Abstract
The mitotic checkpoint system ensures the fidelity of chromosome segregation by preventing the completion of mitosis in the presence of any misaligned chromosome. When activated, it blocks the initiation of anaphase by inhibiting the ubiquitin ligase anaphase-promoting complex/cyclosome (APC/C). Little is known about the biochemical mechanisms by which this system inhibits APC/C, except for the existence of a mitotic checkpoint complex (MCC) inhibitor of APC/C composed of the APC/C activator Cdc20 associated with the checkpoint proteins Mad2, BubR1, and Bub3. We have been studying the mechanisms of the mitotic checkpoint system in extracts that reproduce its downstream events. We found that inhibitory factors are associated with APC/C in the checkpoint-arrested state, which can be recovered from immunoprecipitates. Only a part of the inhibitory activity was caused by MCC [Braunstein I, Miniowitz S, Moshe Y, Hershko A (2007) Proc Natl Acad Sci USA 104:4870–4875]. Here, we show that during exit from checkpoint, rapid disassembly of MCC takes place while APC/C is still inactive. This observation suggested the possible involvement of multiple factors in the regulation of APC/C by the mitotic checkpoint. We have separated a previously unknown inhibitor of APC/C from MCC. This inhibitor, called mitotic checkpoint factor 2 (MCF2), is associated with APC/C only in the checkpoint-arrested state. The inhibition of APC/C by both MCF2 and MCC was decreased at high concentrations of Cdc20. We propose that both MCF2 and MCC inhibit APC/C by antagonizing Cdc20, possibly by interaction with the Cdc20-binding site of APC/C.
Keywords: cell cycle, spindle checkpoint, mitosis
The mitotic (or spindle assembly) checkpoint system delays anaphase onset in the presence of any misaligned chromosome and thus ensures the fidelity of chromosome segregation in mitosis (for reviews, see refs. 1–5). When this checkpoint system is switched on, it inhibits the activity of the ubiquitin–protein ligase anaphase-promoting complex/cyclosome (APC/C). APC/C targets for degradation cell cycle-regulatory proteins such as mitotic cyclins and securin, an inhibitor of anaphase initiation. The activity of APC/C at the end of mitosis requires phosphorylation of several of its subunits and association with the activator protein Cdc20 (for reviews, see refs. 6–8).
Although much genetic information is available on the mitotic checkpoint system, the biochemical mechanisms by which it inhibits APC/C are mostly unknown. One exception was the identification of the mitotic checkpoint complex (MCC), an inhibitor of APC/C that is composed of Cdc20 associated with the checkpoint proteins Mad2, BubR1, and Bub3 (9). MCC is assembled when the mitotic checkpoint system is active, and it is disassembled in exit from checkpoint. It has been recently reported that the disassembly of MCC in exit from checkpoint arrest requires APC/C-dependent ubiquitylation (10, 11), but the targets and role of this ubiquitylation remain unknown.
For biochemical analysis of the mechanisms controlling APC/C by the mitotic checkpoint, we have been using extracts from nocodazole-arrested cells. Such extracts reproduce some of the downstream events of the mitotic checkpoint system, such as the lag kinetics of the degradation of securin (12). We have shown that inhibitory factors are associated with APC/C in stably checkpoint-arrested extracts. The inhibitors were isolated by immunoprecipitation of APC/C followed by elution with high salt. A part of the inhibitory material was identified as MCC. However, it seemed that some other checkpoint-inhibitory factors are also associated with APC/C because only approximately one-half of the inhibitory activity was removed by immunodepletion of MCC (12). We now report evidence for the involvement of multiple factors in the regulation of APC/C by the mitotic checkpoint system. We identified a checkpoint inhibitor of APC/C that is distinct from MCC. Both inhibitors suppress APC/C by antagonizing the action of the activator Cdc20.
Results
The Release of APC/C from Checkpoint Inhibition Is Delayed Relative to the Disassembly of MCC.
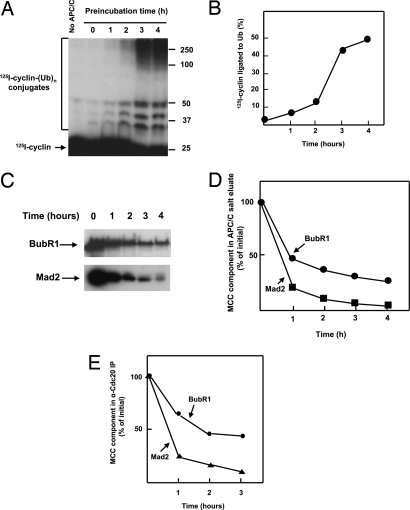
We have noted that only a part of the inhibitory activity associated with APC/C in the checkpoint-arrested state is caused by MCC (12). To examine further the possible existence of different inhibitory factors that regulate APC/C in mitotic checkpoint, we compared the kinetics of the release of APC/C from checkpoint inhibition with that of the decay of MCC. Extracts from nocodazole-arrested HeLa cells were incubated at 23°C in the presence of ATP, and at various times samples were withdrawn, and APC/C was isolated by adsorption to anti-cdc27 beads. The beads were extensively washed at low ionic strength (to prevent the dissociation of APC/C-bound inhibitors by high ionic strength), and the activity of APC/C to ubiquitylate 125I-cyclin was assayed. Assays of APC/C activity were conducted in the presence of E1 and E2C/UbcH10 at low concentration, but without the supplementation of the APC/C activator Cdc20, to avoid possible interference of exogenous Cdc20 with the action of the inhibitors (see below). As shown in Fig. 1 A and B, there was a prolonged lag in the first 1–2 h, followed by marked activation of APC/C after 3–4 h of incubation of extracts. This lag in the activation of isolated APC/C resembles that observed for the degradation of securin (12).
Fig. 1.
Time course of activation of APC/C and disassembly of MCC during exit from mitotic checkpoint. (A) Kinetics of activation of APC/C. Checkpoint extracts were incubated with ATP as described in Methods for the preparation of activated extracts. After incubation for the time periods indicated, APC/C was isolated on anti-Cdc27 beads, and the ligation of 125I-cyclin to ubiquitin was determined in samples of 1-μl beads incubated at 23°C for 20 min without the addition of exogenous Cdc20. The first lane on the left is a sample incubated without a source of APC/C. Numbers on the right indicate the position of molecular mass marker proteins (kDa). (B) Quantitation of results from A. (C) Kinetics of decay of MCC components in salt eluates of APC/C immunoprecipitates. Extracts were incubated for the time periods indicated and then adsorbed to anti-cdc27 beads as in A. Subsequently, beads were subjected to elution with 0.3 M KCl as described in ref. 12. Salt eluates were concentrated by ultrafiltration to one-half of the volume of extracts. Samples of 5 μl were subjected to immunoblotting. (D) Quantitation of results from C. Results were expressed as the percentage of the value at time 0. (E) Kinetics of dissociation of Mad2 and BubR1 from Cdc20. Checkpoint extracts were incubated with ATP for the indicated times as in A and then were subjected to immunoprecipitation with a polyclonal anti-Cdc20 antibody. Immunoprecipitates were analyzed by quantitative immunoblotting.
As suggested, the lag kinetics of exit from mitotic checkpoint could be explained by the decay of labile inhibitors (12). To examine the kinetics of the decay of APC/C-bound MCC, anti-Cdc27 immunoprecipitates from a similar time course experiment were subjected to extraction with high salt (a procedure that dissociates MCC from APC/C), and the amounts of the MCC components BubR1 and Mad2 in salt eluates were determined by immunoblotting. As shown in Fig. 1 C and D, levels of APC/C-bound MCC components decreased rapidly. After 1 h of incubation, the level of BubR1 decreased to approximately one-half of initial and subsequently BubR1 decreased further, although it did not decay completely even after 4 h. Surprisingly, levels of APC/C-bound Mad2 decreased even more rapidly than BubR1: only ≈20% of the initial remained after 1 h of incubation, and the decay of Mad2 was nearly complete after 3–4 h.
The release of APC/C-bound MCC components could be caused by the dissociation of MCC from APC/C or by the disassembly of MCC itself (i.e., dissociation of BubR1 and Mad2 from Cdc20). To examine the kinetics of the disassembly of MCC, extracts incubated for various times were subjected to immunoprecipitation with anti-Cdc20 antibody, and material bound to Cdc20 was immunoblotted for BubR1 and Mad2. As shown in Fig. 1E, in this case, too, rapid dissociation of MCC components from Cdc20 was observed, and again the release of Mad2 preceded that of BubR1. Thus, the release of APC/C-bound MCC reflects the disassembly of MCC to its components.
The rapid initial dissociation of MCC at 1–2 h of incubation (Fig. 1 D and E) precedes the activation of APC/C and occurs during the lag period of this process (Fig. 1 A and B). One possible explanation is that other inhibitors of APC/C decay slower than MCC and thus keep APC/C inactive during the lag period. We therefore searched by direct methods for inhibitor(s) different from MCC.
Resolution of Different Mitotic Checkpoint-Inhibitory Factors.
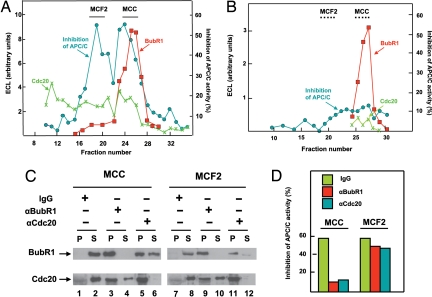
To resolve different factors that inhibit APC/C in mitotic checkpoint, we used extracts after incubation with adenosine 5′-[γ-thio]triphosphate (ATPγS). This treatment stabilized the checkpoint-arrested state, possibly because of stable thiophosphorylation of some proteins (12). Salt eluate of APC/C immunoprecipitates from stably checkpoint-arrested extracts was subjected to ion exchange chromatography on MonoQ. The levels of BubR1 and Cdc20 in different column fractions were determined by quantitative immunoblotting. As shown in Fig. 2A, BubR1 eluted as a sharp peak, whereas Cdc20 was dispersed in different regions. Samples of column fractions were also tested for inhibition of the activity of purified APC/C in the presence of a low concentration of recombinant Cdc20. Two peaks of inhibitory activity could be seen: one coincided partially with BubR1 and another was eluted before most of the BubR1 peak. The inhibitor in the region of BubR1 was identified as MCC by immunoprecipitation (see below). The additional peak of inhibitory activity, separated from most of MCC, was called mitotic checkpoint factor 2 or MCF2.
Fig. 2.
Resolution of inhibitory factors associated with APC/C in the checkpoint-arrested state. (A) Separation of MCC and MCF2 by chromatography on MonoQ. Salt eluate of APC/C immunoprecipitate from checkpoint-arrested extract was subjected to chromatography on MonoQ as described under Methods. Samples of 2 μl of column fractions were tested for inhibition of APC/CCdc20 activity (see Methods). Results are expressed as percentage decrease in activity relative to that without inhibitor. Samples of 3 μl of column fractions were used for quantitative immunoblotting for BubR1 and Cdc20, as described in Methods. MCC eluted at 260 mM NaCl and MCF2 at 210 mM NaCl. The indicated column fractions of MCC and MCF2 were pooled and used for further experiments. (B) Chromatography on MonoQ of salt eluate of APC/C immunoprecipitate from activated extracts. Samples of column fractions were analyzed as in A. The regions where MCC and MCF2 eluted in A are marked by dotted lines. (C) Immunoprecipitation of preparations of MCC and MCF2 with antibodies against Bubr1 and Cdc20. Samples of 30 μl of pooled MCC and MCF2 fractions (from a MonoQ separation similar to that shown in A) were subjected to immunoprecipitation with 2 μg of affinity-purified polyclonal antibodies against BubR1 or Cdc20 adsorbed to 10 μl of Affi-Prep protein A beads (Bio-Rad). The control was a similar amount of nonimmune rabbit IgG. Equal samples of precipitates (P) and supernatants (S) were subjected to immunoblotting with the indicated monoclonal antibodies. The blot on the right was subjected to longer exposure than that on the left, to show more clearly residual BubR1 in MCF2. (D) Effects of immunodepletion on APC/C-inhibitory activities of MCC and MCF2. Samples of 3 μl of the supernatants of the immunoprecipitations shown in C were tested for the inhibition of APC/CCdc20, as described under Methods.
To examine whether MCF2, like MCC, is indeed specific for the checkpoint-arrested state, we have also examined its presence in of salt eluates of APC/C that were isolated from extracts that had exited from checkpoint arrest after prolonged incubation with ATP (“activated” extracts). As shown in Fig. 2B, fractionation of this eluate on MonoQ showed the presence of BubR1, although its amount was approximately one-third of that present in the preparation from “checkpoint-arrested” extracts. There was very little APC/C-inhibitory activity in the region corresponding to the elution position of MCC, indicating that most BubR1 in this preparation is not part of an inhibitory complex. There was also very little inhibitory activity in the region corresponding to the position of MCF2. These results suggest that both MCC and MCF2 are checkpoint-specific inhibitors of APC/C.
To confirm the identity of MCC and to try to gain some information on the composition of MCF2, we have subjected both peaks (from MonoQ separation of checkpoint-stabilized extracts similar to that shown in Fig. 2A) to immunoprecipitation with polyclonal antibodies directed against BubR1 and Cdc20 (Fig. 2C). Immunoprecipitates and supernatants remaining after immunoprecipitation were tested for the presence of these proteins by immunoblotting with respective monoclonal antibodies. A control sample was subjected to similar immunoprecipitation with nonimmune IgG. In the control immunoprecipitation of the MCC peak, BubR1 and Cdc20 remained in the supernatant (Fig. 2C, lanes 1 and 2). Immunoprecipitation with anti-BubR1 precipitated essentially all BubR1 and most of Cdc20 (lanes 3 and 4), whereas anti-Cdc20 precipitated Cdc20 effectively, along with part of BubR1 (lane 5), although significant amounts of BubR1 remained in the supernatant (lane 6). These results showed that a large part of BubR1 and Cdc20 in the MCC region are associated with each other. The observation that this preparation also contained free BubR1 (lane 6) accounted for the slight difference between the elution position of BubR1- and MCC-inhibitory activity in the MonoQ column (Fig. 2A). In fact, we found that the later fractions of the MCC peak were enriched in free BubR1 relative to the earlier fractions (data not shown).
We carried out similar immunoprecipitations of the MCF2 peak, mainly to test the possibility that the Cdc20 that is also present in this region (Fig. 2A) may be a component in this second inhibitory complex. In addition, we wanted to estimate the contribution to inhibitory activity of the small amount of MCC that was carried over to the MCF2 peak. In this case, too, immunoprecipitations with anti-BubR1 and anti-Cdc20 removed practically all respective proteins from the supernatants (Fig. 2C, lanes 10 and 12). As expected from the distribution of Cdc20 and BubR1 in the fractions of the MonoQ column, significant amounts of Cdc20 remained in the supernatant after immunoprecipitation with anti-BubR1 (lane 10).
We next tested APC/C-inhibitory activity in supernatants of the above-described immunoprecipitations. The controls were immunoprecipitations with nonimmune IgG, in which inhibitory activity was expected to remain in the supernatants. As shown in Fig. 2D (Left), immunodepletion of the MCC peak with either anti-BubR1 or anti-Cdc20 removed most inhibitory activity, suggesting that the major APC/C inhibitor in this region is indeed MCC. By contrast, after immunodepletion of MCF2 with anti-BubR1 (Fig. 2D Right) most inhibitory activity remained in the supernatant. The slight reduction of inhibitory activity after BubR1 depletion probably reflects contamination of this preparation by MCC. We also observed that immunodepletion of MCF2 with anti-Cdc20, which effectively removed Cdc20 (Fig. 2C), caused only a modest reduction in APC/C-inhibitory activity, comparable with that observed with BubR1 immunodepletion (Fig. 2D Right). Here again, the slight reduction after Cdc20 depletion possibly reflects the depletion of MCC contaminant in this preparation. These results indicate that Cdc20 is not a component of MCF2 inhibitor.
MCC and MCF2 Antagonize the Influence of Cdc20 on APC/C Activity.
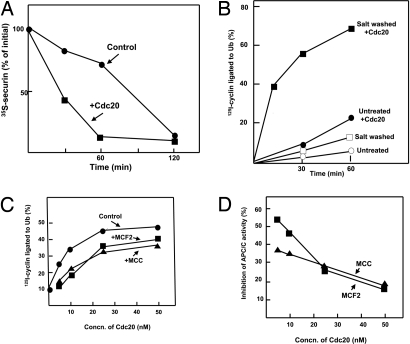
We tried to gain some insight into the mode of the inhibition of APC/C by MCF2 and MCC. The APC/C activator Cdc20 seems to be a primary target of the mitotic checkpoint system because increased levels of Cdc20 overcome mitotic checkpoint arrest in yeast (13–15). To examine whether the checkpoint inhibitors act by antagonizing Cdc20, we first tested the influence of high concentrations of Cdc20 on the degradation of securin in extracts from nocodazole-arrested cells. Because the phosphorylation of Cdc20 by several mitotic and checkpoint protein kinases causes its inactivation (16–19), this experiment was carried out in the presence of the protein kinase inhibitor staurosporine. Staurosporine shortens the lag of securin degradation in such extracts, but it does not eliminate it completely (12). As shown in Fig. 3A, the supplementation of Cdc20 at high concentration to extract incubated with staurosporine markedly accelerated the rate of the degradation of [35S]-securin and abolished the lag. One possible explanation for this result is that Cdc20 at high concentrations competes with checkpoint inhibitors of APC/C on some common target. However, alternative explanations of this experiment, such as that excess CDC20 directly activates the APC/C, which has been kept inactive because checkpoint proteins sequestered endogenous Cdc20, could not be ruled out.
Fig. 3.
High concentrations of Cdc20 overcome the effect of inhibitors on APC/C activity. (A) Effect of Cdc20 on the degradation of securin in checkpoint extracts. The degradation of [35S]-securin in extracts from nocodazole-arrested cells was followed as described in ref. 12, except that 10 μM staurosporine was added to all incubations. Where indicated, 0.1 μM recombinant Cdc20 was supplemented. (B) Influence of high-salt wash of immunoprecipitated APC/C on its sensitivity to stimulation by Cdc20. APC/C from checkpoint extracts was adsorbed to anti-Cdc27 beads. Part of beads was washed with 0.3 M KCl, as described in ref. 12. Another portion (Untreated) was subjected to similar washes without KCl. The ligation of 125I-cyclin to ubiquitin by both preparations was determined in the absence or presence of 50 nM Cdc20, after incubation (23°C with shaking at 1,000 rpm) for the time periods indicated. (C) MCF2 and MCC inhibit APC/C in a manner antagonistic to Cdc20. Ligation of 125I-cyclin to ubiquitin was determined as described in Methods, in the presence of 0.7 μl of highly purified APC/C (including purification on MonoQ) and Cdc20 at the concentrations indicated. Where indicated, samples of 1.5 μl of MCC or MCF2 (Fig. 2A) were added. (D) Results from C were calculated as inhibition of APC/C activity relative to the corresponding sample without inhibitor.
We further investigated this problem by testing the influence of the removal of inhibitors from APC/C on its sensitivity to stimulation by Cdc20. In the experiment shown in Fig. 3B, APC/C was isolated from checkpoint extracts by adsorption to anti-Cdc27 beads. Part of the beads was left untreated, and another part was washed with high salt to remove inhibitors. As shown above (Fig. 1 A and B, time 0 of preincubation), without added Cdc20, “untreated” preparations had very low activity in cyclin ubiquitylation. The supplementation of Cdc20 caused some stimulation of APC/C activity, but the rate of cyclin ubiquitylation was still quite slow (Fig. 3B, Untreated + Cdc20). In the preparation that had been subjected to high-salt wash, the addition of Cdc20 markedly stimulated the rate of cyclin ubiquitylation (Fig. 3B). Thus, the extent of the stimulation of APC/C activity by Cdc20 at 15–30 min of incubation was 2.5-fold in the untreated preparation and ≈10-fold in the salt-washed preparation. These results are compatible with the interpretation that the removal of both MCF2 and MCC by high-salt wash from APC/C bound to anti-Cdc27 beads facilitates the activation of APC/C by Cdc20.
We next examined, in our presently available most purified system, whether high concentrations of Cdc20 can antagonize the action of each inhibitor. Purified APC/C was incubated with MonoQ-separated preparations of MCF2 or MCC in the presence of increasing concentrations of Cdc20, and the rate of cyclin–ubiquitin ligation was determined. The results are shown in Fig. 3C, and their expression as the percentage of inhibition of APC/C activity is shown in Fig. 3D. It may be seen that the extent of the inhibition of APC/C by MCF2 decreased markedly at high concentrations of Cdc20. Thus, with the amount of MCF2 used, inhibition decreased from 55% at 5 nM Cdc20 to 15% at 50 nM Cdc20. The inhibition of APC/C by MCC also decreased significantly at high concentrations of Cdc20, although to a lesser extent than in the case of MCF2. This may be caused by a higher affinity of MCC to its target. The combined results thus suggest that both mitotic checkpoint inhibitors act by competing with Cdc20 at a site necessary for the activation of APC/C, possibly a common binding site on APC/C (see Discussion).
Discussion
In this article we presented evidence indicating the existence of multiple mechanisms for the inhibition of the activity of APC/C by the mitotic checkpoint system, resolved a checkpoint inhibitor from the previously known MCC-inhibitory complex, and showed that both inhibitors antagonize the action of the APC/C activator Cdc20. The existence of multiple mechanisms for APC/C regulation was suggested by the discrepancy between the time course of APC/C activation and MCC disassembly during the exit of extracts from checkpoint inhibition (Fig. 1). Thus, after incubation of extracts for 1–2 h at 23°C, when APC/C was still inactive, ≈80% of Mad2 and ≈50% of BubR1 were already released from Cdc20, which suggested that some additional factor(s) that inhibit APC/C decay slower than MCC during release from checkpoint inhibition. This slowly eliminated factor may be MCF2, the second mitotic checkpoint inhibitor described in this article, or still another regulator. It has been reported that phosphorylation of Cdc20 by mitotic or checkpoint protein kinases inhibits its activity (16–19). It appears reasonable to assume that Cdc20 is dephosphorylated in exit from mitotic checkpoint arrest. We observed that after incubation of extracts for 3–4 h, APC/C isolated by immunoprecipitation has robust ubiquitylation activity without requirement for the supplementation of exogenous Cdc20 (Fig. 1 A and B), which suggests that active, presumably dephosphorylated Cdc20 is associated with APC/C after exit from mitotic checkpoint. If dephosphorylation of Cdc20 indeed occurs at this time, it may be specific and not a part of global dephosphorylation of proteins, because we found that after 3–4 h of incubation of extracts, APC/C is still in its mitotic phosphorylated form, as indicated by the retarded electrophoretic migration of its Cdc27 subunit (data not shown). This problem requires further investigation. At present we note that APC/C is subject to multiple layers of regulation both during and in exit from mitotic checkpoint and that this multiplicity of regulatory mechanisms may ensure strict control of APC/C activity by the mitotic checkpoint system.
We described here the separation from MCC of another mitotic checkpoint inhibitor associated with APC/C, MCF2 (Fig. 2A). Like MCC, MCF2 is also specific to the checkpoint-arrested state (Fig. 2B). The composition of MCF2 is unknown at present and is the subject of continued research effort in our laboratory. It does not contain Cdc20 or BubR1 (Fig. 2C). Protein kinase activity is not required for the action of either MCF2 or MCC, as shown by the observation that both inhibit APC/C activity in the presence of the nonhydrolyzable ATP analogue adenosine 5′-[β,γ-imido]triphosphate (AMP-PNP). Neither MCF2 nor MCC contains significant amounts of protein phosphatase or of checkpoint-specific ubiquitin C-terminal isopeptidase activity (data not shown).
We propose that both MCF2 and MCC inhibit APC/C by antagonizing its activator, Cdc20. This proposal is based on the following observations. (i) The addition of Cdc20 to extracts from nocodazole-arrested cells markedly accelerated the rate of the degradation of securin, provided that protein kinase action was prevented by staurosporine (Fig. 3A). Although this result is subject to alternative explanations (see Results), it is possible that acceleration is caused by the release of APC/C from inhibitors. (ii) When APC/C immunoprecipitated from checkpoint extracts was subjected to high-salt wash, its activity was stimulated by Cdc20 to a much greater extent than that obtained without salt wash (Fig. 3B). A reasonable interpretation is that the removal of inhibitors by high salt allows more efficient stimulation of APC/C by Cdc20. However, we cannot rule out the possibility that high-salt wash removes some other factors that interfere with the interaction of Cdc20 with APC/C. (iii) In our presently available most purified reconstituted system, the extent of the inhibition of APC/C by both MCF2 and MCC was diminished when the concentrations of Cdc20 were increased (Fig. 3C), which suggests competition between the two mitotic checkpoint inhibitors and Cdc20 on a common site. The identity of this common site is not known, but an attractive speculation is that the inhibitors may interact with the Cdc20-binding site of APC/C. MCC may bind to APC/C via its Cdc20 component, which may be converted to an inhibitory form by its association with other MCC components. MCF2 has no Cdc20 component, but it may have another moiety that interacts with the Cdc20-binding site of APC/C. We note that the antagonism between checkpoint inhibitors and Cdc20, reported in this article, may provide explanation for earlier observations that checkpoint arrest in yeast was overcome by increased levels of Cdc20 (13–15).
Although the present work provided some insight into the multiple mechanisms that control the activity of APC/C by the mitotic checkpoint system, it also raised many new questions. What is the mechanism of the disassembly of MCC in exit from checkpoint arrest, and why is Mad2 dissociated from Cdc20 before BubR1? What is the composition of MCF2, and how does it compete with Cdc20? What are the roles of phosphorylation and dephosphorylation of Cdc20 in the mitotic checkpoint? Obviously, much more work is necessary for a more complete understanding of the control of APC/C by the mitotic checkpoint system.
Methods
Extracts from nocodazole-arrested HeLa cells were prepared as described in ref. 12. These were called checkpoint extracts in the text. Extracts stably arrested in mitotic checkpoint (arrested extracts) were prepared by incubation of checkpoint extracts with ATPγS, whereas extracts that had exited from checkpoint (activated extracts) were prepared by incubation with ATP and an ATP-regenerating system, as described in ref. 12. The following procedures have been described in ref. 12:the binding of APC/C from extracts to anti-Cdc27 covalently linked to protein A beads (anti-Cdc27 beads); elution of inhibitors from immunoprecipitated APC/C with 0.3 M KCl; concentration of salt eluates and removal of salt by repeated ultrafiltration. His6-Cdc20 was expressed in baculovirus-infected insect cells and purified as described in ref. 20.
Assay of the Ligation of 125I-Cyclin to Ubiquitin.
Reaction mixtures contained in a volume of 10 μl: 40 mM Tris·HCl (pH 7.6), 5 mM MgCl2, 10% (vol/vol) glycerol, 1 mM DTT, 1 mg/ml BSA, 2 mM ATPγS, 50 μM ubiquitin, 1 μM ubiquitin aldehyde, 10 nM E1, 50 nM E2C/UbcH10, and 1–2 pmol of 125I-labeled cyclin B/protein A (referred to as 125I-cyclin). Cdc20 and a source of APC/C were added as specified in the figure legends. When APC/C was bound to anti-Cdc27 beads, 1 μl of packed beads was added, and reactions were carried out with shaking (1,000 rpm at 23°C) in a Thermomix shaker (Eppendorf) for the time periods indicated. Reaction products were separated on 12.5% SDS/PAGE, and results were quantified by PhosphorImager analysis. Results were expressed as the percentage of 125I-cyclin converted to conjugates with ubiquitin.
Assay of Inhibition of the Activity of APC/CCdc20.
This was similar to the assay of the ligation of 125I-cyclin to ubiquitin as described above, except that the reaction mixture contained soluble purified mitotic APC/C (21), 10 nM Cdc20, 1 μM okadaic acid, and inhibitor as specified in legends to the figures. Incubation was at 30°C for 60 min. The amount of APC/C used was adjusted so that without inhibitor, ≈40% of 125I-cyclin was converted to conjugates with ubiquitin. Results were expressed as the percentage of decrease in activity with inhibitor relative to that without inhibitor.
Chromatography of Inhibitor Preparations on MonoQ.
All operations were carried out at 0–4°C. Salt eluates from APC/C immunoprecipitates of arrested or activated extracts were prepared as described in ref. 12. Approximately 2 ml of salt eluate was applied to a MonoQ HR 5/5 column (GE Healthcare) equilibrated with 50 mM Tris·HCl (pH 7.4), 50 mM NaCl, and 1 mM DTT. The column was washed with 15 ml of the above buffer and then was subjected to elution with a linear gradient of 50–400 mM NaCl in the same buffer at a flow rate of 1 ml/min for 34 min. Fractions of 1 ml were collected into tubes that contained 0.2 mg of BSA. The fractions were numbered from the start of the salt gradient. The fractions were concentrated by ultrafiltration, diluted 10-fold in a buffer consisting of 50 mM Tris·HCl (pH 7.2), 1 mM DTT, and 10% (vol/vol) glycerol and concentrated again to a volume of 60 μl.
Immunoblotting and Immunoprecipitation.
For immunoblotting, we used the monoclonal antibodies described in ref. 12, except for anti-BubR1, which was from BD Transduction Laboratories (612503). Immunoblots were visualized by enhanced chemiluminescence (Pierce) and were quantified with an ImageQuant RT ECL instrument (GE Healthcare). For immunoprecipitation, we used affinity-purified rabbit polyclonal antibodies against BubR1 or Cdc20 (9).
Acknowledgments.
We acknowledge the Laboratory Animal and Hybridoma Facilities at Fox Chase Cancer Center (FCCC) for providing expert technical services. Part of this work was carried out during the stay of A.H. at FCCC on sabbatical leave, partly supported by the FCCC Research Fund. This work was supported by grants from the Israel Cancer Research Fund, Israel Science Foundation, and the Gruss Lipper Foundation (to A.H.) and by National Institutes of Health Grants GM44762, CA99423, and CA75138, Core Grant CA06927, and an appropriation from the Commonwealth of Pennsylvania (to T.J.Y.).
Footnotes
The authors declare no conflict of interest.
References
- 1.Bharadwaj R, Yu H. The spindle checkpoint, aneuploidy, and cancer. Oncogene. 2004;23:2016–2027. doi: 10.1038/sj.onc.1207374. [DOI] [PubMed] [Google Scholar]
- 2.Kops GJBL, Weaver BAA, Cleveland DW. On the road to cancer: Aneuploidy and the mitotic checkpoint. Nat Rev Cancer. 2005;5:773–784. doi: 10.1038/nrc1714. [DOI] [PubMed] [Google Scholar]
- 3.Nasmyth K. How do so few control so many? Cell. 2005;120:739–748. doi: 10.1016/j.cell.2005.03.006. [DOI] [PubMed] [Google Scholar]
- 4.Musacchio A, Salmon ED. The spindle assembly checkpoint in space and time. Nat Rev Mol Cell Biol. 2007;8:379–393. doi: 10.1038/nrm2163. [DOI] [PubMed] [Google Scholar]
- 5.Chan GK, Liu ST, Yen TJ. Kinetochore structure and function. Trends Cell Biol. 2005;15:589–598. doi: 10.1016/j.tcb.2005.09.010. [DOI] [PubMed] [Google Scholar]
- 6.Zachariae W, Nasmyth K. Whose end destruction: Cell division and the anaphase-promoting complex. Genes Dev. 1999;13:2039–2058. doi: 10.1101/gad.13.16.2039. [DOI] [PubMed] [Google Scholar]
- 7.Castro A, Bernis C, Vigneron S, Labbe JC, Lorca T. The anaphase-promoting complex: A key factor in the regulation of the cell cycle. Oncogene. 2005;24:314–325. doi: 10.1038/sj.onc.1207973. [DOI] [PubMed] [Google Scholar]
- 8.Peters JM. The anaphase-promoting complex/cyclosome: A machine designed to destroy. Nat Rev Mol Cell Biol. 2006;7:644–656. doi: 10.1038/nrm1988. [DOI] [PubMed] [Google Scholar]
- 9.Sudakin V, Chan GKT, Yen TJ. Checkpoint inhibition of the APC/C in HeLa cells is mediated by a complex of BubR1, Bub3, Cdc20, and Mad2. J Cell Biol. 2001;154:925–936. doi: 10.1083/jcb.200102093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Stegmeier F, et al. Anaphase initiation is regulated by antagonistic ubiquitination and deubiquitination activities. Nature. 2007;446:876–881. doi: 10.1038/nature05694. [DOI] [PubMed] [Google Scholar]
- 11.Reddy SK, Rape M, Margansky WA, Kirschner MW. Ubiquitination by the anaphase-promoting complex drives spindle checkpoint inactivation. Nature. 2007;446:921–925. doi: 10.1038/nature05734. [DOI] [PubMed] [Google Scholar]
- 12.Braunstein I, Miniowitz S, Moshe Y, Hershko A. Inhibitory factors associated with anaphase-promoting complex/cyclosome in mitotic checkpoint. Proc Natl Acad Sci USA. 2007;104:4870–4875. doi: 10.1073/pnas.0700523104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Schott EJ, Hoyt MA. Dominant alleles of Saccharomyces cerevisiae Cdc20 reveal its role in promoting anaphase. Genetics. 1998;148:599–610. doi: 10.1093/genetics/148.2.599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hwang LH, et al. Budding yeast Cdc20: A target of the spindle checkpoint. Science. 1998;279:1041–1044. doi: 10.1126/science.279.5353.1041. [DOI] [PubMed] [Google Scholar]
- 15.Pan J, Chen RH. Spindle checkpoint regulates Cdc20p stability in Saccharomyces cerevisiae. Genes Dev. 2004;18:1439–1451. doi: 10.1101/gad.1184204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Yudkovsky Y, Shteinberg M, Listovsky T, Brandeis M, Hershko A. Phosphorylation of Cdc20/Fizzy negatively regulates the mammalian cyclosome/APC in the mitotic checkpoint. Biochem Biophys Res Commun. 2000;271:299–304. doi: 10.1006/bbrc.2000.2622. [DOI] [PubMed] [Google Scholar]
- 17.Chung E, Chen RH. Phosphorylation of Cdc20 is required for its inhibition by the spindle checkpoint. Nat Cell Biol. 2003;5:748–753. doi: 10.1038/ncb1022. [DOI] [PubMed] [Google Scholar]
- 18.D'Angiolella V, Mari C, Nocera D, Rametti L, Grieco D. The spindle checkpoint requires cyclin-dependent kinase activity. Genes Dev. 2003;17:2520–2525. doi: 10.1101/gad.267603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tang Z, Shu H, Oncel D, Chen S, Yu H. Phosphorylation of Cdc20 by Bub1 provides a catalytic mechanism for APC/C inhibition by the spindle checkpoint. Mol Cell. 2004;16:387–397. doi: 10.1016/j.molcel.2004.09.031. [DOI] [PubMed] [Google Scholar]
- 20.Eytan E, Moshe Y, Braunstein I, Hershko A. Roles of the anaphase-promoting complex/cyclosome and of its activator Cdc20 in functional substrate binding. Proc Natl Acad Sci USA. 2006;103:2081–2086. doi: 10.1073/pnas.0510695103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Golan A, Yudkovsky Y, Hershko A. The cyclin-ubiquitin ligase activity of cyclosome/APC is jointly activated by protein kinases Cdck1/cyclin B and Plk. J Biol Chem. 2002;277:15552–15557. doi: 10.1074/jbc.M111476200. [DOI] [PubMed] [Google Scholar]