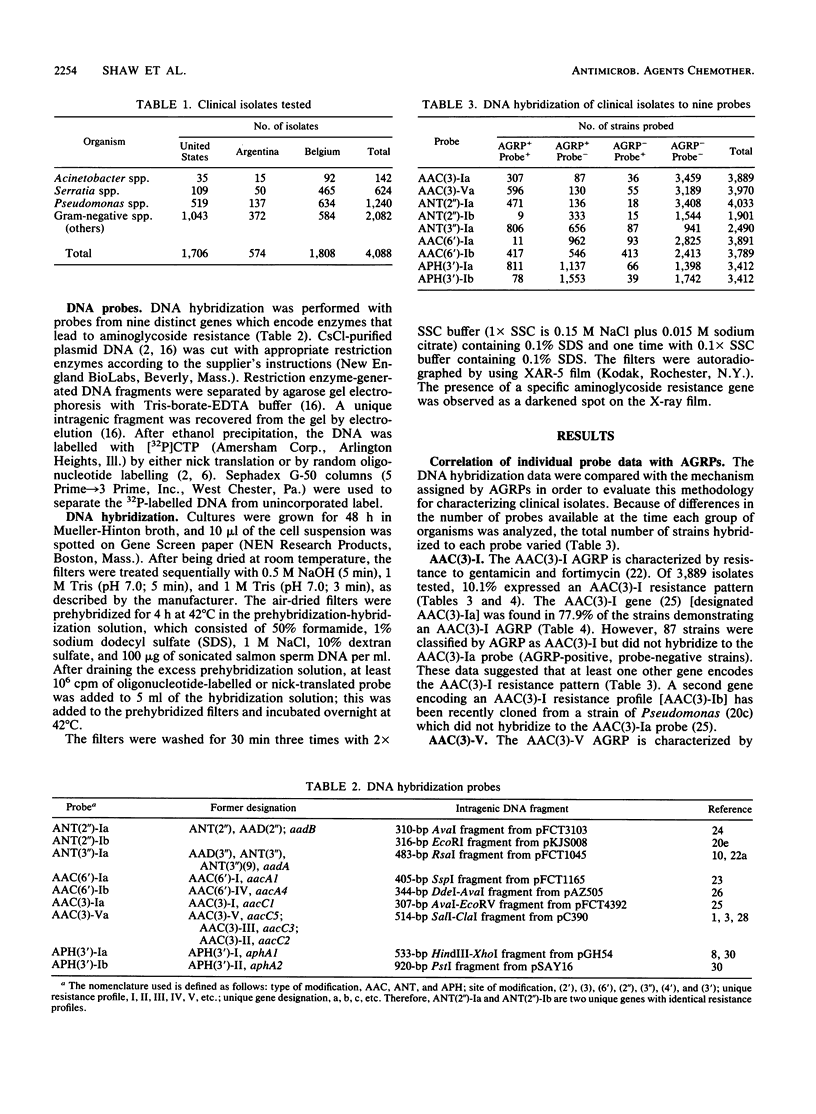
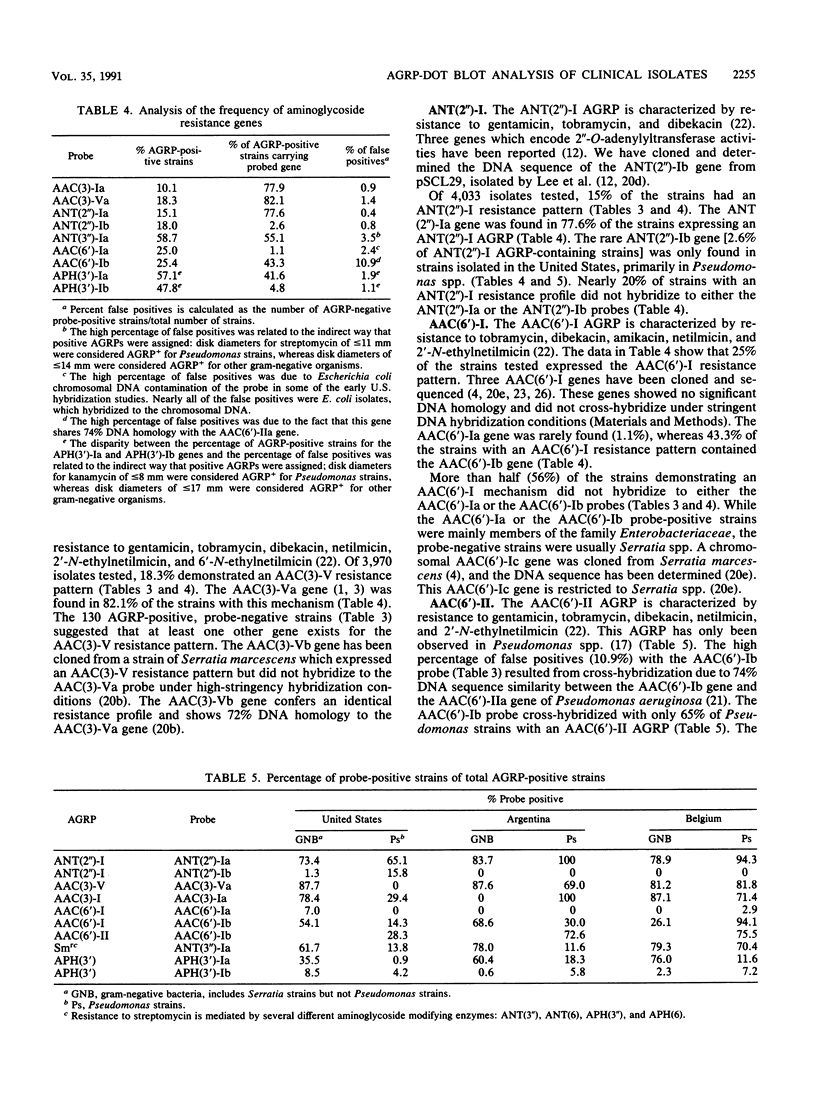
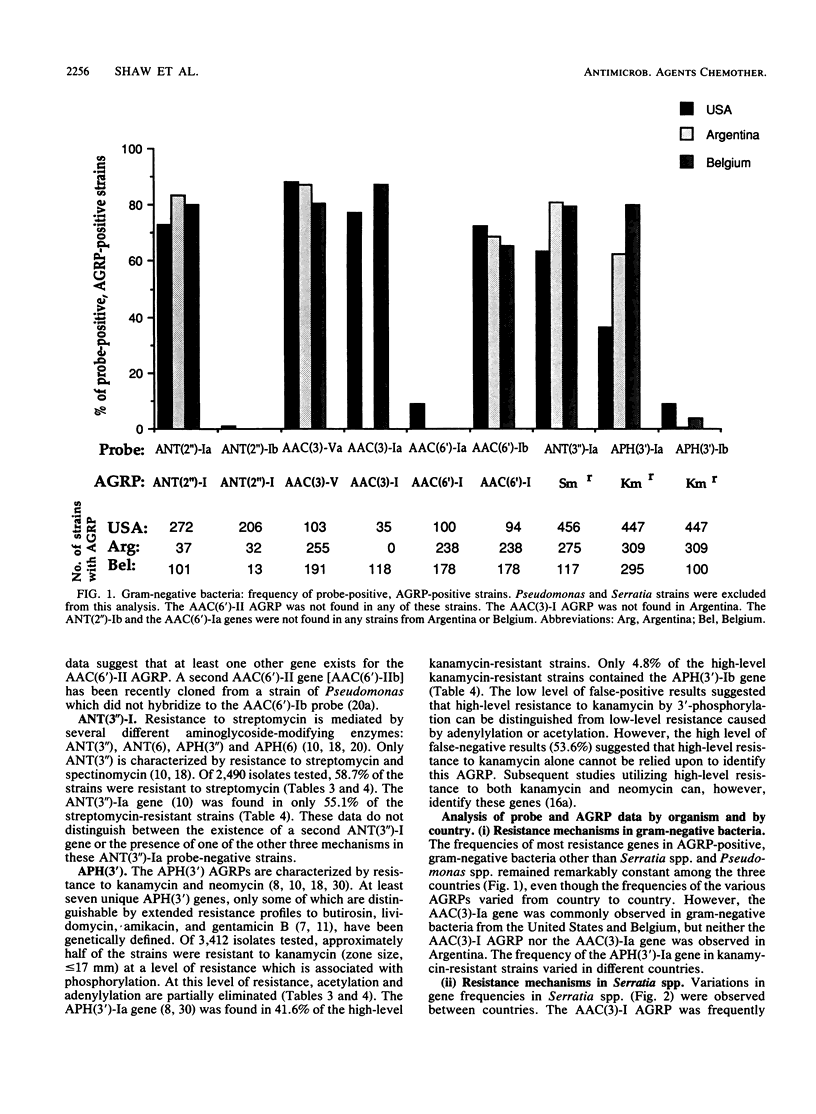
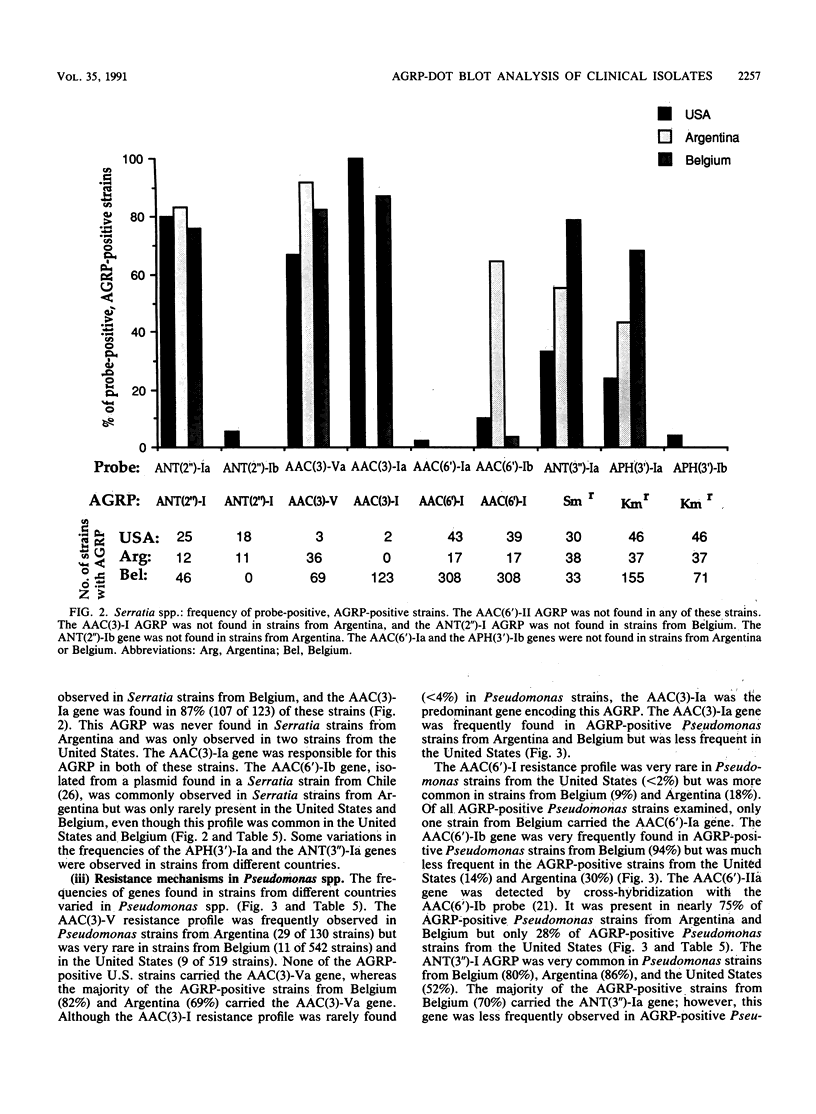
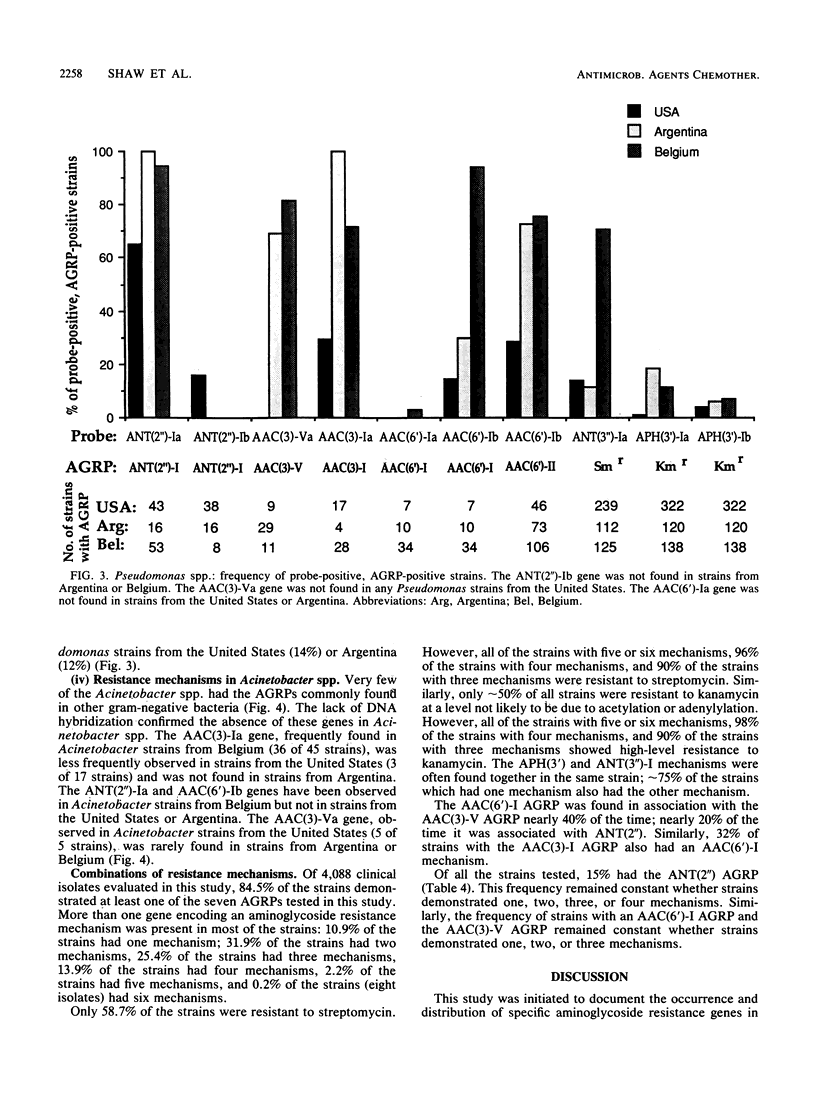
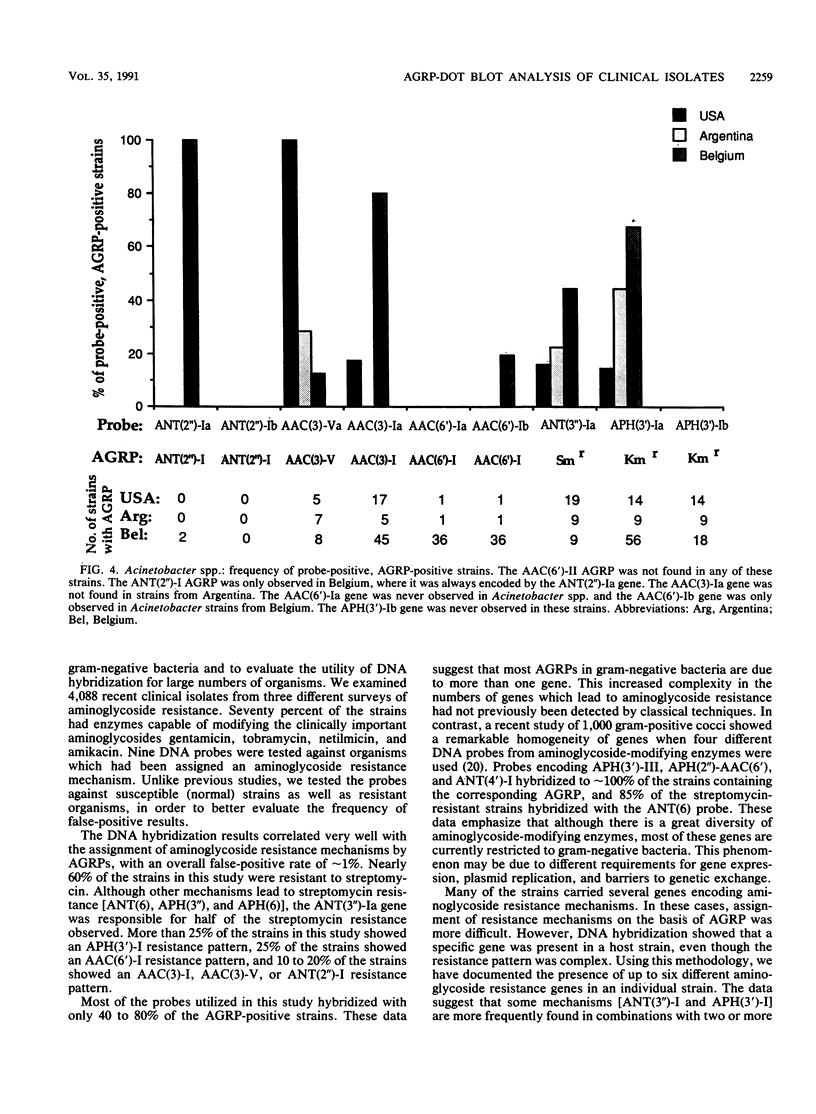
DNA hybridization data and aminoglycoside resistance profiles (AGRPs) were determined for 4,088 clinical isolates from three studies (United States, Belgium, and Argentina). The correlation between susceptibility profiles and hybridization results was determined with nine DNA probes. For each of the seven aminoglycoside resistance profiles which we were able to test, the data suggested at least two distinct genes could encode enzymes which lead to identical resistance profiles. Furthermore, the DNA hybridization data showed that individual strains carried up to six unique aminoglycoside resistance genes. DNA hybridization revealed interesting differences in the frequencies of these genes by organism and by country.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Allmansberger R., Bräu B., Piepersberg W. Genes for gentamicin-(3)-N-acetyl-transferases III and IV. II. Nucleotide sequences of three AAC(3)-III genes and evolutionary aspects. Mol Gen Genet. 1985;198(3):514–520. doi: 10.1007/BF00332949. [DOI] [PubMed] [Google Scholar]
- Barg N. L. Construction of a probe for the aminoglycoside 3-V-acetyltransferase gene and detection of the gene among endemic clinical isolates. Antimicrob Agents Chemother. 1988 Dec;32(12):1834–1838. doi: 10.1128/aac.32.12.1834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Champion H. M., Bennett P. M., Lewis D. A., Reeves D. S. Cloning and characterization of an AAC(6') gene from Serratia marcescens. J Antimicrob Chemother. 1988 Nov;22(5):587–596. doi: 10.1093/jac/22.5.587. [DOI] [PubMed] [Google Scholar]
- Chaslus-Dancla E., Glupcznski Y., Gerbaud G., Lagorce M., Lafont J. P., Courvalin P. Detection of apramycin resistant Enterobacteriaceae in hospital isolates. FEMS Microbiol Lett. 1989 Oct 15;52(3):261–265. doi: 10.1016/0378-1097(89)90208-5. [DOI] [PubMed] [Google Scholar]
- Feinberg A. P., Vogelstein B. A technique for radiolabeling DNA restriction endonuclease fragments to high specific activity. Anal Biochem. 1983 Jul 1;132(1):6–13. doi: 10.1016/0003-2697(83)90418-9. [DOI] [PubMed] [Google Scholar]
- Gaynes R., Groisman E., Nelson E., Casadaban M., Lerner S. A. Isolation, characterization, and cloning of a plasmid-borne gene encoding a phosphotransferase that confers high-level amikacin resistance in enteric bacilli. Antimicrob Agents Chemother. 1988 Sep;32(9):1379–1384. doi: 10.1128/aac.32.9.1379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grindley N. D., Joyce C. M. Genetic and DNA sequence analysis of the kanamycin resistance transposon Tn903. Proc Natl Acad Sci U S A. 1980 Dec;77(12):7176–7180. doi: 10.1073/pnas.77.12.7176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hollingshead S., Vapnek D. Nucleotide sequence analysis of a gene encoding a streptomycin/spectinomycin adenylyltransferase. Plasmid. 1985 Jan;13(1):17–30. doi: 10.1016/0147-619x(85)90052-6. [DOI] [PubMed] [Google Scholar]
- Lambert T., Gerbaud G., Courvalin P. Transferable amikacin resistance in Acinetobacter spp. due to a new type of 3'-aminoglycoside phosphotransferase. Antimicrob Agents Chemother. 1988 Jan;32(1):15–19. doi: 10.1128/aac.32.1.15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee S. C., Cleary P. P., Gerding D. N. More than one DNA sequence encodes the 2''-O-adenylyltransferase phenotype. Antimicrob Agents Chemother. 1987 Apr;31(4):667–670. doi: 10.1128/aac.31.4.667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Licitra C. M., Brooks R. G., Terry P. M., Shaw K. J., Hare R. S. Use of plasmid analysis and determination of aminoglycoside-modifying enzymes to characterize isolates from an outbreak of methicillin-resistant Staphylococcus aureus. J Clin Microbiol. 1989 Nov;27(11):2535–2538. doi: 10.1128/jcm.27.11.2535-2538.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lovering A. M., Bywater M. J., Holt H. A., Champion H. M., Reeves D. S. Resistance of bacterial pathogens to four aminoglycosides and six other antibacterials and prevalence of aminoglycoside modifying enzymes, in 20 UK centres. J Antimicrob Chemother. 1988 Dec;22(6):823–839. doi: 10.1093/jac/22.6.823. [DOI] [PubMed] [Google Scholar]
- Lovering A. M., White L. O., Reeves D. S. AAC(1): a new aminoglycoside-acetylating enzyme modifying the Cl aminogroup of apramycin. J Antimicrob Chemother. 1987 Dec;20(6):803–813. doi: 10.1093/jac/20.6.803. [DOI] [PubMed] [Google Scholar]
- O'Brien T. F., Pla M. P., Mayer K. H., Kishi H., Gilleece E., Syvanen M., Hopkins J. D. Intercontinental spread of a new antibiotic resistance gene on an epidemic plasmid. Science. 1985 Oct 4;230(4721):87–88. doi: 10.1126/science.2994226. [DOI] [PubMed] [Google Scholar]
- Ounissi H., Derlot E., Carlier C., Courvalin P. Gene homogeneity for aminoglycoside-modifying enzymes in gram-positive cocci. Antimicrob Agents Chemother. 1990 Nov;34(11):2164–2168. doi: 10.1128/aac.34.11.2164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaw K. J., Cramer C. A., Rizzo M., Mierzwa R., Gewain K., Miller G. H., Hare R. S. Isolation, characterization, and DNA sequence analysis of an AAC(6')-II gene from Pseudomonas aeruginosa. Antimicrob Agents Chemother. 1989 Dec;33(12):2052–2062. doi: 10.1128/aac.33.12.2052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimizu K., Kumada T., Hsieh W. C., Chung H. Y., Chong Y., Hare R. S., Miller G. H., Sabatelli F. J., Howard J. Comparison of aminoglycoside resistance patterns in Japan, Formosa, and Korea, Chile, and the United States. Antimicrob Agents Chemother. 1985 Aug;28(2):282–288. doi: 10.1128/aac.28.2.282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tenover F. C., Filpula D., Phillips K. L., Plorde J. J. Cloning and sequencing of a gene encoding an aminoglycoside 6'-N-acetyltransferase from an R factor of Citrobacter diversus. J Bacteriol. 1988 Jan;170(1):471–473. doi: 10.1128/jb.170.1.471-473.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tenover F. C., Gootz T. D., Gordon K. P., Tompkins L. S., Young S. A., Plorde J. J. Development of a DNA probe for the structural gene of the 2"-O-adenyltransferase aminoglycoside-modifying enzyme. J Infect Dis. 1984 Nov;150(5):678–687. doi: 10.1093/infdis/150.5.678. [DOI] [PubMed] [Google Scholar]
- Tenover F. C., Phillips K. L., Gilbert T., Lockhart P., O'Hara P. J., Plorde J. J. Development of a DNA probe from the deoxyribonucleotide sequence of a 3-N-aminoglycoside acetyltransferase [AAC(3)-I] resistance gene. Antimicrob Agents Chemother. 1989 Apr;33(4):551–559. doi: 10.1128/aac.33.4.551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tran van Nhieu G., Collatz E. Primary structure of an aminoglycoside 6'-N-acetyltransferase AAC(6')-4, fused in vivo with the signal peptide of the Tn3-encoded beta-lactamase. J Bacteriol. 1987 Dec;169(12):5708–5714. doi: 10.1128/jb.169.12.5708-5714.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vliegenthart J. S., Ketelaar-van Gaalen P. A., van de Klundert J. A. Nucleotide sequence of the aacC2 gene, a gentamicin resistance determinant involved in a hospital epidemic of multiply resistant members of the family Enterobacteriaceae. Antimicrob Agents Chemother. 1989 Aug;33(8):1153–1159. doi: 10.1128/aac.33.8.1153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young S. A., Tenover F. C., Gootz T. D., Gordon K. P., Plorde J. J. Development of two DNA probes for differentiating the structural genes of subclasses I and II of the aminoglycoside-modifying enzyme 3'-aminoglycoside phosphotransferase. Antimicrob Agents Chemother. 1985 May;27(5):739–744. doi: 10.1128/aac.27.5.739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van de Klundert J. A., Vliegenthart J. S., van Doorn E., Bongaerts G. P., Molendijk L., Mouton R. P. A simple method for the identification of aminoglycoside-modifying enzymes. J Antimicrob Chemother. 1984 Oct;14(4):339–348. doi: 10.1093/jac/14.4.339. [DOI] [PubMed] [Google Scholar]



