Abstract
Transforming growth factor β (TGFβ) controls a diverse set of cellular processes by activating TGFβ type I (TβRI) and type II (TβRII) serine-threonine receptor kinases. Canonical TGFβ signaling is mediated by Smad2 and Smad3, which are phosphorylated in their SXS motif by activated TβRI. The 90-kDa heat-shock protein (Hsp90) is a molecular chaperone facilitating the folding and stabilization of many protein kinases and intracellular signaling molecules. Here, we present evidence identifying a critical role for Hsp90 in TGFβ signaling. Inhibition of Hsp90 function by using small-molecule inhibitors such as 17-allylamino-17-demethoxygeldanamycin (17AAG), and also at the genetic level, blocks TGFβ-induced signaling and transcriptional responses. Furthermore, we identify TβRI and TβRII as Hsp90-interacting proteins in vitro and in vivo and demonstrate that inhibition of Hsp90 function increases TβR ubiquitination and degradation dependent on the Smurf2 ubiquitin E3 ligase. Our data reveal an essential level of TGFβ signaling regulation mediated by Hsp90 by its ability to chaperone TβRs and also implicate the use of Hsp90 inhibitors in blocking undesired activation of TGFβ signaling in diseases.
Keywords: Smad, Smurf, tumor suppression, degra
TGFβ signals through heteromeric complexes of transmembrane serine-threonine kinase receptors to control diverse developmental processes and the pathogenesis of many diseases. Within the receptor complex, the TGFβ type II receptor (TβRII) is constitutively active and phosphorylates the TGFβ type I receptor (TβRI) on serine-threonine residues in the GS domain in response to TGFβ. Activated TβRI then phosphorylates Smad2 and Smad3 (collectively Smad2/3) in the distal C-terminal SXS motif. Smad2/3 phosphorylation subsequently controls a cascade of downstream events, including hetero-oligomeric complex formation with Smad4, and the nuclear accumulation of this complex, which ultimately regulates gene transcription in conjunction with a variety of transcriptional cofactors (1, 2). Activated TβRI can also lead to the phosphorylation of non-Smad targets, such as the ERK, c-Jun NH2-terminal kinase, and p38 MAP kinase (3, 4).
Recent progress shows that TGFβ receptors are regulated by internalization and ubiquitin-mediated down-regulation as a means to control signaling (5–7). However, although TGFβ receptors are essential in the activation of all TGFβ downstream responses, via both Smad and non-Smad signaling pathways, research has primarily focused on the regulation of Smads. Thus, how TβRs are regulated is less well understood, and few proteins that directly interact with the receptors have been identified (8).
The 90-kDa heat-shock protein (Hsp90) is an abundant molecular chaperone that functions by facilitating protein folding and stabilization. Hsp90 chaperones a variety of signaling proteins involved in cancer, including protein kinases and other proteins involved in cell growth, survival, and differentiation (9, 10). The regulatory domain of Hsp90 contains an ATP-binding site. Upon ATP binding and hydrolysis, the Hsp90–client complex associates with cochaperones, such as CDC37, to facilitate client stabilization (11, 12). In contrast, in its ADP-bound form, Hsp90 associates with different cochaperones, such as Hsp70 and p60Hop, which results in ubiquitin-mediated degradation of the client.
Because many Hsp90 clients are involved in tumorigenesis, excitement exists around the development of Hsp90 inhibitors as pathway-directed cancer drugs. Geldanamycin (GM), a naturally occurring benzoquinone ansamycin, inhibits Hsp90's ATP-dependent association with cochaperones and thus its activity as a molecular chaperone (11, 12). Such pharmacological inhibition of Hsp90 resembles its ADP-bound conformation, which favors and results in ubiquitin-mediated degradation of clients, including ErbB2 and AKT (12). 17AAG, a synthetic analogue of GM in clinical trials, as well as the structurally distinct nonansamycin antibiotic radicicol, also inhibit Hsp90 function by this mechanism.
Although TGFβ signaling is strictly controlled in normal cells, aberrant TGFβ responses are frequent in human diseases, including cancers, fibrosis, and autoimmune and cardiovascular ailments (13, 14), suggesting that the TGFβ pathway might harbor key targets for drug design. In an effort to evaluate anticancer effects of Hsp90 inhibitors, we inadvertently found that Hsp90 inhibitors blocked TGFβ antiproliferative signaling. Thus, we sought to determine whether Hsp90 might regulate TGFβ signaling. Here, we present data identifying a critical level of TGFβ-signaling regulation mediated by Hsp90. Inhibition of Hsp90 by 17AAG compromises TGFβ-mediated transcriptional responses by enhancing TβR ubiquitination and degradation in a Smurf2 ubiquitin E3 ligase-dependent manner, thus preventing Smad2/3 activation. TβRI and TβRII specifically interact with Hsp90 and are clients of this cellular chaperone.
Results
Hsp90 Inhibitors Block TGFβ-Induced Transcription.
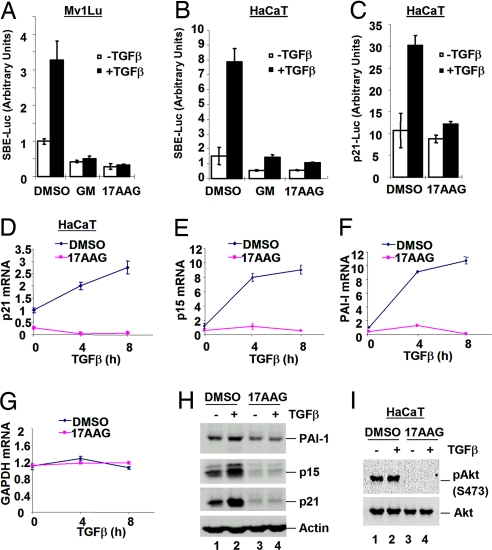
Because Hsp90 inhibitors inhibit various signaling pathways involved in tumorigenesis, we assessed whether GM and 17AAG could block TGFβ-induced transcription. We first evaluated the effect of GM/17AAG on TGFβ responses by using SBE-luc, a synthetic TGFβ-responsive reporter gene dependent on Smad activation, in epithelial cells. In control cells, TGFβ increased SBE-luc activity in Mv1Lu lung epithelial cells (Fig. 1A) and HaCaT keratinocytes (Fig. 1B), respectively. Treatment of cells with GM or 17AAG completely abolished TGFβ-induced responses (Fig. 1 A and B).
Fig. 1.
Hsp90 inhibitors block TGFβ-induced transcription. (A and B) GM and 17AAG decrease TGFβ-induced SBE-luc activity. Mv1Lu (A) and HaCaT (B) cells were transfected with SBE-luc reporter. Cells were treated for 30 min with 10 μM GM or 17AAG before and during a 16-h TGFβ treatment as indicated. Luciferase assays are described in Materials and Methods. (C) 17AAG decreases TGFβ-induced p21-promoter activity. HaCaT cells were transfected with p21-luc reporter. Cells were treated with 1 μM 17AAG for 30 min before and during a 16-h TGFβ treatment before luciferase assay. (D) 17AAG abolishes Smad-dependent gene transcription. HaCaT cells were treated with 0.5 μM 17AAG for 1 h before and during 0-, 4-, or 8-h TGFβ treatment as indicated. Cells were harvested for RNA preparation and quantitative RT-PCR to assess p21 mRNA levels. (E–G) Quantitative RT-PCR analysis of p15 (E), PAI-I (F), and GAPDH (G) mRNA. (H) 17AAG abolishes TGFβ-induced protein expression. HaCaT cells were treated with 1 μM 17AAG for 5 h before and during a 15-h TGFβ treatment as indicated. Cells were harvested for Western blotting with anti-PAI-I, anti-p15, anti-p21, and anti-actin antibodies. (I) 17AAG abolishes endogenous Akt phosphorylation at S473 in HaCaT cells.
Smad2/3 mediate the transcriptional regulation of cyclin-dependent kinase inhibitors p15 (15, 16) and p21 (17, 18), which results in TGFβ-induced growth arrest. We assessed the effect of 17AAG on TGFβ-induced transcription from the natural p21 promoter. In HaCaT cells transfected with p21-luc, TGFβ induced an ≈3-fold increase in p21-luc activity as expected, and 17AAG prevented TGFβ-induced activation of the p21 promoter (Fig. 1C). In accordance, quantitative RT-PCR analysis revealed that the induction of endogenous p21 and p15 mRNA was abolished by 17AAG, suggesting that 17AAG inhibits TGFβ-induced gene transcription (Fig. 1 D and E).
Because Smads also up-regulate transcription of the extracellular matrix component plasminogen activator inhibitor-1 (PAI-1) (19, 20), we examined the level of PAI-1 mRNA. As shown in Fig. 1F, 17AAG efficiently blocks TGFβ-induced increases in PAI-1 mRNA level. As a control, GAPDH mRNA levels remained fairly constant in 17AAG-treated and control cells (Fig. 1G), suggesting that the effect of 17AAG on TGFβ-induced mRNA expression is specific and not the result of cytotoxicity or general transcriptional repression in the cell. In addition, the effect of 17AAG on TGFβ-induced gene transcription correlates with a 17AAG-mediated decrease in levels of corresponding proteins (Fig. 1H). Finally, the same 17AAG treatment abolished S473 phosphorylation of endogenous AKT, a well known Hsp90 client, demonstrating the efficacy of 17AAG treatment (Fig. 1I). Thus, 17AAG blocks TGFβ-induced transcriptional responses, which suggests that Hsp90 may positively regulate TGFβ signaling.
Inhibition of Hsp90 Blocks Smad2/3 SXS Phosphorylation.
TGFβ stimulates Smad2/3 phosphorylation, which controls a cascade of downstream events. To begin to investigate how GM/17AAG blocks TGFβ-induced transcription, and consequently where Hsp90 may regulate TGFβ signaling, we used several well established approaches to compromise Hsp90 function and analyzed the effect on TGFβ-induced Smad2/3 phosphorylation.
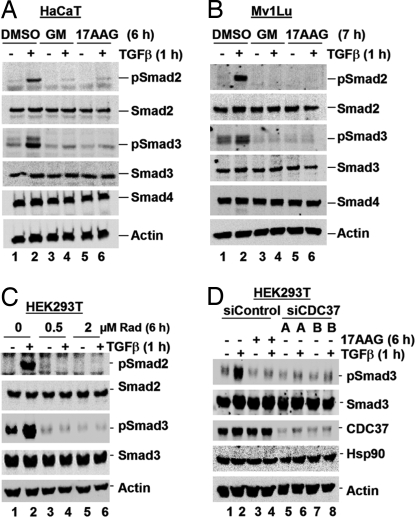
Initially, GM or 17AAG (10 μM) was used to treat HaCaT and Mv1Lu cells. Western blot analysis of whole-cell lysates revealed that GM and 17AAG treatments completely abolish TGFβ-induced phosphorylation of endogenous Smad2/3 in HaCaT (Fig. 2A, lanes 4 and 6) and Mv1Lu cells (Fig. 2B, lanes 4 and 6). Further, 17AAG could inhibit TGFβ-induced Smad2/3 phosphorylation at a concentration as low as 500 nM in Mv1Lu cells [supporting information (SI) Fig. S1A, lane 10], 100 nM in HaCaT cells (Fig. S1B Right, lane 8), and 1 μM in HEK293T cells (Fig. S1C, lane 8) (see also Figs. S1–S7). The ability of 17AAG to block TGFβ-induced Smad2/3 phosphorylation is not restricted to these cells because we observed a similar phenomenon in a range of cell lines including MCF10A, HepG2, HeLa, and COS-7 (data not shown).
Fig. 2.
Inhibition of Hsp90 blocks Smad2/3 SXS phosphorylation. (A and B) GM/17AAG abolishes TGFβ-induced Smad2/3 activation. HaCaT (A) and Mv1Lu (B) cells were cultured with 10 μM 17AAG or GM for the indicated time, with TGFβ added for the last 1 h. Cells were harvested for Western blotting with anti-Smad, anti-phospho-Smad, and anti-actin antibodies. (C) Radicicol (Rad) abolishes TGFβ-induced Smad2/3 activation. HEK293T cells were treated with DMSO or Rad (0.5 μM or 2 μM) for 6 h, with TGFβ added for the last 1 h. Western blotting was performed as in A. (D) CDC37 depletion abolishes TGFβ-induced Smad3 activation. HEK293T cells were transfected with siControl or siCDC37 (A or B) for 48 h and treated with TGFβ for 1 h before harvest and Western blotting by using anti-Smad3, anti-phospho-Smad3, anti-CDC37, anti-Hsp90, and anti-actin antibodies. Where indicated, 17AAG was added to siControl-transfected cells for 5 h before TGFβ treatment.
To verify that GM/17AAG-mediated loss of TGFβ-induced Smad phosphorylation is due to compromised Hsp90 function, we used two additional means to inhibit Hsp90 and measured the effect on Smad2/3 activation. Cells were treated with radicicol, a macrolactone class Hsp90 inhibitor that is structurally distinct from GM/17AAG (11). Radicicol treatment (0.5 or 2 μM) resulted in the loss of TGFβ-induced Smad2/3 phosphorylation in HEK293T (Fig. 2C) and HaCaT cells (data not shown).
Because knockdown of the Hsp90 essential cochaperone CDC37 can cause a similar outcome to GM/17AAG treatment (21), we assessed the effect of siRNA-mediated CDC37 knockdown on TGFβ-induced Smad3 phosphorylation. As shown in Fig. 2D, both siCDC37 oligos (A and B) decreased the CDC37 protein level by ≈90%, compared with siControl. Significantly, siCDC37 caused a loss of TGFβ-induced Smad3 phosphorylation comparable with that seen when siControl-transfected cells were treated with 17AAG (Fig. 2D, lanes 2, 4, 6, and 8).
Because structurally distinct inhibitors (GM/17AAG and radicicol) and CDC37 knockdown all dramatically reduce Smad2/3 phosphorylation, Hsp90 activity may be required for TGFβ-induced Smad2/3 activation.
Hsp90 Inhibitors Do Not Block TGFβ Signaling at the Smad Level.
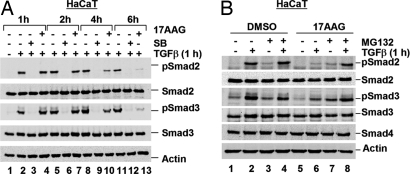
Having established that several Hsp90 inhibitors block TGFβ-induced Smad2/3 activation (Fig. 2), we used 17AAG for our subsequent experiments. We began to investigate how 17AAG causes loss of Smad2/3 phosphorylation and compromised TGFβ-induced transcription. We reasoned that 17AAG might inhibit TβR kinase activity or promote degradation of R-Smads or TβRs. To distinguish between these possibilities, we first determined how long 17AAG, compared with the TβRI kinase inhibitor SB431542 (SB), took to reduce TGFβ-induced Smad2/3 phosphorylation. Treatment with SB immediately blocked TGFβ-induced Smad2/3 phosphorylation, as expected (Fig. 3A) (22). In sharp contrast, a 1-h 17AAG treatment did not significantly alter TGFβ-induced Smad2/3 phosphorylation in HaCaT (Fig. 3A, lanes 2 and 4) or Mv1Lu cells (Fig. S2). Instead, TGFβ-induced phospho-Smad2/3 levels decreased in the presence of 17AAG over time, completely disappearing after 6 h of treatment (Fig. 3A, lanes 11 and 13). In accordance, TGFβ-induced Smad2 nuclear translocation, which is dependent on SXS phosphorylation, was reduced after just 1 h of SB treatment, but only after 6 h of 17AAG treatment (data not shown). Thus, 17AAG takes significantly longer than SB to block Smad2/3 phosphorylation and nuclear accumulation, and the two compounds may act via different mechanisms to block TGFβ signaling.
Fig. 3.
Hsp90 inhibitors do not block TGFβ signaling at the Smad level. (A) SB and 17AAG inhibit Smad phosphorylation at different rates. HaCaT cells were treated with SB or 17AAG for the indicated time, with TGFβ added for the last 1 h. Cells were harvested for Western blotting as in Fig. 2A. (B) 17AAG-induced loss of TGFβ-mediated Smad activation is restored by MG132. HaCaT cells were treated with 1 μM 17AAG or DMSO for 6 h, with TGFβ added for the last 1 h. Cells were cotreated with MG132 in parallel with 17AAG where indicated, and Western blotting was performed as in Fig. 2A.
17AAG stimulates degradation of several Hsp90 clients, including ErbB2 (11, 12). Thus, because 17AAG may not inhibit TβR kinase activity, we investigated whether 17AAG might promote degradation of Smad2/3 or TβRs to result in the loss of Smad2/3 phosphorylation. Cells were treated with 17AAG in the presence or absence of the proteasome inhibitor MG132 before TGFβ stimulation. TGFβ-induced Smad2/3 phosphorylation was profoundly reduced by 17AAG, but this 17AAG-mediated reduction was almost completely restored by simultaneous treatment with MG132 (Fig. 3B, lane 8). A similar observation was made in Mv1Lu cells (Fig. S3). This finding suggests that 17AAG might decrease Smad phosphorylation via a mechanism dependent on proteasome-mediated degradation. Furthermore, because 17AAG does not alter the level of phosphomimetic Smad2SD (data not shown), the degradation event is not at the Smad level, but occurs upstream of phospho-Smad2/3.
Hsp90 Inhibitors Cause TGFβ Receptor Degradation.
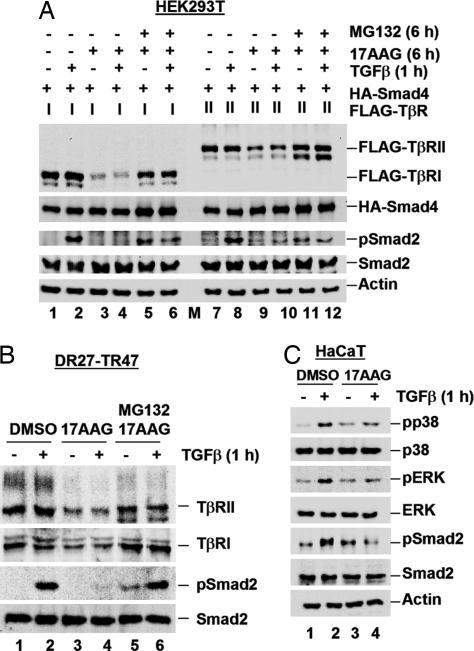
TGFβ receptors are targets of ubiquitin-mediated degradation (6, 7). Thus, we next examined the consequence of 17AAG on TβR stability. In transfected HEK293T cells, FLAG-TβRI and FLAG-TβRII levels were dramatically reduced in the presence of 17AAG regardless of TGFβ stimulation (Fig. 4A, lanes 3, 4, 9, and 10). As internal controls, coexpressed HA-Smad4 and endogenous Smad2 levels remained fairly constant. The 17AAG-induced decrease in TβR levels was restored by MG132 treatment (Fig. 4A, lanes 5, 6, 11, and 12). Similarly, in mink lung epithelial DR27-TR47 cells, which harbor inactive endogenous TβRII yet stably express wild-type FLAG-TβRII (23), endogenous TβRI and stably expressed TβRII levels were decreased after 17AAG treatment, paralleling 17AAG-induced loss of phospho-Smad2, although endogenous Smad2 levels remained constant (Fig. 4B, lanes 3 and 4). The decrease in endogenous TβRs and phospho-Smad2 was rescued by MG132 treatment (Fig. 4B, lanes 5 and 6). These data suggest that 17AAG treatment may compromise TβR stability and, conversely, that Hsp90 may stabilize TβRs.
Fig. 4.
Hsp90 inhibitors cause TβR degradation. (A) 17AAG-mediated reduction of TβR levels is restored by MG132. HEK293T cells cotransfected with FLAG-TβR and HA-Smad4 were treated with 2 μM 17AAG for 6 h, in parallel with MG132 as indicated, with TGFβ added for the last 1 h. Lysates were analyzed by Western blotting with anti-FLAG, anti-HA, anti-Smad2, anti-phospho-Smad2, and anti-actin antibodies. M, molecular weight marker lane. (B) 17AAG-mediated reduction of endogenous TβR levels is restored by MG132. DR27-TR47 cells were treated as in A and harvested for Western blotting with anti-TβRI, anti-FLAG, anti-Smad2, anti-phospho-Smad2, and anti-actin antibodies. (C) 17AAG treatment attenuates TGFβ-induced MAPK phosphorylation. HaCaT cells were treated with 1 μM 17AAG for 6 h, with TGFβ added for the last 1 h. Cells were harvested for Western blotting with anti-Smad2, anti-phospho-Smad2, anti-p38, anti-phospho-p38, anti-ERK, anti-phospho-ERK, and anti-actin antibodies.
If the primary effect of 17AAG is to destabilize TβRs, we anticipated that it would also block TGFβ-dependent activation of non-Smad targets, such as the MAPKs p38 and ERK (4). Indeed, in HaCaT control cells, TGFβ induced phospho-p38 and phospho-ERK (Fig. 4C, lane 2), but this induction was lost in cells treated with 17AAG (Fig. 4C, lane 4). The ability of 17AAG to inhibit TGFβ/TβR-mediated activation of non-Smad MAPK targets further suggests that Hsp90 could positively regulate TGFβ signaling at the receptor level.
TβRs Interact With Hsp90 in Vitro and in Vivo.
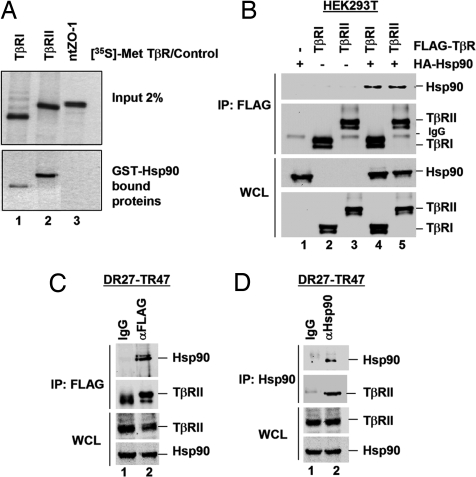
Because Hsp90 inhibition decreases TβR stability, we next determined whether TβRI and TβRII might interact with Hsp90 and potentially be Hsp90 clients. Using an in vitro translation system, TβRs were labeled with [35S]-Met, and their ability to bind to GST-Hsp90 was assessed. As shown in Fig. 5A, TβRI and TβRII both bound GST-Hsp90, whereas the tight junction protein ZO-1 (as a control) did not. Furthermore, in transfected HEK293T cells, TβRI and TβRII specifically coimmunoprecipitated Hsp90 (Fig. 5B, lanes 4 and 5), and this interaction was independent of TGFβ stimulation (Fig. S4). Finally, to determine the physiological relevance of TβR–Hsp90 interactions, we carried out endogenous coimmunoprecipitations by using the DR27-TR47 cells used in Fig. 4B. Immunoprecipitation and Western blot analysis revealed that stably expressed TβRII and endogenous Hsp90 coimmunoprecipitate (Fig. 5 C and D). The data in Fig. 5 suggest that Hsp90 specifically interacts with TβRI and TβRII in vitro and in vivo. Coupled with our data showing that loss of Hsp90 function decreases TβR levels (Fig. 4) and blocks TGFβ-induced Smad2/3 activation (Fig. 2) and transcription (Fig. 1), this result suggests that Hsp90 controls TGFβ signaling as an essential component for stabilizing TβRs.
Fig. 5.
TβRI and TβRII specifically interact with Hsp90. (A) TβRs interact with Hsp90 in vitro. [35S]Met-labeled TβRs and ZO-1 N terminus (ntZO-1) were incubated with GST-Hsp90 on glutathione-Sepharose. Hsp90-bound proteins were resolved by SDS/PAGE and visualized by autoradiography. (B) TβRs interacts with Hsp90 in vivo. HEK293T cells were cotransfected with HA-Hsp90 and FLAG-TβR. FLAG-TβR-bound Hsp90 was identified by anti-FLAG immunoprecipitation (IP) and anti-HA Western blotting. WCL, whole-cell lysate. (C and D) Endogenous Hsp90 and stably expressed FLAG-TβRII interact. DR27-TR47 lysates were subject to anti-FLAG (C) or anti-Hsp90 (D) IP. FLAG-TβRII-bound Hsp90 and Hsp90-bound FLAG-TβRII were detected by anti-Hsp90 (C) and anti-FLAG (D) immunoblotting, respectively.
Smurf2 Is Essential for 17AAG-Induced TβR Degradation.
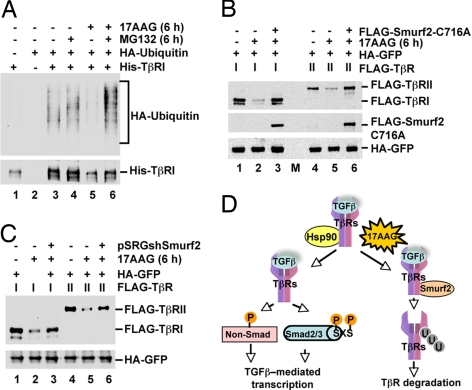
Because TβRs are regulated by ubiquitin-mediated degradation, we tested whether 17AAG could promote TβR ubiquitination. His-TβRI was precipitated from HEK293T cells transfected with His-TβRI and HA-ubiquitin by using Ni-NTA-agarose, and its ubiquitination was analyzed by anti-ubiquitin Western blotting. Treatment with 17AAG alone decreased His-TβRI levels, as expected. Thus, less ubiquitination was detected, compared with that associated with His-TβRI from control cells (Fig. 6A, lanes 3 and 5). However, the addition of MG132 partially restored TβRI levels in the presence of 17AAG and facilitated the detection of TβR ubiquitination. Notably, 17AAG significantly increased His-TβRI ubiquitination (Fig. 6A, lanes 4 and 6). This finding suggests that 17AAG promotes the ubiquitination, and thus the ensuing proteasomal degradation, of TβRI.
Fig. 6.
Smurf2 is essential for 17AAG-induced TβR degradation. (A) 17AAG increases TβRI ubiquitination. HEK293T cells were cotransfected with His-TβRI and HA-ubiquitin and treated for 6 h with 17AAG and/or MG132 as indicated. His-TβRI was precipitated with Ni-NTA agarose, and TβRI ubiquitination was determined by anti-His and anti-ubiquitin immunoblotting. (B) Dominant-negative Smurf2(C716A) prevents 17AAG-induced TβR degradation. HEK293T cells were cotransfected with FLAG-TβR, HA-GFP, and FLAG-Smurf2(C716A) as indicated. Cells were treated with 2 μM 17AAG for 6 h as specified and harvested for Western blotting with anti-FLAG and anti-HA antibodies. M, molecular weight marker lane. (C) Smurf2 depletion reduces 17AAG-induced TβR degradation. HEK293T cells were cotransfected with FLAG-TβR, HA-GFP, and shSmurf2 as indicated. Cells were treated and Western blotting was performed as described in B. (D) Working model for the regulation of TGFβ signaling by Hsp90. Hsp90 protects TβRs from degradation, ensuring that activated receptors promote TGFβ-mediated transcription via Smad and non-Smad targets. Inhibition of Hsp90 by 17AAG promotes Smurf2-mediated TβR-ubiquitination and degradation, abolishing TGFβ-mediated transcription.
We finally sought to identify the E3 ubiquitin ligase that could mediate TβRI, and presumably TβRII, ubiquitination and degradation in the absence of functional Hsp90. Although carboxyl terminus of Hsc70 interacting protein (CHIP) promotes ubiquitin-mediated degradation of some Hsp90 clients (24), it did not alter TβR levels in our system (data not shown). Thus, we examined Smurf proteins because they are E3 ubiquitin ligases for TβRs (6, 7). First we assessed the consequence of the established dominant-negative effect of Smurf2(C716A) on the ability of 17AAG to degrade TβRs. Significantly, although TβRI and TβRII levels were massively reduced by 17AAG, as anticipated (Fig. 6B, lanes 2 and 5, respectively), expression of Smurf2(C716A) restored TβR levels in 17AAG-treated cells to the level observed in control cells (Fig. 6B, lanes 3 and 6). This result suggests that Smurf2(C716A) dominant-negatively blocks 17AAG-stimulated TβR degradation. Next, we generated an effective shSmurf2 construct (pSRGshSmurf2) to knock down Smurf2 (Fig. S5) and found that shSmurf2 significantly reversed 17AAG-induced degradation of TβRs (Fig. 6C, lanes 3 and 6), further suggesting that Smurf2 is required for 17AAG-stimulated TβR degradation. In accordance, 17AAG promoted the binding of Smurf2 to both TβRI and TβRII (Fig. S6).
In summary, Hsp90 appears to be essential for the stability of TGFβ receptors and consequently for successful TGFβ signaling and the implementation of critical TGFβ-mediated transcriptional responses. Inhibition of Hsp90 function, using small-molecule inhibitors such as 17AAG, leads to an increase in Smurf2 binding to TβR (Fig. S6) and subsequent TβR ubiquitination and degradation (Fig. 6D).
Discussion
Aberrant TGFβ responses are frequent in human diseases, suggesting that the TGFβ-signaling pathway might harbor key targets for drug design. We have identified Hsp90 inhibitors, including 17AAG, which is in clinical trials, as inhibitors of TGFβ signaling. Subsequently, we have revealed a critical level of TGFβ-signaling regulation mediated by the Hsp90 chaperone. We show that TβRs are Hsp90-interacting proteins (Fig. 5), and we present a role for Hsp90 in TGFβ signaling via its ability to stabilize TβRs (Figs. 4 and 6). The role of functional Hsp90 in promoting the stability of TβRs, and thus the integrity of the TGFβ response, is demonstrated by enhanced TβR degradation on pharmacological inhibition of Hsp90 by 17AAG, a derivative of the ansamycin antibiotic geldanamycin (Figs. 4 and 6). Further, the inhibition of Hsp90 by geldanamycin, the macrolactone class Hsp90 inhibitor Radicicol, and also via genetic means by siRNA-mediated knockdown of the Hsp90 cochaperone CDC37 all abolished TβR-mediated Smad2/3 activation (Fig. 2).
Recent work hints that Hsp90 may be involved in TGFβ superfamily signaling. Endoglin, an ancillary receptor for several TGFβ superfamily ligands in endothelial cells, acts as a scaffold protein to enhance the well established Hsp90–eNOS interaction and stabilize eNOS during endothelial cell function (25). Interestingly, endoglin aids Hsp90's chaperone activity, rather than being a chaperone client itself. While this article was in preparation, Pei et al. (26) presented genetic data suggesting that Hsp90 may act in concert with Squint, a member of the nodal-related factors of the TGFβ superfamily, to protect zebrafish embryos against the developmental defect cyclopia. No direct involvement of Hsp90 in nodal signaling was demonstrated, and no Hsp90 client was identified. However, this study supports our finding of an essential role for Hsp90 in TGFβ signaling during evolution. It is possible that Squint receptors are the Hsp90 client in this zebrafish study. Indeed, mammalian type I activin and BMP receptors also appear to be client proteins of Hsp90 because 17AAG caused significant reduction in the levels of all ALKs (Fig. S7). Our study now shows a definitive role for Hsp90, and conversely for the use of its inhibitors, in the regulation of TGFβ superfamily signaling. It also has been reported that TGFβ induces Hsp90 expression in chicken embryo cells, implicating the intriguing possibility of a positive-feedback regulation (27, 28). However, we did not observe a TGFβ-mediated increase in endogenous Hsp90 protein level in mammalian cells (Fig. 2D).
In contrast to many Hsp90 clients, whose ubiquitin-mediated degradation is mediated by CHIP (24), CHIP did not alter TβR levels in response to 17AAG. Notably, consistent with their function as E3 ubiquitin ligases for TβRs (6, 7), Smurf proteins promote TβR ubiquitination upon Hsp90 inhibition. Expression of dominant-negative Smurf2(C716A) and shRNA-mediated knockdown of Smurf2 blocked 17AAG-induced TβR degradation (Fig. 6). This observation presents Smurf family proteins as a potential new group of ubiquitin E3 ligases involved in targeting Hsp90 clients for degradation.
It has been reported that TβRs are internalized to EEA1 endosomes in a clathrin-dependent manner, where they associate with SARA to promote TGFβ signaling, or via the lipid raft-caveolar pathway, where they associate with Smurf2 and undergo ubiquitin-mediated degradation (5). Our data suggest that Hsp90 may protect TβRs from the lipid raft-caveolar pathway and ensuing Smurf2-mediated degradation, whereas the inhibition of Hsp90 chaperone activity may promote this degradation. It also is conceivable that Hsp90 facilitates trafficking and maturation of TβRs before they reach the plasma membrane. In preliminary experiments, the Golgi export inhibitor Brefeldin A had no effect on the TβR–Hsp90 interaction. Furthermore, at least a portion of the TβRs bound to Hsp90 were sensitive to EndoH, an enzyme that cleaves the N-linked glycoproteins not yet fully processed by the Golgi (29), suggesting that immature TβRs are able to interact with Hsp90 (data not shown).
Hsp90 accounts for >1% of cellular protein under normal conditions and is considerably elevated under stressful conditions and in tumor cells (12). TGFβ has dual and opposing roles in the progression of tumorigenesis, acting as both a tumor suppressor and a significant stimulator of tumor progression, invasion, and metastasis (30). We demonstrate that 17AAG blocks TGFβ-induced transcription of CDK inhibitors p15 and p21 (Fig. 1), which could be important where 17AAG is used to treat cancer. Small-molecule inhibitors of Hsp90 could turn off beneficial TGFβ-induced anti-proliferative effects in normal cells and in early stage cancer, despite the potentially beneficial effect of these inhibitors in inhibiting an unfavorable TGFβ-induced pro-invasive response in late-stage tumorigenesis. Significantly, because Hsp90 inhibitors target TGFβ signaling at the receptor level, they can block TGFβ responses mediated via Smads and non-Smad targets (4), as we observed for p38 and ERK (Fig. 4C).
In conclusion, our data identify a critical layer of TGFβ-signaling regulation mediated by Hsp90 as a result of its ability to chaperone TβRs. Inhibiting Hsp90 function leads to ubiquitin-mediated degradation of TβRs in a Smurf2 E3 ligase-dependent manner, thus terminating the TGFβ–Smad-signaling cascade and preventing TGFβ-mediated transcription. Coupled with the fact that 17AAG is in clinical trials, our data present the genuine possibility that small-molecule inhibitors of Hsp90 may represent a class of drugs for treating certain TGFβ-related diseases, including metastatic cancer and fibrosis.
Materials and Methods
Expression Plasmids.
FLAG-TβRI, FLAG-TβRII, HA-Smad4, and FLAG-Smurf2(C716A) have been described previously (31–33). His-tagged TβRI was generated by subcloning into pXF2RH. HA-ubiquitin was a gift from Bert O'Malley (Baylor College of Medicine). Full-length Hsp90α was obtained by PCR (forward primer, GAAGGATCCCACCATGCCTGAGGAAACCCAGAC; reverse primer, GGAGTCGACTTAGTCTACTTCTTCCATGCGT) and cloned into pXF3H (HA-tag). pXF2RH, pXF2F, and pXF3H were derived from the CMV-driven vector pRK5 (Genentech).
Cell Culture and Treatment with Small Molecules.
HEK293T cells were cultured in DMEM/10% FBS. HaCaT and Mv1Lu cells were cultured in MEM/10% FBS. DR27-TR47 cells (23) were cultured in MEM/10% FBS with 200 μg/ml G418. HEK293T and HaCaT cells were transfected by using Lipofectamine (Invitrogen), and Mv1Lu cells were transfected by using Lipofectin (Invitrogen).
Geldanamycin, 17AAG, Radicicol, and SB431542 (Calbiochem) were dissolved as 1 mM stocks in DMSO. Treatments were carried out in reduced-serum media (0.2% FBS), and TGFβ was added where indicated to a final concentration of 2 ng/ml. Where specified, cells were treated with the proteasome inhibitor MG132 (Calbiochem) at a final concentration of 20 μM.
GST-Binding Assay.
[35S]Met-labeled TβRs and an N-terminal fragment of ZO-1 were generated by using a TNT kit (Promega). In vitro translation products were precleared by GST on glutathione-Sepharose (Amersham) and incubated with Glutathione Sepharose-bound GST-Hsp90. After washing, GST-Hsp90-binding products were analyzed by SDS/PAGE and autoradiography. Recombinant Hsp90 was generated by purification of bacterially expressed GST-fusion protein.
RNA Interference.
CDC37 siRNA duplexes were siCDC37-A (sense strand, 5′-GCGUGUGGGACCACAUUGATT) and siCDC37-B (5′-GGAGGUGAGGGAGCAGAAATT) (Sigma). HEK293T cells were transfected with siCDC37 or siControl (at 100 nM) by using Lipofectamine 2000 (Invitrogen). After 48 h, cells were treated with or without TGFβ for 1 h, and 2 μM 17AAG for 6 h as indicated, before harvest for Western blotting. To construct the Smurf2 shRNA vector, oligos (target sequence, 5′-GCCCACACTTGCTTCAATC) were cloned into pSRG as described previously (34).
Ni-NTA Precipitation, Immunoprecipitation, and Western Blotting.
Immunoprecipitation and Ni-NTA precipitation were performed essentially as described in ref. 33. Precipitated proteins and whole-cell lysates were subjected to Western blotting using the following antibodies: anti-FLAG (M2; Sigma), anti-HA (Mouse 1.1; Covance), anti-His (Serotec), anti-Hsp90 (SPA-830; Stressgen), anti-β-actin (Sigma), anti-ubiquitin (from Lily Feng, Baylor College of Medicine), anti-p38 (P39520; Transduction Laboratories), anti-phospho-p38 (P19820; Transduction Laboratories), anti-ERK (M12320; Transduction Laboratories), anti-phospho-ERK (9106; Cell Signaling), anti-TβRI (3712; Cell Signaling), anti-Akt (9272; Cell Signaling), anti-phospho-Akt-S473 (9271; Cell Signaling), anti-Smad4 (B8; Santa Cruz Biotechnology), anti-PAI-I (H-135; Santa Cruz Biotechnology), anti-p15 (C-20; Santa Cruz Biotechnology), anti-p21 (C-19; Santa Cruz Biotechnology), and anti-CDC37 (E-4; Santa Cruz Biotechnology). Anti-Smad2/3 and anti-phospho-Smad2/3 antibodies were described previously (35).
Transcription Reporter Assays.
Plasmid SBE-luc and p21-luc (gifts from Bert Vogelstein) were used to assay TGFβ-induced transcription in Mv1Lu and HaCaT cells. Transfections, TGFβ treatment, and reporter assays were carried out as described previously (33). Cells were pretreated with 17AAG or GM for 30 min before incubation with TGFβ in the presence of 17AAG or GM as indicated.
Quantitative RT-PCR.
Total RNAs were prepared by using TRIzol (Invitrogen) from HaCaT cells pretreated with 0.5 μM 17AAG (or DMSO as control) before culture with 2 ng/ml TGFβ in the presence or absence of 0.5 μM 17AAG for 0, 4, and 8 h. Quantitative RT-PCR was performed by using assay-on-demand kits (ABI) (34). mRNA levels of target genes were normalized against 18S RNA. Each target was measured in triplicate, and data were analyzed by using Microsoft Excel.
Supplementary Material
Acknowledgments.
We thank Drs. Miercio Pereira (Tufts University, Boston, MA) and Joan Massagué (Sloan–Kettering Institute, New York, NY) for DR27-TR47 cells, Dr. Ed Leof (Mayo Clinic, Rochester, MN) for anti-P-Smad3 antibody, Dr. Lily Feng (in memoriam) for anti-ubiquitin antibody, Dr. Bert O'Malley (Baylor College of Medicine, Houston, TX) for HA-ubiquitin, and Dr. Bert Vogelstein (Johns Hopkins University, Baltimore, MD) for SBE-luc and WWP(p21)-luc. We are grateful to Dr. Yao-Yun Liang for technical assistance with quantitative RT-PCR and helpful suggestions. This research was supported by National Institutes of Health Grants R01DK073932 (to X.L.) and R01GM63773, R01CA108454, P50HL083794, and P50DK064233 (to X.-H.F.); an American Heart Association (Texas Affiliate) Postdoctoral Fellowship (to K.H.W.); and a National Institutes of Health Breast Specialized Program of Research Excellence Career Development Award (to X.L.). X.-H.F is a Leukemia and Lymphoma Society Scholar.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/cgi/content/full/0800163105/DCSupplemental.
References
- 1.Massague J, Seoane J, Wotton D. Smad transcription factors. Genes Dev. 2005;19:2783–2810. doi: 10.1101/gad.1350705. [DOI] [PubMed] [Google Scholar]
- 2.Feng X-H, Derynck R. Specificity and versatility in TGFβ signaling through Smads. Annu Rev Cell Dev Biol. 2005;21:659–693. doi: 10.1146/annurev.cellbio.21.022404.142018. [DOI] [PubMed] [Google Scholar]
- 3.Derynck R, Zhang YE. Smad-dependent and Smad-independent pathways in TGFβ family signalling. Nature. 2003;425:577–584. doi: 10.1038/nature02006. [DOI] [PubMed] [Google Scholar]
- 4.Moustakas A, Heldin C-H. Non-Smad TGFβ signals. J Cell Sci. 2005;118:3573–3584. doi: 10.1242/jcs.02554. [DOI] [PubMed] [Google Scholar]
- 5.Di Guglielmo GM, Le Roy C, Goodfellow AF, Wrana JL. Distinct endocytic pathways regulate TGFβ receptor signalling and turnover. Nat Cell Biol. 2003;5:410–421. doi: 10.1038/ncb975. [DOI] [PubMed] [Google Scholar]
- 6.Ebisawa T, et al. Smurf1 interacts with TGFβ type I receptor through Smad7 and induces receptor degradation. J Biol Chem. 2001;276:12477–12480. doi: 10.1074/jbc.C100008200. [DOI] [PubMed] [Google Scholar]
- 7.Kavsak P, et al. Smad7 binds to Smurf2 to form an E3 ubiquitin ligase that targets the TGFβ receptor for degradation. Mol Cell. 2000;6:1365–1375. doi: 10.1016/s1097-2765(00)00134-9. [DOI] [PubMed] [Google Scholar]
- 8.Le Roy C, Bose R, Wrana JL. In: Smad Signal Transduction. Dijke PT, Heldin C-H, editors. New York: Springer; 2006. pp. 177–191. [Google Scholar]
- 9.Citri A, et al. Hsp90 recognizes a common surface on client kinases. J Biol Chem. 2006;281:14361–14369. doi: 10.1074/jbc.M512613200. [DOI] [PubMed] [Google Scholar]
- 10.Pratt WB, Toft DO. Regulation of signaling protein function and trafficking by the Hsp90/Hsp70-based chaperone machinery. Exp Biol Med. 2003;228:111–133. doi: 10.1177/153537020322800201. [DOI] [PubMed] [Google Scholar]
- 11.Neckers L, Schulte T, Mimnaugh E. Geldanamycin as a potential anti-cancer agent: Its molecular target and biochemical activity. Invest New Drugs. 1999;17:361–373. doi: 10.1023/a:1006382320697. [DOI] [PubMed] [Google Scholar]
- 12.Isaacs J, Xu W, Neckers L. Hsp90 as a molecular target for cancer therapeutics. Cancer Cell. 2003;3:213–217. doi: 10.1016/s1535-6108(03)00029-1. [DOI] [PubMed] [Google Scholar]
- 13.Waite KA, Eng C. From developmental disorder to heritable cancer: It's all in the BMP/TGFβ family. Nat Rev Genet. 2003;4:763–773. doi: 10.1038/nrg1178. [DOI] [PubMed] [Google Scholar]
- 14.Akhurst RJ. TGFβ signaling in health and disease. Nat Genet. 2004;36:790–792. doi: 10.1038/ng0804-790. [DOI] [PubMed] [Google Scholar]
- 15.Feng X-H, Lin X, Derynck R. Smad2, Smad3 and Smad4 cooperate with Sp1 to induce p15Ink4B transcription in response to TGFβ. EMBO J. 2000;19:5178–5193. doi: 10.1093/emboj/19.19.5178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Seoane J, et al. TGFβ influences Myc, Miz-1 and Smad to control the CDK inhibitor p15Ink4B. Nat Cell Biol. 2001;3:400–408. doi: 10.1038/35070086. [DOI] [PubMed] [Google Scholar]
- 17.Seoane J. p21Waf1/Cip1 at the switch between the anti-oncogenic and oncogenic faces of TGFβ. Cancer Biol Ther. 2004;3:226–227. doi: 10.4161/cbt.3.2.717. [DOI] [PubMed] [Google Scholar]
- 18.Pardali K, et al. Role of Smad proteins and transcription factor Sp1 in p21Waf1/Cip1 regulation by TGFβ. J Biol Chem. 2000;275:29244–29256. doi: 10.1074/jbc.M909467199. [DOI] [PubMed] [Google Scholar]
- 19.Stroschein S, Wang W, Luo K. Cooperative binding of Smad proteins to two adjacent DNA elements in the plasminogen activator inhibitor-1 promoter mediates TGFβ-induced Smad-dependent transcriptional activation. J Biol Chem. 1999;274:9431–9441. doi: 10.1074/jbc.274.14.9431. [DOI] [PubMed] [Google Scholar]
- 20.Dennler S, et al. Direct binding of Smad3 and Smad4 to critical TGFβ-inducible elements in the promoter of human plasminogen activator inhibitor-type 1 gene. EMBO J. 1998;17:3091–4100. doi: 10.1093/emboj/17.11.3091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Shang L, Tomasi TB. The Hsp90-CDC37 chaperone complex is required for signaling by type I and II interferons. J Biol Chem. 2006;281:1876–1884. doi: 10.1074/jbc.M509901200. [DOI] [PubMed] [Google Scholar]
- 22.Inman GJ, et al. SB-431542 is a potent and specific inhibitor of TGFβ superfamily type I activin receptor-like kinase receptors ALK4, ALK5, and ALK7. Mol Pharmacol. 2002;62:65–74. doi: 10.1124/mol.62.1.65. [DOI] [PubMed] [Google Scholar]
- 23.Ming M, Ewen M, Pereira ME. Trypanosome invasion of mammalian cells requires activation of the TGFβ signaling pathway. Cell. 1995;82:287–296. doi: 10.1016/0092-8674(95)90316-x. [DOI] [PubMed] [Google Scholar]
- 24.Murata S, Chiba T, Tanaka K. CHIP: A quality-control E3 ligase collaborating with molecular chaperones. Int J Biochem Cell Biol. 2003;35:572–578. doi: 10.1016/s1357-2725(02)00394-1. [DOI] [PubMed] [Google Scholar]
- 25.Toporsian M, et al. A role for endoglin in coupling eNOS activity and regulating vascular tone revealed in hereditary hemorrhagic telangiectasia. Circ Res. 2005;96:684–692. doi: 10.1161/01.RES.0000159936.38601.22. [DOI] [PubMed] [Google Scholar]
- 26.Pei W, Williams PH, Clark M, Stemple D, Feldman B. Environmental and genetic modifiers of squint penetrance during zebrafish embryogenesis. Dev Biol. 2007;308:368–378. doi: 10.1016/j.ydbio.2007.05.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Takenaka I, Hightower LE. TGFβ1 rapidly induces Hsp70 and Hsp90 molecular chaperones in cultured chicken embryo cells. J Cell Physiol. 1992;152:568–577. doi: 10.1002/jcp.1041520317. [DOI] [PubMed] [Google Scholar]
- 28.Takenaka I, Hightower LE. Regulation of chicken Hsp70 and Hsp90 family gene expression by TGFβ1. J Cell Physiol. 1993;155:54–62. doi: 10.1002/jcp.1041550108. [DOI] [PubMed] [Google Scholar]
- 29.Wells R, Yankelev H, Lin H, Lodish HF. Biosynthesis of the type I and type II TGFβ receptors: Implications for complex formation. J Biol Chem. 1997;272:11444–11451. doi: 10.1074/jbc.272.17.11444. [DOI] [PubMed] [Google Scholar]
- 30.Akhurst R, Derynck R. TGFβ signaling in cancer–a double-edged sword. Trends Cell Biol. 2001;11:S44–S51. doi: 10.1016/s0962-8924(01)02130-4. [DOI] [PubMed] [Google Scholar]
- 31.Feng X-H, Filvaroff EH, Derynck R. TGFβ-induced down-regulation of cyclin A expression requires a functional TGF-beta receptor complex. Characterization of chimeric and truncated type I and type II receptors. J Biol Chem. 1995;270:24237–24245. doi: 10.1074/jbc.270.41.24237. [DOI] [PubMed] [Google Scholar]
- 32.Lin X, Liang M, Feng X-H. Smurf2 is a ubiquitin E3 ligase mediating proteasome-dependent degradation of Smad2 in TGFβ signaling. J Biol Chem. 2000;275:36818–36822. doi: 10.1074/jbc.C000580200. [DOI] [PubMed] [Google Scholar]
- 33.Lin X, et al. Activation of TGFβ signaling by SUMO-1 modification of tumor suppressor Smad4/DPC4. J Biol Chem. 2003;278:18714–18719. doi: 10.1074/jbc.M302243200. [DOI] [PubMed] [Google Scholar]
- 34.Lin X, et al. PPM1A functions as a Smad phosphatase to terminate TGFβ signaling. Cell. 2006;125:915–928. doi: 10.1016/j.cell.2006.03.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wrighton KH, et al. Small C-terminal domain phosphatases dephosphorylate the regulatory linker regions of Smad2 and Smad3 to enhance TGFβ signaling. J Biol Chem. 2006;281:38365–38375. doi: 10.1074/jbc.M607246200. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.