Abstract
Promising anti-breast cancer agents derived from substituted quinolines were discovered. The quinolines were readily synthesized in large scale from a sequence of reactions starting from 4-acetamidoanisole. The Michael addition product was isolated as the reaction intermediate in the ring closing reaction of 4-amino-5-nitro-2-(3-trifluoromethylphenyloxy)anisole with methyl vinyl ketone leading to 6-methoxy-4-methyl-8-nitro-5-(3-trifluoromethylphenyloxy)quinoline (14). The amino function of 8-amino-6-methoxy-4-methyl-5-(3-trifluoromethylphenyloxy)quinoline, prepared from 14, was connected to various side chains via alkylation with N-(3-iodopropyl)phthalimide, Michael addition with acrylonitrile, and reductive amination with various heterocycle carboxaldehydes, such as imidazole-4-carboxaldehyde, thiophene-2-carboxaldehyde, and 2-furaldehyde. Effects of the substituted quinolines on cell viability of T47D breast cancer cells using trypan blue exclusion assay were examined. The results showed that the IC50 value of 6-methoxy-8-[(2-furanylmethyl)amino]-4-methyl-5-(3-trifluoromethylphenyloxy)quinoline is 16 ± 3 nM, the lowest IC50 out of all the quinolines tested. IC50 values of three other quinolines are in the nanomolar range, a desirable range for pharmacological testing.
Keywords: synthesis of substituted quinolines, anti-breast-cancer agents, T47D breast cancer cells
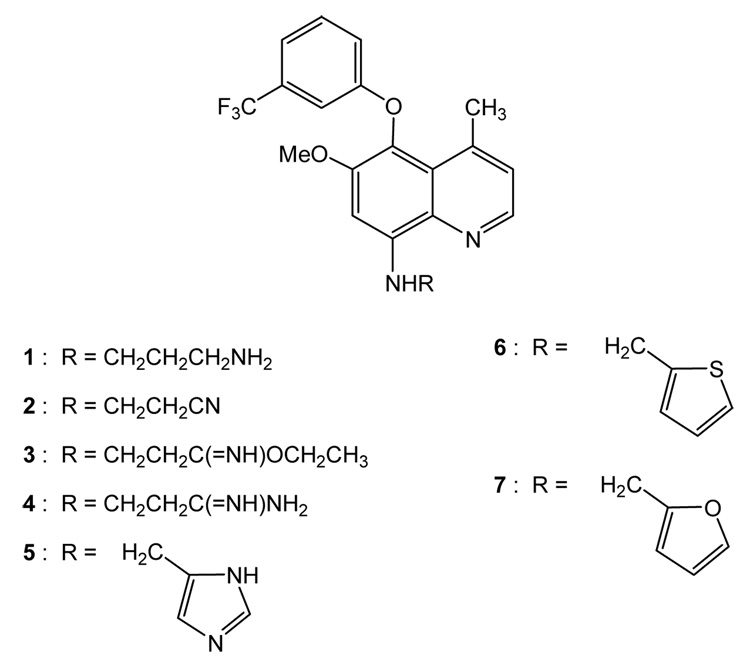
Quinolines are known for their anti-malarial,1–3 leishmanicidal,4 antibacterial5 and anticancer activities.6–9 Recently, quinolines were examined in ATP-binding cassette drug transporter inhibition,6 targeting tumor hypoxia,7 modulation of multidrug resistance,8 and tyrosine kinase inhibition.9 Based on these literature results, we investigated substituted quinolines in search of novel anti-breast cancer compounds. After our initial anticancer screening, we focused on substituted quinolines with a skeletal structure derived from 8-amino-5-(aryloxy)-6-methoxy-4-methylquinoline,1 by derivatizing its C8-amino side chain. We report herein syntheses of new quinolines possessing amine, nitrile, imidate, amidine and heterocyclic functionalities in the C8-amino side chain, and their potent anti-breast cancer activities against T47D breast cancer cells. The synthetic substituted quinolines are summarzied in Figure 1.
Figure 1.
Structural formulas of substituted quinolines
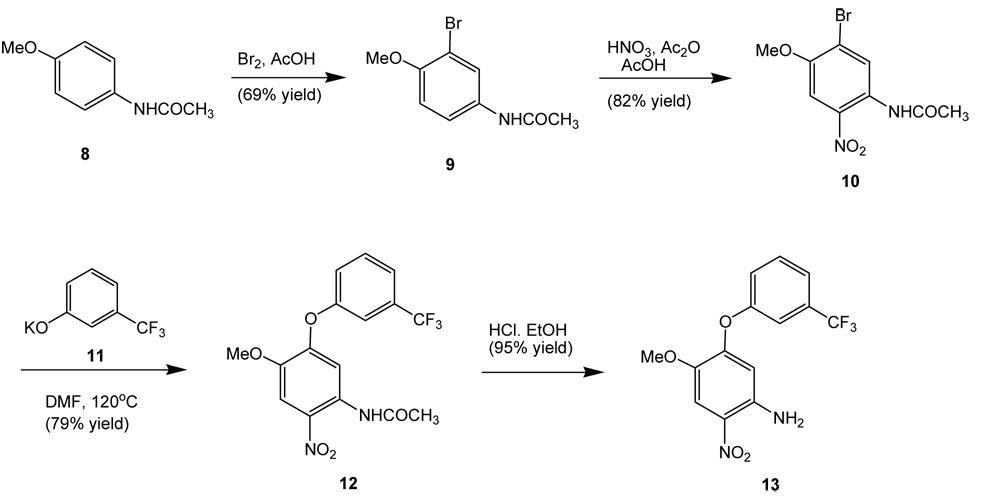
We utilized a similar synthetic method leading to 5-(aryloxy)-4-methylprimaquine1,10 by starting with 4-acetamidoanisole (8) via sequential C2 and C5 functionalizations followed by a ring closing reaction. Hence, 2-bromo-4-acetamino-5-nitroanisole (10)10 was prepared from the bromination of compound 8 at C2 followed by nitration at C5. Displacement of bromide 6 with potassium 3-trifluoromethylphenoxide (11) in N,N-dimethylformamide (DMF) at 120°C gave 4-acetamino-5-nitro-2-(3-trifluoromethylphenyloxy)anisole (12). Removal of the acetyl protecting group of 12 with hydrochloric acid in ethanol afforded 4-amino-5-nitro-2-(3-trifluoromethylphenyloxy)anisole (13).
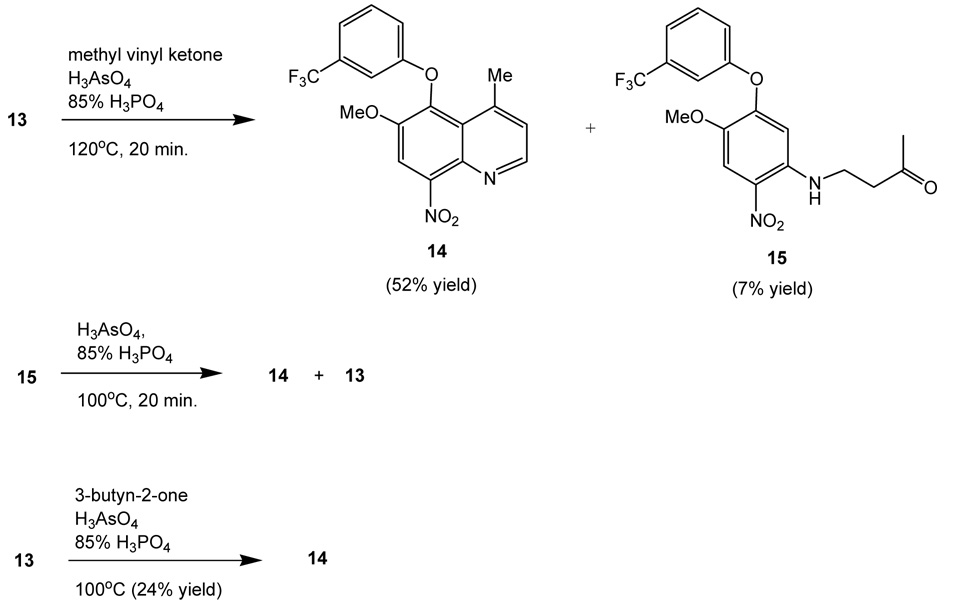
Various reaction conditions were studied to maximize the yield of the construction of the quinoline ring from compound 13 with methyl vinyl ketone.2 Treatment of 13 with vinyl methyl ketone, arsenic acid and 85% phosphoric acid at 100 °C after 20 minutes,8 a mixture of desired quinoline 14 and 1,4-adduct 15 along with starting material 13 was obtained in a ratio of 1:2:1. When the reaction was carried out at 120 °C for 20 minutes, a 7:1:1 ratio of compounds 14:15:13 was achieved (Scheme 2). The crude products were separated by silica gel column chromatography to give a 52% yield of compound 14, 7% yield of compound 15, and 7% recovery of 13. A longer reaction time resulted in decomposition of the products and yields were not improved. The use of excess of vinyl methyl ketone did not improve the yield either. The 1,4-adduct, 15, can be treated with arsenic acid and phosphoric acid under similar reaction conditions as that mentioned above to give quinoline 14 and amine 13 along with starting material 15 in a ratio of ~7:1:1. Hence, uses of a large excess of arsenic acid and 85% phosphoric acid may minimize the formation of intermediate 15. The results suggest that adduct 15 is the reaction intermediate leading to quinoline 14, but it also underwent reversed Michael addition reaction to provide amine 13 and vinyl methyl ketone. Since conjugated alkynones had been used in the construction of quinolines from aromatic amines,4,11 amine 13 was treated with 3-butyn-2-one and arsenic acid and phosphoric acid at 100°C. Desired product 14 was isolated in a 24% yield along with 28% of recovery of starting material 13. The 1,4-adduct was not detected, and various unidentifiable oligomers were obtained. The lower yield may contribute to the ease of polymerization of the terminal alkynone or the decomposition of the intermediate 1,4-adduct.
Scheme 2.
Preparation of quinoline 14.
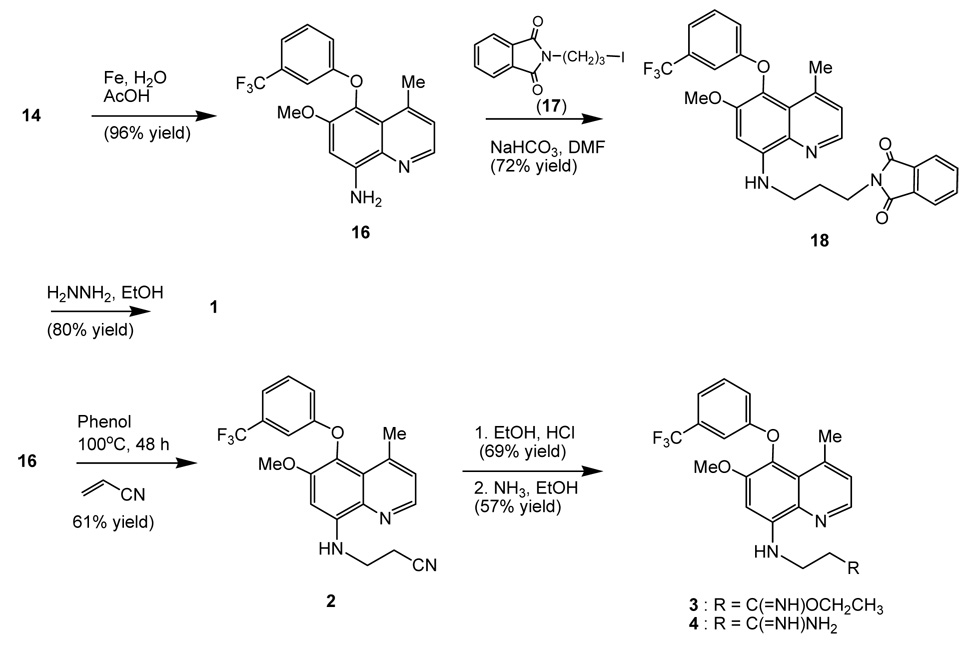
Quinoline 14 was converted to compound 16, which serves as the precursor to produce various substituted quinolines depicted in Figure 1. Hence, reduction of the nitro function of 14 with iron powder in acetic acid-water under reflux gave a 96% yield of amino quinoline 16 (Scheme 3). Alkylation of 16 with iodide 1712 and sodium bicarbonate in DMF produced compound 18 which upon treatment with hydrazine in refluxing ethanol afforded quinoline 1.
Scheme 3.
Synthesis of substituted quinolines 1 ~ 4.
Quinolines 2 – 4 were synthesized through a Michael addition reaction of arylamine 16 with acrylonitrile in phenol13 at 100°C in a sealed tube and gave adduct 2 (61% yield). No reaction was found when 16 was treated with acrylonitrile in refluxing ethanol.14 Ethanolysis of quinoline 2 with HCl (gas) in ethanol15 produced ethyl imidate 3, which upon treatment with NH3 (gas) in ethanol16 at 50°C for 6 h afforded amidine 4 (57% yield) along with 13% of recovered starting material 3.
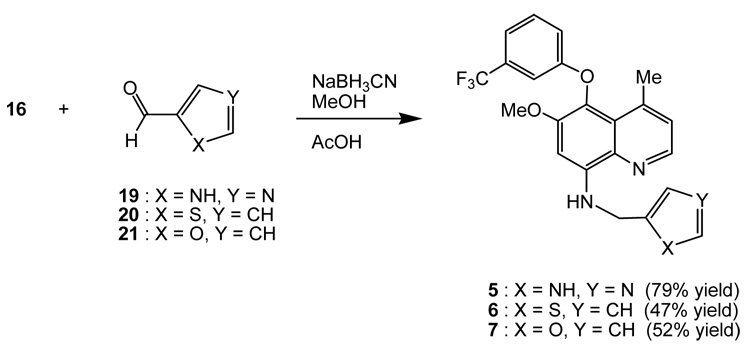
Quinolines 5 – 7 were synthesized via a reductive amination of amine 16 and aldehydes (Scheme 4). Treatment of amine 16 with aldehyde 19 in methanol-acetic acid followed by sodium cyanoborohydride17 furnished quinoline 5. Similar treatment of amine 16 with aldehydes 20 and 21 separately afforded quinolines 6 and 7, respectively. Aldehyde 19 was prepared from the oxidation of 4-hydroxymethylimidazole with manganese dioxide in methanol (99% yield).18
Scheme 4.
Synthesis of substituted quinolines 5 ~ 7.
The effects of quinolines 1 – 7 on cell viability of T47D breast cancer cells using trypan blue exclusion assay were examined. The trypan blue exclusion assay provides a rapid and effective means in screening multiple drugs.19 Assays for each compound were conducted in triplicate and the statistical significances are shown along with the IC50 values in Table 1. The results showed that the IC50 value of quinoline 7 is 16 ± 3 nM, the lowest IC50 out of all the quinolines tested. The weak inhibition of quinoline 6 may due to the oxidation of sulfur of the thiophene moiety during the two-day incubation with the T47D breast cancer cells. IC50 values of 1, 2, and 4 are in the nanomolar range, a desirable range for pharmacological testing. Compounds 16 and 18 did not inhibit T47D cell viability even at 10 µM concentration.
Table 1.
Cell Viability using Trypan Blue Exclusion. T47D breast cancer cells were treated with various concentrations of 1 – 7 for 2 days. A cell suspension was mixed with trypan blue dye and then visually examined to determine whether cells take up or exclude dye. The number of live cells (excluded dye) was quantified and IC50 value for each compound was determined.
| Compound | 1 | 2 | 3 | 4 | 5 | 6 | 7 |
|---|---|---|---|---|---|---|---|
| IC50 value (nM) | 119 ± 21 | 378 ± 79 | 1974 ± 404 | 519 ± 102 | 1276 ± 246 | 3732 ± 696 | 15.6 ± 3.0 |
In conclusion, various substituted quinolines were synthesized from a tandem Michael addition followed by electrophilic aromatic substitution reaction of substituted aniline with vinyl methyl ketone, reduction of the nitro function, and alkylation of the resulting amine moiety. The synthetic sequence is short and amenable to large scale synthesis. Several of these compounds possess potent anticancer activities against T47D breast cancer cells in nM ranges. In particular, quinoline 7 showed an IC50 value of 16 ± 3 nM, and is worthy of further pharmacological studies. The absence of asymmetric center in these molecules alleviates the chiral synthesis and purification of enantiomers for bioevaluation. Variation of substituents of these lead compounds along with the mechanism of action of their anticancer activity is being studied and will be reported in due course.
Supplementary Material
Supplementary data Synthetic procedure, analysis data, cell line and cell culture, and protocols for cell-viability assay. Supplementary data associated with this article can be found, in the online version, at doi:
Scheme 1.
Preparation of 4-amino-5-nitro-2-(3-trifluoromethylphenyloxy)anisole (13)
Acknowledgements
We gratefully acknowledge financial support by EYRO113421 to D.J.T., NIH R01AG025500, NSF CHE-0555341 and American Heart Association 0750115Z to D.H.H., and NIH P20RR017686 to T.A.N.
Footnotes
This manuscript is dedicated to Professor E. J. Corey on the occasion of his 80th birthday.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.LaMontagne MP, Markovac A, Khan MS. J. Med. Chem. 1982;25:964. doi: 10.1021/jm00350a016. [DOI] [PubMed] [Google Scholar]
- 2.LaMontagne MP, Blumbergs P, Strube RE. J. Med. Chem. 1982;25:1094. doi: 10.1021/jm00351a017. [DOI] [PubMed] [Google Scholar]
- 3.Wiesner J, Ortmann R, Jomaa H, Schlitzer M. Angew. Chem. Int. Ed. 2003;42:5274. doi: 10.1002/anie.200200569. [DOI] [PubMed] [Google Scholar]
- 4.Dade J, Provot O, Moskowitz H, Mayrargue J, Prina E. Chem. Pharm. Bull. 2001;49:480. doi: 10.1248/cpb.49.480. [DOI] [PubMed] [Google Scholar]
- 5.Veyssier P, Bryskier A. Antimicrobial Agents. 2005:941–963. [Google Scholar]
- 6.Cnubben NHP, Wortelboer HM, van Zanden JJ, Rietjens IMCM, van Bladeren PJ. Expert Opinion on Drug Metabol. & Toxicol. 2005;1:219. doi: 10.1517/17425255.1.2.219. [DOI] [PubMed] [Google Scholar]
- 7.Boyle RG, Travers S. Anti-cancer Agents in Med. Chem. 2006;6:281. doi: 10.2174/187152006777698169. [DOI] [PubMed] [Google Scholar]
- 8.Kawase M, Motohashi N. Curr. Drug Targets. 2003;4:31. doi: 10.2174/1389450033347064. [DOI] [PubMed] [Google Scholar]
- 9.Levitt ML, Koty PP. Invest. New Drugs. 1999;17:213. doi: 10.1023/a:1006372102543. [DOI] [PubMed] [Google Scholar]
- 10.Lauer WM, Rondestvedt C, Arnold RT, Drake NL, van Hook J, Tinker J. J. Am. Chem. Soc. 1946;68:1546. doi: 10.1021/ja01212a045. [DOI] [PubMed] [Google Scholar]
- 11.von Dieter Sicker Es, Wilde H. Helv. Chim. Acta. 1996;79:658. [Google Scholar]
- 12.Kaji E, Zen S. Bull. Chem. Soc. Japan. 1973;46:337. [Google Scholar]
- 13.Butskus PF. Zhurnal Obshchei Khimii. 1961;31:764. [Google Scholar]
- 14.Klenke B, Stewart M, Barrett MP, Brun R, Gilbert IH. J. Med. Chem. 2001;44:3440. doi: 10.1021/jm010854+. [DOI] [PubMed] [Google Scholar]
- 15.McElvain SM, Nelson JW. J. Am. Chem. Soc. 1942;64:1825. [Google Scholar]
- 16.Easson APT, Pyman FL. J. Chem. Soc. 1931:2991. [Google Scholar]
- 17.Borch RF, Bernstein MD, Durst HD. J. Am. Chem. Soc. 1971;93:2897. [Google Scholar]
- 18.McNab H. J. Chem. Soc., Perkin Trans. 1: Org. & Bio-Org. Chem. 1987;3:653. [Google Scholar]
- 19.Lanzilli G, Fuggetta MP, Tricarico M, Cottarelli A, Serafino A, Falchetti R, Ravagnan G, Turriziani M, Adamo R, Franzese O, Bonmassar E. Int. J. Oncol. 2006;28:641. [PubMed] [Google Scholar]
- 20.Deng R, Zhong J, Dong Z, Wang J, Ding D, Shi Y, Wang S, Yang J, Guo B, Gao X. Yaoxue Xuebao. 1984;19:343. NMR spectral data are not reported. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary data Synthetic procedure, analysis data, cell line and cell culture, and protocols for cell-viability assay. Supplementary data associated with this article can be found, in the online version, at doi: