Abstract
Cerebral responses to putative pheromones and objects of sexual attraction were recently found to differ between homo- and heterosexual subjects. Although this observation may merely mirror perceptional differences, it raises the intriguing question as to whether certain sexually dimorphic features in the brain may differ between individuals of the same sex but different sexual orientation. We addressed this issue by studying hemispheric asymmetry and functional connectivity, two parameters that in previous publications have shown specific sex differences. Ninety subjects [25 heterosexual men (HeM) and women (HeW), and 20 homosexual men (HoM) and women (HoW)] were investigated with magnetic resonance volumetry of cerebral and cerebellar hemispheres. Fifty of them also participated in PET measurements of cerebral blood flow, used for analyses of functional connections from the right and left amygdalae. HeM and HoW showed a rightward cerebral asymmetry, whereas volumes of the cerebral hemispheres were symmetrical in HoM and HeW. No cerebellar asymmetries were found. Homosexual subjects also showed sex-atypical amygdala connections. In HoM, as in HeW, the connections were more widespread from the left amygdala; in HoW and HeM, on the other hand, from the right amygdala. Furthermore, in HoM and HeW the connections were primarily displayed with the contralateral amygdala and the anterior cingulate, in HeM and HoW with the caudate, putamen, and the prefrontal cortex. The present study shows sex-atypical cerebral asymmetry and functional connections in homosexual subjects. The results cannot be primarily ascribed to learned effects, and they suggest a linkage to neurobiological entities.
Keywords: amygdala, homosexuality, cerebral lateralization, cerebral connectivity, magnetic resonance volumetry
One of the more controversial questions in the neurobiology of human behavior relates to the mechanisms of sexual orientation. This issue received increasing interest over the last decade and was further substantiated by recent results from imaging studies of cerebral activation in homo- and heterosexual subjects. During judgment of face attractiveness and when viewing sexually arousing films the cerebral response was found to be invariant to the preferred sexual stimulus/face [male in heterosexual women (HeW) and homosexual men (HoM), and female in heterosexual men (HeM) and homosexual women (HoW)] and located in certain core regions of the reward circuitry and the motor cortex (1, 2). Furthermore, in a series of PET activation studies during smelling of putative pheromones we detected a sex differentiated activation of the anterior hypothalamus in HeM and HeW (3) and a sex-atypical (almost reciprocal) pattern of activation in HoM and HoW (4, 5). While intriguing, none of these studies provided conclusions about the underlying mechanisms because they imaged perceptional processes, which could be innate, as well as learned. By indicating a link between sexually dimorphic regions of the midbrain and the sexual orientation, they fueled, however, the on-going discussion about the neurobiology of sexual orientation and raised several new questions. One is whether the sexual dimorphism reported in the literature (6) could be sex-atypical in homosexual subjects even with respect to factors that are not directly associated with behavior. Another is whether possible differences between homo- and heterosexual subjects could be present also in the cerebral circuits outside those strictly involved in reproduction.
In the present study we addressed these issues by investigating age-matching groups of homo- and heterosexual men and women with respect to two separate parameters, both unlikely to be directly affected by learned patterns and behavior, and both showing sex-dimorphic characteristics in previous studies. The first parameter was cerebral and cerebellar asymmetry measured with magnetic resonance (MR) volumetry. The second parameter was the functional connectivity from the right and left amygdalae, analyzed on the basis of PET measurements of regional cerebral blood flow (rCBF) during rest and passive smelling of unscented air. Our choice to measure hemispheric volumes was based on survey of the current literature providing some indications of a sex-atypical lateralization in HoM, and to a certain extent also in HoW during certain neuropsychological tests. One example is studies of dichotic listening showing a more pronounced right–ear preference in HeM compared with HoM (and also HeW), in whom no significant lateralization was detected (7, 8). Another is the report that HoM, like HeW, have a relatively larger anterior commissure compared with HeM (9), providing a possible anatomical substrate for higher interhemispheric connections (10). Furthermore, HoM are, like HeW, reported to outperform HeM in certain verbal tests. Such tests involve language circuits, which according to some studies are more symmetrical in women (11, 12). HoM also show an inferior performance in visuospatial tasks, and particularly in tests of mental rotation and navigation strategy (13–15). These functions are processed primarily by the right parietal lobe, which is relatively larger in men than in women (16, 17). The data from HoW are sparse. HoW score like HeW on most cognitive measures except for verbal fluency and mental rotation, two tests in which they perform more like HeM (13, 14). They are reported to have a slightly stronger right-ear preference during dichotic listening than HeW, and some studies show similarities with HeM also in click-evoked otoacoustic emissions (CEOAEs) (13, 16, 18). Together, these data provide a rationale for possible global hemispheric differences with respect to sex and sex orientation.
The choice to measure amygdala connectivity was based on several reports about sex differentiated amygdala lateralization in processing of emotional memories (with an activation of the right amygdala in men, and the left amygdala in women) (19, 20). Furthermore, Kilpatrick et al. (21) recently found that the amygdala exhibits sex-differentiated functional connections during rest: in men, the connections were mainly displayed from the right amygdala and targeted to the sensorimotor cortex, striatum, and pulvinar, whereas in women they were more pronounced from the left amygdala and targeted to the subgenual cortex and the hypothalamus. Because measurements of the resting state functional connectivity are independent of user, perceptive, cognitive, or behavior-related tasks, they lend themselves to studies of more crude potential neurobiological correlates to sex and sexual orientation. In addition, the amygdala is a key structure in the limbic networks and exhibits high density of estrogen and androgen receptors (22, 23).
The present study was carried out under the hypothesis that:
The hemispheric volumes are symmetrical in HeW but not HeM;
in HeW the amygdala is functionally connected primarily with the subgenual cortex and the hypothalamus, in HeM with the sensorimotor cortex and the striatum;
the side difference in hemispheric volumes, as well as the pattern of amygdala connectivity, could be sex-atypical in homosexual subjects.
Results
Hemispheric Volumes.
HeM and HoW had significantly asymmetrical hemispheric volumes, with larger right hemisphere (P = 0.0063 for HeM and P < 0.001 for HoW; paired t tests). In contrast, no asymmetry was detected in HeW (P = 0.6054) or in HoM (P = 0.8749) [Table 1 and supporting information (SI) Fig. S1]. The ANOVA showed a significant overall group difference (P = 0.0008; F = 6.168, df = 3). Fisher's post hoc test revealed that the asymmetry in HeM was significant in relation to HeW (P = 0.0005) and HoM (P = 0.0010). Likewise, the asymmetry index in HoW was significant in relation to HeW and HoM (P = 0.0244 and P = 0.0344, respectively). No difference was found between the HeM and HoW, or between HeW and HoM. The observed differences in asymmetry were not related to a particular hemisphere (Table 1). A post hoc evaluation detected asymmetry exceeding two standard deviations of that in HeW (who were hypothesized to have symmetrical volumes) in 1 HeW, 4 HoM, 7 HoW, and 12 HeM.
Table 1.
Volumes of interest
| Group | No. | R cerebral hemisphere, cm3 | L cerebral hemisphere, cm3 | AI, cerebral hemispheres | R cerebellar volume,cm3 | L cerebellar volume, cm3 | AI, cerebellar hemispheres |
|---|---|---|---|---|---|---|---|
| HeM | 25 | 624 ± 43 | 612 ± 41 | 0.012 ± 0.02* | 68.2 ± 6.4 | 68.4 ± 6.6 | −0.003 ± 0.007 |
| HeW | 25 | 581 ± 37 | 581 ± 36 | −0.001 ± 0.005 | 68.7 ± 7.4 | 68.4 ± 7.9 | 0.004 ± 0.029 |
| HoM | 20 | 608 ± 46 | 609 ± 47 | −0.0004 ± 0.009 | 67.6 ± 6.6 | 67.5 ± 5.5 | 0.0004 ± 0.025 |
| HoW | 20 | 548 ± 34 | 543 ± 33 | 0.008 ± 0.007† | 65.6 ± 7.5 | 65.8 ± 6.7 | 0.002 ± 0.020 |
The numbers indicate means and standard deviations. R, right; L, left; AI, asymmetry index [(R − L)/(R + L)].
*, P = 0.0005 in relation to HeW and 0.0010 in relation to HoM;
†, P = 0.0244 in relation to HeW and P = 0.0344 in relation to HoM.
The cerebellar hemispheres were symmetrical in all four populations (P = 0.871, F = 0.236, df = 3; one-way ANOVA), without any group difference (Table 1). The inter-rater correlations was 0.85, P < 0.001, for cerebral hemispheres, and 0.93, P < 0.001, for the cerebellar hemispheres. The corresponding intra-rater correlations were 0.88 and 0.95 (P < 0.001).
Functional Connectivity.
Only positive covariations are reported because only one significant negative covariation was observed [detected in HeM, with respect to the right amygdala, and located in the occipital cortex, (z = 4.5; size 5.3, Talairach coordinates 6, −80, 16)].
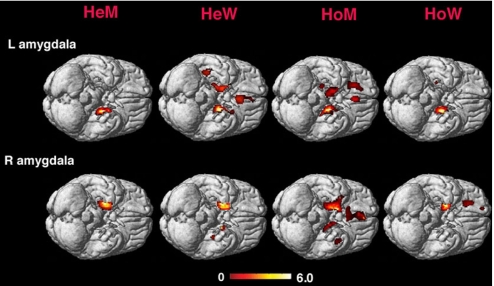
HeW had more widespread connections from the left amygdala, HeM from the right amygdala (Table 2). Furthermore, in HeW connections were displayed with the contralateral amygdala, the anterior cingulate and subcallosum, and the hypothalamus, whereas in HeM the connectivity clusters covered the putamen and caudate, and portions of the agranular insular cortex. Both HeW and HeM showed covariation with portions of the temporal neocortex (Tables 2 and 3 and Fig. 1).
Table 2.
Significant covariations from the left amygdala
| Region | HeW |
HeM |
HoM |
HoW |
||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| z level | Size, cm3 | Coordinates | z level | Size,cm3 | Coordinates | z level | Size,cm3 | Coordinates | z level | Size,cm3 | Coordinates | |
| L amygdala | inf | 9.6 | −18, 2, −18* | inf | 4.6 | −18, 2, −18 | inf | 6.5 | −18, 2, −10† | inf | 5.1 | −18, 2, −10 |
| −26, 2, −2 | ||||||||||||
| R amygdala | 4.2 | 2.0 | 22, −16, −12‡ | 4.4 | 1.8 | 8, 6, −12‡ | ||||||
| Cingulate | 5.7 | 1.4 | −16, 45, −4 | 4.0 | 1.2 | −30, 14, 2§ | ||||||
| 5.2 | 2.6 | −18, 32, −12 | 3.6 | 0.8 | −2, 44, −12 | |||||||
| L superior and middle temporal gyrus | 4.2 | 1.1 | −40, −2, −4 | |||||||||
| 4.4 | 1.0 | −34, −76, 12 | ||||||||||
| Superior collicle | 3.9 | 2.5 | −14, −32, −10 | |||||||||
Clusters detected at T = 3.0, corrected P < 0.05. Talairach coordinates denote local maxima. R, right; L, left; inf, infinite.
*Includes the anterior cingulate, subcallosum, and hypothalamus, and a minor portion of the superior temporal gyrus.
†Covers the hypothalamus, the subcallosum, and the anterior cingulate.
‡Covers the piriform cortex.
§Subcallosum.
Table 3.
Significant covariations from the right amygdala
| Region | HeW |
HeM |
HoM |
HoW |
||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| z level | Size, cm3 | Coordinates | z level | Size, cm3 | Coordinates | z level | Size, cm3 | Coordinates | z level | Size, cm3 | Coordinates | |
| R amygdala | inf | 5.0 | 14, 4, −14 | inf | 2.4 | 16, 4, −14* | inf | 12.0 | 18, 4, −14† | inf | 3.4 | 16, 4, −14* |
| L amygdala + hipp + piri-form cortex | 5.0 | 3.4 | −26, 4, −14‡ | 5.4 | 0.8 | −34, 6, −8† | ||||||
| R orbitofrontal cortex | 4.5 | 1.6 | 22, 32, −10 | |||||||||
| L putamen + insular cortex | 3.6 | 3.0 | −38, 0, −8 | |||||||||
| L middle temporal gyrus | 5.0 | 2.6 | −42, −22, −6 | 5.0 | 1.8 | −42, −22, −6 | ||||||
| L middle frontal gyrus | 4.6 | 3.2 | −26, 54, 24 | |||||||||
| 3.6 | 1.6 | −32, 36, 40 | ||||||||||
| L superior frontal gyrus | 3.9 | 1.6 | −16, 64, 16 | |||||||||
| 2.8 | 0.7 | −12, 54, 24 | ||||||||||
Threshold at T = 3.0 and P < 0.05 uncorrected; italics indicate P < 0.1 uncorrected. Talairach coordinates denote local maxima. R, right; L, left; inf, infinite; hipp, hippocampus.
*Includes the putamen.
†Includes the anterior cingulate, subcallosum, and hypothalamus, and a minor portion of the superior temporal gyrus.
‡Covers the piriform cortex.
Fig. 1.
Covariations with the respective amygdala seed region in hetero- and homosexual subjects. The Sokoloff scale indicates T values. Clusters detected at T = 3.0 are superimposed on the standard MR image of the brain.
The connectivity pattern in homosexual subjects was almost reciprocal in relation to the same-sex controls. First, in HoM the connections were more widespread from the left amygdala, in HoW from the right amygdala (Fig. 1). Second, HoM, just as HeW, displayed connections with the contralateral amygdala, the anterior cingulate, the subcallosum, and the hypothalamus. In HoW, on the other hand, connections were displayed with the putamen and the orbitofrontal and prefrontal cortex, but not with the contralateral amygdala or the cingulate cortex (Tables 2 and 3 and Fig. 1).
All four groups also showed functional connections with the temporal neocortex ipsilateral to the amygdala seed region.
Group comparisons confirmed these findings: HeW, as well as HoM, showed a greater connectivity with the contralateral amygdala and the cingulate cortex compared with both HeM and HoW. In relation to HoW, HoM showed, in addition, a greater connectivity with the cerebellum, as did HeW in relation to HeM. HeM and HoW, on the other hand, showed significantly more pronounced connections with the frontal lobe cortex including the postcentral gyrus, with putamen, and the parietal cortex (inferior parietal lobe and the posterior cingulate) compared with both HeW and HoM (Tables 4 and 5 and Fig. S2). No significant differences were detected between the HeW and HoM, nor between the HeM and HoW.
Table 4.
Group differences in connectivity pattern, left amygdala
| Region | z level | Size, cm3 | Coordinates | z level | Size, cm3 | Coordinates | z level | Size, cm3 | Coordinates | z level | Size, cm3 | Coordinates |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Group difference | HeW − HeM | HoM − HeM | HeW − HoW | HoM − HoW | ||||||||
| Anterior cingulate (subcallosum) | 3.8 | 2.4 | −16, 32, −1 | 6.5 | 4.2 | −20, 2, −18 | 4.3 | 2.0 | 34, 8, −32 | 4.0 | 2.0 | −20, 62, 12 |
| −22, 0, −18 | 3.5 | 1.6 | 20, 32, −4 | 3.7 | 4.8 | −16, 34, −12 | ||||||
| R amygdala | 4.5 | 2.9 | 30, 10, −12* | 3.6 | 2.0 | 3, 5, −15* | 3.6 | 1.3 | 20, −18, −12 | |||
| 24, 2, 2 | ||||||||||||
| R parahippocampus | 3.1 | 0.3 | 36, −20, −24 | |||||||||
| L parahippocampus | 3.5 | 2.0 | −40, −30, −4 | |||||||||
| Cerebellum | 3.2 | 1.4 | −23, −50, −24 | 3.3 | 2.8 | −36, −54, −28 | ||||||
| Group difference | HeM − HeW | HeM − HoM | HoW − HeW | HoW − HoM | ||||||||
| L amygdala | 4.0 | 2.0 | 6.2 | 4.1 | −18, 2, −18 | |||||||
| Superior frontal gyrus | 0, 22, 52 | No significant difference | ||||||||||
| Parietal cortex | 4.4 | 4.4 | 38, −66, 40 | 3.5 | 2.0 | −18, –66, 12† | 4.2 | 4.8 | −38, –64, 28 | |||
| 56, −26, 13‡ | ||||||||||||
| Postcentral gyrus + putamen + insular cortex | 4.1 | 11.1 | −48, −12, 1 | |||||||||
Significances were calculated by using threshold at T = 3.0, and P < 0.05 uncorrected. HoM − HeW not significant (ns); HoW − HeM ns; HeW − HoM ns; HeM − HoW ns. Talairach coordinates denote local maxima. R, right; L, left.
*Covers the insular and the piriform cortex.
†Posterior cingulate.
‡Includes the postcentral gyrus.
Table 5.
Group differences in connectivity pattern, right amygdala
| Region | z level | Size, cm3 | Coordinates | z level | Size, cm3 | Coordinates | z level | Size, cm3 | Coordinates | z level | Size, cm3 | Coordinates |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Group difference | HeW − HeM | HoM − HeM | HeW − HoW | HoM − HoW | ||||||||
| Anterior cingulate (subcallosum) | 4.4 | 1.3 | 16, 4, −12 | No significant difference | ||||||||
| Hypothalamus + portion of L amygdala | 3.8 | 1.6 | −6, −26, 2 | 3.6 | 2.4 | −10, 5, −6 | ||||||
| 3.3 | 1.4 | −10, 2, 2 | ||||||||||
| L amygdala | 4.2 | 1.6 | −30, −6, −18 | |||||||||
| Superior collicle | 3.8 | 1.6 | 0, −27, 2 | |||||||||
| Occippital cortex | 4.0 | 5.4 | 20, −70, 8 | |||||||||
| Group difference | HeM − HeW | HeM − HoM | HoW − HeW | HoW − HoM | ||||||||
| R amygdala | ns | 5.3 | 3.2 | 16, 6, −14 | ||||||||
| Precentral gyrus + putamen | 3.5 | 2.4 | 46, 14, 36 | |||||||||
| 26, 0, 12 | ||||||||||||
| Orbitofr. + putamen + caudate | 5.3 | 3.3 | 10, 38, −8 | |||||||||
| −14, 7, 12 | ||||||||||||
| Frontopolar cortex | 3.4 | 1.1 | −14, 62, 0 | 3.6 | 3.8 | −18, 62, 2 | 4.3 | 6.2 | −18, 52, 34 | |||
| 8, 62, 22 | 3.2 | 16, 64, 16 | ||||||||||
| Pulvinar | 3.2 | 1.6 | 7, −22, 16 | |||||||||
| Parietal cortex | 3.5 | 3.0 | −12, −54, 22 | 3.7 | 3.7 | 38, −18, 20 | ||||||
Talairach coordinates denote local maxima. HoW − HeM not significant (ns); HeM − HoW ns; HeM − HoM ns; HoM − HeW ns; HeW − HoM ns. Orbitofr., orbitofrontal cortex.
Careful positioning of the amygdala volumes of interest (VOIs) on the separate reformatted PET images showed almost identical locations, and no systematic positioning shifts between the study groups.
We found no group differences in respiratory frequency (df 3, F = 1.0, P = 0.39), or amplitude (df 3, F = 2.1, P = 0.11), (Table S1).
Discussion
Using combined PET and MRI experiments, we found sexual dimorphism with respect to hemispheric asymmetry and the functional connections from the right and left amygdala. We also found that the hemispheric ratios, as well as the patterns of amygdala connectivity, were sex atypical in homosexual subjects, with HoM exhibiting more female patterns and HoW showing more male-like features (albeit less pronounced).
Methodological Issues.
The investigated parameters were selected to be methodologically robust and to minimize perceptional and active cognitive interference. By carrying out MR volumetry of cerebral hemispheres, which have easily identifiable demarcations, a possible bias ascribable to cumbersome structural landmarks was minimized. The variation was further reduced by inclusion of only right-handed subjects, and by use of age-matched populations (no correction for aging effects was, thus, needed).
Left-handedness is more prevalent among HoM compared to HeM, and left-handed subjects are reported to have a less significant cerebral asymmetry than those who are strictly right-handed. A more representative population of HoM with respect to handedness would, therefore, probably show an even more pronounced symmetry. The odds for non-right-handedness are much lower in HoW (24). Consequently, the restriction to right-handed homosexual population in the present study is unlikely to explain the results.
Standard deviations were generally low and not larger in HeW and HoM than in HeM and HoW; failure to detect asymmetry can, thus, not simply be attributed to a larger variability. The present study was not designed to show how possible regional asymmetries were distributed; thus, the symmetrical hemispheric volumes in HeW and HoM could, theoretically, result from multiple regional asymmetries pointing in opposite directions. This possibility does not, however, invalidate the detected group differences.
The amygdala VOIs showed no systematic positioning shifts between the study groups. Neither were there any indications that homo- and heterosexual men and women were engaged in systematically different cognitive processes during the scans, or that they reacted differently to the experimental situation (judged by their reports, and the respiratory measurements). We, therefore, believe that the observed connectivity patterns reflected true biological differences.
Functional connectivity was in the present study calculated during rest when the subjects were lying in the scanner and breathing unscented air (without sniffing). A potential difficulty with cueing the subject to concentrate on breathing room air is that this act may constitute a task and that therefore the functional connectivity may not represent true “resting” condition. However, the major purpose of the present study was to investigate how the rCBF covaries between the amygdala and the rest of the brain during a condition not associated with perceptive, emotional, or cognitive tasks, which could be linked to sexual orientation or behavior. By prompting subjects to concentrate on breathing the room air we aimed to minimize variations caused by spontaneous reflections or judgments. A caveat with such approach is that individual differences in respiratory frequency and amplitude could affect the rCBF in parts of the piriform cortex and cerebellum (25, 26). Calculation of respiratory frequency and amplitude showed, however, no significant group differences (Table S1). Furthermore, respiration-related group differences in calculated functional connectivity would be primarily expected in the ventral tegmentum–pontocerebellar connections and not in the contralateral amygdala, the anterior cingulate, subcallosum, striatum, and frontal cortex. The present findings were also congruent with the major observations of Kilpatrick et al. (21), although they did not report significant connections between the two amygdalae. Bilateral amygdala connections were, however, found in females by Zald and colleagues (27), whereas Irwin et al. (28) failed to detect them in their control material, perhaps because the results were based on a mixed population of males and females.
According to the statistical parametric mapping (SPM) method applied for functional connectivity the material was sufficient to generate inference at group level, implying that each individual was representative for his/her designated group (29, 30). The implications of the data will, therefore, be discussed at the group, and not the individual, level. With respect to MRI measurements it is noteworthy that the degree of asymmetry was in the range of those reported in previous studies, whereas the standard deviations were even smaller, although our material was relatively limited (31–33).
Possible Mechanisms and Implications.
Despite different methodological approaches, most studies suggest that cerebral asymmetry is more pronounced in men. The degree and direction of asymmetry is, however, spatially variable.
In general, the asymmetry is rightward in frontal and temporal lobe and leftward in the posterior temporal and occipitoparietal regions (17), and in both regions more prominent in men (32–37). In addition, in analysis of the cytoarchitecture of the primary visual cortex Amunts et al. (36) recently found right more than left cortical cell density, which was, again, more prominent in men. Among studies explicitly comparing the entire hemispheres most, although not all, suggest that the right hemisphere is larger in men (32, 34, 38, 39). The present findings from MR volumetry fit well with these observations, as well as with anecdotal reports about functional lateralization in homosexual subjects, men in particular. One possibility is that they reflect more pronounced interhemisphere connections in HeW and HoM; women are reported to have a larger anterior commissure than men (40, 41), and Allen and Gorski (9) found that this structure was larger in HoM compared to HeM. Another possibility is that the functions of the two hemispheres are not as sharply differentiated in HeW and HoM.
HeW and HoM displayed more pronounced between-amygdala connections and greater connections with the anterior cingulate, the subcallosum, and the hypothalamus. This connectivity pattern provides a strong substrate for processing of external stimuli that are relayed by the two amygdalae and represents a possible pathway for their functional interconnection in HeW and HoM. The remarkable similarity between HeW and HoM in the connectivity pattern deserves special attention. The amygdala has a key role in emotional reactions to external stimuli, including stress; the subcallosum and the anterior cingulate, on the other hand, are highly involved in mediation of mood and anxiety-related processes (42). Affective disorders are 2–3 times more common in women than men, and the tight functional connections between the amygdala and cingulate in women is currently discussed as a possible neurobiological substrate for their higher vulnerability (43), in addition to the effects of estrogen and testosterone. Interestingly, the incidence of depression and suicide attempts is elevated in homosexual subjects, and HoM in particular (44, 45). Although the underlying mechanisms are likely to be multifactorial and include social pressure, the presently observed similarity with HeW vis-à-vis the amygdala connectivity motivates further evaluations.
The sensorimotor cortex and striatum displayed stronger connections in HeM and HoW. These regions have been associated with attending to and acting into the external environment (the fight and flight reactions). Fight and flight reactions are reported to be more common in men (46, 47).
The mechanisms behind the present observations are unknown. In accordance with discussions about the sexual dimorphism of the brain, three factors have to be taken into account: environmental effects, genetics, and sex hormonal influences.
Sex difference in brain size has been shown to be present at birth (48), and some volumetric data suggest that sex differences in hemispheric asymmetry exist already in the human fetus (49, 50), although other studies failed to detect them (51, 52). Adult patterns of cerebral asymmetry (53), as well some features of regional sexual dimorphism, are detected already in children (54). Cerebral maturation continues after puberty, especially in boys (31), providing a substrate for effects of social/environmental factors. However, to attribute such effects to the present results would require a detailed comprehension of how specific environmental factors relate to the four groups investigated, and how they affect various cerebral circuits. In the light of currently available information this can only be speculative. Of note is that at variance with previous studies in homosexual subjects the present data were not directly dependent on perception or behavior. Thus, although repetitive sex- (or sexual orientation-) specific preferred strategies may, theoretically, have influenced the results, such systematic effects have, to the best of our knowledge, not been reported, and seem unlikely.
As to the genetic factors, the current view is that they may play a role in male homosexuality, but they seem to be insignificant for female homosexuality (55). Genetic factors, therefore, appear less probable as the major common denominator for all group differences observed here.
Mechanisms behind homosexuality are often discussed in terms of an under-exposure to prenatal androgens in HoM and over-exposure in HoW (56). In animal experiments manipulation of testosterone has been shown to alter development of the androgen receptor neurons by influencing anatomical connections and patterns of programmed cell death (57). Interestingly, male rhesus monkeys are found to have more androgen receptors in the right hemisphere, whereas the distribution is symmetrical in females (58). In rats, male cerebral asymmetry is established, in part, by early androgen exposure, because castration at birth blocks the normal rightward brain asymmetry. The symmetry in females, on the other hand, can be reversed to the male pattern by neonatal ovariectomy (59). To what extent these data are relevant for humans remains to be clarified.
The present study does not allow narrowing of potential explanations, which are probably multifactorial, including interplay between pre- and postnatal testosterone and estrogen, the androgen and estrogen receptors, and the testosterone-degrading enzyme aromatase. It nevertheless contributes to the ongoing discussion about sexual orientation by showing that homosexual men and women differed from the same-sex controls and showed features of the opposite sex in two mutually independent cerebral variables, which, in contrast to those studied previously, were not related to sexual attraction. The observations cannot be easily attributed to perception or behavior. Whether they may relate to processes laid down during the fetal or postnatal development is an open question. These observations motivate more extensive investigations of larger study groups and prompt for a better understanding of the neurobiology of homosexuality.
Methods
Subjects.
Twenty-five HeM (age 30 ± 4 years), 25 HeW (age 31 ± 4 years), 20 HoM (age 32 ± 7 years), and 20 HoW (age 31 ± 5 years) were included. All subjects participated in the MR study, and 50 of them (13 HeM, 13 HeW, 12 HoM, and 12 HoW) also participated in the PET studies. All of the subjects were right-handed (60), healthy, and HIV negative. The heterosexual men and women all scored 0, the homosexual men 6, and the homosexual women on average 5.5 on the Kinsey heterosexual/homosexual scale (0 = maximally heterosexual, 6 = maximally homosexual) (61). In addition to scoring themselves on the Kinsey scale (which is based on self-identification), the subjects also participated in interviews regarding three dimensions of sexual orientation (fantasy, romantic attraction, and sexual behavior) over consecutive 5-year historical time periods, from age 16 to the present (5, 62). All decisions about subjects' sexual orientation were made in ignorance of the subjects' PET and MR data. The Ethics Committee of the Karolinska Institutet approved the study.
MRI.
Structural images were acquired on a GE 1.5-T scanner including 3D-weighted T1 SPGR images with 1-mm sections according to a previously described protocol (63). Homologous VOIs were delineated manually by using MRIcro software (www.sph.sc.edu/comd/rorden/mricro.html) on original, unreformatted T1 images by two investigators who were uninformed about the identity of the subjects, their sex, and their sexual identity. The values presented in Results and Table 1 were generated by investigator 1, who analyzed all of the data (investigator 2 analyzed data from 15 subjects in each cohort). The cerebral hemispheres were delineated on every second coronal slice of the individual MR images. The same coronal section was displayed in parallel windows, to avoid overlapping demarcation. Cerebral hemispheres were divided at the midline in the coronal plane by a hand-drawn line connecting the measured midpoint of the corpus callosum with the midpoint of the hypothalamus, third ventricle, cerebral aqueduct, and so on (38). The respective VOI included ventricles and the cortical and subcortical structures, and it ended in the caudal direction at the level of the superior colliculum (Fig. S1). Thus, the subcortical regions, brainstem, and cerebellum were separated from the remaining brain and not included. The cerebellar hemispheres were delineated separately according to Ciumas and Savic (64). Fifteen pairs of randomly selected right and left cerebral and cerebellar hemispheres were chosen for repeated measurements by two independent investigators, to allow assessment of inter-rater and inter-trial reliability.
Asymmetry in hemispheric volumes (right vs. left hemisphere) was first tested in each group with paired t tests (P < 0.0125). Possible group differences were then tested with respect to asymmetry indices [(right side − left side)/right side + left side/2] using one-way ANOVA and Fisher's post hoc test (P < 0.05), separately for the cerebral and cerebellar hemispheres. The inter- and intra-rater variability was calculated by using simple regression (P < 0.05).
PET.
The PET data were, in accordance with the report of Kilpatrick et al. (21), derived from multiple PET studies. These studies were carried out during perception of various odorants presented in glass jars under the nose, and conducted by I.S. and colleagues at the Karolinska University Hospital (4, 5) on a scanner with a spatial resolution (FWHM) of 3.8 mm. During the baseline condition, which was the only condition used for the present analyses, subjects were instructed to relax, with eyes closed and ears plugged, and just breathe the unscented room air while an open and empty jar was presented 10 mm under the nose. Before PET scans subjects were trained to breathe normally without sniffing or hyperventilating. They were asked to restrain from active thinking during the scans, and gave verbal output in how they succeeded after each scan. rCBF was measured by using [15O]H2O during each scan, which lasted for 60 sec. The baseline scan was repeated three times, and always separated from any sensory task scan by at least 10 min, as previously described (4, 5). For detailed description about scanning procedure, see SI Text.
Functional connectivity was defined as the extent to which normalized rCBF in seed VOIs covaried with pixel-based rCBF values across the investigated subjects. The normalized rCBF was extracted from circular (5-mm) VOIs covering the right and left amygdala (center of gravity in Talairach coordinates −18, +2, −16 and −15, +4, −14). These coordinates provided a symmetrical coverage of both amygdalae, as the standard brain is slightly rotated in Talairach space. Significant covariations (T = 3.0, corrected P < 0.05) were calculated by using the entire brain as search space (multisubject condition and covariate analysis within SPM2). Between group comparisons were carried out at T = 3.0, and P < 0.05 uncorrected, hypothesizing more pronounced symmetry in women, and thus more pronounced connections with the contralateral amygdala; furthermore, based on Kilpatrick et al. (21), we expected more pronounced connections with the anterior cingulate and subcallosum in HeW than in HeM, and more pronounced connections with the sensorimotor cortex and the basal ganglia in HeM than in HeW.
Supplementary Material
Acknowledgments.
We thank Dr. Carolina Ciumas for calculations of amygdala connectivity and Lovisa Kasström and Martin Paucar for drawing of VOIs. Financial support was provided by the Swedish Medical Research Council, the Karolinska Institute, and the Center for Gender Related Medicine at the Karolinska Institute.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/cgi/content/full/0801566105/DCSupplemental.
References
- 1.Kranz F, Ishai A. Face perception is modulated by sexual preference. Curr Biol. 2006;16:63–68. doi: 10.1016/j.cub.2005.10.070. [DOI] [PubMed] [Google Scholar]
- 2.Ponseti J, et al. Homosexual women have less grey matter in perirhinal cortex than heterosexual women. PLoS ONE. 2007;2:e762. doi: 10.1371/journal.pone.0000762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Savic I. Processing of odorous signals in humans. Brain Res Bull. 2001;54:307–312. doi: 10.1016/s0361-9230(00)00439-1. [DOI] [PubMed] [Google Scholar]
- 4.Savic I, Berglund H, Lindström P. Brain response to putative pheromones in homosexual men. Proc Natl Acad Sci USA. 2005;102:7356–7361. doi: 10.1073/pnas.0407998102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Berglund H, Lindström P, Savic I. Brain response to putative pheromones in lesbian women. Proc Natl Acad Sci USA. 2006;103:8269–8274. doi: 10.1073/pnas.0600331103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cahill L. Why sex matters for neuroscience. Nat Rev Neurosci. 2006;7:477–484. doi: 10.1038/nrn1909. [DOI] [PubMed] [Google Scholar]
- 7.Hiscock M, et al. Is there a sex difference in human laterality? I. An exhaustive survey of auditory laterality studies from six neuropsychology journals. J Clin Exp Neuropsychol. 1994;16:423–435. doi: 10.1080/01688639408402653. [DOI] [PubMed] [Google Scholar]
- 8.McCormick CM, Witelson SF. Functional cerebral asymmetry and sexual orientation in men and women. Behav Neurosci. 1994;108:525–531. doi: 10.1037//0735-7044.108.3.525. [DOI] [PubMed] [Google Scholar]
- 9.Allen LS, Gorski RA. Sexual orientation and the size of the anterior commissure in the human brain. Proc Natl Acad Sci USA. 1992;89:7199–7202. doi: 10.1073/pnas.89.15.7199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Aboitiz F, et al. The anatomical substrates for language and hemispheric specialization. Biol Res. 1995;28:45–50. [PubMed] [Google Scholar]
- 11.Shaywitz BA, et al. Sex differences in the functional organization of the brain for language. Nature. 1995;373:607–609. doi: 10.1038/373607a0. [DOI] [PubMed] [Google Scholar]
- 12.Miller JW, et al. Gender differences in handedness and speech lateralization related to early neurologic insults. Neurology. 2005;65:1974–1975. doi: 10.1212/01.wnl.0000188900.91741.ea. [DOI] [PubMed] [Google Scholar]
- 13.Rahman Q, Cockburn A, Govier E. A comparative analysis of functional cerebral asymmetry in lesbian women, heterosexual women, and heterosexual men. Arch Sex Behav. 2007 Jan 6; doi: 10.1007/s10508-006-9137-0. [DOI] [PubMed] [Google Scholar]
- 14.Rahman Q, Abrahams S, Wilson GD. Sexual-orientation-related differences in verbal fluency. Neuropsychology. 2003;17:240–246. doi: 10.1037/0894-4105.17.2.240. [DOI] [PubMed] [Google Scholar]
- 15.Wegesin DJ. Relation between language lateralisation and spatial ability in gay and straight women and men. Laterality. 1998;3:227–239. doi: 10.1080/713754303. [DOI] [PubMed] [Google Scholar]
- 16.Rahman Q, Wilson GD. Large sexual-orientation-related differences in performance on mental rotation and judgment of line orientation tasks. Neuropsychology. 2003;17:25–31. [PubMed] [Google Scholar]
- 17.Toga AW, Thompson PM. Mapping brain asymmetry. Nat Rev Neurosci. 2003;4:37–48. doi: 10.1038/nrn1009. [DOI] [PubMed] [Google Scholar]
- 18.McFadden D, Champlin CA. Comparison of auditory evoked potentials in heterosexual, homosexual, and bisexual males and females. J Assoc Res Otolaryngol. 2000;1:89–99. doi: 10.1007/s101620010008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Canli T, et al. Sex differences in the neural basis of emotional memories. Proc Natl Acad Sci USA. 2002;99:10789–10794. doi: 10.1073/pnas.162356599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hamann S, Canli T. Individual differences in emotion processing. Curr Opin Neurobiol. 2004;14:233–238. doi: 10.1016/j.conb.2004.03.010. [DOI] [PubMed] [Google Scholar]
- 21.Kilpatrick LA, et al. Sex-related differences in amygdala functional connectivity during resting conditions. Neuroimage. 2006;30:452–461. doi: 10.1016/j.neuroimage.2005.09.065. [DOI] [PubMed] [Google Scholar]
- 22.Osterlund MK, Hurd YL. Estrogen receptors in the human forebrain and the relation to neuropsychiatric disorders. Prog Neurobiol. 2001;64:251–267. doi: 10.1016/s0301-0082(00)00059-9. [DOI] [PubMed] [Google Scholar]
- 23.Swaab DF. Sexual differentiation of the human brain: relevance for gender identity, transsexualism and sexual orientation. Gynecol Endocrinol. 2004;19:301–312. doi: 10.1080/09513590400018231. [DOI] [PubMed] [Google Scholar]
- 24.Lippa RA. Handedness, sexual orientation, and gender-related personality traits in men and women. Arch Sex Behav. 2003;32:103–114. doi: 10.1023/a:1022444223812. [DOI] [PubMed] [Google Scholar]
- 25.Johnson BN, Mainland JD, Sobel N. Rapid olfactory processing implicates subcortical control of an olfactomotor system. J Neurophysiol. 2003;90:1084–1094. doi: 10.1152/jn.00115.2003. [DOI] [PubMed] [Google Scholar]
- 26.Sobel N, et al. Odorant-induced and sniff-induced activation in the cerebellum of the human. J Neurosci. 1998;18:8990–9001. doi: 10.1523/JNEUROSCI.18-21-08990.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zald DH, Donndelinger MJ, Pardo JV. Elucidating dynamic brain interactions with across-subjects correlational analyses of positron emission tomographic data: The functional connectivity of the amygdala and orbitofrontal cortex during olfactory tasks. J Cereb Blood Flow Metab. 1998;18:896–905. doi: 10.1097/00004647-199808000-00010. [DOI] [PubMed] [Google Scholar]
- 28.Irwin W, et al. Amygdalar interhemispheric functional connectivity differs between the non-depressed and depressed human brain. Neuroimage. 2004;21:674–686. doi: 10.1016/j.neuroimage.2003.09.057. [DOI] [PubMed] [Google Scholar]
- 29.Friston KJ, Penny W. Posterior probability maps and SPMs. Neuroimage. 2003;19:1240–1249. doi: 10.1016/s1053-8119(03)00144-7. [DOI] [PubMed] [Google Scholar]
- 30.Mechelli A, et al. Effective connectivity and intersubject variability: Using a multisubject network to test differences and commonalities. Neuroimage. 2002;17:1459–1469. doi: 10.1006/nimg.2002.1231. [DOI] [PubMed] [Google Scholar]
- 31.Paus T. Mapping brain maturation and cognitive development during adolescence. Trends Cogn Sci. 2005;9:60–68. doi: 10.1016/j.tics.2004.12.008. [DOI] [PubMed] [Google Scholar]
- 32.Yucel M, et al. Hemispheric and gender-related differences in the gross morphology of the anterior cingulate/paracingulate cortex in normal volunteers: An MRI morphometric study. Cereb Cortex. 2001;11:17–25. doi: 10.1093/cercor/11.1.17. [DOI] [PubMed] [Google Scholar]
- 33.Raz N, et al. Regional brain changes in aging healthy adults: general trends, individual differences and modifiers. Cereb Cortex. 2005;15:1676–1689. doi: 10.1093/cercor/bhi044. [DOI] [PubMed] [Google Scholar]
- 34.Kovalev VA, Kruggel F, von Cramon DY. Gender and age effects in structural brain asymmetry as measured by MRI texture analysis. Neuroimage. 2003;19:895–905. doi: 10.1016/s1053-8119(03)00140-x. [DOI] [PubMed] [Google Scholar]
- 35.Luders E, et al. Hemispheric asymmetries in cortical thickness. Cereb Cortex. 2006;16:1232–1238. doi: 10.1093/cercor/bhj064. [DOI] [PubMed] [Google Scholar]
- 36.Amunts K, et al. Gender-specific left-right asymmetries in human visual cortex. J Neurosci. 2007;27:1356–1364. doi: 10.1523/JNEUROSCI.4753-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Good CD, et al. Cerebral asymmetry and the effects of sex and handedness on brain structure: A voxel-based morphometric analysis of 465 normal adult human brains. Neuroimage. 2001;14:685–700. doi: 10.1006/nimg.2001.0857. [DOI] [PubMed] [Google Scholar]
- 38.Filipek PA, et al. The young adult human brain: An MRI-based morphometric analysis. Cereb Cortex. 1994;4:344–360. doi: 10.1093/cercor/4.4.344. [DOI] [PubMed] [Google Scholar]
- 39.Barrick TR, et al. Automatic analysis of cerebral asymmetry: an exploratory study of the relationship between brain torque and planum temporale asymmetry. Neuroimage. 2005;24:678–691. doi: 10.1016/j.neuroimage.2004.09.003. [DOI] [PubMed] [Google Scholar]
- 40.Zaidel E, Aboitiz F, Clarke J. Sexual dimorphism in interhemispheric relations: anatomical-behavioral convergence. Biol Res. 1995;28:27–43. [PubMed] [Google Scholar]
- 41.Allen LS, et al. Sex differences in the corpus callosum of the living human being. J Neurosci. 1991;11:933–942. doi: 10.1523/JNEUROSCI.11-04-00933.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Phillips ML, et al. Neurobiology of emotion perception II: Implications for major psychiatric disorders. Biol Psychiatry. 2003;54:515–528. doi: 10.1016/s0006-3223(03)00171-9. [DOI] [PubMed] [Google Scholar]
- 43.Wang J, et al. Gender difference in neural response to psychological stress. Soc Cogn Affect Neurosci. 2007;2:227–239. doi: 10.1093/scan/nsm018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Fergusson DM, Horwood LJ, Beautrais AL. Is sexual orientation related to mental health problems and suicidality in young people? Arch Gen Psychiatry. 1999;56:876–880. doi: 10.1001/archpsyc.56.10.876. [DOI] [PubMed] [Google Scholar]
- 45.Wang J, et al. High prevalence of mental disorders and comorbidity in the Geneva Gay Men's Health Study. Soc Psychiatry Psychiatr Epidemiol. 2007;42:414–420. doi: 10.1007/s00127-007-0190-3. [DOI] [PubMed] [Google Scholar]
- 46.Klein LC, Corwin EJ. Seeing the unexpected: How sex differences in stress responses may provide a new perspective on the manifestation of psychiatric disorders. Curr Psychiatry Rep. 2002;4:441–448. doi: 10.1007/s11920-002-0072-z. [DOI] [PubMed] [Google Scholar]
- 47.Korte SM, et al. The Darwinian concept of stress: Benefits of allostasis and costs of allostatic load and the trade-offs in health and disease. Neurosci Biobehav Rev. 2005;29:3–38. doi: 10.1016/j.neubiorev.2004.08.009. [DOI] [PubMed] [Google Scholar]
- 48.Dekaban AS. Changes in brain weights during the span of human life: Relation of brain weights to body heights and body weights. Ann Neurol. 1978;4:345–356. doi: 10.1002/ana.410040410. [DOI] [PubMed] [Google Scholar]
- 49.de Lacoste MC, Horvath DS, Woodward DJ. Possible sex differences in the developing human fetal brain. J Clin Exp Neuropsychol. 1991;13:831–846. doi: 10.1080/01688639108405101. [DOI] [PubMed] [Google Scholar]
- 50.Chi JG, Dooling EC, Gilles FH. Left-right asymmetries of the temporal speech areas of the human fetus. Arch Neurol. 1977;34:346–348. doi: 10.1001/archneur.1977.00500180040008. [DOI] [PubMed] [Google Scholar]
- 51.Gilmore JH, et al. Regional gray matter growth, sexual dimorphism, and cerebral asymmetry in the neonatal brain. J Neurosci. 2007;27:1255–1260. doi: 10.1523/JNEUROSCI.3339-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Hering-Hanit R, et al. Asymmetry of fetal cerebral hemispheres: In utero ultrasound study. Arch Dis Child Fetal Neonatal Ed. 2001;85:F194–F196. doi: 10.1136/fn.85.3.F194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Caviness VS, Jr, et al. Regulation of normal proliferation in the developing cerebrum potential actions of trophic factors. Exp Neurol. 1996;137:357–366. doi: 10.1006/exnr.1996.0037. [DOI] [PubMed] [Google Scholar]
- 54.Giedd JN, et al. Quantitative magnetic resonance imaging of human brain development: Ages 4–18. Cereb Cortex. 1996;6:551–560. doi: 10.1093/cercor/6.4.551. [DOI] [PubMed] [Google Scholar]
- 55.Lyons MJ, et al. A twin study of sexual behavior in men. Arch Sex Behav. 2004;33:129–136. doi: 10.1023/b:aseb.0000014327.11433.66. [DOI] [PubMed] [Google Scholar]
- 56.Ellis L, Burke D, Ames MA. Sexual orientation as a continuous variable: a comparison between the sexes. Arch Sex Behav. 1987;16:523–529. doi: 10.1007/BF01541716. [DOI] [PubMed] [Google Scholar]
- 57.Chung WC, Swaab DF, De Vries GJ. Apoptosis during sexual differentiation of the bed nucleus of the stria terminalis in the rat brain. J Neurobiol. 2000;43:234–243. [PubMed] [Google Scholar]
- 58.Sholl SA, Kim KL. Androgen receptors are differentially distributed between right and left cerebral hemispheres of the fetal male rhesus monkey. Brain Res. 1990;516:122–126. doi: 10.1016/0006-8993(90)90905-q. [DOI] [PubMed] [Google Scholar]
- 59.Diamond MC. Hormonal effects on the development or cerebral lateralization. Psychoneuroendocrinology. 1991;16:121–129. doi: 10.1016/0306-4530(91)90074-4. [DOI] [PubMed] [Google Scholar]
- 60.Oldfield RC. The assessment and analysis of handedness: The Edinburgh inventory. Neuropsychologia. 1971;9:97–113. doi: 10.1016/0028-3932(71)90067-4. [DOI] [PubMed] [Google Scholar]
- 61.Kinsey AC, Pomeroy WR, Martin CE. Sexual Behavior in the Human Male. Philadelphia: Saunders; 1948. [Google Scholar]
- 62.Chung YB, Katayama M. Assessment of sexual orientation in lesbian/gay/bisexual studies. J Homosex. 1996;30:49–62. doi: 10.1300/J082v30n04_03. [DOI] [PubMed] [Google Scholar]
- 63.Ciumas C, Savic I. Structural changes in patients with primary generalized tonic and clonic seizures. Neurology. 2006;67:683–686. doi: 10.1212/01.wnl.0000230171.23913.cf. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.