Abstract
Ascorbic acid in its reduced form is not transported across the capillary endothelial cell blood-brain barrier. This is thought to be due to absence of the SVCT2, a specific transporter for ascorbate. To assess this directly we prepared primary cultures of mouse cortical microvascular endothelial cells. When still in the capillaries, these cells did not express the SVCT2 protein as assessed by immunocytochemistry and by immunoblotting. However, during several days in culture, they developed SVCT2 expression and showed ascorbate transport rates comparable to those in immortalized endothelial cell lines. SVCT2 expression was inversely proportional to cell density, was enhanced by culture at low physiologic plasma ascorbate concentrations, was inhibited by ascorbate concentrations expected in the brain interstitium, and was stimulated by cobalt ions. Expression of the SVCT2 was associated with ascorbate-dependent maturation and release of type IV collagen by the cells in culture. Although the SVCT2 is induced by culture of cortical capillary endothelial cells, its absence in vivo remains perplexing, given the need for intracellular ascorbate to facilitate type IV collagen maturation and release by endothelial cells.
Section: Structural Organization of the Brain
Keywords: SVCT2; ascorbate transport; cobalt, collagen; cortical capillary endothelial cells
Vitamin C, or ascorbic acid, is an important antioxidant and enzyme co-factor in the central nervous system, where it has several functions, including antioxidant protection (Rice2000), peptide amidation (Eipper et al., 1983), myelin formation (Eldridge et al., 1987), enhancement of synaptic activity (Qiu et al., 2007), and protection against glutamate toxicity (Rebec et al., 1994). In contrast to other organs, however, very little ascorbate appears to enter the brain directly across capillary endothelial cells forming the blood-brain barrier (BBB). Rather, the vitamin is transported from the blood into the cerebrospinal fluid through the choroid plexus (Agus et al., 1997; Spector1977) and by specialized cells lining the third ventricle (García et al., 2005). Plasma concentrations of ascorbate are 30–60 µM, and these are increased to 200–400 µM in the cerebrospinal fluid through this mechanism (Reiber et al., 1993; Spector et al., 1973). Transport of ascorbate into neurons further increases the gradient, since neurons contain between 1–10 mM ascorbate, depending on the brain region studied (Rice2000). This two-step gradient of ascorbate is generated by a specific ascorbate transporter termed the sodiumdependent vitamin C transporter, type 2 (SVCT2). This transporter, the sequence and structure/function characteristics of which were determined in 1999 (Tsukaguchi et al., 1999), mediates sodium- and energy dependent movement of ascorbate across the plasma membrane and into most non-epithelial cells in the organism.
Transport of ascorbate across the endothelial cell BBB occurs (Agus et al., 1997; Lam et al., 1986), but is much slower than is movement of dehydroascorbate (DHA) across the BBB (Agus et al., 1997). DHA, the two-electron oxidized form of ascorbate, is transported by the GLUT-type glucose transporters across the BBB (Agus et al., 1997; Rumsey et al., 1997; Vera et al., 1993). It is then rapidly taken up on GLUT-type transporters by neurons and other cells in the brain and converted to ascorbate within cells (Wilson2005). In vivo evidence for lack of effective ascorbate transport across the BBB derives from direct measurements in mice injected with radiolabeled ascorbate (Agus et al., 1997) and from studies showing that parenteral DHA, but not ascorbate, administration decreases infarct size after middle cerebral artery occlusion in mice (Huang et al., 2001). Further evidence for lack of the SVCT2 in cerebrovascular endothelial cells is that these cells contain neither SVCT2 mRNA nor SVCT2 protein when studied by in situ hybridization and immunostaining, respectively (García et al., 2005). Undetectable SVCT2 in brain capillary endothelial cells brings up questions of whether and how ascorbate enters these cells. Indeed, one of the major functions of endothelial cells is to deposit type IV collagen to form the basement membrane of the capillaries (Gould et al., 2005). Intracellular ascorbate is required for the hydroxylation of proline and lysine in pro-collagen to form mature folded collagen that can be secreted from the cell (May et al., 2005). Thus, the regulation of ascorbate transport in capillary endothelial cells is of importance in assessing their function to form and maintain the integrity of brain capillaries.
To study regulation of ascorbate transport across the BBB, we examined primary culture cortical capillary endothelial cells (CCECs). These cells can be prepared from mouse cortical microvessels with little contamination by perivascular cells and maintained in culture (Wu et al., 2003). As expected, we found that these cells lacked the SVCT2 protein when resident in the capillaries. However, they rapidly developed it in culture after several days. Understanding the mechanism by which these cells acquire ascorbate transport due to the SVCT2 might shed light on regulation of this transporter. Therefore, we examined factors affecting the development of SVCT2 expression and its association with ascorbate-dependent type IV collagen synthesis and release in these cells.
1. Results
1.1 Development of the SVCT2 in brain capillary endothelial cells
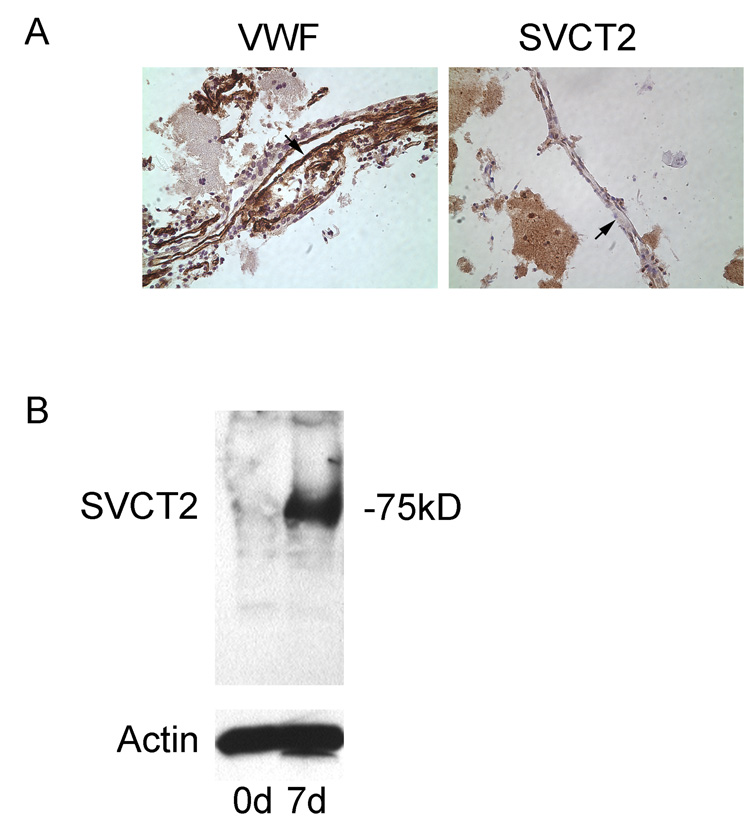
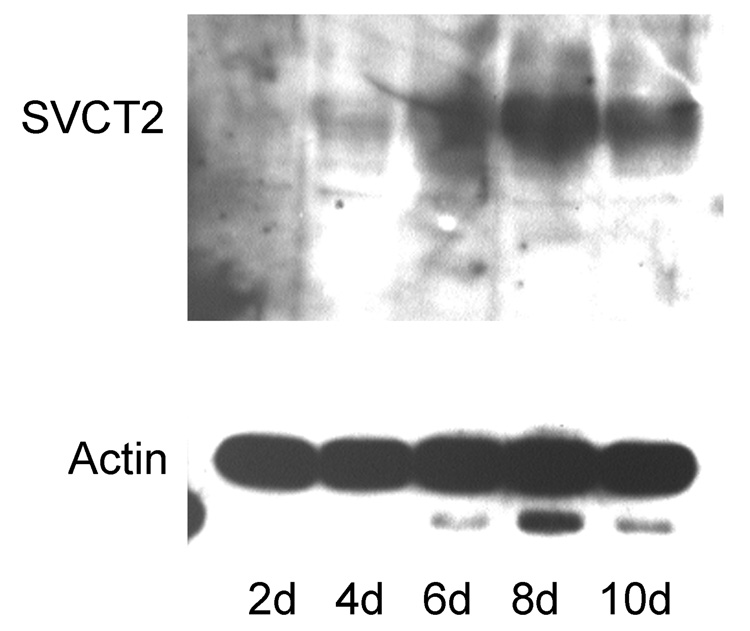
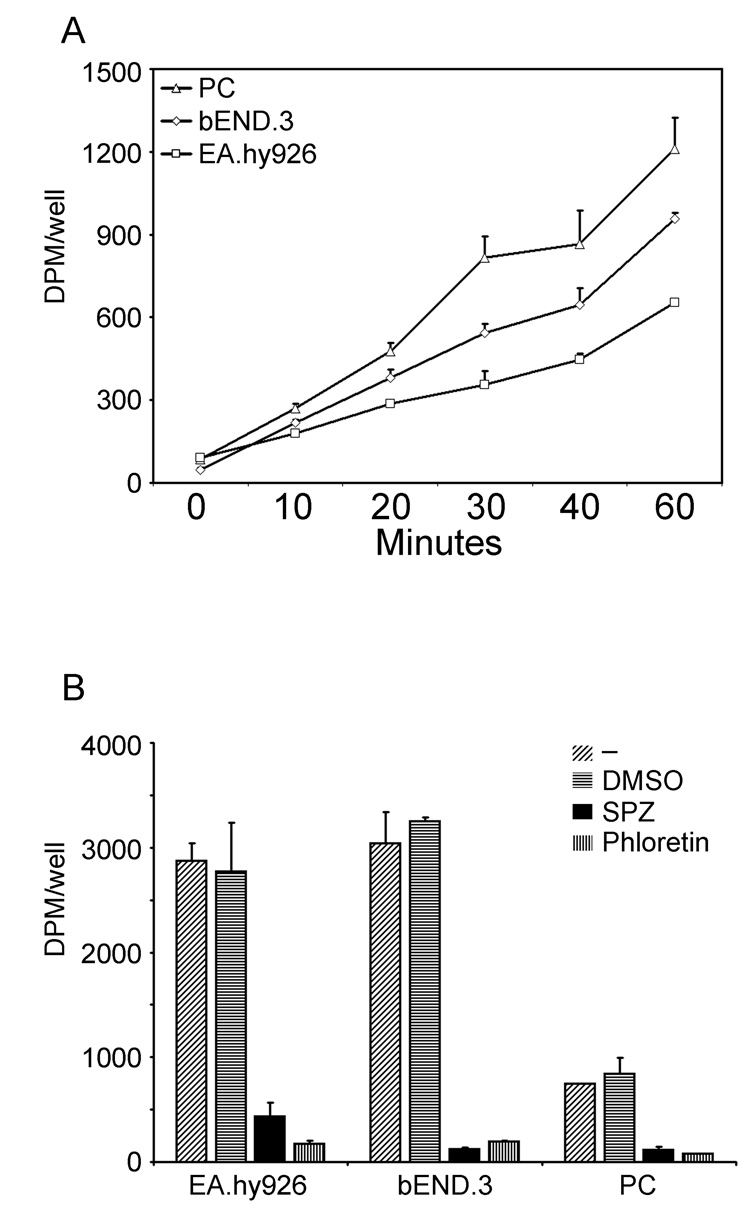
Freshly prepared cerebrovascular microcapillaries were immunostained with von Willebrand factor and the SVCT2. As shown in the left-hand panel of Fig. 1A, von Willebrand factor stained cells lining capillaries in these preparations, confirming them as endothelial cells. However, there was no staining of such cells by the SVCT2 antibody, despite clear staining of supporting cells and nearby neurons. Immunoblots of endothelial cell-rich capillaries confirmed these results, in that there was no specific staining of the SVCT2 on day zero of culture (Fig. 1B, “0d”). On the other hand, when the purified cortical microvessels were plated onto rat tail collagen-coated tissue culture plates, the endothelial cells migrated out of the vessels and groups of cells formed small islands expressing the spindle-shaped microvascular endothelial morphology. By day 7 there was appearance of a ~75 kDa band on the immunoblot representing the SVCT2 (Fig. 1B, “7d”). This increase in SVCT2 staining was time-dependent. It appeared about day 2 of culture and increased to a maximum by day 8 of culture (Fig. 2). The increase in SVCT2 on immunoblotting was associated with ascorbate transport that was similar in rates to that seen in both EA.hy926 cells and bEND.3 cells, which are immortalized endothelial cell lines (Fig. 3A). Transport of ascorbate was sensitive in each cell type to inhibition by phloretin and sulfinpyrazone, which are known inhibitors of ascorbate transport in other cells (Fig. 3B). In CCECs, ascorbate transport had an apparent Km of 41 ±12 µM (data not shown).
Fig. 1. Absence of the SVCT2 protein in brain microcapillaries.
Panel A: Immunostaining. Freshly isolated cortical microcapillaries were immunostained for von Willebrand factor in the left image, and for the SVCT2 in the right image. Arrowheads point to the endothelial cell-lined capillaries. Panel B: Immunoblotting. Rinsed, concentrated cortical capillary vessels were blotted for the SVCT2 at day zero (0d) and day 7 (7d) in culture. The location of the SVCT2 as a 75 kDa band on electrophoresis is noted.
Fig. 2. Time-dependent appearance of the SVCT2 in CCECs in culture.
Immunostaining of the SVCT2 in cells cultured for various times (upper panel). Actin staining of the capillary endothelial cell cultures shown in Panel A to confirm that similar protein amounts were loaded in each lane (lower panel).
Fig. 3. Appearance of specific ascorbate transport in CCECs in culture.
Panel A. Time-dependent ascorbate transport measurements in CCECs (PC) and in two immortalized endothelial cell lines, EA.hy926 and bEND.3. Panel B: Specificity of ascorbate transport in brain primary culture endothelial cells not treated with an agent (-), treated with the concentration of dimethylsulfoxide required to dissolve the two inhibitors (DMSO), and treated with either 1 mM sulfinpyrazone (SPZ) or 100 µM phloretin just before the transport assay. Results are corrected for cell number and are representative of 3 such experiments performed.
1.2 Regulation of the SVCT2
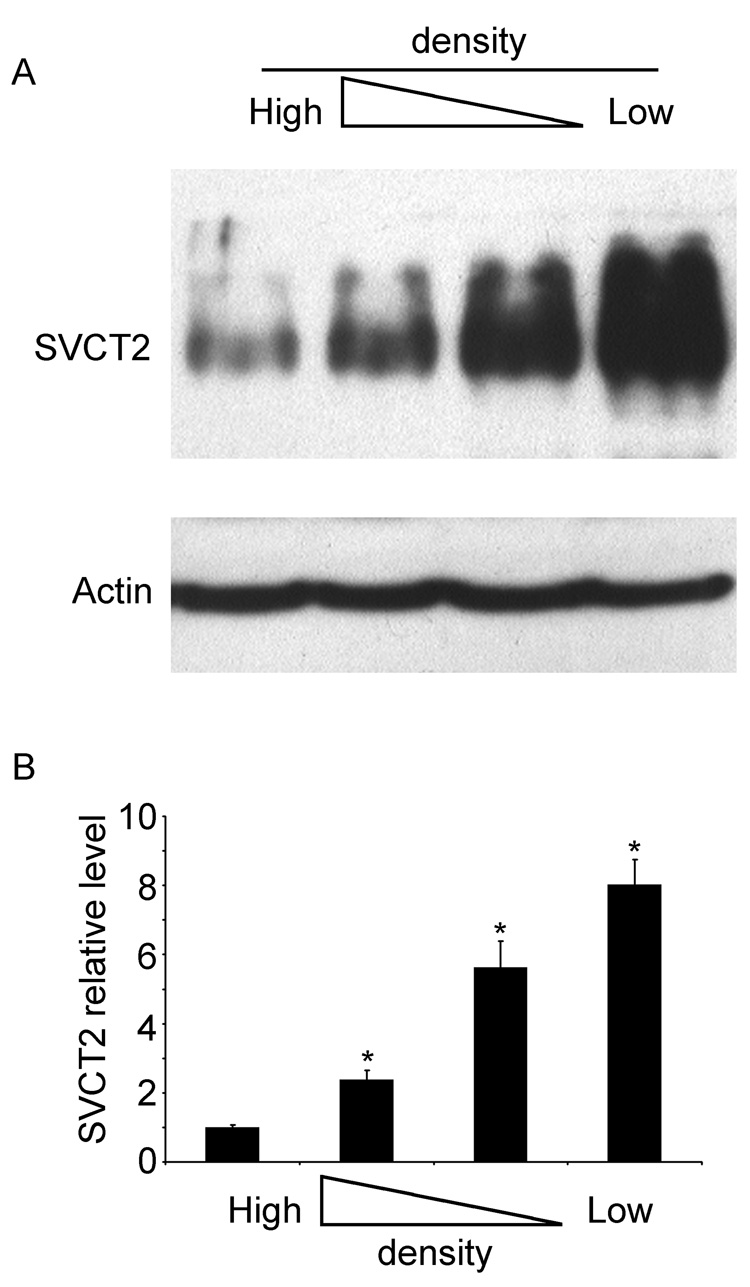
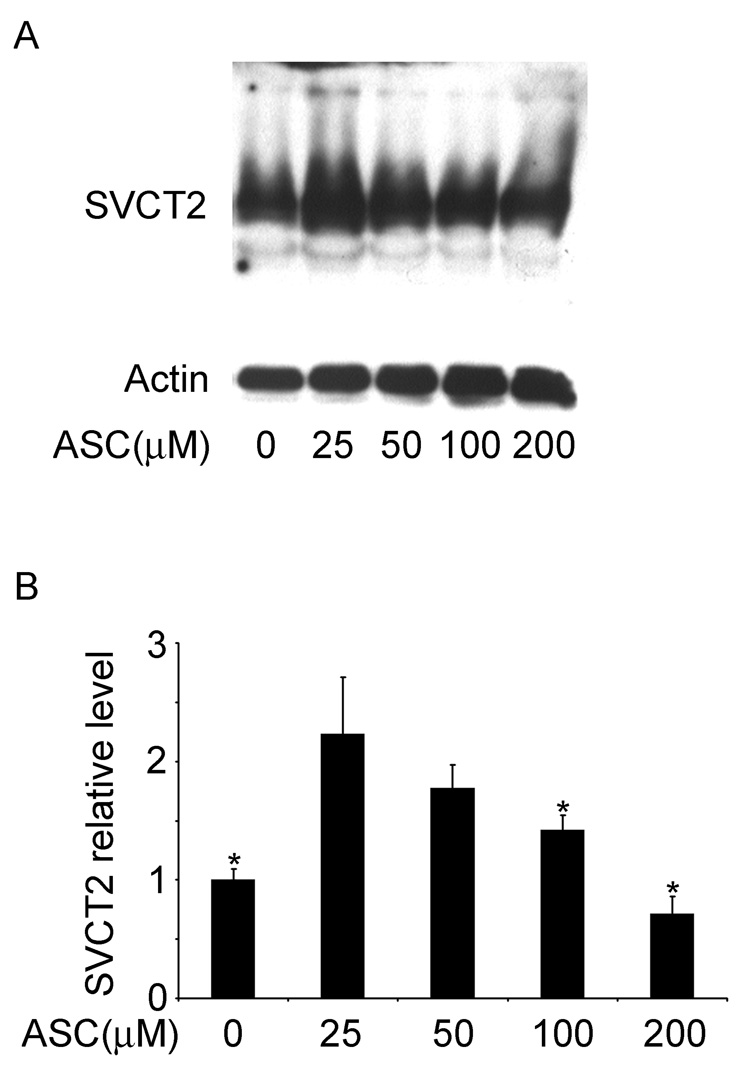
Regulation of the SVCT2 was evaluated by study of its response to cell density, to changes in extracellular ascorbate concentrations, and to cobalt. As shown in Fig. 4, the SVCT2 content of brain capillary endothelial cells was inversely proportional to the density of plating. This is evident in the representative immunoblot shown in Fig. 4A, as well as when 4 immunoblots were assessed by densitometry (Fig. 4B). Following treatment with ascorbate in culture, SVCT2 protein showed a biphasic response (Fig. 5), in that it increased almost 2-fold in cells treated with a low physiologic concentration of ascorbate (25 µM), but decreased as the concentration of ascorbate was further increased, such that there was a suppression of SVCT2 expression of about 40% at 200 µM ascorbate.
Fig. 4. Inhibition of SVCT2 protein expression at with culture of CCECs at high cell densities.
CCECs were cultured at the same plating density in different sized wells for 7 days, followed by protein extraction for immunoblotting of the SVCT2. Panel A shows a representative immunoblot, below which is β-actin expression in the same gel shown to confirm equal loading of protein in the gel lanes. Panel B is a densitometric analysis of 4 experiments performed, with an asterisk (*) indicating p < 0.05.
Fig. 5. Dependence of SVCT2 expression on medium ascorbate concentrations in CCECs.
Cells were cultured for 7 days supplemented with the ascorbate concentrations noted before they were taken for gel electrophoresis and immunoblotting. Panel A shows a representative immunoblot and Panel B shows the densitometric readings of 3 immunoblots with fractional densities normalized to actin noted above each bar. An asterisk (*) indicates p < 0.05 compared to the sample treated with 25 mM ascorbate (N = 4 experiments).
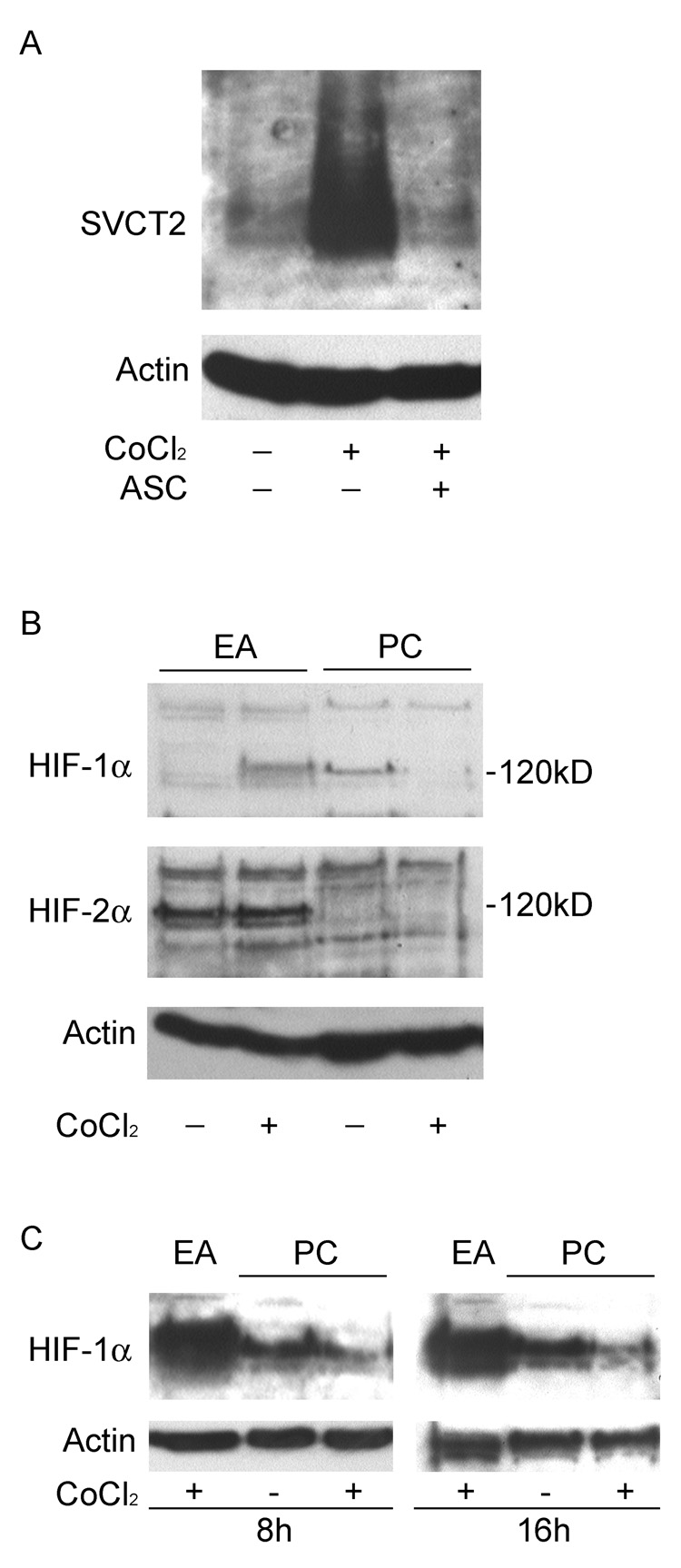
Since ascorbate is known to be involved in the regulation of the transcription factor HIF-1α in endothelial cells (Vissers et al., 2007), we tested whether cobalt, which increases HIF-1α in most cells, could also regulate the SVCT2. When primary endothelial cells were exposed to cobalt chloride (250 µM) for 48 h in culture, a marked increase in SVCT2 was observed (Fig. 6A). Addition of 400 µM ascorbate during cobalt treatment prevented the effect of cobalt on the expression of SVCT2 (Fig. 6A), suggesting that HIF-1α might regulate SVCT2 protein expression. As expected, cobalt induced the expression of HIF-1α, but not HIF-2α in EA.hy926 endothelial cells (Fig. 6B, two left-hand gel lanes). On the other hand, whereas CCECs had basal HIF-1α expression, this was significantly attenuated by cobalt treatment (Fig. 6B, two right-hand gel lanes). HIF-2α was not detected in CCECs (Fig. 6B). Since the effect of cobalt on HIF-1α expression might be transient, we also carried out immunoblots after 8 and 16 h of cobalt treatment, but inhibition of HIF-1α was again observed (Fig. 6C).
Fig. 6. Induction of SVCT2 by cobalt chloride is independent of HIF.
Panel A: Effect of cobalt exposure on SVCT2 expression. Primary endothelial cells (PC) were cultured for 5 days, and exposed to 250 µM cobalt alone or in combination with 400 µM ascorbate for additional 2 days. Confluent EA.hy926 cells (EA) were used as positive controls for HIF expression. The protein extracts were subjected to immunoblot analysis using SVCT2-specific antibody. Panel B: Expression level of HIF-α in cobalt treated CCECs. The cells were cultured as described above, and the whole-cell extracts were analyzed by immunoblot analysis using HIF-1α- or HIF-2α-specifc antibodies. Panel C: Early time course of cobalt (250 µM) on HIF-1α protein expression in EA.hy926 cells and in primary culture cells. The bottom panel in all the gels shows β-actin expression to confirm equal loading of protein in the gel lanes.
1.3 Ascorbate stimulation of type IV collagen maturation
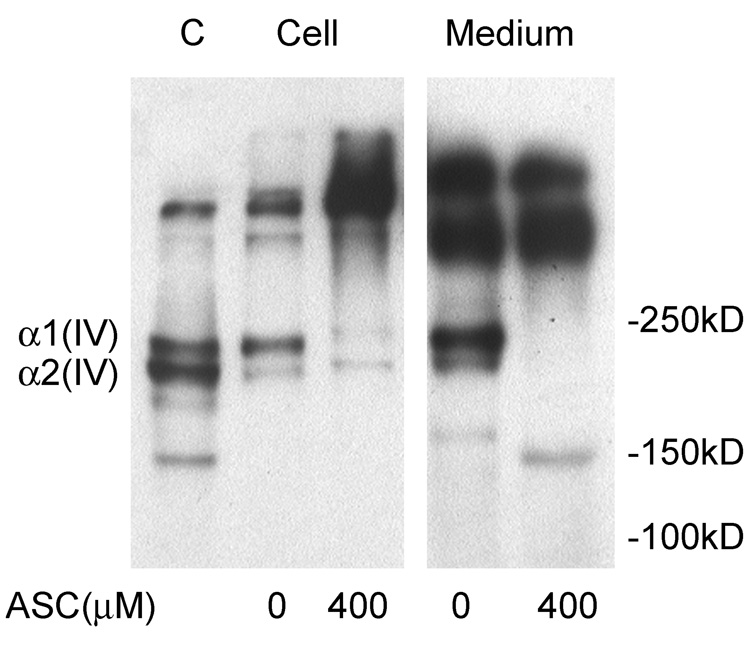
A functional consequence of SVCT2-dependent ascorbate uptake into brain capillary endothelial cells was investigated by measuring intra- and extracellular type IV collagen generation in immunoblots of cultured cells. As shown in Fig. 7, both brain capillary endothelial and EA.hy926 cells cultured in the absence of ascorbate contained monomers of type IV collagen (α1 and α2) (Rautavuoma et al., 2004). The identity of the band migrating at 150 kDa is unknown, but is likely a degradation product. There was also a small amount of larger collagen triple helix or filaments that migrated at high apparent molecular weight. Culture of CCECs for 3 days with 400 µM ascorbate added the first day caused a decrease in both type IV collagen subunits within the cells and a corresponding increase in larger apparent molecular weight material. This would be expected if ascorbate enhanced proline/lysine hydroxylation and this facilitated the folding of collagen subunits into triple helices or filaments that could not be dissociated under the conditions of electrophoresis. Corresponding changes were observed in the incubation medium, where ascorbate treatment abolished accumulation of the two monomeric sub-types. Thus, the presence of ascorbate in CCECs known to have the SVCT2 enhances the maturation of type IV collagen.
Fig. 7. Generation of type IV collagen in cultured endothelial cells.
EA.hy926 endothelial cells (EA, lane 1) in culture were used as a control for generation of type IV collagen sub-types. CCECs (PC) at 7 days in culture were treated overnight without or with ascorbate as indicated. Both cells and medium were taken for immunoblotting of type IV collagen. Collagen sub-types are identified based on their relative migration in the gels.
2. Discussion
In agreement with previous in situ hybridization (Astuya et al., 2005; García et al., 2005) and immunocytochemical (García et al., 2005) studies, the SVCT2 was not present in endothelial cells lining cortical capillaries in the mouse, either by direct staining or by immunoblotting. However, when the cells were purified and placed into culture, the SVCT2 was readily detected after several days and was associated with ascorbate transport rates comparable to immortalized endothelial cell lines. Appearance of the SVCT2 was dependent on cell density, responsive to ascorbate concentrations in the medium, associated with the expected maturation and release of type IV collagen, but not dependent on HIF-1α expression.
Why cells forming the blood-brain barrier lack the SVCT2 in vivo but develop it ex vivo in culture is unclear. Moreover, this phenomenon is not unique to CCECs, since cortical astrocytes have also been shown not to express SVCT2 mRNA (Astuya et al., 2005; Berger et al., 2000) or protein (García et al., 2005) in the brain, but readily develop specific ascorbate transport in culture (Berger et al., 2000; Korcok et al., 2000). Exposure of cells to increased oxidant stress in culture seems a likely cause. This is supported by studies in mice in which middle cerebral artery occlusion increased the SVCT2 in both neurons and astrocytes in the areas surrounding infarcted tissue that showed evidence of increased oxidant stress (Berger et al., 2003).
Additional evidence in support of SVCT2 induction by oxidant stress derives from our studies with cobalt. Cobalt markedly augmented SVCT2 expression in CCECs, an effect that was prevented by culturing cells with ascorbate. Cobalt and other divalent cation metals have been shown in many cell types to increase protein levels of the transcription factor HIF-1α, which in turn can induce a large number of genes related to cell proliferation (Salnikow et al., 2005). However, in CCECs the effect of cobalt to increase SVCT2 protein appears to be independent of HIF-α, because cobalt did not enhance HIF-1α / HIF-2α expression. This contrasts with our results in the EA.hy926 endothelial cell line, in which cobalt did increase HIF-1α protein. It also differs from the results of Vissers and colleagues (Vissers et al., 2007) who showed in primary cultures of human umbilical vein endothelial cells that both cobalt- and hypoxia increased expression of HIF-1α. Why cobalt did not increase HIF-1α in CCECs is unclear, but it makes it very unlikely the cobalt-induced increase in SVCT2 is due to HIF-1α in these particular cells. Thus, the stimulation of SVCT2 expression by cobalt must be due to some other mechanism. Salnikow, et al. (Salnikow et al., 2005) have proposed that divalent cations such as cobalt and nickel increase HIF-1α protein by oxidizing and thus depleting cellular ascorbate. Ascorbate is the preferred electron donor to the prolyl-4-hydoxylase (EC 1.14.11.2) that is responsible for hydroxylating HIF-1α, which then targets it for destruction in the ubiquitin-proteosome system (Cash et al., 2007). Without ascorbate, this hydroxylation would not occur and HIF-1α would accumulate. Thus, a plausible explanation for the cobalt effect on SVCT2 and its reversal by ascorbate could relate to depletion of intracellular ascorbate. These cells probably had very low levels of ascorbate after 7 days in culture and thus would be particularly susceptible to cobalt.
Another possible cause of induction of the SVCT2 in culture could relate to changes in intracellular ascorbate. We consistently observed a biphasic result, in which culture of CCECs in ascorbate concentrations in the physiologic range enhanced SVCT2 protein expression, whereas culture at concentrations above 100 µM suppressed SVCT2 protein expression by about 40%. Although bathed in plasma on one side, brain capillary endothelial cells would be exposed to interstitial ascorbate concentrations presumably similar to those in the CSF of 200–400 µM. Thus, at least part of the up-regulation of the SVCT2 protein and function when the cells are put in culture could be due to changes in intracellular ascorbate, with low concentrations inducing the SVCT2 and high concentrations suppressing it. Wilson and colleagues have previously shown that culture in high concentrations of ascorbate suppresses ascorbate transport in primary cultures of cerebral astrocytes (Wilson et al., 1990), which supports the notion that ascorbate can regulate its own transporter.
Still another possibility for enhanced SVCT2 expression in culture is that decreased cell contact during the initial phases of culture increases synthesis of the SVCT2. It has previously been shown that sub-confluent cultures of BAE-2 endothelial cells have increased ascorbate uptake (Saitoh et al., 1997). Our results confirm these observations and show further that this is due to increased amounts of the SVCT2.
In most capillary beds, endothelial cells deposit the type IV collagen that helps to form the basement membrane of the vessel. Ascorbate-dependent hydroxylation of proline and lysine on non-helical type IV collagen monomers facilitates inter-chain disulfide formation that is required to form a triple-helix comprised of two α1(IV) chains and one α2(IV) chain (Yoshikawa et al., 2001). Our results show that the same process also occurs within CCECs cultured in the presence of ascorbate. The Km of the prolyl hydroxylase involved in proline hydroxylation is about 300 µM (Majamaa et al., 1986), which suggests relatively high intracellular ascorbate concentrations are required to optimize collagen hydroxylation. Indeed, our previous studies in cultured EA.hy926 cells indicated that collagen synthesis was optimal at intracellular ascorbate concentrations of greater than 2 mM (May et al., 2005). It would seem that the SVCT2 is required to maintain such a concentration gradient. Although uptake of DHA on glucose transporters with subsequent reduction to ascorbate within cells can at least transiently generate a substantial gradient of ascorbate across the plasma membrane (Mendiratta et al., 1998), this mechanism cannot maintain millimolar intracellular ascorbate concentrations at the low DHA concentrations in blood. For example, human erythrocytes lack the SVCT2 (May et al., 2007), but have a large number of glucose transporters (Sogin et al., 1980) and readily reduce DHA to ascorbate (May et al., 1996), yet they have the same intracellular ascorbate concentration as the surrounding plasma (Evans et al., 1982; Mendiratta et al., 1998). Further, mice lacking the SVCT2 due to targeted deletion of its gene have areas of cerebral hemorrhage typical of capillary fragility (Sotiriou et al., 2002), such as associated with low levels of type IV collagen (Gould et al., 2005). If cerebral capillary endothelial cells lack the SVCT2 in vivo, why knockout of the SVCT2 should affect their ability to generate type IV collagen remains an enigma.
3. Experimental Procedures
3.1 Materials
Sigma/Aldrich Chemical Co. (St. Louis, MO) supplied the ascorbic acid, DHA, and other analytic chemicals used in the studies. Perkin-Elmer Life and Analytical Sciences, Inc. (Boston, MA) supplied the L-[1-14C]ascorbic acid, which was dissolved in deionized water containing 0.1 mM acetic acid and stored in multiple aliquots at −20 °C until use.
3.2 Culture of immortalized endothelial cells
EA.hy926 cells were originally provided by Dr. Cora Edgell (University of North Carolina, Chapel Hill, NC) and were cultured in Dulbecco’s minimal essential medium that contained 10% (v/v) fetal bovine serum and HAT media supplement (Sigma/Aldrich Chemical Co., St. Louis, MO). Addition of the latter resulted in final medium concentrations of 5 mM hypoxanthine, 20 µM aminopterin, and 0.8 mM thymidine. The murine brain endothelial cell line bEND.3 was obtained from American Type Culture Collection and was cultured under the same conditions as EA.hy926 cells, except that HAT supplement was omitted.
3.3 Isolation of mouse cortical capillary endothelial cells (CCEC)
Isolation of CCEC was accomplished following a modified protocol of Song and Pachter (Song et al., 2003). Briefly, up to 10 C57/Bl6 mice (2–3 weeks old) were sacrificed by cervical dislocation, brain cortical regions were removed and meninges were trimmed. The tissue was homogenized in a Dounce homogenizer (10 strokes) in ice-cold phosphate buffered saline (12.5 mM sodium phosphate, pH 7.4) and the resulting homogenate was centrifuged at 1000 rpm for 10 min. After removal of the supernatant, the pellet was suspended in 10 ml of 14% (wt/vol) dextran solution and was centrifuged at 7000 rpm for 10 min. The foamy myelin layer and dextran supernatant were removed by gentle aspiration and the pellet was suspended in 5 ml of collagenase solution (Ca2+/Mg2+-containing Hanks balanced salt solution, #14025, Gibco) supplemented with 1 mg/ml collagenase (Type 1, #CO130, Sigma, St. Louis, MO), 20 units/ml deoxyribonuclease-I, and 3% (v/v) fetal bovine serum) at 37 °C for 90 min with occasional agitation. By the end of the digestion, the suspension was homogenized (10 strokes). The resulting vessels, which were mainly short fragments containing only a few endothelial cells, were centrifuged and the pellet was incubated with anti-mouse PECAM-1-precoated Dynabeads (Dynal, Lake Success, NY) at 4 °C for 30 min with constant rotation. Bead-bound vessel fragments were then separated, and suspended in rat brain microvascular endothelial cell growth medium obtained from Cell Applications, Inc. (San Diego, CA). This medium also contained 20% (v/v) fetal bovine serum. The cells were cultured in 12-well plates that had been pre-coated with type I collagen prepared from rat tails. After 7 days in culture, 2 brains were sufficient to obtain ~90% confluent cells in one well of a 12-well plate.
3.4 Assay of ascorbate transport
Endothelial cells at near confluence in 12-well plates were rinsed with cell incubation buffer (15 mm Hepes, 135 mm NaCl, 5 mm KCl, 1.8 mm CaCl2, 0.8 mm MgCl2, pH 7.4) and incubated for 20 min at 37°C in 1 ml of the same medium that also contained 0.05 µCi of L-[1-14C]ascorbic acid (specific activity 4 mCi/mmol). Uptake was terminated rinsing the cells with ice-cold PBS. Cells were dissolved in 1 ml of lysis buffer (1 M NaOH), and the incorporated radioactivity was assayed by liquid scintillation spectrometry. Data represent means ± SD of two experiments with each determination done in duplicate.
3.5 Polyacrylamide gel electrophoresis and immunoblotting
For SVCT2 and type IV collagen immunoblotting, cultured cells in 12-well plates were rinsed once in PBS and solubilized in a lysis buffer that consisted of 150 mM NaCl, 1% Nonidet P40 (v/v), 0.5% sodium deoxycholate (w/v), 0.1% sodium dodecyl sulfate (SDS, w/v). To prevent proteolysis, this buffer also contained 0.1 mg/ml phenylmethylsulfonyl fluoride, and leupeptin, pepstatin, and aprotinin, each at 0.01 mg/ml. The lysate was mixed, and after centrifugation for 10 s at 13,000 × g, the supernatant was combined with an equal volume of sample buffer, which consisted of 125 mM Tris-HCl, 20% (v/v) glycerol, 4% (w/v) SDS, 10% (v/v) mercaptoethanol, and 0.0025% bromphenol blue (w/v), pH 6.8. Solubilized protein was subjected to SDS polyacrylamide gel electrophoresis for the SVCT2 (10% gel) and type IV collagen (5% gel) according to the method of Laemmli (Laemmli1970). Electrophoresis and transfer to poly(vinylidine difluoride) membrane, was performed as previously described (Due et al., 1995). The SVCT2 was probed with a 1:200 dilution of an affinity-purified goat polyclonal antibody that was specific for the SVCT2 transporter (sc-9926, Santa Cruz Biotechnology, Inc., Santa Cruz, CA). Type IV collagen was detected using a 1:8000 dilution of affinity-purified polyclonal rabbit antibody against mouse type IV collagen (T59106R, Biodesign International, Saco, ME). For HIF-1α and HIF-2α immunoblotting, the cells were homogenized in lysis buffer (6.2 M urea, 10% glycerol, 5 mM dithiothreitol, and 1% SDS plus protease inhibitors). Whole cell extract was separated by 7.5% SDS-polyacrylamide gel electrophoresis. Primary antibodies were rabbit anti-hypoxia-inducible factor-1α (HIF-1α) and anti-HIF-2α polyclonal antibodies reactive to mouse at 1:200 dilution (sc-10790 and sc-28706, Santa Cruz Biotechnology, Inc., Santa Cruz, CA).β-Actin served as a control for the amount of sample loaded and was detected in immunoblots using an antibody to actin at a dilution of 1:400 (SC-1616-R, Santa Cruz Biotechnology, Inc., Santa Cruz, CA). The secondary antibody for all immunoblots was a 1:5000 dilution of anti-goat or anti-rabbit IgG conjugated to horseradish peroxidase (Sigma-Aldrich, Inc., St. Louis, MO). Bands were stained using ECL Plus Western blotting reagents (RPN 2132, Amersham Biosciences, Piscataway, NJ). Band locations were determined using pre-stained molecular weight markers. Immunoblotting results were confirmed at least 3 times.
3.6 Immunocytochemistry
Cortical microvessels prepared as described above were fixed in formalin and embedded in paraffin. Sections (3 µm) were mounted, dewaxed, rehydrated and immunostained with primary and secondary antibodies. Following immunostaining, sections were counterstained with hematoxylin. Von Willebrand factor (Factor VIII) was detected with rabbit anti-mouse Von Willebrand factor (sc-14014, Santa Cruz) at 1:100 dilution, and SVCT2 was detected with goat anti-mouse SVCT2 antibody at a 1:100 dilution.
3.7 Data analysis
Statistical significance was determined by analysis of variance with post-hoc testing using the software program Sigma Stat 2.0 (Jandel Scientific, San Rafael, CA). Significance was based on a p value of < 0.05.
Acknowledgments
This work was supported by NIH grant DK 50435.
Abbreviations used
- BBB
blood-brain barrier
- CCEC
cortical capillary endothelial cells
- DHA
dehydroascorbic acid
- Hepes
N-2-hydroxyethylpiperazine-N′-2-ethanesulfonic acid
- KRH
Krebs-Ringer Hepes
- SVCT2
sodium-dependent vitamin C transporter 2
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Agus DB, Gambhir SS, Pardridge WM, Speilholz C, Baselga J, Vera JC. Vitamin C crosses the blood-brain barrier in the oxidized form through the glucose transporters. J. Clin. Invest. 1997;100:2842–2848. doi: 10.1172/JCI119832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Astuya A, Caprile T, Castro M, Salazar K, García MD, Reinicke K, Rodríguez F, Vera JC, Millán C, Ulloa V, Low M, Martínez F, Nualart F. Vitamin C uptake and recycling among normal and tumor cells from the central nervous system. J. Neurosci. Res. 2005;79:146–156. doi: 10.1002/jnr.20326. [DOI] [PubMed] [Google Scholar]
- Berger UV, Hediger MA. The vitamin C transporter SVCT2 is expressed by astrocytes in culture but not in situ. Neuroreport. 2000;11:1395–1399. doi: 10.1097/00001756-200005150-00009. [DOI] [PubMed] [Google Scholar]
- Berger UV, Lu XC, Liu W, Tang Z, Slusher BS, Hediger MA. Effect of middle cerebral artery occlusion on mRNA expression for the sodium-coupled vitamin C transporter SVCT2 in rat brain. J. Neurochem. 2003;86:896–906. doi: 10.1046/j.1471-4159.2003.01891.x. [DOI] [PubMed] [Google Scholar]
- Cash TP, Pan Y, Simon MC. Reactive oxygen species and cellular oxygen sensing. Free Radic. Biol. Med. 2007;43:1219–1225. doi: 10.1016/j.freeradbiomed.2007.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Due AD, Zhi-Chao Q, Thomas JM, Buchs A, Powers AC, May JM. Role of the C-terminal tail of the GLUT1 glucose transporter in its expression and function in Xenopus laevis oocytes. Biochemistry. 1995;34:5462–5471. doi: 10.1021/bi00016a017. [DOI] [PubMed] [Google Scholar]
- Eipper BA, Mains RE, Glembotski CC. Identification in pituitary tissue of a peptide alpha-amidation activity that acts on glycine-extended peptides and requires molecular oxygen, copper, and ascorbic acid. Proc. Natl. Acad. Sci. U. S. A. 1983;80:5144–5148. doi: 10.1073/pnas.80.16.5144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eldridge CF, Bunge MB, Bunge RP, Wood PM. Differentiation of axon-related Schwann cells in vitro. I. Ascorbic acid regulates basal lamina assembly and myelin formation. J. Cell Biol. 1987;105:1023–1034. doi: 10.1083/jcb.105.2.1023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans RM, Currie L, Campbell A. The distribution of ascorbic acid between various cellular components of blood, in normal individuals, and its relation to the plasma concentration. Br. J. Nutr. 1982;47:473–482. doi: 10.1079/bjn19820059. [DOI] [PubMed] [Google Scholar]
- García ML, Salazar K, Millán C, Rodríguez F, Montecinos H, Caprile T, Silva C, Cortes C, Reinicke K, Vera JC, Aguayo LG, Olate J, Molina B, Nualart F. Sodium vitamin C cotransporter SVCT2 is expressed in hypothalamic glial cells. Glia. 2005;50:32–47. doi: 10.1002/glia.20133. [DOI] [PubMed] [Google Scholar]
- Gould DB, Phalan FC, Breedveld GJ, van Mil SE, Smith RS, Schimenti JC, Aguglia U, van der Knaap MS, Heutink P, John SW. Mutations in Col4a1 cause perinatal cerebral hemorrhage and porencephaly. Science. 2005;308:1167–1171. doi: 10.1126/science.1109418. [DOI] [PubMed] [Google Scholar]
- Huang J, Agus DB, Winfree CJ, Kiss S, Mack WJ, McTaggart RA, Choudhri TF, Kim LJ, Mocco J, Pinsky DJ, Fox WD, Israel RJ, Boyd TA, Golde DW, Connolly ES., Jr Dehydroascorbic acid, a blood-brain barrier transportable form of vitamin C, mediates potent cerebroprotection in experimental stroke. Proc. Natl. Acad. Sci. USA. 2001;98:11720–11724. doi: 10.1073/pnas.171325998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korcok J, Yan R, Siushansian R, Dixon SJ, Wilson JX. Sodium-ascorbate cotransport controls intracellular ascorbate concentration in primary astrocyte cultures expressing the SVCT2 transporter. Brain Res. 2000;881:144–151. doi: 10.1016/s0006-8993(00)02829-8. [DOI] [PubMed] [Google Scholar]
- Laemmli UK. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970;227:680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lam DK, Daniel PM. The influx of ascorbic acid into the rat's brain. Q. J. Exp. Physiol. 1986;71:483–489. doi: 10.1113/expphysiol.1986.sp003007. [DOI] [PubMed] [Google Scholar]
- Majamaa K, Gunzler V, Hanauske-Abel HM, Myllylä R, Kivirikko KI. Partial identity of the 2-oxoglutarate and ascorbate binding sites of prolyl 4-hydroxylase. J. Biol. Chem. 1986;261:7819–7823. [PubMed] [Google Scholar]
- May JM, Qu ZC. Transport and intracellular accumulation of vitamin C in endothelial cells: relevance to collagen synthesis. Arch. Biochem. Biophys. 2005;434:178–186. doi: 10.1016/j.abb.2004.10.023. [DOI] [PubMed] [Google Scholar]
- May JM, Qu ZC, Qiao H, Koury MJ. Maturational loss of the vitamin C transporter in erythrocytes. Biochem. Biophys. Res. Commun. 2007;360:295–298. doi: 10.1016/j.bbrc.2007.06.072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- May JM, Qu ZC, Whitesell RR, Cobb CE. Ascorbate recycling in human erythrocytes: Role of GSH in reducing dehydroascorbate. Free Radic. Biol. Med. 1996;20:543–551. doi: 10.1016/0891-5849(95)02130-2. [DOI] [PubMed] [Google Scholar]
- Mendiratta S, Qu Z-C, May JM. Erythrocyte ascorbate recycling: Antioxidant effects in blood. Free Radic. Biol. Med. 1998;24:789–797. doi: 10.1016/s0891-5849(97)00351-1. [DOI] [PubMed] [Google Scholar]
- Qiu S, Li L, Weeber EJ, May JM. Ascorbate transport by primary cultured neurons and its role in neuronal function and protection against excitotoxicity. J. Neurosci. Res. 2007;85:1046–1056. doi: 10.1002/jnr.21204. [DOI] [PubMed] [Google Scholar]
- Rautavuoma K, Takaluoma K, Sormunen R, Myllyharju J, Kivirikko KI, Soininen R. Premature aggregation of type IV collagen and early lethality in lysyl hydroxylase 3 null mice. Proc. Natl. Acad. Sci. U. S. A. 2004;101:14120–14125. doi: 10.1073/pnas.0404966101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rebec GV, Pierce RC. A vitamin as neuromodulator: Ascorbate release into the extracellular fluid of the brain regulates dopaminergic and glutamatergic transmission. Prog. Neurobiol. 1994;43:537–565. doi: 10.1016/0301-0082(94)90052-3. [DOI] [PubMed] [Google Scholar]
- Reiber H, Ruff M, Uhr M. Ascorbate concentration in human cerebrospinal fluid (CSF) and serum. Intrathecal accumulation and CSF flow rate. Clin. Chim. Acta. 1993;217:163–173. doi: 10.1016/0009-8981(93)90162-w. [DOI] [PubMed] [Google Scholar]
- Rice ME. Ascorbate regulation and its neuroprotective role in the brain. Trends Neurosci. 2000;23:209–216. doi: 10.1016/s0166-2236(99)01543-x. [DOI] [PubMed] [Google Scholar]
- Rumsey SC, Kwon O, Xu GW, Burant CF, Simpson I, Levine M. Glucose transporter isoforms GLUT1 and GLUT3 transport dehydroascorbic acid. J. Biol. Chem. 1997;272:18982–18989. doi: 10.1074/jbc.272.30.18982. [DOI] [PubMed] [Google Scholar]
- Saitoh Y, Nagao N, O'Uchida R, Yamane T, Kageyama K, Muto N, Miwa N. Moderately controlled transport of ascorbate into aortic endothelial cells against slowdown of the cell cycle, decreasing of the concentration or increasing of coexistent glucose as compared with dehydroascorbate. Mol. Cell. Biochem. 1997;173:43–50. doi: 10.1023/a:1006879001316. [DOI] [PubMed] [Google Scholar]
- Salnikow K, Kasprzak KS. Ascorbate depletion: a critical step in nickel carcinogenesis? Environ. Health Perspect. 2005;113:577–584. doi: 10.1289/ehp.7605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sogin DC, Hinkle PC. Binding of cytochalasin B to human erythrocyte glucose transporter. Biochemistry. 1980;19:3417–3420. doi: 10.1021/bi00564a041. [DOI] [PubMed] [Google Scholar]
- Song L, Pachter JS. Culture of murine brain microvascular endothelial cells that maintain expression and cytoskeletal association of tight junction-associated proteins. In Vitro Cell Dev. Biol. Anim. 2003;39:313–320. doi: 10.1290/1543-706X(2003)039<0313:COMBME>2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- Sotiriou S, Gispert S, Cheng J, Wang YH, Chen A, Hoogstraten-Miller S, Miller GF, Kwon O, Levine M, Guttentag SH, Nussbaum RL. Ascorbic-acid transporter Slc23a1 is essential for vitamin C transport into the brain and for perinatal survival. Nature Med. 2002;8:514–517. doi: 10.1038/0502-514. [DOI] [PubMed] [Google Scholar]
- Spector R. Vitamin homeostasis in the central nervous system. N. Engl. J. Med. 1977;296:1393–1398. doi: 10.1056/NEJM197706162962409. [DOI] [PubMed] [Google Scholar]
- Spector R, Lorenzo AV. Ascorbic acid homeostasis in the central nervous system. Am. J. Physiol. 1973;225:757–763. doi: 10.1152/ajplegacy.1973.225.4.757. [DOI] [PubMed] [Google Scholar]
- Tsukaguchi H, Tokui T, Mackenzie B, Berger UV, Chen X-Z, Wang YX, Brubaker RF, Hediger MA. A family of mammalian Na+-dependent L-ascorbic acid transporters. Nature. 1999;399:70–75. doi: 10.1038/19986. [DOI] [PubMed] [Google Scholar]
- Vera JC, Rivas CI, Fischbarg J, Golde DW. Mammalian facilitative hexose transporters mediate the transport of dehydroascorbic acid. Nature. 1993;364:79–82. doi: 10.1038/364079a0. [DOI] [PubMed] [Google Scholar]
- Vissers MC, Gunningham SP, Morrison MJ, Dachs GU, Currie MJ. Modulation of hypoxia-inducible factor-1 alpha in cultured primary cells by intracellular ascorbate. Free Radic. Biol. Med. 2007;42:765–772. doi: 10.1016/j.freeradbiomed.2006.11.023. [DOI] [PubMed] [Google Scholar]
- Wilson JX. Regulation of vitamin C transport. Annu. Rev. Nutr. 2005;25:105–125. doi: 10.1146/annurev.nutr.25.050304.092647. [DOI] [PubMed] [Google Scholar]
- Wilson JX, Jaworski EM, Kulaga A, Dixon SJ. Substrate regulation of ascorbate transport activity in astrocytes. Neurochem. Res. 1990;15:1037–1043. doi: 10.1007/BF00965751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu Z, Hofman FM, Zlokovic BV. A simple method for isolation and characterization of mouse brain microvascular endothelial cells. J. Neurosci. Methods. 2003;130:53–63. doi: 10.1016/s0165-0270(03)00206-1. [DOI] [PubMed] [Google Scholar]
- Yoshikawa K, Takahashi S, Imamura Y, Sado Y, Hayashi T. Secretion of non-helical collagenous polypeptides of alpha1(IV) and alpha2(IV) chains upon depletion of ascorbate by cultured human cells. J. Biochem. (Tokyo) 2001;129:929–936. doi: 10.1093/oxfordjournals.jbchem.a002939. [DOI] [PubMed] [Google Scholar]