Abstract
The influence of past land use on the present-day diversity of stream invertebrates and fish was investigated by comparing watersheds with different land-use history. Whole watershed land use in the 1950s was the best predictor of present-day diversity, whereas riparian land use and watershed land use in the 1990s were comparatively poor indicators. Our findings indicate that past land-use activity, particularly agriculture, may result in long-term modifications to and reductions in aquatic diversity, regardless of reforestation of riparian zones. Preservation of habitat fragments may not be sufficient to maintain natural diversity in streams, and maintenance of such biodiversity may require conservation of much or all of the watershed.
Conservation of species diversity at local, regional, and continental scales has received increasing attention as human disturbance and modification of ecosystems increase. Our understanding of the magnitude of species decline is clearest for vertebrates in terrestrial, marine, and lake ecosystems (1–4). In contrast, empirical evidence of extirpations and extinctions of invertebrate species in lotic (running water) ecosystems is comparatively sparse (1–9). Worldwide, many rivers and streams have been profoundly modified by urban and agricultural development, impoundment, channelization, resource-extraction projects, and pollution. In many regions, such as the southern Appalachian Mountains, reforestation of previously cleared watersheds is occurring as agriculture becomes less important to the local economy (10, 11). This process of reforestation allows us to ask: to what extent are the effects of human disturbance reversible, and how long does recovery take? Although recovery and restoration of the physical habitat is often possible, the degree to which biological communities can recover from long-term disturbance is still relatively unknown.
Stream ecologists have long recognized the strong dependence of streams on the surrounding terrestrial environment (12–15). The riparian zone bordering streams serves as a buffer between the stream and the surrounding watershed and is also the primary source of organic matter for many small streams in forested biomes (12–15). Conditions in the riparian zone, therefore, strongly influence stream hydrology, substrate characteristics, temperature regimes, and water chemistry, which in turn affect all trophic levels. Considerable emphasis has been placed on protection or revegetation of riparian zones as a tactic for preserving aquatic ecosystems (16, 17). The presence of natural vegetation in riparian zones has been shown to improve stream hydrology, water quality, and reduce sedimentation in disturbed watersheds (18–20). However, by emphasizing restoration of riparian zones, land managers assume that stream conditions across the whole catchment can be mitigated by attention only to land adjacent to the stream. This assumption is not supported by recent studies (21, 22).
The overall objective of the present study was to investigate relationships between land use and invertebrate and fish diversity in streams. We used two approaches in the study. The first was to compare diversity in streams that drain agricultural land to diversity in streams that drain forested land. The second was to examine the land-use history associated with the streams to look for clues that might help explain present-day diversity patterns. To achieve these aims, we investigated 24 tributary watersheds ranging from 1,750 to 40,700 ha in size in two river basins, the Little Tennessee and the French Broad Rivers, in western North Carolina. Of the 12 watersheds chosen within each basin, 6 were currently primarily forested and 6 were agricultural. Land use in these 24 watersheds was assessed by determining the percentage of the watershed in forest at seven spatial scales for the 1950s and 1990s and was calculated from Geographic Information System overlays constructed from topographic maps, aerial photographs, and satellite imagery from the 1950s and 1990s. The seven spatial scales selected included both different riparian widths and longitudinal distances along the stream continuum as follows: (i) land use over the entire watershed; (ii) land use within a 30-m riparian zone of the stream (for the entire length of the stream); (iii) land use within a 100-m riparian zone of the stream (for the entire length of the stream); (iv) land use within a 30-m riparian zone of the stream (up to 1 km upstream of the sampling site); (v) land use within a 30-m riparian zone of the stream (up to 2 km upstream of the sampling site); (vi) land use within a 100-m riparian zone of the stream (up to 1 km upstream of the sampling site); and (vii) land use within a 100-m riparian zone of the stream (up to 2 km upstream of the sampling site).
At each of the 24 streams, random benthic invertebrate samples were collected in 1995–1996 from riffles along a 10-m reach. A modified quantitative kick net (0.4 m2; 250-μm mesh) was used to collect five samples, and a qualitative sample was taken from a range of microhabitats within the reach. Fish were sampled by electroshocking and seining a 50-m reach, including a riffle-pool complex. Fish samples were taken at each site during spring and fall of 1995 and 1996. Comparisons of diversity and land-use data were made with multiple regression models, and stepwise regression analysis was used to identify the combination of history and spatial land use acquired from the Geographic Information System that best explained the diversity of stream invertebrates and fishes. Invertebrate assemblages for each of the 24 streams were also compared by detrended correspondence analysis (DCA; ref. 23).
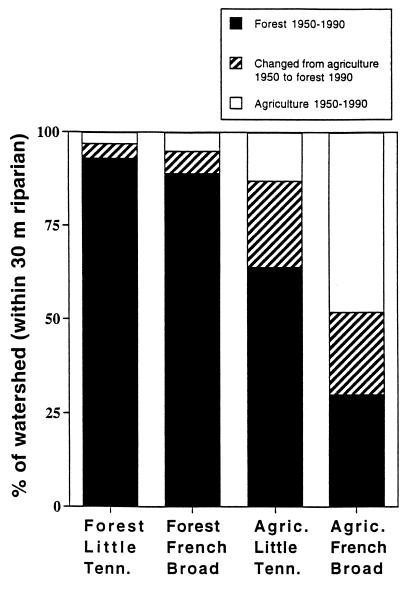
Streams in 1990s forested watersheds were generally >90% forested in the 1950s. However, streams in 1990s agricultural watersheds in the Little Tennessee Basin averaged ≈60% forest in the 1950s, whereas those in the French Broad Basin averaged ≈30% forest (Fig. 1).
Figure 1.
Percentage of watershed (within a 30-m riparian zone) in different land uses in the 1950s and 1990s. Each column represents six watersheds characterized by 1990s land use in the basins of the Little Tennessee and the French Broad Rivers (data assessed from the Geographic Information System).
In both river basins, significant differences in both faunal diversity and assemblage composition were observed between agricultural and forested streams. Invertebrate taxonomic richness and other analogs of diversity [Margalef’s Index and the number of Ephemeroptera, Plecoptera, and Trichoptera taxa (EPT)] were significantly greater in forested streams than in agricultural streams in both river basins (Table 1). In contrast, invertebrate density did not differ significantly between current land-use types. Fish assemblages showed a different trend; the total number of fish species, Margalef’s index, and total abundance were significantly greater in agricultural streams than in forested ones (Table 1). Fish diversity was greater where trout were absent and where species tolerant of sedimentation were favored. We found a significant negative correlation between fish-species diversity and trout abundance (n = 24 streams; P < 0.001; >99% of trout were introduced rainbow and brown trout, and <1% were native brook trout). Substrate analysis of percentage of fine sediments indicated greater quantities in agricultural than forested streams (M. Paul and J. Meyer, personal communication), and sedimentation seemed to be linked to a reduced abundance of fishes belonging to the crevice-spawning reproductive guild (G.S.H., unpublished data).
Table 1.
Mean diversity for forested and agricultural streams in the Little Tennessee and French Broad Rivers
| Diversity indices | Forest (L. Tennessee) | Forest (Fr. Broad) | Agriculture (L. Tennessee) | Agriculture (Fr. Broad) | Land use
|
River basin
|
||
|---|---|---|---|---|---|---|---|---|
| F | P | F | P | |||||
| Invertebrates | ||||||||
| Taxonomic richness | 59.3 ± 3.6 | 59.7 ± 7.9 | 48.7 ± 3.6 | 39.0 ± 5.4 | 8.25 | ∗∗ | 0.73 | n.s. |
| Margalef’s index | 7.9 ± 0.4 | 8.1 ± 0.9 | 6.2 ± 0.4 | 5.2 ± 0.6 | 12.86 | ∗∗ | 0.46 | n.s. |
| EPT | 40.5 ± 3.4 | 45.2 ± 6.7 | 32.0 ± 3.4 | 25.0 ± 4.4 | 9.34 | ∗∗ | 0.06 | n.s. |
| NCBI† | 2.7 ± 0.1 | 2.5 ± 0.1 | 3.3 ± 0.2 | 3.4 ± 0.2 | 28.14 | ∗∗ | 0.02 | n.s. |
| Invertebrate density | 1858 ± 496 | 1441 ± 211 | 2635 ± 758 | 3015 ± 1958 | 1.17 | n.s. | 0.01 | n.s. |
| Fishes | ||||||||
| Species richness | 14.5 ± 3.3 | 11.7 ± 1.7 | 23.2 ± 1.2 | 16.8 ± 2.3 | 9.22 | ∗∗ | 4.56 | ∗ |
| Margalef’s index | 4.4 ± 0.9 | 3.7 ± 0.5 | 6.7 ± 0.4 | 4.7 ± 0.6 | 6.33 | ∗ | 4.04 | n.s. |
| Fish abundance | 1096 ± 256 | 757 ± 149 | 2212 ± 354 | 1772 ± 377 | 12.76 | ∗∗ | 1.70 | n.s. |
| Fish + invertebrate | ||||||||
| Species richness | 73.8 ± 4.3 | 71.3 ± 7.2 | 71.8 ± 3.3 | 56.2 ± 7.2 | 2.21 | n.s. | 2.48 | n.s. |
Mean diversities are given ±SE (n = 6). Results of two-way ANOVA are shown, with Tukey’s test for land use (all forest vs. agriculture combined) and river basin (all Little Tennessee vs. French Broad) treatments. (∗, P < 0.05; ∗∗, P < 0.01; n.s., not significant; NCBI, North Carolina Biotic Index.)
Regressions of diversity and watershed conditions across time and space showed that land use in the 1950s was usually the best indicator of present-day diversity. When data from both basins were combined, the best single model for explaining invertebrate taxonomic richness was land use across the entire watershed in the 1950s (Table 2). A stepwise regression of DCA Axis 1 values was carried out against the 14 time and space Geographic Information System values for percentage of each watershed in forest (r2 = 0.56; F = 28.42; P < 0.001). Therefore when considered separately, the French Broad Basin, which experienced greater agricultural development in the past, generally showed stronger links to the past than agricultural watersheds in the Little Tennessee Basin.
Table 2.
Multiple regression analyses of measures of diversity against percentage of the watershed in forest at 14 different spatial scales and at two time periods
| Basins | Time and spatial watershed scales | r2 | F | P | |
|---|---|---|---|---|---|
| Invertebrates | |||||
| Taxonomic richness | Combined river basins | 1950-WS | 0.56 | 27.9 | ∗∗ |
| French Broad River | 1950-WS | 0.67 | 21.09 | ∗∗ | |
| Little Tennessee River | 1950-1k-30 | 0.28 | 4.03 | n.s. | |
| Margalef’s index | Combined river basins | 1950-WS-30 | 0.59 | 31.2 | ∗∗ |
| French Broad River | 1950-WS | 0.69 | 22.77 | ∗∗ | |
| Little Tennessee River | 1950-1k-100 | 0.53 | 11.23 | ∗∗ | |
| EPT | Combined river basins | 1950-WS-30 | 0.51 | 22.9 | ∗∗ |
| French Broad River | 1950-WS | 0.69 | 22.94 | ∗∗ | |
| Little Tennessee River | 1950-WS | 0.25 | 3.37 | n.s. | |
| NCBI† | Combined river basins | 1950-WS-30 | 0.51 | 22.3 | ∗∗ |
| French Broad River | 1950-WS-100 | 0.73 | 27.04 | ∗∗ | |
| Little Tennessee River | 1990-1k-100 | 0.40 | 6.88 | ∗ | |
| Invertebrate density | Combined river basins | 1990-1k-100 | 0.23 | 6.8 | ∗ |
| French Broad River | 1990-1k-30 | 0.36 | 5.86 | ∗ | |
| Little Tennessee River | 1990-1k-100 | 0.22 | 2.87 | n.s. | |
| Fishes | |||||
| Species richness | Combined river basins | 1950-2k-30 | 0.37 | 12.7 | ∗∗ |
| French Broad River | 1990-1k-100 | 0.47 | 9.08 | ∗ | |
| Little Tennessee River | 1950-WS | 0.53 | 11.33 | ∗∗ | |
| Margalef’s index | Combined river basins | 1950-2k-30 | 0.27 | 8.3 | ∗∗ |
| French Broad River | 1990-1k-100 | 0.32 | 4.79 | n.s. | |
| Little Tennessee River | 1950-WS | 0.45 | 8.30 | ∗ | |
| Fish abundance | Combined river basins | 1950-1k-100 | 0.46 | 19.4 | ∗∗ |
| French Broad River | 1950-2k-100 | 0.40 | 6.88 | ∗ | |
| Little Tennessee River | 1950-2k-100 | 0.67 | 20.82 | ∗∗ | |
| Total fish + invertebrate taxa | Combined river basins | 1950-WS | 0.46 | 18.4 | ∗∗ |
| French Broad River | 1950-WS | 0.56 | 12.82 | ∗∗ | |
| Little Tennessee River | 1990-WS-30 | 0.07 | 0.75 | n.s. |
Combined river basins analysis consists of data for 24 watersheds, whereas French Broad and Little Tennessee River data are for 12 watersheds in their respective basins. Only best single variable models are shown. (WS, land use over the entire watershed; 1k, land use up to 1 km upstream from the sampling reach; 2k, land use up to 2 km upstream from the sampling reach; 30, land use within a 30-m riparian buffer zone of the stream; 100, land use within a 100-m riparian buffer zone of the stream; *P < 0.05; **P < 0.01; n.s., not significant.)
North Carolina Biotic Index
Land-use conditions in the 1950s in the 30-m riparian zone were the best predictors of invertebrate diversity, as measured by Margalef’s Index, the North Carolina Biotic Index, and EPT values (which account for disturbance-sensitive taxa) in combined basins (Table 2). Again, when analyzed separately, land use in the historically more developed French Broad Basin showed consistently stronger regression values than in the Little Tennessee Basin. Invertebrate density was only weakly correlated with land-use patterns; the strongest predictor was land use in the 1990s in the 100-m riparian zone, within 1 km upstream of the sampling sites (Table 2). The best single variable models for fish species richness, diversity (Margalef’s index), and abundance in both the combined basins and in the Little Tennessee were 1950s land use at various spatial scales. However, species richness and diversity in the French Broad basin alone were best explained by more localized land-use data in the 1990s (Table 2). Finally, 1950s watershed conditions best explained combined fish and invertebrate diversity across all watersheds.
These findings support our assertion that in currently forested watersheds, historic land-use data may be more useful indicators than present land use in predicting taxonomic diversity. Furthermore, our findings indicate that large-scale and long-term agricultural disturbances in a watershed limit the recovery of stream diversity for many decades.
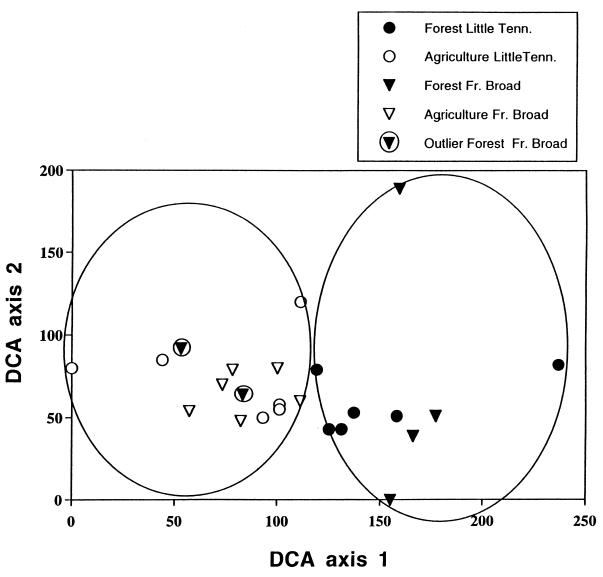
Legacies of land use also help to explain the current composition of invertebrate assemblages in the study streams. Multivariate analysis incorporating invertebrate assemblage data for all streams shows that forested and agricultural streams differ in taxonomic composition, with the exception of two forested streams (Fig. 2). Both of these streams drain watersheds that were 92% forested in the 1990s, but the invertebrate assemblages more closely resemble those of agricultural streams (Fig. 2). The best predictor of invertebrate composition in these two forested streams was land use in the 1950s in the 30-m riparian zone up to 2 km upstream of the sampling site. In the 1950s, the two forested streams were embedded in a landscape with high percentages of riparian agriculture (43% and 44%). These two streams were also anomalous with respect to fish species composition, having assemblages more similar to the agricultural streams than other forested streams. These forested sites contained 15 and 14 fish species, 12 of which they held in common. Of these 12 species, 5 occurred at no other forested stream, whereas 4 of these 5 were recorded in at least one agricultural stream. Sculpin and trout were absent at both of these streams and were essentially absent from five of six agricultural streams, but they were abundant at the other French Broad forested streams. The mean Jaccard similarity coefficient between these two anomalous streams and the other French Broad forested streams was 0.16, and for these streams and the French Broad agricultural streams it was 0.32. The two means were significantly different (t test; df = 78; P < 0.001), indicating that these forested streams were more similar to agricultural than to other forested streams.
Figure 2.
DCA of invertebrate assemblages based on presence/absence data for the 24 watersheds in two river basins. Streams are grouped into two general clusters, forested and agricultural streams (as indicated by the ellipses). Two outlier forested streams within the agricultural cluster represent streams that today lie in forested watersheds but were in partially agricultural watersheds in the 1950s.
Reforestation of the riparian zone over the last 47 years has resulted in little effective recovery of the fauna of these streams to predisturbance conditions. Current stream restoration philosophy and policy supports the idea that recovery of stream fauna can occur relatively rapidly after short-term natural and human disturbances when riparian conditions are returned to a predisturbance state (24–26). Our data suggest that recovery requires decades.
The difference in response between invertebrate and fish diversity may be caused by a stronger dependence of invertebrates (especially EPT taxa) on the presence of a relatively stable, sediment-free streambed (27–29). As well as differences in overall fish diversity, we found that fishes dependent on the streambed for foraging or breeding (e.g., some minnows, sculpins, and darters) were replaced in agricultural streams by species that dwell in the water column or those that clean sediment from their nests (e.g., other minnows and sunfishes).
Our findings challenge assumptions about both the maintenance and future recovery of biodiversity in disturbed stream ecosystems. Studies of the recovery of stream assemblages after short-term catastrophic disturbances (e.g., experimental manipulation, floods, logging, construction, and point-source pollution) have often shown relatively rapid recovery of biotic communities (30–35), and these findings have provided the cornerstone of accepted theory and policy. However, high impact or sustained anthropogenic disturbance, such as sustained agriculture, may profoundly alter biotic communities, and the effects of this disturbance may be persistent. Few studies have assessed recovery from prolonged disturbance or scrutinized changes from a multiple-watershed perspective.
Current land-management practices often operate on the assumption that economic activity within a catchment can proceed as long as riparian zones are preserved (36, 37). Riparian zones have been used effectively to mitigate the adverse effects of many land-use practices, but our understanding of the linkages among ecological processes that shape biodiversity, biotic communities, and watershed conditions is far from complete. In addition to understanding the value of intact riparian zones, our results support the view that conservation of natural ecosystems may require preservation of the entire watershed—not just fragments of it as many current policies assume. In terrestrial systems, the influence of forest-fragment size on biodiversity has been investigated intensively (38). In contrast, this issue has been largely ignored in stream systems; however, our results indicate that the amount of forest and possibly forest size may be critical in influencing stream biota.
Our findings provide new insights into possible causes of variability in the diversity and composition of aquatic assemblages. Data from studies of multiple streams are often highly variable and difficult to interpret. Our results suggest that some of this variability may be a legacy of land use, which is often unrecorded or unknown.
Finally, our study provides evidence of the importance of past land use as a determinant of present species diversity in streams. Exploitation and development of natural watersheds is continuing worldwide. We suggest that disturbance of these systems, which in our study involved the conversion of forest to agriculture, may result in substantial long-term modifications and reductions in natural biodiversity. Realization of the potential alteration or loss of biodiversity from watershed-wide land use should provide a warning for conservation organizations and policy makers alike.
Acknowledgments
We thank B. Bennett, R. H. Jones, M. McTammany, L. Martin, J. L. Meyer, M. Neatrour, M. J. Paul, H. R. Pulliam, K. Simon, J. L. Tank, P. Wagner, and J. R. Webster for constructive comments. J. Harper, H. Pape, and M. Scott provided assistance with data collection and analysis. This research was supported by National Science Foundation Division of Environmental Biology Grants 9011661 and 9416803.
ABBREVIATIONS
- DCA
detrended correspondence analysis
- EPT
number of Ephemeroptera, Plecoptera, and Trichoptera taxa
Footnotes
This paper was submitted directly (Track II) to the Proceedings Office.
References
- 1.Groombridge B, editor. World Conservation Monitoring Centre. Global Biodiversity: Status of the Earth’s Living Resources. London: Chapman & Hall; 1992. [Google Scholar]
- 2.Wilson E O. The Diversity of Life. Cambridge, MA: Harvard Univ. Press; 1992. [Google Scholar]
- 3.Warren M L, Jr, Burr B M. Fisheries. 1994;19:6–18. [Google Scholar]
- 4.Williams J E, Johnson J E, Hendrickson D A, Contreras-Balderas S, Williams J D, Navarro-Mendoza M, McAllister D E, Deacon J E. Fisheries. 1989;14:2–20. [Google Scholar]
- 5.Schindler D W. Bioscience. 1989;39:426. [Google Scholar]
- 6.Allan J D, Flecker A S. Bioscience. 1993;43:32–43. [Google Scholar]
- 7.Vitousek P M, Mooney M H A, Lubchenco J, Melillo J M. Science. 1997;277:494–499. [Google Scholar]
- 8.Williams J D, Warren M L, Jr, Cummings K S, Harris J L, Neves R J. Fisheries. 1993;18:6–22. [Google Scholar]
- 9.Taylor C A, Warren M L, Jr, Fitzpatrick J F, Jr, Hobbs H H, III, Jezerinac R F, Pflieger W L, Robison H W. Fisheries. 1996;21:25–38. [Google Scholar]
- 10.Hackney C T, Adams S M, Martin W H, editors. Biodiversity of the Southeastern United States: Aquatic Communities. New York: Wiley; 1992. [Google Scholar]
- 11.Benz G W, Collins D E, editors. Southeast Aquatic Research Institute. Aquatic Fauna in Peril: The Southeastern Perspective. Decatur, AL: Southeast Aquatic Research Institute; 1996. , Special Publication 1. [Google Scholar]
- 12.Cummins K W. Bioscience. 1974;24:631–641. [Google Scholar]
- 13.Hynes H B N. Verh Int Ver Theor Angew Limnol. 1975;19:1–15. [Google Scholar]
- 14.Vannote R L, Minshall G W, Cummins K W, Sedel J R, Cushing C E. Can J Fish Aquat Sci. 1980;37:130–137. [Google Scholar]
- 15.Wallace J B, Eggert S L, Meyer J L, Webster J R. Science. 1997;277:102–104. [Google Scholar]
- 16.Osborne L L, Kovacic D A. Freshwater Biol. 1993;29:243–258. [Google Scholar]
- 17.Waters T F. Sediment in Streams: Sources, Biological Effects and Control. Bethesda: American Fisheries Soc.; 1995. [Google Scholar]
- 18.Schlosser J R, Karr J R. Water Resour Bull. 1981;17:233–240. [Google Scholar]
- 19.Lowrance R, Todd R, Fail J, Jr, Hendrickson O, Jr, Leonard R, Asmussen L. Bioscience. 1984;24:374–377. [Google Scholar]
- 20.Peterjohn W T, Correll D L. Ecology. 1984;65:1466–1475. [Google Scholar]
- 21.Roth N E, Allan J D, Erickson D L. Land Ecol. 1996;11:141–156. [Google Scholar]
- 22.Wang L, Lyons J, Kanehl P, Gatti R. Fisheries. 1997;22:6–12. [Google Scholar]
- 23.McCune B, Mefford M J. multivariate analysis of ecological data. Gleneden Beach, OR: MjM Software; 1997. [Google Scholar]
- 24.Doppelt B, Scurlock M, Frissell C, Karr J. Entering the Watershed: A New Approach to Save America’s River Ecosystems. Washington, DC: Island; 1993. [Google Scholar]
- 25.Harper D M, Ferguson A J D, editors. The Ecological Basis for River Management. New York: Wiley; 1995. [Google Scholar]
- 26.Naiman R J, editor. Watershed Management: Balancing Sustainability and Environmental Change. New York: Springer; 1992. [Google Scholar]
- 27.Crouse M R, Callahan C A, Malueg K W, Dominguez S E. Trans Am Fish Soc. 1981;110:281–286. [Google Scholar]
- 28.Alexander G R, Hansen E A. North Am J Fish Manage. 1983;3:365–372. [Google Scholar]
- 29.Copper C M. J Freshwater Ecol. 1987;4:101–113. [Google Scholar]
- 30.Ide F P. J Fish Res Board Can. 1967;24:769–805. [Google Scholar]
- 31.Fisher S G, Gray L J, Grimm N B, Busch D E. Ecol Monogr. 1982;52:93–110. [Google Scholar]
- 32.Reice S R. Oecologia. 1985;67:90–97. doi: 10.1007/BF00378456. [DOI] [PubMed] [Google Scholar]
- 33.Johnson S L, Vaughn C C. Freshwater Biol. 1995;34:531–540. [Google Scholar]
- 34.Matthaei C D, Uehlinger U, Meyer E I, Fruitiger A. Freshwater Biol. 1996;35:233–248. [Google Scholar]
- 35.Wallace J B, Vogel D S, Cuffney T F. J North Am Benthological Soc. 1986;5:115–126. [Google Scholar]
- 36.Newbold J D, Erman D C, Roby K B. Can J Fish Aquat Sci. 1980;37:1076–1085. [Google Scholar]
- 37.Growns I O, Davis J A. Aust J Mar Freshwater Res. 1991;42:689–706. [Google Scholar]
- 38.Forman R T, Galli A E, Leck C F. Oecologia. 1976;26:1–8. doi: 10.1007/BF00345649. [DOI] [PubMed] [Google Scholar]