Abstract
OBJECTIVE—To evaluate the efficacy, safety, and tolerability of pregabalin across the effective dosing range, to determine differences in the efficacy of three times daily (TID) versus twice daily (BID) dosage schedules, and to use time-to-event analysis to determine the time to onset of a sustained therapeutic effect using data from seven trials of pregabalin in painful diabetic peripheral neuropathy (DPN).
RESEARCH DESIGN AND METHODS—Data were pooled across seven double-blind, randomized, placebo-controlled trials using pregabalin to treat painful DPN with dosages of 150, 300, and 600 mg/day administered TID or BID. Only one trial included all three of these dosages, and TID dosing was used in four. All studies shared fundamental selection criteria, and treatment durations ranged from 5 to 13 weeks.
RESULTS—Pooled analysis showed that pregabalin significantly reduced pain and pain-related sleep interference associated with DPN (150, 300, and 600 mg/day administered TID vs. placebo, all P ≤ 0.007). Only the 600 mg/day dosage showed efficacy when administered BID (P ≤ 0.001). Pain and sleep interference reductions associated with pregabalin appear to be positively correlated with dosage; the greatest effect was observed in patients treated with 600 mg/day. Kaplan-Meier analysis revealed that the median time to onset of a sustained (≥30% at end point) 1-point improvement was 4 days in patients treated with pregabalin at 600 mg/day, 5 days in patients treated with pregabalin at 300 mg/day, 13 days in patients treated with pregabalin at 150 mg/day, and 60 days in patients receiving placebo. The most common treatment-emergent adverse events were dizziness, somnolence, and peripheral edema.
CONCLUSIONS—Treatment with pregabalin across its effective dosing range is associated with significant, dose-related improvement in pain in patients with DPN.
The prevalence of diabetic neuropathy is as high as 50% in patients who have had diabetes for 25 years (1), and painful diabetic peripheral neuropathy (DPN) occurs in up to 26% of all people with diabetes (2). Symptoms range from mild dysesthesias to severe unremitting pain that can profoundly impact patients’ lives (3,4).
Medications of several different classes are used to treat painful DPN with varying degrees of efficacy, safety, and tolerability. The antiepileptic agents gabapentin and pregabalin have attained widespread use in the treatment of painful DPN. These agents bind to the auxiliary α2-δ subunit of the voltage-sensitive calcium channel, thereby decreasing Ca2+ influx at nerve terminals and modulating neurotransmitter release (5).
There are seven double-blind, randomized, placebo-controlled trials in painful DPN with pregabalin (6–12), five of which are published in full (7–11). The effective dosing range for treatment of neuropathic pain syndromes is 150 to 600 mg/day, administered either three times daily (TID) or twice daily (BID). Among the seven trials, dosages of 150, 300, and 600 mg/day were used, but only one trial included all three of these dosages. Thus, individually, the seven trials present an incomplete picture of the effective dosing range. In addition, TID dosing was used in the first four trials, whereas the three most recent trials of pregabalin in painful DPN used BID dosing.
The objective of the current report is to use the pooled data from these seven trials to evaluate the efficacy, safety, and tolerability of pregabalin across the effective dosing range. We also use these data to determine differences in the efficacy of TID and BID dosing schedules. Finally, we use a time-to-event analysis of the pooled data to determine the time to onset of a sustained therapeutic effect across the range of doses.
RESEARCH DESIGN AND METHODS—
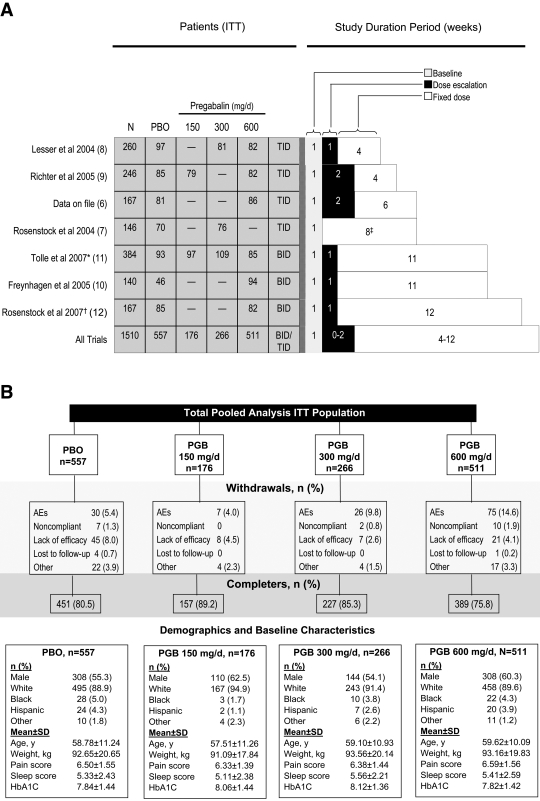
Study treatment durations ranged from 5 to 13 weeks (Fig. 1A). Four trials used TID dosing, three used BID dosing, and all but one trial (pregabalin 300 mg/day vs. placebo) used escalation to assigned fixed dosing over a period of 1 to 2 weeks. Patients were randomized to placebo or fixed-dosage pregabalin at 150, 300, or 600 mg/day. One trial included a 75-mg/day dosage arm (8); results for this dosage are not presented here, as 75 mg/day is considered to be a nontherapeutic dosage of pregabalin in painful DPN. One trial (10) studied both DPN and postherpetic neuralgia patients administered flexible-dosage pregabalin (150–600 mg/day), fixed-dosage pregabalin (600 mg/day), or placebo. To ensure consistency for the purposes of this analysis, only the DPN patients who received fixed-dosage pregabalin (28% of the total cohort) were included in this analysis.
Figure 1—
A: Dosage arms of seven trials contributing to this pooled analysis, ITT populations. *Trial used a modified ITT population: 11 patients were withdrawn by Ministry of Health/European Committee during a partial clinical hold. AEs, adverse events; PBO, placebo; PGB, pregablin. †Trial included 338 patients total, 96 of whom had painful DPN and were assigned to a fixed dosage of 600 mg/day pregabalin. Postherpetic neuralgia patients from this trial were not included in the present analysis nor were DPN patients assigned to flexible-dosage pregabalin. ‡No dose escalation. PBO, placebo. B: Pooled studies patient disposition with baseline demographics and characteristics.
Each of the studies shared fundamental inclusion criteria, including ≥18 years of age, an average pain score ≥4 (on an 11-point, Likert-like numeric rating scale [NRS]: 0 = “no pain” to 10 = “worst possible pain”) over a 7-day baseline period, and a score ≥40 mm on the 0- to 100-mm visual analog scale of the Short-Form McGill Pain Questionnaire at screening and randomization (baseline and randomization in one study). All patients in each trial were required to have A1C levels ≤11%. Prior therapeutic failure of gabapentin was an exclusion criterion in three studies (6–8). All patients provided informed consent before participation, and all studies were conducted in compliance with the ethics principles originating in or derived from the Declaration of Helsinki, internal review board requirements, or good clinical practices guidelines.
The primary efficacy measure in each study was end point mean pain score (on the 11-point NRS) derived from entries in patients’ daily pain diaries. A supplemental responder analysis using two definitions of response—patients with ≥50% and with ≥30% reductions in mean pain scores from baseline—was also performed. The studies included several secondary efficacy measures. End point mean sleep-interference score was derived from daily sleep diaries in which patients rated daily how much their pain had interfered with their sleep (also done using an 11-point NRS, with 0 = “pain does not interfere with sleep” to 10 = “pain completely interferes with sleep”). Each study included the Patient Global Impression of Change, in which patients rate their improvement on a 7-point scale ranging from “very much worse” to “very much improved” (6–12). These measures were also analyzed to determine whether there were significant differences versus placebo between BID and TID dosing regimens.
Finally, time to onset of sustained and clinically meaningful pain relief was investigated across the seven studies. This was defined as the first day on which patients demonstrated a ≥1-point reduction in mean pain score in patients with a ≥30 and ≥50% reduction in mean pain score at end point. These two criteria were imposed to ensure clinically meaningful and durable pain relief based on evidence that in studies with a similar design, a ≥30% improvement from baseline corresponds to a patient global impression of change of “much improved” or “very much improved” at study end point (13). Baseline pain scores in a typical clinical trial with painful DPN patients are between 6 and 6.5 on the 0- to 10-point scale; therefore, a 30% improvement is ∼2 points. We defined the “event” of interest in this time-to-event analysis as the time to the first ≥1-point reduction in the daily pain score, recognizing that any such criterion could be considered arbitrary. Time to onset of sustained pain relief was evaluated by applying the Kaplan-Meier procedure, and comparisons with placebo were made using the log rank test.
Safety measures included incidence of adverse events, physical and neurologic examinations, 12-lead electrocardiogram, vital signs, weight change, and clinical laboratory testing including A1C.
For the pooled analysis, all statistical testing of efficacy measures was performed on the intent-to-treat (ITT) population. End point mean pain scores using last observation carried forward and sleep interference were analyzed with ANCOVA (with a term for baseline values and a term for treatment), whereas other secondary efficacy measures (responders) were analyzed using a logistic regression model (with a term for baseline values and a term for treatment).
RESULTS—
A total of 1,510 patients represented the ITT population in the seven studies: 557 received placebo, and 953 received pregabalin. Ninety percent of patients were white, and 58% were male. Mean age was 59 years, mean weight was 93 kg, and mean baseline pain score was 6.5 (Fig. 1B).
Efficacy
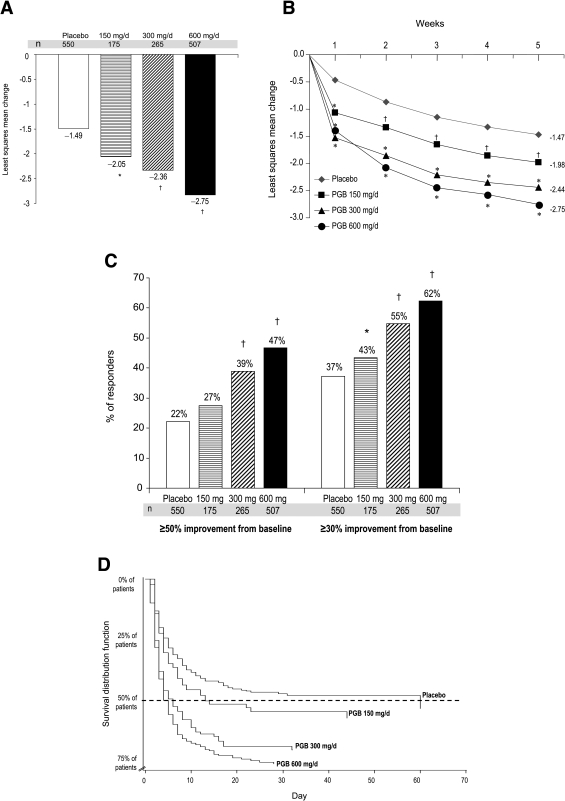
Significant reductions in end point least-squares mean pain scores were observed for all three dosages investigated (P = 0.007 for 150 mg/day and P < 0.0001 for 300 and 600 mg/day vs. placebo) (Fig. 2A and B). Pain reductions associated with pregabalin appear to be positively correlated with dosage, with the greatest effect observed in patients treated with 600 mg/day. The proportions of patients experiencing ≥50 or ≥30% reductions in pain levels (responders) were significantly greater in the pregabalin groups than in the placebo group (Fig. 2C) and were dose related.
Figure 2—
A: Change from baseline to end point in least-squares mean pain score based on last observation carried forward analysis. Patient population comprised of patients who had both baseline and end point assessments (numbers in some groups are therefore smaller than in the ITT population). Significant reductions in end point least-squares mean pain score were observed for all three dosages investigated: −2.05, −2.36, and −2.75 points for patients receiving pregabalin 150, 300, and 600 mg/day vs. −1.49 for patients receiving placebo (*P = 0.007 for 150 mg/day and †P < 0.0001 for 300 and 600 mg/day vs. placebo). B: Change from baseline to week 5 in least-squares mean pain score. Reductions were observed for all three dosages investigated: −1.98, −2.44, and −2.75 points for patients receiving pregabalin 150, 300, and 600 mg/day vs. −1.47 for patients receiving placebo (*P < 0.0001 vs. placebo; †P < 0.01 vs. placebo). C: Proportion of patients meeting ≥50% improvement and ≥30% improvement from baseline in mean pain score at end point based on last observation carried forward analysis. Patient population is comprised of patients who had both baseline and end point assessments (numbers in some groups are therefore smaller than in the ITT population). Among patients receiving 150, 300, and 600 mg/day pregabalin, 27, 39, and 47%, respectively, reported pain reductions ≥50% from baseline to end point, while 22% of placebo patients reported comparable reductions (pregabalin 300 and 600 mg/day, †P < 0.0001 vs. placebo). Using the ≥30% improvement criterion, a level of improvement deemed clinically meaningful (31), 43, 55, and 62% of patients treated with 150, 300, and 600 mg/day pregabalin, respectively, were responders vs. 37% of patients who received placebo (*P = 0.0455 for 150 mg/day, †P = 0.0001 for 300, and ‡P < 0.0001 for 600 mg/day vs. placebo). D: Survival curve analysis of the time to onset of meaningful pain relief, defined as the first day on which patients demonstrated a sustained ≥1 point in mean pain score where sustained is defined as a ≥30% reduction in mean pain score at end point. The median time to onset of a sustained (≥30% at end point) 1-point improvement was 4 days in patients treated with pregabalin at 600 mg/day, 5 days in patients treated with pregabalin at 300 mg/day, 13 days in patients treated with pregabalin at 150 mg/day, and 60 days in patients receiving placebo. Hazard ratios were 1.44 for pregabalin at 150 mg/day (P = 0.013), 1.84 for pregabalin at 300 mg/day (P < 0.0001), and 2.26 for pregabalin at 600 mg/day (P < 0.0001). PGB, pregablin.
The number needed to treat for these data are as follows: pregabalin 600 mg/day 4.04 (95% CI 3.3–5.3), pregabalin 300 mg/day 5.99 (4.2–10.4), and pregabalin 150 mg/day 19.06 (CI for the absolute risk reduction contains 0, rendering the CI for the number needed to treat difficult to interpret).
More patients treated with pregabalin reported global health status improvements than patients treated with placebo, as measured by the Patient Global Impression of Change. Eighty percent of pregabalin 600 mg/day patients, 74% of 300 mg/day patients, and 65% of 150 mg/day patients were improved, compared with 54% of placebo patients (300 and 600 mg/day, P < 0.0001).
In each of the above analyses, both BID and TID regimens of 600 mg/day pregabalin were significantly superior to placebo (P < 0.0001 for all comparisons of BID dosing to placebo and TID dosing to placebo). For 300 mg/day, only the TID dosing regimen was significantly superior to placebo (P < 0.0001 for all comparisons); however, the 300 mg/day BID dosage group was included in only one of the seven studies.
Kaplan-Meier analysis revealed that the median time to onset of sustained (≥30% improvement from baseline) pain relief was 4 days in patients treated with pregabalin at 600 mg/day, 5 days in patients treated with pregabalin at 300 mg/day, 13 days in patients treated with pregabalin at 150 mg/day, and 60 days in patients receiving placebo (Fig. 2D). Comparison of the pregabalin treatment groups with placebo by log rank test confirmed that time to onset of clinically meaningful pain relief was statistically significantly more rapid than with placebo (P < 0.0001 for pregabalin doses of 300 and 600 mg/day and P = 0.01 for 150 mg/day). The median time to onset of sustained (≥50% improvement from baseline) pain relief was 6 days in patients treated with pregabalin at 600 mg/day and 12 days in patients treated with pregabalin at 300 mg/day. Comparison of the pregabalin treatment groups with placebo by log rank test confirmed that time to onset of clinically meaningful pain relief was statistically significantly more rapid than with placebo (P < 0.0001 for pregabalin doses of 300 and 600 mg/day and P = NS for 150 mg/day).
Mean sleep interference scores at end point were also significantly improved in all three pregabalin groups, with 150, 300, and 600 mg/day showing reductions of −1.92, −2.32, and −2.62, respectively, compared with −1.32 for placebo (P = 0.003 for 150 mg/day and P < 0.0001 for 300 and 600 mg/day vs. placebo). As with change in mean pain score, improvement in sleep interference appeared to be positively correlated with dosage.
Safety and tolerability
Incidence of treatment-emergent adverse events (TEAEs) appeared to be related to dosage, with most of the common TEAEs having the greatest incidence among patients receiving 600 mg/day (Table 1). There was no consistent pattern in TEAE incidence rates by dosing regimen, with some TEAEs, such as peripheral edema and weight gain, having greater incidence in the BID dosing groups relative to the TID dosing groups, while other TEAEs, such as dizziness and somnolence, occurred with greater frequency in the TID than the BID groups. For all treatment groups, TEAEs were generally mild to moderate. The discontinuation rate due to adverse events was greatest in the 600 mg/day group (Fig. 1B). Serious TEAEs occurred in 3.4, 2.3, and 4.9% of patients receiving 150, 300, and 600 mg/day pregabalin and in 3.4% of patients receiving placebo. The most common serious TEAEs reported by both pregabalin and placebo patients were of cardiovascular nature and were not considered associated with treatment.
Table 1—
Common TEAEs and discontinuations occurring in ≥5% of any treatment group (ordered by greatest percentage of adverse events in the pregabalin 600 mg/day group)
| Placebo
|
Pregabalin
|
|||||||
|---|---|---|---|---|---|---|---|---|
| 150 mg/day
|
300 mg/day
|
600 mg/day
|
||||||
| n (%) | Discontinuation | n (%) | Discontinuation | n (%) | Discontinuation | n (%) | Discontinuation | |
| n | 557 | 176 | 266 | 511 | ||||
| Adverse event | ||||||||
| Dizziness | 26 (4.7) | 0.7 | 12 (6.8) | 1.1 | 62 (23.3) | 3.4 | 142 (27.8) | 6.8 |
| Peripheral edema* | 40 (7.2) | 0.5 | 10 (5.7) | 1.1 | 26 (9.8) | 1.5 | 82 (16.0) | 2.7 |
| Somnolence | 16 (2.9) | 0.4 | 9 (5.1) | 0.6 | 38 (14.3) | 3.0 | 68 (13.3) | 4.3 |
| Weight gain | 5 (0.9) | 0 | 8 (4.5) | 0 | 10 (3.8) | 0.4 | 45 (8.8) | 1.0 |
| Asthenia | 12 (2.2) | 0.2 | 4 (2.3) | 0.6 | 13 (4.9) | 2.3 | 44 (8.6) | 2.0 |
| Headache | 38 (6.8) | 1.1 | 12 (6.8) | 0.6 | 16 (6.0) | 0.8 | 35 (6.8) | 2.5 |
| Dry mouth | 7 (1.3) | 0 | 3 (1.7) | 0.6 | 13 (4.9) | 0.8 | 30 (5.9) | 1.8 |
| Accidental injury | 16 (2.9) | 0 | 4 (2.3) | 0 | 7 (2.6) | 0.4 | 26 (5.1) | 0.6 |
| Vertigo | 5 (0.9) | 0.4 | 3 (1.7) | 0 | 8 (3.0) | 1.5 | 25 (4.9) | 1.4 |
| Nausea | 29 (5.2) | 0.9 | 4 (2.3) | 0.6 | 8 (3.0) | 1.1 | 23 (4.5) | 2.0 |
| Pain | 18 (3.2) | 0.4 | 9 (5.1) | 0.6 | 8 (3.0) | 0.4 | 20 (3.9) | 0 |
| Infection | 35 (6.3) | 0 | 14 (8.0) | 0 | 23 (8.6) | 0.8 | 17 (3.3) | 0.4 |
| Edema | 0 | 0 | 4 (2.3) | 0 | 13 (4.9) | 0.4 | 10 (2.0) | 0 |
Data are % unless otherwise indicated.
One patient in the 600-mg group had both edema and peripheral edema.
Over the course of 5 to 13 weeks of treatment, the incidence of clinically meaningful weight gain (defined using a Food and Drug Administration–guided criterion of ≥7% weight increase from baseline to end point) for pregabalin versus placebo was dose related: 2.01% for pregabalin at 150 mg/day (P = 0.14 [95% CI −0.47 to 3.03%]), 2.12% for pregabalin at 300 mg/day (P = 0.04 [−0.09 to 2.86%]), and 3.88% for pregabalin at 600 mg/day (P < 0.0001 [1.76–4.54%]) groups, compared with 0.73% for the placebo group. The odds of weight gain compared with placebo are 2.3-fold for pregabalin at 150 mg/day (P = 0.14 [0.77–6.60%]), 2.8-fold for pregabalin at 300 mg/day (P = 0.04 [1.06–7.47%]), and 6.2-fold for pregabalin at 600 mg/day (P < 0.0001 [2.82–13.67%]). Mean changes in weight from baseline to end point for pregabalin-treated patients versus placebo control subjects were 0.76 kg for pregabalin at 150 mg/day (P = 0.02 [0.08–1.11 kg]), 1.86 kg for pregabalin at 300 mg/day (P < 0.0001 [1.26–2.14 kg]), and 2.04 kg for pregabalin at 600 mg/day (P < 0.0001 [1.54–2.22 kg]); the mean change was 0.16 kg for placebo. The incidence of ≥7% weight gain by study duration across all pregabalin doses is as follows: 5 weeks, 2.8%; 8 weeks, 6.8%; and 12–13 weeks, 7.9%.
There was a dose-related increase in edema and peripheral edema (Table 1). The presence of edema across doses and by severity is as follows: pregabalin at 150 mg/day, mild 64.3%, moderate 28.6%, and severe 7.1%; pregabalin at 300 mg/day, mild 56.4%, moderate 41%, and severe 2.6%; pregabalin at 600 mg/day, mild 63.4%, moderate 33%, and severe 1.1%; and placebo, mild 81%, moderate 19%, and severe 0%.
In all pregabalin-treated groups, 15.2% had edema or peripheral edema, 6.0% had a ≥7% weight increase, and 2.3% had both weight increase and edema. In comparison, in the placebo-administered group, 7.3% had edema or peripheral edema, 1.5% had a ≥7% weight increase, and 0.2% had both.
There were no clinically meaningful changes in laboratory values from baseline to end point reported in the studies. There were neither statistically significant nor clinically meaningful changes from baseline to end point in A1C values (% of total Hb) in pregabalin-treated patients and in control subjects over 5 to 13 weeks of treatment: pregabalin at 150 mg/day, 0.07% (95% CI −0.07 to 0.24); pregabalin at 300 mg/day, 0.01% (−0.01 to 0.26); pregabalin at 600 mg/day, 0.08% (−0.09 to 0.13); and placebo, 0.03% (−0.05 to 0.11).
CONCLUSIONS—
In this pooled analysis of patients with painful DPN from seven randomized, controlled trials spanning the effective dose range, pregabalin was shown to significantly reduce pain associated with DPN. The pooled analysis, in contrast to individual reports, revealed efficacy of the 150-mg dose; however, efficacy with BID dosing was only present with the 600-mg dose. In addition, time to event analysis revealed that pregabalin was associated with a dose-related, rapid onset of sustained pain relief.
Several anticonvulsants with diverse mechanisms of action have been subjected to large-scale randomized controlled trials assessing therapeutic efficacy in the treatment of painful DPN. These agents, which include topiramate (14–16), lamotrigine (17), oxcarbazepine (18,19), and gabapentin (20–22) have shown varying efficacy in clinical trials.
In contrast, pregabalin has shown efficacy in six of seven clinical trials. Among the seven trials, one included 150-, 300-, and 600-mg/day treatment arms, two included two of these dosages, and four included only one of these dosages (Fig. 1A). Pooling data from all treatment arms in this analysis adds to our knowledge of the efficacy, safety, and tolerability of pregabalin for treatment of painful DPN. In terms of efficacy, there was an evident dose response, with the greatest efficacy observed among patients treated with 600 mg/day. Equally evident from this pooled analysis—but not from examination of the trials individually, as 150 mg/day was not significantly efficacious in any individual study—was the observation that patients treated with pregabalin at its lowest effective dosage for chronic neuropathic pain, 150 mg/day, experienced statistically significant improvements in their pain and pain-related sleep interference and responded to pregabalin (≥30% improvement) in proportions significantly greater than placebo.
Time to onset analysis of the pooled data revealed dose-related, rapid onset of durable pain relief. By day 4, 50% of the subjects taking 600 mg/day had a sustained (≥30% at end point) 1-point improvement in pain score, while a response of this magnitude was achieved by day 5 in the 300 mg/day group. All seven studies showed statistically significant differences between pregabalin and placebo by week 1 (6–10,12) or week 2 (11); however, these analyses do not necessarily imply that the response is clinically meaningful. This approach also does not provide insight into the durability of the response in individual patients. Although not frequently used in pain therapeutic trials (23), the time to onset analysis used here provides numerical and graphic (Fig. 2D) information that is clinically relevant for patient and clinician, specifically the likelihood of a predetermined clinical response (the hazard ratio) and the time to this response. The analysis in this report was complicated by the different dose escalation schedules of the individual studies. Since time to onset was determined from the start of dose escalation and not the time point when an effective therapeutic dose was attained, the analysis may have overestimated the time to sustained efficacy. Future studies should incorporate this analytic technique prospectively.
The present analyses revealed that dosing schedule (BID vs. TID) apparently made no meaningful difference for patients treated with 600 mg/day, as both regimens were highly statistically significant versus placebo. This finding is in contrast to studies of postherpetic neuralgia in which BID efficacy was demonstrated across a range of doses from 150 to 600 mg/day (24). The basis for this difference in efficacy between disease states is not known, although it may be related, in part, to the permitted use of concomitant pain medication in all postherpetic neuralgia pregabalin clinical trials (24–26). However, the 150- and 300-mg/day doses were used BID in only one study in this pooled analysis (15), and further studies are required to definitively address this question.
The dose-related increase in efficacy was accompanied by a dose-related increase in incidence of most adverse events. Similarly, the rate of discontinuation due to an adverse event was dose related. Dizziness, somnolence, and peripheral edema were the most common adverse events. While there was a consistent increase in the incidence of dizziness across doses, the incidence of somnolence was similar in the 300- and 600-mg/day doses. Examination of adverse events by dosing regimen, i.e., BID versus TID, for each pregabalin daily dosage did not reveal any consistent patterns favoring one regimen over the other. There was a dose-related increase in peripheral edema (Table 1). Edema was not an exclusion criterion in any study; however, clinical judgment is warranted when pregabalin is used in patients with preexisting edema. Pregabalin was not associated with cardiovascular complications, rarely led to discontinuations, and was not associated with laboratory changes suggestive of renal or hepatic failure. The incidence of reported weight gain was not only dose related but also dependent on duration of exposure. The underlying cause of the weight gain is not known and does not appear to be related to the presence of peripheral edema. There was no evidence that the weight increase compromised glycemic control; the pooled analysis showed no clinically meaningful changes in A1C values in any dose cohort in studies with treatment durations of 5 to 13 weeks. Long-term studies are warranted to address this question.
In conclusion, the current pooled analysis of seven randomized, controlled clinical trials in patients with painful DPN showed that over the effective dose range, pregabalin not only significantly reduced pain associated with DPN but was also associated with rapid onset of sustained pain relief. The improvement in pain relief was accompanied by a dose-related incidence of adverse events.
Acknowledgments
This research was sponsored by Pfizer.
Administrative and copyediting support was provided by Adelphi Inc. and also by Pfizer.
R.F. has served as a consultant to Pfizer.
The costs of publication of this article were defrayed in part by the payment of page charges. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C Section 1734 solely to indicate this fact.
References
- 1.Pirart J: Diabetes mellitus and its degenerative complications: a prospective study of 4,400 patients observed between 1947 and 1973. Diabete Metab 3:245–256, 1977 [PubMed] [Google Scholar]
- 2.Davies M, Brophy S, Williams R, Taylor A: The prevalence, severity, and impact of painful diabetic peripheral neuropathy in type 2. Diabetes Care 29:1518–1522, 2006 [DOI] [PubMed] [Google Scholar]
- 3.Galer BS, Gianas A, Jensen MP: Painful diabetic polyneuropathy: epidemiology, pain description, and quality of life. Diabetes Res Clin Pract 47:123–128, 2000 [DOI] [PubMed] [Google Scholar]
- 4.Boulton AJ, Vinik AI, Arezzo JC, Bril V, Feldman EL, Freeman R, Malik RA, Maser RE, Sosenko JM, Ziegler D: Diabetic neuropathies. Diabetes Care 28:956–962, 2005 [DOI] [PubMed] [Google Scholar]
- 5.Dooley DJ, Taylor CP, Donevan S, Feltner D: Ca2+ channel alpha2delta ligands: novel modulators of neurotransmission. Trends Pharmacol Sci 28:75–82, 2007 [DOI] [PubMed] [Google Scholar]
- 6.Pfizer Protocol No. 1008–040: A placebo-controlled trial of pregabalin and amitriptyline for treatment of painful diabetic peripheral neuropathy [article online], 2007. Available from http://www.clinicalstudyresults.org/drugdetails/?drug_name_id=203&sort=c.company_name&page=2&drug_id=1952. Accessed 28 June 2007
- 7.Rosenstock J, Tuchman M, La Moreaux L, Sharma U: Pregabalin for the treatment of painful diabetic peripheral neuropathy: a double-blind, placebo-controlled trial. Pain 110:628–638, 2004 [DOI] [PubMed] [Google Scholar]
- 8.Lesser H, Sharma U, La Moreaux L, Poole RM: Pregabalin relieves symptoms of painful diabetic neuropathy: a randomized controlled trial. Neurology 63:2104–2110, 2004 [DOI] [PubMed] [Google Scholar]
- 9.Richter RW, Portenoy R, Sharma U, LaMoreaux L, Bockbrader H, Knapp LE: Relief of painful diabetic peripheral neuropathy with pregabalin: a randomized, placebo-controlled trial. J Pain 6:253–260, 2005 [DOI] [PubMed] [Google Scholar]
- 10.Freynhagen R, Strojek K, Griesing T, Whalen E, Balkenohl M: Efficacy of pregabalin in neuropathic pain evaluated in a 12-week, randomised, double-blind, multicentre, placebo-controlled trial of flexible- and fixed-dose regimens. Pain 115:254–263, 2005 [DOI] [PubMed] [Google Scholar]
- 11.Tölle T, Freynhagen R, Versavel M, Trostmann U, Young JP Jr: Pregabalin for relief of neuropathic pain associated with diabetic neuropathy: a randomized, double-blind study. Eur J Pain 12:203–213, 2008 [DOI] [PubMed] [Google Scholar]
- 12.Rosenstock J, Arezzo J, Pauer L, LaMoreaux L, Barrett J, Durso-De Cruz E, Pfizer Global R&D: Pregabalin as treatment of painful diabetic peripheral neuropathy (DPN): nerve conduction and analgesic effect in a 13-week double-blind, placebo-controlled trial (Poster). Pain 8(Suppl. 1):S27, 2007 [Google Scholar]
- 13.Farrar JT, Young JP Jr, LaMoreaux L, Werth JL, Poole RM: Clinical importance of changes in chronic pain intensity measured on 11-point numerical pain rating scale. Pain 94:149–158, 2001 [DOI] [PubMed] [Google Scholar]
- 14.Thienel U, Neto W, Schwabe SK, Vijapurkar U: Topiramate in painful diabetic polyneuropathy: findings from three double-blind placebo-controlled trials. Acta Neurol Scand 110:221–231, 2004 [DOI] [PubMed] [Google Scholar]
- 15.Raskin P, Donofrio PD, Rosenthal NR, Hewitt DJ, Jordan DM, Xiang J, Vinik AI; CAPSS-141 Study Group: Topiramate vs placebo in painful diabetic neuropathy: analgesic and metabolic effects. Neurology 63:865–873, 2004 [DOI] [PubMed] [Google Scholar]
- 16.Freeman R, McIntosh KA, Vijapurkar U, Thienel U: Topiramate and physiologic measures of nerve function in polyneuropathy. Acta Neurol Scand 115:222–231, 2007 [DOI] [PubMed] [Google Scholar]
- 17.Vinik AI, Tuchman M, Safirstein B, Corder C, Kirby L, Wilks K, Quessy S, Blum D, Grainger J, White J, Silver M: Lamotrigine for treatment of pain associated with diabetic neuropathy: results of two randomized, double-blind, placebo-controlled studies. Pain 128:169–179, 2007 [DOI] [PubMed] [Google Scholar]
- 18.Grosskopf J, Mazzola J, Wan Y, Hopwood M: A randomized, placebo-controlled study of oxcarbazepine in painful diabetic neuropathy. Acta Neurol Scand 114:177–180, 2006 [DOI] [PubMed] [Google Scholar]
- 19.Beydoun A, Shaibani A, Hopwood M, Wan Y: Oxcarbazepine in painful diabetic neuropathy: results of a dose-ranging study. Acta Neurol Scand 113:395–404, 2006 [DOI] [PubMed] [Google Scholar]
- 20.Backonja M, Beydoun A, Edwards KR, Schwartz SL, Fonseca V, Hes M, LaMoreaux L, Garofalo E: Gabapentin for the symptomatic treatment of painful neuropathy in patients with diabetes mellitus: a randomized controlled trial. JAMA 280:1831–1836, 1998 [DOI] [PubMed] [Google Scholar]
- 21.Backonja M, Glanzman RL: Gabapentin dosing for neuropathic pain: evidence from randomized, placebo-controlled clinical trials. Clin Ther 25:81–104, 2003 [DOI] [PubMed] [Google Scholar]
- 22.Gorson KC, Schott C, Herman R, Ropper AH, Rand WM: Gabapentin in the treatment of painful diabetic neuropathy: a placebo controlled, double blind, crossover trial. J Neurol Neurosurg Psychiatry 66:251–252, 1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Freeman R, Raskin P, Hewitt DJ, Vorsanger GJ, Jordan DM, Xiang J, Rosenthal NR: Randomized study of tramadol/acetaminophen versus placebo in painful diabetic peripheral neuropathy. Curr Med Res Opin 23:147–161, 2007 [DOI] [PubMed] [Google Scholar]
- 24.van Seventer R, Feister HA, Young JP, Jr Stoker M, Versavel M, Rigaudy L: Efficacy and tolerability of twice-daily pregabalin for treating pain and related sleep interference in postherpetic neuralgia: a 13-week, randomized trial. Curr Med Res Opin 22:375–384, 2006 [DOI] [PubMed] [Google Scholar]
- 25.Dworkin RH, Corbin AE, Young JP, Jr, Sharma U, LaMoreaux L, Bockbrader H, Garofalo EA, Poole RM: Pregabalin for the treatment of postherpetic neuralgia: a randomized, placebo-controlled trial. Neurology 60:1274–1283, 2003 [DOI] [PubMed] [Google Scholar]
- 26.Sabatowski R, Galvez R, Cherry DA, Jacquot F, Vincent E, Maisonobe P, Versavel M: Pregabalin reduces pain and improves sleep and mood disturbances in patients with post-herpetic neuralgia: results of a randomised, placebo-controlled clinical trial. Pain 109:26–35, 2004 [DOI] [PubMed] [Google Scholar]