Abstract
OBJECTIVE—To determine the time course of changes in glucagon and insulin secretion in children with recently diagnosed type 1 diabetes.
RESEARCH DESIGN AND METHODS—Glucagon and C-peptide concentrations were determined in response to standard mixed meals in 23 patients with type 1 diabetes aged 9.4 ± 4.6 years, beginning within 6 weeks of diagnosis, and every 3 months thereafter for 1 year.
RESULTS—Glucagon secretion in response to a physiologic stimulus (mixed meal) increased by 37% over 12 months, while C-peptide secretion declined by 45%. Fasting glucagon concentrations remained within the normal (nondiabetic) reference range.
CONCLUSIONS—Postprandial hyperglucagonemia worsens significantly during the first year after diagnosis of type 1 diabetes and may represent a distinct therapeutic target. Fasting glucagon values may underestimate the severity of hyperglucagonemia. The opposing directions of abnormal glucagon and C-peptide secretion over time support the link between dysregulated glucagon secretion and declining β-cell function.
Patients with diabetes frequently have a deficient glucagon response to hypoglycemia and exhibit an inappropriately high glucagon response to a meal. This dysregulation has been attributed in part to a lack of intraislet insulin, but the exact pathophysiologic mechanisms remain controversial (1). Glucagon excess and its contribution to postprandial hyperglycemia have become treatment targets, especially in the field of type 2 diabetes. In this study, we describe the time course of development of abnormal glucagon secretion paralleled by decreasing insulin secretion in a cohort of recently diagnosed patients with type 1 diabetes.
RESEARCH DESIGN AND METHODS
Children with new-onset type 1 diabetes were recruited for a randomized, placebo-controlled trial of low-dose oral interferon-α for preservation of β-cell function (the primary outcome of which will be published in a separate report). Patients had a mean age of 9.4 ± 4.6 years, were enrolled within 6 weeks of diagnosis, and were free of other significant illness (n = 23, 30% female, 91% Caucasian). Study approval was obtained from the institutional review board at the National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK) at the National Institutes of Health (NIH). Parents gave written informed consent and minors gave written assent before enrollment.
Mixed meal testing was performed at screening and every 3 months thereafter. Patients fasted after midnight before testing. For subjects on insulin pumps, basal rates were continued but morning bolus injections were withheld. For patients on multiple daily injections of insulin, morning doses were withheld. The meal consisted of Boost High Protein, 7 ml/kg (maximum 400 ml), ingested over 5 min. Blood samples were drawn at −10, 0, 30, 60, 90, and 120 min.
Plasma glucagon concentrations were determined using a radioimmunoassay at Mayo Medical Laboratories. The normal range in healthy individuals was <60 ng/l, with an interassay coefficient of variation (CV) of 18% and an intra-assay CV of 15%. C-peptide concentrations were determined with a competitive chemiluminescence assay at the NIH Clinical Center laboratory. The normal range in healthy individuals was 0.3–1.3 nmol/l, with an intra-assay CV of 3.4% (at 1.45 nmol/l) and inter-assay CVs of 7.7 and 8.3% at 0.37 and 1.98 nmol/l, respectively. The lower limit of detection was 0.17 nmol/l.
Area under the curve (AUC) for glucagon and C-peptide were calculated using the trapezoidal method. The mean of values obtained at −10 and 0 min was used as the baseline value. Net glucagon and C-peptide secretion following the mixed meal were expressed as AUC.
Statistical analyses were performed using SAS version 9.1 (SAS Institute, Cary, NC). Results were described using frequency distributions, Pearson's correlation coefficients, and repeated-measures ANOVA using PROC MIXED for testing change over time. Opposing change (i.e., positive for glucagon vs. negative for C-peptide, or vice versa) was assessed using a sign test based on the sign of the product of the slopes over time for glucagon and C-peptide. All tests were two-sided, with statistical significance defined as P ≤ 0.05. Data are presented as means ± SEM.
RESULTS
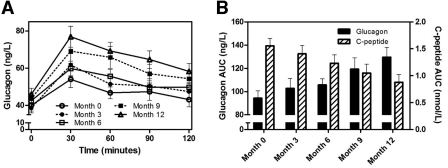
There were no differences between treatment groups (interferon vs. placebo) for glucagon or C-peptide AUC; therefore, all 23 subjects were combined for subsequent analyses. Subjects showed an increase in glucagon secretion following the mixed meal, with peak glucagon levels at 30 min (Fig. 1A). Glucagon AUC progressively increased over the 12-month observation period (P = 0.0008), whereas C-peptide AUC progressively declined (P < 0.0001) (Fig. 1B). The magnitude of change was similar for both hormones (37% rise in glucagon vs. 45% decline in C-peptide AUC over 12 months). Of the 23 subjects, 18 (78%) showed opposing changes in glucagon and C-peptide AUC over time (P = 0.01).
Figure 1—
Glucagon secretion after a mixed meal (A) and glucagon and C-peptide AUC after a mixed meal (B) during the first year after diagnosis of type 1 diabetes. Data are means ± SEM.
The percent rise in meal-stimulated C-peptide [100 × (peak − fasting)/fasting] declined over time, from 147 ± 22% at month 0 to 85 ± 12% at month 12. In contrast, the percent rise in meal-stimulated glucagon increased over time, from 63 ± 12% at month 0 to 82 ± 9% at month 12. Fasting glucagon rose from 40 ± 3 ng/l at month 0 to 46 ± 3 ng/l at month 12. No significant correlations were found between glucagon AUC and A1C, insulin dose, or insulin dose-adjusted A1C (data not shown).
CONCLUSIONS
The phenomenon of elevated glucagon in response to oral glucose or mixed meal testing has been described cross-sectionally in patients with type 1 and type 2 diabetes, generally of several years duration (2–5). More recently, Porksen et al. (6) reported a 17% rise in post–mixed meal glucagon (measured as a single value at 90 min) from 1 to 12 months after diagnosis of type 1 diabetes. In the present study, we demonstrate a progressive, 37% rise in meal-stimulated glucagon secretion over 12 months after diagnosis of type 1 diabetes, paralleled by a 45% decline in C-peptide secretion. Fasting glucagon rose only 15% over 12 months and remained within the normal range of the assay, suggesting that hyperglucagonemia may not be recognized if only fasting blood samples are obtained.
As seen in previous studies (7,8), the ability of β-cells to secrete insulin in response to a stimulus declined over time in our patients with type 1 diabetes. In contrast, the ability of α-cells to secrete glucagon in response to a stimulus increased over time. This suggests that α-cell secretory reserve is not diminished by the ongoing autoimmune process in type 1 diabetes. Although the pathophysiology of glucagon excess during hyperglycemia in type 1 diabetes is not well understood, the opposing directions of abnormal glucagon and C-peptide secretion over time support the link between dysregulated glucagon secretion and declining β-cell function.
These data represent the most detailed time course to date of the progression of abnormal glucagon secretion in type 1 diabetes. We did not observe a significant correlation between glucagon AUC and A1C or insulin dose; however, this may be related to small sample size. Nevertheless, the timing and rapid progression shown here suggest that excess glucagon secretion may represent a distinct therapeutic target early in the course of type 1 diabetes.
Acknowledgments
This research was supported in part by the Intramural Research Program of the National Institutes of Health, National Institute of Diabetes and Digestive and Kidney Diseases.
Parts of this study were presented in abstract form at the 68th annual meeting of the American Diabetes Association, San Francisco, California, 6–10 June 2008.
The costs of publication of this article were defrayed in part by the payment of page charges. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
References
- 1.Gromada J, Franklin I, Wollheim CB: Alpha-cells of the endocrine pancreas: 35 years of research but the enigma remains. Endocr Rev 28:84–116, 2007 [DOI] [PubMed] [Google Scholar]
- 2.Sperling MA, Aleck K, Voina S: Suppressibility of glucagon secretion by glucose in juvenile diabetes. J Pediatr 90:543–547, 1977 [DOI] [PubMed] [Google Scholar]
- 3.Gerich JE, Lorenzi M, Karam JH, Schneider V, Forsham PH: Abnormal pancreatic glucagon secretion and postprandial hyperglycemia in diabetes mellitus. JAMA 234:159–155, 1975 [PubMed] [Google Scholar]
- 4.Ternand C, Go VL, Gerich JE, Haymond MW: Endocrine pancreatic response of children with onset of insulin-requiring diabetes before age 3 and after age 5. J Pediatr 101:36–39, 1982 [DOI] [PubMed] [Google Scholar]
- 5.Henkel E, Menschikowski M, Koehler C, Leonhardt W, Hanefeld M: Impact of glucagon response on postprandial hyperglycemia in men with impaired glucose tolerance and type 2 diabetes mellitus. Metabolism 54:1168–1173, 2005 [DOI] [PubMed] [Google Scholar]
- 6.Porksen S, Nielsen LB, Kaas A, Kocova M, Chiarelli F, Orskov C, Holst JJ, Ploug KB, Hougaard P, Hansen L, Mortensen HB: Meal-stimulated glucagon release is associated with postprandial blood glucose level and does not interfere with glycemic control in children and adolescents with new-onset type 1 diabetes. J Clin Endocrinol Metab 92:2910–2916, 2007 [DOI] [PubMed] [Google Scholar]
- 7.Steele C, Hagopian WA, Gitelman S, Masharani U, Cavaghan M, Rother KI, Donaldson D, Harlan DM, Bluestone J, Herold KC: Insulin secretion in type 1 diabetes. Diabetes 53:426–433, 2004 [DOI] [PubMed] [Google Scholar]
- 8.Steffes MW, Sibley S, Jackson M, Thomas W: Beta-cell function and the development of diabetes-related complications in the diabetes control and complications trial. Diabetes Care 26:832–836, 2003 [DOI] [PubMed] [Google Scholar]